Abstract
Context
Evidence-based treatment trials for adolescents with anorexia nervosa are few.
Objective
To evaluate the relative efficacy of family-based treatment (FBT) and adolescent focused individual therapy (AFT) for adolescents with anorexia nervosa on full remission.
Design
Randomized controlled trial.
Setting
Stanford University and The University of Chicago (April 2005 until March 2009)
Participants
One hundred and twenty one participants, ages 12 through 18 years with DSM-IV diagnosis of anorexia nervosa except for not requiring ammenorhea.
Interventions
Twenty four outpatient hours of treatment over 12 months of FBT or AFT. Participants were assessed at baseline, end of treatment (EOT), 6 months and 12 months follow-up post treatment.
Main outcome measures
Full remission from anorexia nervosa defined as normal weight (>95% of expected gender, age, weight for height) and mean global Eating Disorder Examination (EDE) score within 1 standard deviation of published means. Secondary outcome measures included partial remission rates (>85% of expected weight for height plus those who were fully remitted) and changes in Body Mass Index (BMI) percentile and eating related psychopathology.
Results
There were no differences in full remission between treatments at EOT. However, at both 6 and 12 month follow-up FBT was significantly superior to AFT on this measure. FBT was significantly superior for partial remission at EOT but not at follow-up. In addition, BMI percentile at EOT was significantly superior for FBT, but this effect was not found at follow-up. Participants in FBT also had greater changes on the EDE at EOT than those in AFT, but there were no differences at follow-up.
Conclusions
Although both treatments led to considerable improvement and were similarly effective in producing full remission at EOT, FBT was more effective in facilitating full remission at both follow-up points.
Clinical Trials Registry
Effectiveness of Family-Based Versus Individual Psychotherapy in Treating Adolescents With Anorexia Nervosa (NCT00149786)
Anorexia Nervosa (AN) with an incidence rate of 73.9 per 100,000 and a prevalence among adolescent females of 0.48%- 0.70% is a serious disorder affecting both psychological and physical health.1-4 Physical health impacts in adolescents include growth retardation, pubertal delay or interruption, and peak bone mass reduction.5 The aggregate mortality rate of AN is approximately 5.6% per decade,6, 7 with about half of the deaths due to cardiac failure and half suicide. Common co-morbid psychological conditions are depressive disorders, anxiety disorders, including obsessive-compulsive disorder, and personality disorders.8-12
Although various forms of individual and family therapy are used in the treatment of adolescents with AN, most have not been systematically examined.13 Hence there is little guidance for providing evidence based interventions for either adolescents or adults with AN.13 For adolescents with AN, there are only six randomized clinical trials (RCTs) published to date.14-19 One model of a commonly used psychological approach—adolescent focused individual therapy (AFT)--is a psycho-dynamically informed individual psychotherapy focusing on enhancing autonomy, self-efficacy, individuation and assertiveness while also including collateral parent meetings to support individual treatment.17, 20 This model was examined in one modest clinical trial that suggested that the approach was likely effective.17 Another approach is a family-based treatment (FBT) that promotes parental control of weight restoration while enhancing familial functioning as it relates to adolescent development.15, 16, 19, 21-24 Two small studies suggest that FBT may be more efficacious than individually-based therapy.14, 17
The purpose of the current study was to conduct an RCT comparing these two outpatient psychosocial treatments for adolescents with AN. We hypothesized that FBT, by empowering parents to directly address the behaviors maintaining weight loss in their children would be more effective than the individually-based psychological approach (AFT) in normalizing weight and psychological processes associated with AN. Our primary outcome was full remission from AN defined as having achieved an ideal body weight (IBW) of greater than 95% expected for gender, age, and height25 and an Eating Disorder Examination (EDE) global score within one standard deviation of community norms.26 Secondary outcomes were rates of partial remission (all participants with weights > than 85% IBW expected for height, gender, and age), changes in percent BMI adjusted for age and gender, and changes in EDE.
PATIENTS AND METHODS
Design
This two site study (The University of Chicago and Stanford University) randomized 121 participants to either FBT or AFT. Randomization was performed separately for each site by a biostatistician in the Data and Coordinating Center (DCC) under independent management from either intervention site. Efron’s biased coin design was used to balance treatment within sites. Participants were stratified within sites based on current use of psychiatric medication.27 Participants were assigned to therapists who conducted both forms of treatment to control for non-specific therapist effects. Therapists were 5 PhD psychologists and 2 child psychiatrists all with previous experience treating eating disorders. Three 2-day workshops were held to train therapists in manualized FBT and AFT. The first workshop was held prior to beginning recruitment, the second six months after the first participants were randomized, and the third workshop was held one year later. Experts, who are also authors of this report (JDL, DLG for FBT, AM for AFT), trained the therapists and supervised them weekly. Therapists treated 3 pilot cases satisfactorily with each treatment prior to treating randomized cases. This study protocol was approved by the institutional review boards at the respective sites. Treatment took place in clinics for children and adolescent eating disorders located at each university.
Participants
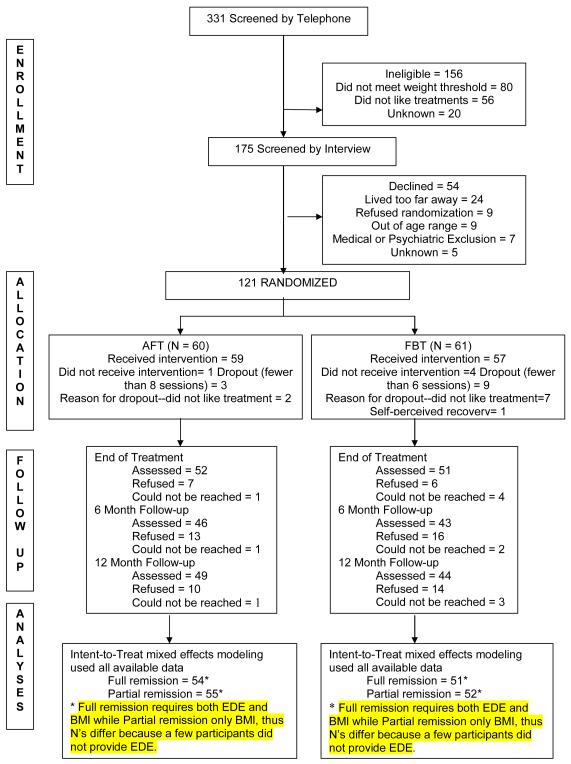
Participants were recruited from October 2004 through March 2007 by advertising to clinicians, organizations and clinics treating eating disorders. After telephone screening (N= 331) to determine eligibility, 175 (53%) were invited for an assessment interview (see Figure 1). The study was described in detail to participants and parents and consent obtained (assent for adolescents younger than 18 years of age) before assessments were conducted. Participants were eligible if they were between the ages of 12 and 18 years, living with their parents, or legal guardians, and met the DSM-IV criteria for AN excluding the amenorrhea criterion.28, 29 Weight thresholds (IBW < 86%) for study entry were calculated using the CDC weight charts, growth curve trajectories and Metropolitan Life charts.25, 30 Participants meeting the binge eating and purging subtype and adolescents on a stable dose of antidepressant or anxiolytic medications for a period of two months who still met entry criteria were eligible. Participants were excluded from the study if there was a current psychotic disorder, dependence on drugs or alcohol, physical condition known to influence eating or weight (e.g. diabetes mellitus, pregnancy), or previous treatment with FBT or AFT. Seven potential participants were excluded for medical or psychiatric reasons. Both adolescent participants and their families were required to be available for the one year treatment duration. Sixty-nine percent (121) of eligible participants agreed to randomization.
FIGURE 1.
CONSORT DIAGRAM
Treatments
Family-Based Treatment (FBT)
FBT is a 3 phase treatment.31 In the first phase therapy is characterized by attempts to absolve the parents from the responsibility of causing the disorder, and by complimenting them on the positive aspects of their parenting. Families are encouraged to work out for themselves how best to help restore the weight of their child with AN. In Phase 2, parents are helped to transition eating and weight control back to the adolescent in an age appropriate manner. The third phase focuses on establishing of a healthy adolescent relationship with the parents. Twenty-four one hour sessions were provided over the one year period.
Adolescent Focused Therapy (AFT)
AFT (originally described by Robin et al., as Ego-Oriented Individual Therapy) posits that individuals with AN manifest ego deficits and confuse self-control with biological needs.17, 20 Patients learn to identify and define their emotions, and later, to tolerate affective states rather than numbing themselves with starvation. In Phase 1, the therapist establishes rapport, assesses motivation and formulates the patient’s psychological concerns. The therapist actively encourages the patient to stop dieting and to gain weight by setting weight goals and emphasizing the need to change these behaviors. The importance of weight gain is discussed and actively encouraged throughout treatment until the patient is weight restored. The therapist interprets behavior, emotions, and motives, and helps the patient distinguish emotional states from bodily needs and asks the patient to accept responsibility for food related issues as opposed to relinquishing authority to others (e.g. parents). Phase 2 focuses on encouraging separation and individuation and increasing the ability to tolerate negative affect. Phase 3 focuses on termination. AFT sessions are 45 minutes in duration for a total of 32 sessions over the treatment year (24 contact hours). Collateral meetings are held with parents alone to assess parental functioning, advocate for the patient’s developmental needs, and update parents on progress are included in this treatment model. Up to eight sessions were used for this purpose.
Assessment and Procedures
Assessment included diagnostic evaluation for co-morbid psychiatric disorders, weight, and eating disorder related symptoms and psychopathology. There were 4 assessment points: pre-treatment, end of treatment (EOT), 6 and 12 month follow up. Independent assessors not involved in treatment delivery conducted all assessments.
Measures
An a priori definition of full remission used in this study is the proportion of participants that achieve a combination of a minimum of 95% of expected IBW for gender, age, and height as determined by CDC growth charts25 (http://www.cdc.gov/growthcharts/percentile_data_files.htm) and scores within 1 standard deviation from global mean EDE published norms (1.59). 32, 33 26 Normalization of weight in this range approximates the typical set point for menstrual return in most females, the weight where growth is likely to resume, and the weight where bone loss may begin to be reversed.34-37 The normalization of the global EDE score to 1 standard deviation (SD) of community norms sets the risk related to eating and weight concerns to community averages.38 Partial remission rates included all participants who achieved a weight >85% of expected IBW for age, height and gender and therefore also includes those who achieved full remission as well as those with weight greater than 95% IBW but with elevated EDE scores. This definition of partial remission is similar to “intermediate outcome” using Morgan-Russell criteria. and is reported here to allow comparison to other studies of adolescent AN.14, 16-18
Eating Disorder Examination (EDE).39
The EDE is a standardized validated investigator-based interview that measures the severity of the characteristic psychopathology of eating disorders in adolescents including the frequency of key behaviors and the severity of psychopathology.40-41
Weight
Weight and height was assessed before every therapy session in both treatment protocols. For all major assessments, the participant was weighed in a hospital gown on a balance beam scale that was regularly re-calibrated. BMI percentiles, adjusted for age and gender were used as the outcome measure (http://www.cdc.gov/growthcharts/percentile_data_files.htm).42, 43 BMI percentiles below 10% are considered to be consistent with anorexia nervosa.44 An average BMI percentile of 50 would be the expected average in a group of normally developing adolescents.
Schedule for Affective Disorders and Schizophrenia for School-Aged Children (6-18 years) (K-SADS).45
The K-SADS-PL is a widely used interview for detecting psychiatric disorders in children and adolescents. Both parents and adolescents were interviewed to achieve summary ratings.
Participant Safety
Participants were assessed at approximately weekly intervals throughout the study by physicians with extensive experience in medical treatment of adolescents with AN. If a participant became medically unstable (hypothermic (< 36.3°), bradycardic (< 50 or QTc >0.45), orthostatic (pulse increase > 35, systolic blood pressure decrease greater than 10 mm hg) or weight fell below 75% IBW), hospitalization for medical stabilization was required according to the guidelines of the Society of Adolescent Medicine and the American Academy of Pediatrics.46
Statistical Analyses
Statistical analysis for this study was performed by the DCC. Sample size calculation was based on prior studies.19 We calculated that a sample of 120 participants, 60 per site, 30 per site in each treatment group and employing a 5% two-tailed test would yield 84% power to detect a moderate main effect (Cohen’s d of .5). The primary outcome analysis was based upon the intent-to-treat principle and utilized the definitions of full remission and partial remission described above.
For the analyses of repeated measures, we employed a method widely known as mixed effects modeling or growth modeling.47-50 We used maximum likelihood estimation implemented in Mplus, which is a widely used program for statistical modeling with latent variables.51 The mixed effects analyses were conducted including all data from individuals in the sample (see Figure 1). The fully remitted or partially remitted (0 = no; 1 = yes) at three assessment points (0, 6, 12 months) were used as repeated measures in the analyses. We treated these repeated measures as categorical in the analyses and allowed for nonlinear trend across the three time points. As predictors of longitudinal trends of remission, we used treatment assignment status (FBT = 0.5; AFT = −0.5), site (Site 1 = 0.5; Site 2 = −0.5), treatment by site interaction (i.e., Treatment × Site), and the baseline EDE (centered at the mean to control for baseline differences on this variable as indicated on Table 1). Based on mixed effect model estimates, the difference between FBT and AFT conditions in terms of the remission status at each follow-up point was calculated. This method was chosen instead of reporting the overall rate of change given that there was no variation at baseline (i.e., nobody was remitted). Longitudinal trends of full and partial remission rates, based on the observed means are shown in Figure 2.
TABLE 1.
Demographics and Baseline Clinical Characteristics
| Chicago | Stanford | TOTAL | ||||
|---|---|---|---|---|---|---|
| AFT | FBT | AFT | FBT | AFT | FBT | |
| Age1 | 14.7(1.6) | 14.4(1.8) | 14.8(1.4) | 13.8(1.6) | 14.7(1.5) | 14.1(1.7) |
| Comorbidity Depression disorders Anxiety disorders OCD ADHD PTSD Phobia Tic Adjustment disorder |
9 (31%) 5 2 2 1 1 0 0 0 |
4(12%) 4 2 0 0 0 0 0 0 |
10(32%) 5 2 2 0 1 0 0 1 |
8(28%) 6 2 1 1 0 0 1 0 |
32% 10 4 4 1 2 0 0 1 |
20% 10 4 1 1 0 0 1 0 |
| Duration of illness ( in months) |
8.9 (7.8) |
11.6 (8.5) |
11.6 (9.5) |
13.0 (8.6) |
10.3 (8.7) |
12.3 (8.5) |
| Ethnicity Asian Black Caucasian Hispanic Other % minority2 |
0 (0%) 0 (0%) 27(93%) 1(3%) 1(3%) 2 (7%) |
1(3%) 0(0%) 27(84%) 3(9%) 1(3%) 5(16%) |
6(19%) 1(3%) 20(64%) 2(6%) 2(6%) 11(35%) |
6(21%) 0(0%) 18(62%) 3(10%) 2(7%) 11(38%) |
6(10%) 1(2%) 47(78%) 3(5%) 3(5%) 13(22% |
7(12%) 0(0%) 45(74%) 6(10%) 3(5%) 16(26%) |
| Gender % male |
3(10%) |
4(12%) |
1(3%) |
3(10%) |
4(7%) |
7(11%) |
| Intact family3 | 23(79%) | 30(94%) | 23(74%) | 19(66%) | 46(77%) | 49(80%) |
| Medication use4 | 9(31%) | 8(25)% | 2(6%) | 1(3%) | 11 (18%) | 9(15%) |
| Parent education (years)5 |
17.8(2.6) | 16.3(2.6) | 16.1(3.3) | 17.1(2.6) | 17.0(3.1) | 16.7(2.6) |
| Previous Hospitalizations 6 |
7(24%) | 6(19%) | 22(71%) | 19(66%) | 29(48%) | 25(41%) |
| BMI percentile for age and sex |
5.3 (7.6) | 7.7 (9.2) | 5.0 (7.6) | 6.8(5.5) | 5.2(7.55) | 7.2 (7.6) |
| Global EDE 7 | 2.0(1.6) | 1.7(1.2) | 2.1(1.5) | 1.3(1.4) | 2.1(1.5) | 1.5(1.3) |
| Sample Size | 29 | 32 | 31 | 29 | 60 | 61 |
Treatment: F(1,117)=4.6 p=.04
Center: F(1,117)=11.4 p=.001
Center: F(1,117)=5.1 p=.03
Center: F(1,117)=12.5 p=.001
Center X treatment F(1,117)=6.1 p=.02
Center: F(1,117)=33.5 p=.000
Treatment F(1,117)=10.1,p=.03
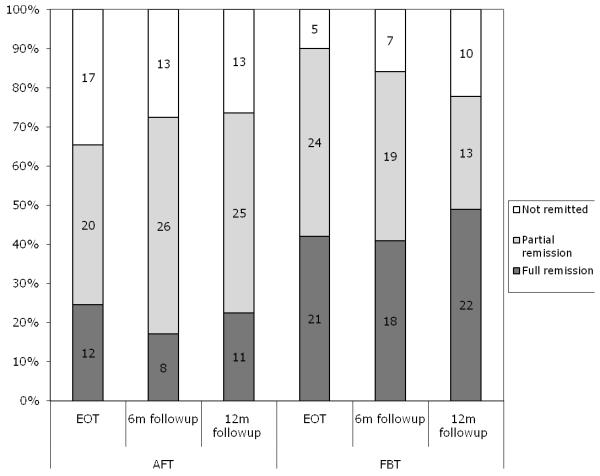
Figure 2.
Observed Partial and Full Remission Rates by Treatment Assignment (End of Treatment N=49 (AFT); N=50 (FBT); 6 Month Follow-up N=47 (AFT), N=44 (FBT); 12 Month Follow-up N=49 (AFT), N=45 (FBT))
Analysis of continuous outcomes (BMI age-and-gender-adjusted percentiles and global EDE) used a similar approach: mixed modeling was used to estimate the treatment differences at each time point using treatment, site and the interaction as predictors and controlling for the baseline values.
Treatment and site differences for participant characteristics, dropout status, assessment completion, were calculated using a 2 way ANOVA with site, treatment and their interaction as independent measures. Nonparametric measures such as number of minutes of therapy and number of days of hospitalization were compared using Mann- Whitney U statistic.
Effect size in this study is reported as Number Needed to Treat (NNT). NNT is defined as the number of patients one would expect to treat with one treatment to have one more success than if they had all been treated with the other treatment. Equivalent treatments result in an NNT of 1. The larger the NNT, the less effective the treatment is in comparison. Kraemer et al. (2003) report NNT values of 8-9 as small, 3-6 as medium and 2-3 as large, in correspondence to the cut-points referenced by Cohen for effect size.52 For categorical variables NNT is the reciprocal of the percent difference between groups. For continuous measures, NNT is calculated according to standard formulas.53, 54
RESULTS
Participant Characteristics
Participants averaged 14.4 (SD 1.6) years of age with a mean ideal body weight of 82% and body mass index (BMI) of 16.1 (SD 1.1) using CDC growth charts. The majority of the sample was female (91%) with a mean duration of illness of 11.3 (SD 8.6) months. Twenty-six percent (N = 31) of the participants reported a current co-morbid psychiatric disorder by KSADS and 17% (N=20) were taking psychotropic medications at baseline. Seventy-nine percent (N=95) were from intact families. Twenty-four percent of the participants were ethnic minorities (self-reported). Forty-five percent (N=54) had been hospitalized for AN or medical problems associated with AN prior to randomization (see Table 1).
Randomization
There were few differences between treatment groups on baseline sociodemographic variables, however the global EDE was significantly higher in AFT (Table 2) and participants in FBT were slightly younger than those in AFT. Site differences included significantly more ethnic minorities at Stanford; higher rates of baseline medication use at Chicago, higher rates of intact families at Chicago and higher rates of previous hospitalization at Stanford.
Table 2.
Change in Outcome Over Time (Remission Status, Weight (BMI percentile), and Eating Related Psychopathology) Based on Mixed Effects Model Estimates
| Measure | Baseline Adjusted Estimated Mean and Standard Error |
Baseline Adjusted Mean Difference (FBT-AFT) and 95 % Confidence Intervals |
Test of Significance (t-values)3 |
P value | Number- Needed-to- Treat (Effect Size) |
|
|---|---|---|---|---|---|---|
| AFT | FBT | |||||
| Full remission 1 | ||||||
| End of Treatment | 22.6% (5.7) | 41.8% (6.7) | 19.3 % (−0.2, 41.0) | t(104)=1.9 | .055 | 5 |
| 6 month follow-up | 18.3% (5.2) | 39.9% (7.0) | 21.6 % (1.6, 44.7) | t(104)=2.2 | .029 | 5 |
| 12 month follow-up | 23.2% (5.7) | 49.3% (7.2) | 26.2 % (4.8, 47.7) | t(104)=2.5 | .024 | 4 |
| Partial remission 2 | ||||||
| End of Treatment | 66.9% (7.4) | 89.1% (9.3) | 22.2% (3.9, 30.3) | t(106)=2.3 | .023 | 5 |
| 6 month follow-up | 73.7% (7.9) | 82% (8.6) | 8.3% (−9.5, 19.2) | t(106)=1.0 | .316 | 12 |
| 12 month follow-up | 75.3% (7.6) | 77.7% (8.9) | 2.3% (−16.3, 14.9) | t(106)=0.3 | .779 | 43 |
| BMI percentile for age and gender |
||||||
| End of Treatment | 23.4 (2.8) | 31.4 (2.8) | 8.0 (0.1, 15.9) | t(117)=2.0 | .048 | 5 |
| 6 month follow-up | 29.1 (3.4) | 31.4 (3.5) | 2.3 ( −7.4, 12.0) | t(117)=0.5 | .640 | 19 |
| 12 month follow-up | 29.0 (3.4) | 32.2 (3.4) | 3.2 ( −6.4, 12.8) | t(117)=0.7 | .510 | 14 |
| Eating Disorder Examination |
||||||
| End of Treatment | 1.20 (0.15) | 0.71 (0.16) | −0.49 ( −0.93, −0.06) | t(117)=−2.2 | .027 | 4 |
| 6 month follow-up | 1.01 (0.16) | 0.78 (0.17) | −0.24 (−0.70, 0.22) | t(117)=−1.0 | .307 | 10 |
| 12 month follow-up | 1.04 (0.16) | 0.79 (0.16) | −0.25( −0.69, 0.19) | t(117)=1.1 | .263 | 9 |
only those that achieved 95% IBW adjusted for age and gender and Total EDE within 1 SD of normal
> 85% IBW for adjusted for age, gender, —includes those who achieved full remission
The t-values reported are calculated by dividing the mixed effects model estimates by estimated standard errors (both based on maximum likelihood estimation). Corresponding p-values can be obtained from the standard t distribution critical value table.
Treatment delivery and study retention
Treatment time did not differ between groups. Participants assigned to FBT completed 84% of total therapy time compared with 92% for AFT. We used treatment time rather than number of sessions in this analysis because sessions were not equal in duration in both treatments (60 minute sessions for FBT and 45 minute sessions in AFT).The Spearman correlation between treatment time in each group and full remission was not significant. Study dropout (failure to complete study assessment) was 14% at EOT and 22% at follow-up (see Figure 1 for details). There was a significant difference in assessment follow-up rates between the two intervention sites at all time points (End of Treatment W(1) = 4.0, p = .046; 6 month follow-up W(1) = 10.6, p = .001; 12 month follow-up W(1) = 7.9, p = .005) with one site completing 68% and the other 89% of planned assessments.
Hospitalization during the treatment phase
More participants were hospitalized in AFT (N=32, 37%) than FBT (N=9, 15%) (W(1)=1.4, p=.020). For those hospitalized, the median number of days until first hospitalization was 17 days for AFT and 32 days for FBT, but there was not a significant difference between the groups. Fifty nine percent (13/22) of AFT and 44% (4/9) of FBT hospitalizations were in the 1st 4 weeks of treatment. Stanford had higher hospitalization rates than Chicago (43% compared to 8% W(1)=13.1, p = .000). The median number of days in hospital was 10 for AFT and 12 for FBT, and weight gain while in hospital was a median of 1.7 kg for AFT and 1.0 kg for FBT. Three hospitalizations were related to suicidal thoughts or behavior and the remainder were for medical stabilization.
Outcomes
Based on mixed effects analysis estimates, full remission rates between treatments (see Figure 2 and Table 2) did not differ statistically at EOT (FBT = 42%; AFT =23%, p = .055, Number Needed to Treat (NNT) = 5 ); however, at 6 month follow-up (FBT = 40%, AFT = 18%, p =.029, NNT = 5) and 12 month follow-up (FBT =49%, AFT = 23%, p = .024, NNT = 4), FBT was statistically superior to AFT. Rates of partial remission (see Figure 2 and Table 2) were greater in FBT than AFT at EOT (FBT = 89%, AFT = 67%, p= .023, NNT= 5), but did not differ at follow-up.
Treatment effects on age and gender adjusted BMI percentile were greater in FBT than AFT (mean difference=8.0, CI= −0.1,15.9, p = .048, NNT =5) at EOT, but not at follow-up. Treatment effects on EDE were greater in FBT than AFT (mean difference=−0.49, CI= −0.93,−0.06, p= .030, NNT= 4) at EOT, but not at follow-up (see Table 2).
Of the 33 subjects who achieved full remission at EOT, 29 (10 AFT, 19 FBT) were also assessed at the 12 month follow-up. Six out of the 29 had relapsed 1 year after EOT: 2 (10%) from FBT and 4 (40%) from AFT. Of the 77 subjects who achieved partial remission at EOT, 71 (31 AFT and 40 FBT) were available for assessment at 12 month follow-up. Nine of the 71 had relapsed by 12 month follow-up: 7 (18%) from FBT and 2 (6%) from AFT. Relapse rates cannot be detected on Figure 2 as the numbers and percentages reported at follow-up time points are totals that include newly remitted subjects as well as those that remained remitted from EOT.
There were no significant site by treatment interaction effects on the primary or secondary outcomes.
During the follow-up period, 50 subjects (29 AFT, 21 FBT) received additional therapy in the community. In AFT 29 (57%) subjects received Individual Therapy, 9 (18%) subjects received Family Therapy and 9 (18%) had ED-related hospitalizations. In FBT, 18 (38%) subjects received individual therapy, 8 (17%) received family therapy and 4(8%) were hospitalized for an ED-related condition. There were no significant differences between the two treatments.
COMMENT
Among the strengths of this study were the relatively large sample size, use of manualized, treatments, and therapists trained in both approaches through workshops and supervision by experts.31, 55 Assessments were conducted independent of treatment and utilized well characterized measures. Treatment attrition and study dropout were relatively low. In addition, we employed growth curve modeling in our analyses to avoid the restrictive assumptions of repeated measures analysis and to make use of all available data without listwise deletion of data. This also allowed us to avoid parameter biases inherent in last observation carried forward methods.56, 57 We employed clinically meaningful thresholds for full and partial remission. In addition, we utilized age and gender adjusted BMI percentiles appropriate for analyzing weight outcomes in this age group.42
Both treatments led to considerable improvements with no difference on the primary outcome variable, full remission, at EOT, though the moderate NNT (5) suggest that the failure to detect a statistical superiority for FBT may have been due to limited power. There were also no differences between the two groups on treatment dropout, average amount of treatment received, or utilization of treatment after the end of treatment. During the follow-up period, however, FBT became statistically superior to AFT. This may have been due in part to differences in relapse from full remission, 10% for FBT and 40% for AFT as well a more subjects reaching full remission thresholds in FBT. Weight gain appeared faster for FBT as assessed by age and gender adjusted BMI percentile though this effect was no longer found at follow-up. FBT participants were also hospitalized significantly less often.
The results of this study can be compared to the two previous studies comparing FBT to individually-based therapies. The first study14 is best understood as a relapse prevention trial because all participants in the adolescent cohort comparable to those in our study (N = 21) were treated in hospital to approximately 90% IBW prior to receiving either FBT or individual therapy.14 Initially both groups of patients lost considerable weight; however, those who received FBT did not lose as much and regained weight faster and to a greater degree than those in individual therapy. At the end of one year of outpatient treatment, the mean IBW of the group assigned to FBT was 92.8% (±8) while the individual therapy group had a mean IBW of 80.1% (±15). Sixty percent of the adolescents who received FBT were in the “good” Morgan Russell (MR) outcome group that requires weight to 85% IBW, menstruation, and psychological improvement58 (similar to our full remission group) while 90% were in either the “good” or “intermediate” group (similar to the partial remission group used here) at EOT. For those participants assigned to individual therapy, 10% were in the MR good outcome group and 20% were in the MR intermediate group by percent IBW. It is noteworthy that the individual treatment used by Russell and colleagues was supportive in nature and not specifically tailored to adolescents.14 This may account for the better performance of AFT in our study.
Robin and colleagues in a study of 37 adolescents with AN compared a family therapy similar to FBT (Behavioral Family Systems Therapy)59 to a more adolescent focused individual therapy (Ego-Oriented Individual Therapy--EOIT) similar to AFT.17 The current study’s findings are consistent with those in Robin et al (1999). FBT was found to be superior in promoting weight gain and menstrual return both at EOT and at follow-up. A small majority (52.6%) of those in family therapy and 41.2% of participants in individual therapy achieved the 50th percentile BMI (the outcome closest to full remission used here) at EOT. At one year follow-up, the percent that reached this threshold was 66.7% in family therapy and 46.7% in EOIT. In this moderately scaled study, significant differences were not found on any of these categorical outcomes.
Although FBT outperformed AFT on several important clinically significant measures, AFT “caught up” in terms of age and gender adjusted BMI percentile and global EDE scores during the follow-up period. From a clinical perspective, there are cases where parents are unwilling or unable to participate in FBT where AFT would likely be a good alternative. Further, there are few providers who practice FBT and dissemination of this treatment remains a challenge in non specialized treatment settings. It is noteworthy that although AFT is primarily an individually based therapy, it involves parent meetings without the child to support the goals of the individual sessions as would be usual in most child or adolescent psychiatric treatment.55, 60
Despite considerable improvements in many participants in the study, a substantial portion of participants remained clinically concerning either in terms of low weight or continued eating related cognitions or both. Future studies should address how to improve outcomes for these groups. On the other hand, relapse, especially weight relapse is a common problem in the treatment of AN;61-63 therefore, one of the most important findings of this study is the low rate of relapse (10%) from full remission in FBT.19
Most of the limitations of the current study are those commonly found in RCTs of treatment. The sample size, though comparatively large for adolescent AN studies, remains modest. Participants were recruited from referrals to university-based treatment centers for child and adolescent eating disorders. Participants, though meeting diagnostic criteria by weight for AN, were not severely under weight at the start of treatment and may therefore differ from some community samples. Availability of expert medical consultation, medical surveillance of participants during treatment, medical hospitalization for acutely medically ill participants, and expectation effects of participation in a treatment study may also have contributed to outcome. The study was undertaken in research centers known for work in FBT and a possible bias because of this could have effected results despite efforts to limit this possibility through the study design and an independent data center. The study follow-up is limited to only 12 months post treatment. A longer-term follow-up of the participants would determine if the effects of the treatment are maintained or if additional differences between them emerge over time.14, 17
The findings of this study together with the existing smaller-scale studies, suggests that FBT is superior to AFT for adolescent AN, though AFT remains an important alternative treatment for families that would prefer a largely individual treatment 64 Additional studies are needed comparing FBT to other credible treatments, including cognitive behavioral treatment and other forms of family therapy to delineate the best approach to treating adolescent AN.
Acknowledgments
Funding support for this study was provided by NIH grant R01-MH-070621 to Dr. Lock and NIH grant R01-MH-070620 to Dr. Le Grange. Drs. Lock, Le Grange, Agras, Moye, and Jo take responsibility for the accuracy of the data and the data analyses. We thank Angela Celio-Doyle, PhD, Catherine Glunz, MD, Renee Hoste, PhD, Sarah Fischer, PhD, Angela Smyth, MD, Lydia Kruge, BA, Kristen Anderson, AM, Jamie Peisel, BA, Blaine Washington, BA, Rebecca Peebles, MD, Margo Thienemann, MD, Kara Fitzpatrick, Ph.D, Mary Sanders, PhD, Judy Beenhakker, MS,and Sarah Forsberg, BA for their contributions in executing this study.
Contributor Information
James Lock, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Stanford, CA 94305.
Daniel Le Grange, Department of Psychiatry and Behavioral Neuroscience, The University of Chicago.
W. Stewart Agras, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
Ann Moye, Bloomfield Hills, MI.
Susan W. Bryson, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine.
Booil Jo, Department of Psychiatry and Behavioral Sciences Stanford University School of Medicine.
REFERENCES
- 1.Hoek H. Review of epidemiological studies of eating disorders. Int Rev Psychiatry. 1993;5:61–74. [Google Scholar]
- 2.Hoek H, Hoeken Dv. Review of prevalence and incidence of eating disorders. Int J Eat Disord. 2003;34:383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 3.Hoek H, van Harten PN, Hermans KM, et al. The incidence of anorexia nervosa on Curacao. Am J. Psychiatry. 2005;162:748–752. doi: 10.1176/appi.ajp.162.4.748. [DOI] [PubMed] [Google Scholar]
- 4.van Son G, van hoeken D, Aad I, et al. Time trends in the incidence of eating disorders: a primary care study in the Netherlands. Int J Eat Disord. 2006;39:565–569. doi: 10.1002/eat.20316. [DOI] [PubMed] [Google Scholar]
- 5.Rome E, Ammerman S. Medical complications of eating disorders: An update. Journal of Adolecent Health. 2003;33:418–426. doi: 10.1016/j.jadohealth.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PF. Mortality in anorexia nervosa. American Journal of Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 7.Herzog DB, Greenwood DN, Dorer DJ, et al. Mortality in eating disorders: A descriptive study. International Journal of Eating Disorders. 2000;28:20–26. doi: 10.1002/(sici)1098-108x(200007)28:1<20::aid-eat3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Herzog DB, Nussbaum KM, Marmor AK. Comorbidity and outcome in eating disorders. Psychiatr Clin North Am. 1996;19(4):843–59. doi: 10.1016/s0193-953x(05)70385-3. [DOI] [PubMed] [Google Scholar]
- 9.Casper R, Hedeker D, McClough J. Personality dimensions in eating disorders and their relevance for subtyping. J Am Acad Child Adolesc Psychiatry. 1992;31:830–840. doi: 10.1097/00004583-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Anderluch M, Tchanturia K, Rabe-Hesketh S, Treasure JL. Childhood obsessive compulsive personality traits in adult women with eating disorders: Defining a broader eating disorder phenotype. Am J Psychiatry. 2003;160:242–247. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 11.Godart N, Flament M, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: A review. Int J Eat Disord. 2002:32. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 12.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: comorbidity and chronology of appearance. European Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 13.Bulik CM, Berkman N, Kimberly A, et al. Anorexia nervosa: a systematic review of randomized clinical trials. Int J Eat Disord. 2007;40:310–320. doi: 10.1002/eat.20367. [DOI] [PubMed] [Google Scholar]
- 14.Russell GF, Szmukler GI, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry. 1987;44(12):1047–56. doi: 10.1001/archpsyc.1987.01800240021004. [DOI] [PubMed] [Google Scholar]
- 15.Le Grange D, Eisler I, Dare C, Russell G. Evaluation of family treatments in adolescent anorexia nervosa: a pilot study. Int J Eat Disord. 1992;12(4):347–357. [Google Scholar]
- 16.Eisler I, Dare C, Hodes M, et al. Family therapy for adolescent anorexia nervosa: the results of a controlled comparison of two family interventions. J Child Psychol Psychiatry. 2000;41(6):727–36. [PubMed] [Google Scholar]
- 17.Robin A, Siegal P, Moye A, et al. A controlled comparison of family versus individual therapy for adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1482–1489. doi: 10.1097/00004583-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Gowers S, Clark A, Roberts C, et al. Clinical effectiveness of treatments for anorexia nervosa in adolescents. Br J Psychiatry. 2007;191:427–435. doi: 10.1192/bjp.bp.107.036764. [DOI] [PubMed] [Google Scholar]
- 19.Lock J, Agras WS, Bryson S, Kraemer H. A comparison of short- and long-term family therapy for adolescent anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:632–639. doi: 10.1097/01.chi.0000161647.82775.0a. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick K, Moye A, Hostee R, et al. Adolescent Focused Therapy for Adolescent Anorexia Nervosa. Journal of Contemporary Psychotherapy. submitted. [Google Scholar]
- 21.Dare C, Eisler I. Family therapy for anorexia nervosa. In: Garner DM, Garfinkel P, editors. Handbook of Treatment for Eating Disorders. Guilford Press; New York: 1997. pp. 307–324. [Google Scholar]
- 22.Dare C, Eisler I, Russell G, et al. Psychological therapies for adults with anorexia nervosa: Randomized controlled trial of outpatient treatments. Br J Psychiatry. 2001;178:216–221. doi: 10.1192/bjp.178.3.216. [DOI] [PubMed] [Google Scholar]
- 23.Eisler I, Simic M, Russell G, Dare C. A randomized controlled treatment trial of two forms of family therapy in adolesdent anorexia nervosa: a five-year follow-up. J Child Psychol Psychiatry. 2007;48:552–60. doi: 10.1111/j.1469-7610.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 24.Lock J, Couturier J, Agras WS. Comparison of long term outcomes in adolescents with anorexia nervosa treated with family therapy. American Journal of Child and Adolescent Psychiatry. 2006;45:666–672. doi: 10.1097/01.chi.0000215152.61400.ca. [DOI] [PubMed] [Google Scholar]
- 25.Control CfD . CDC Growth Charts for the United States: Development and Methods. Center for Disease Control; 2002. [Google Scholar]
- 26.Alison D. Handbook of Assessment Methods for Eating Behavior and Weight-Related Problems. Sage; Thousand Oaks, CA: 1995. [Google Scholar]
- 27.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 28.Workgroup for the Classification of Eating Disorders in Children and Adolescents Classification of child and adolescent eating disturbances. Int J Eat Disord. 2007;40:S117–S122. doi: 10.1002/eat.20458. [DOI] [PubMed] [Google Scholar]
- 29.Roberto C, Steinglass J, Mayer L, et al. The cliinical significance of amenorrhea as a diagnostic criterion for anorexia nervosa. Int J Eat Disord. 2008;41:559–563. doi: 10.1002/eat.20534. [DOI] [PubMed] [Google Scholar]
- 30.Company MLI Metropolitan height and weight tables. Stat Bull Metropolitan Life Insurance Company. 1983;64:1–9. 1983. [PubMed] [Google Scholar]
- 31.Lock J, Le Grange D, Agras WS, Dare C. Treatment manual for anorexia nervosa: A family-based approach. Guilford Publications, Inc.; New York: 2001. [Google Scholar]
- 32.Couturier J, Lock J. What constitutes remission in adolecent anorexia nervosa: a review of various conceptualizations and a quantitative analysis. Int J Eat Disord. 2006;39:175–183. doi: 10.1002/eat.20224. [DOI] [PubMed] [Google Scholar]
- 33.Couturier J, Lock J. What is recovery in adolescent anorexia nervosa? Int J Eat Disord. 2006;39:550–555. doi: 10.1002/eat.20309. [DOI] [PubMed] [Google Scholar]
- 34.Swenne I. Weight requirements for return of menstruation in teenage girls with eating disorders, weight loss and secondary amenorrhoea. Acta Paediatr. 2004;93:1449–1455. doi: 10.1080/08035250410033303. [DOI] [PubMed] [Google Scholar]
- 35.Swenne I. Weight requirements for catch-up growth in girls with eating disorders and onset of weight loss before menarche. Int J Eat Disord. 2005;38:340–345. doi: 10.1002/eat.20182. [DOI] [PubMed] [Google Scholar]
- 36.Golden N, Jacobson M, Schebendach J, et al. Resumption of menses in anorexia nervosa. Archives of Pediatrics and Adolescent Medicine. 1997;151:16–21. doi: 10.1001/archpedi.1997.02170380020003. [DOI] [PubMed] [Google Scholar]
- 37.Modan-Moses D, Yaroslavsky A, Novikov I, et al. Stunting of growth as a major feature of anorexia nervosa in male adolescents. Pediatrics. 2003;111:270–276. doi: 10.1542/peds.111.2.270. [DOI] [PubMed] [Google Scholar]
- 38.Couturier J, Lock J. Denial and minimization in adolescent anorexia nervosa. Int J Eat Disord. 2006;39:175–183. doi: 10.1002/eat.20224. [DOI] [PubMed] [Google Scholar]
- 39.Fairburn CG, Cooper I. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, Assessment, and Treatment. 12th edition Guilford Press; New York: 1993. [Google Scholar]
- 40.Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. British Journal of Psychiatry. 1989;154:807–12. doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- 41.Passi V, Bryson S, Lock J. Assessment of eating disorders in adolescents with anorexia nervosa: Self-report versus interview. Int J Eat Disord. 2003;33:45–54. doi: 10.1002/eat.10113. [DOI] [PubMed] [Google Scholar]
- 42.Hebebrand J, Himmelmann G, Hesecker H, et al. The use of percentiles for the body mass index in anorexia nervosa: diagnostic, epidemiological, and therapeutic considerations. Int J Eat Disord. 1996;19:359–369. doi: 10.1002/(SICI)1098-108X(199605)19:4<359::AID-EAT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Hebebrand J, Casper R, Treasure JL, Schweiger U. The need to revise the diagnostic criteria for anorexia nervosa. J. Neural Transmission. 2004;111:827–840. doi: 10.1007/s00702-004-0136-9. [DOI] [PubMed] [Google Scholar]
- 44.Hebebrand J, Wehmeier P, Remschmidt H. Weight criteria for diagnosis of anorexia nervosa. Am J Psychiatry. 2000;157:6. doi: 10.1176/appi.ajp.157.6.1024. [DOI] [PubMed] [Google Scholar]
- 45.Orvaschel H, Puig-Antich J, Chambers W, et al. Retrospective assessment of pre-pubertal major depression with the Kiddie-SADS-E. J Am Acad Child Adolesc Psychiatry. 1982;21:392–392. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- 46.Golden N, Katzman D, Kreipe R, et al. Eating disorders in adolescents: position paper of the Society for Adolescent Medicine:Medical Indications for Hospitalization in an Adolescent with an Eating Disorder. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 47.Diggle P, Liang K, Zeger S. The analysis of longitudinal data. Oxford University Press; Oxford: 1994. [Google Scholar]
- 48.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 49.Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55:107–122. [Google Scholar]
- 50.Raudenbush S, Bryk A. Hierarchical linear models: applications and data analysis methods. Sage; Thousand Oaks: 2002. [Google Scholar]
- 51.Muthén L, Muthén BO. Mplus user’s guide. 1998-2009. [Google Scholar]
- 52.Cohen J. Statistical Power Analysis for Behavioral Science. Second Edition. Lawrence Earlbaum; Hillsdale, New Jersey: 1988. ed. [Google Scholar]
- 53.Kraemer H, Morgan G, Leech N, et al. Measure of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Kraemer H, Kupfer D. Size of Treatment Effects and Their Importance to Clinical Research and Practice. Biol Psychiatry. 2006;58:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Fitzpatrick K, Moye A, Hostee R, et al. Adolescent Focused Therapy for Adolescent Anorexia Nervosa. Journal of Contemporary Psychotherapy. in press. [Google Scholar]
- 56.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of LOCF and MMRM approaches. Pharmaceutical Statistics. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 57.Simpson HP, Cheng J, Huppert J, et al. Statistical choices can affect inferences about treatment efficacy: A case study from obsessive-compulsive disorder research. Journal of Psychiatric Research. 2008;42:631–638. doi: 10.1016/j.jpsychires.2007.07.012. E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan H, Russell G. Clinical assessment of anorexia nervosa: The Morgan-Russell outcome assessment schedule. Br J Psychiatry. 1988;152:367–371. doi: 10.1192/bjp.152.3.367. [DOI] [PubMed] [Google Scholar]
- 59.Robin A. Behavioral family systems therapy for adolescents with anorexia nervosa. In: Kazdin A, Weisz J, editors. Evidence-based psychotherapies for children and adolescents. Guilford Press; New York: 2003. pp. 358–373. [Google Scholar]
- 60.Lock J. Treating adolescents with eating disorders in the family context: Empirical and theoretical considerations. Child and Adolescent Psychiatric Clinics of North America. 2002;11:331–342. doi: 10.1016/s1056-4993(01)00009-8. [DOI] [PubMed] [Google Scholar]
- 61.Howard W, Evans K, Quintero-Howard C, et al. Predictors of succes or failure of transition to day hospital treatment for inpatients with anorexia nervosa. Am J Psychiatry. 1999;156:1697–1702. doi: 10.1176/ajp.156.11.1697. [DOI] [PubMed] [Google Scholar]
- 62.Carter J, Blackmore E, Sutandar-Pinnock K, Woodside D. Relapse in anorexia nervosa: a survival analysis. Psychol Med. 2004;34:671–679. doi: 10.1017/S0033291703001168. [DOI] [PubMed] [Google Scholar]
- 63.Walsh BT, Kaplan AS, Attia E, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized clinical trial. JAMA. 2006;295:2605–2612. doi: 10.1001/jama.295.22.2605. [DOI] [PubMed] [Google Scholar]
- 64.National Institute for Clinical Excellence (N.I.C.E.) Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa, and binge eating disorder. British Psychological Society; 2004. [PubMed] [Google Scholar]