Abstract
Background
Higher fish or higher docosahexaenoic acid (DHA) intake normally correlates positively with higher plasma DHA level, but recent evidence suggests that the positive relationship between intake and plasma levels of DHA is less clear in the elderly.
Methods
We compared the metabolism of 13C-DHA in six healthy elderly (mean - 77 y old) and six young adults (mean - 27 y old). All participants were given a single oral dose of 50 mg of uniformly labelled 13C-DHA. Tracer incorporation into fatty acids of plasma triglycerides, free fatty acids, cholesteryl esters and phospholipids, as well as apparent retroconversion and β-oxidation of 13C-DHA were evaluated 4 h, 24 h, 7d and 28d later.
Results
Plasma incorporation and β-oxidation of 13C-DHA reached a maximum within 4 h in both groups, but 13C-DHA was transiently higher in all plasma lipids of the elderly 4 h to 28d later. At 4 h post-dose, 13C-DHA β-oxidation was 1.9 times higher in the elderly, but over 7d, cumulative β-oxidation of 13C-DHA was not different in the two groups (35% in the elderly and 38% in the young). Apparent retroconversion of 13C-DHA was well below 10% of 13C-DHA recovered in plasma at all time points, and was 2.1 times higher in the elderly 24 h and 7d after tracer intake.
Conclusions
We conclude that 13C-DHA metabolism changes significantly during healthy aging. Since DHA is a potentially important molecule in neuro-protection, these changes may be relevant to the higher vulnerability of the elderly to cognitive decline.
Background
The consumption of fish containing eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids protects against cardiovascular disease risk [1] and is possibly associated with lower risk of cognitive decline [2,3]. Higher fish or higher EPA and DHA intake normally correlates positively with higher plasma EPA and DHA levels [4], but recent evidence suggests that the positive relationship between intake and plasma levels of EPA and DHA is less clear in the elderly [5-9]. For instance, EPA was 50 - 100% higher in plasma phospholipids (PL) and total lipids of the elderly than in young adults consuming the same EPA-enriched supplement [6-8]. Similarly, a DHA-enriched supplement increased DHA 42% more in plasma total lipids in healthy elderly compared to young adults [9]. Higher EPA and DHA concentrations is also reported in erythrocyte lipids of the elderly [10]. Several prospective epidemiological studies report that higher erythrocyte omega-3 fatty acids is associated with better cognitive function [11] or with lower risk of cognitive decline [12-16] in later life.
In the elderly, increased concentrations of omega-3 fatty acid in plasma significantly predict less cognitive decline over 3 years [17]. Unlike saturated and monounsaturated fatty acids, synthesis of DHA from its omega-3 precursor, alpha-linolenic acid, is extremely limited in humans [18]. Thus, it is recommended that DHA be obtained from dietary sources. DHA is a potential key molecule in neuro-protection because it is a major component of synaptic membranes and is involved in membrane repair and fluidity, cell signalling, initiation of anti-inflammatory processes and gene expression [19-22].
Tracing metabolism of carbon 13 (13C)-DHA may provide useful information about age-related changes in DHA metabolism in humans [23-25]. In young adults given an oral dose of 250 - 280 mg 13C-DHA, 13C enrichment peaked at 2 h post-dose in plasma triglycerides (TG) when the tracer was given in the TG form, but at 6 h post-dose when esterified to phosphatidylcholine [24,25]. Brossard et al. also reported 1.4% apparent retroconversion of 13C-DHA to 13C-docosapentaenoate (13C-DPA) and 13C-EPA 3 d after giving the tracer [24].
Neither the impact of aging on 13C-DHA metabolism nor β-oxidation of 13C-DHA has yet been investigated in humans but both may influence the somewhat higher blood EPA and DHA commonly seen in the healthy elderly [5-10]. Hence, our aim here was to evaluate the incorporation of 13C-DHA into plasma lipids, its apparent retroconversion to DPA and EPA, and its β-oxidation in a group of young adults ~27 y old versus a group of healthy elderly ~77 y old.
Methods
All procedures reported here were approved by the Human Ethics Research Committee of the Health and Social Sciences Center -- Sherbrooke University Geriatrics Institute, which is the committee mandated to oversee human experimentation at our institution. All study participants gave informed written consent.
13C-DHA tracer study
Six young participants and six elderly were recruited (Table 1). All participants were non-smokers, were not pregnant or lactating, not using medication to control diabetes, liver disease, renal disease, hypertension, anemia or low serum albumin and had no other clinical evidence of malnutrition. An upper limit of 3.0% DHA in plasma total lipids was set to exclude those individuals who were probably already consuming fish oil supplements providing EPA and DHA or who were habitually eating relatively high amounts of fish. The participants' medical histories were taken by a registered nurse.
Table 1.
Baseline characteristics (means ± SD) of the two groups
| Young (n = 6) | Elderly (n = 6) | p * | |
|---|---|---|---|
| Age (years) | 26.8 ± 2.6 | 76.5 ± 2.7 | 0.004 |
| Male/Female | 3/3 | 2/4 | 0.337 |
| Weight (kg) | 84.0 ± 20.1 | 64.9 ± 9.7 | 0.078 |
| Body Mass Index (kg/m2) | 26.9 ± 5.9 | 24.9 ± 3.5 | 0.670 |
| Plasma Triglycerides (mmol/L) | 0.71 ± 0.18 | 0.94 ± 0.28 | 0.078 |
| Plasma Total Cholesterol (mmol/L) | 4.32 ± 0.58 | 5.26 ± 0.33 | 0.016 |
| Plasma Fatty Acidsa(%) | |||
| Sum Saturates | 32.7 ± 2.1 | 29.3 ± 2.7 | 0.055 |
| Sum Monounsaturates | 24.6 ±3.2 | 26.6 ± 2.7 | 0.200 |
| Linoleate | 32.5 ± 5.0 | 30.9 ± 1.9 | 0.630 |
| Arachidonate | 6.3 ± 0.4 | 8.3 ± 1.3 | 0.013 |
| α-Linolenate | < 0.1 | 0.5 ± 0.6 | 0.059 |
| Eicosapentaenoate | 0.6 ± 0.4 | 1.2 ± 0.3 | 0.012 |
| Docosapentaenoate | 0.1 ± 0.2 | 0.3 ± 0.3 | 0.211 |
| Docosahexaenoate | 1.7 ± 0.4 | 1.4 ± 0.8 | 0.872 |
aPlasma total lipids
*Mann-Whitney test
The 13C tracer used in this study was non-radioactive, safe for human research, uniformly labelled ( > 98%), and of high chemical purity [26]. At a breakfast scheduled in the Metabolic Unit of the Research Center on Aging, all participants received a muffin and a yogurt into which we added a single 50 mg dose of 13C-DHA as the methyl ester. The macronutrient composition of the 360 kCal muffin was: 13 g of fat containing 2 g saturated fat, 4.8 g of protein, 58.5 g of carbohydrates, 2.8 g of fibre and 28.9 g of sugar. Complete fatty acid profile of the muffin was not performed at the time of the experiment. The composition of the yogurt was 0 g of fat, 4 g of protein, 5.5 g of carbohydrates and 4.5 g of sugar for a total of 37.5 kCal. The composition of 200 mL orange juice provided 100 kCal, 1 g of protein, 23 g of carbohydrates and 20 g of sugar. The breakfast was consumed by all participants within 15 minutes after giving the tracer.
To follow appearance of 13CO2 coming from 13C-DHA β-oxidation, breath samples were collected at baseline and 4 h, 24 h and 7d after the tracer was consumed. To collect the breath samples, the participants breathed in a purpose-built device consisting of a perforated plastic bag attached to a mouthpiece (Easysampler, Quintron Instrument Company, Milwaukee, WI) where vacuum tubes were inserted to collect a sample of the expired breath [27,28]. Breath sample collection takes less than 30 sec. Our preliminary data showed that to effectively follow 13C-DHA metabolism in the blood, samples should be collected over a 4 wk period after tracer intake, by which time remaining 13C-DHA in plasma has essentially returned to baseline [29]. Enrichment of 13C-DHA in plasma lipid classes was performed by gas chromatography-combustion-isotope ratio mass spectrometry as previously described [30]. The 13C/12C values of the samples and the reference were used to calculate the δ per mil values which were designated thereafter as atom percent excess (APE). Calculation of 13C in DHA, DPA, EPA and α-linolenic acid (ALA) from APE values was done according to Brossard et al. [31]. Enrichment of 13C in breath CO2 following the ingestion of the 13C tracer was analyzed by isotope ratio mass spectrometry (Europa 20 - 20, Sercon Ltd, Crewe, Cheshire, UK) as previously described [28]. 5% CO2/N2 was the reference gas and He was the carrier gas (Praxair Canada Inc. Mississauga, ON, Canada). Percent dose recovered from β-oxidation of 13C-DHA in the breath was calculated as described by Freemantle et al. [27]. Cumulative oxidation of 13C-DHA was determined by calculating area under the curve between each time points using GraphPad Prism 5 software (San Diego, CA, USA) followed by summing the areas at each time.
Analytical methodology
Total lipids from 0.5 ml plasma were extracted into chloroform-methanol and a mixture of esterified (TG, CE or PL) and non-esterified (FFA) heptadecanoate internal standard was added for quantification. The lipid classes were separated by thin layer chromatography using petroleum ether: ethyl ether: methanol: acetic acid (85:15:1:2.5) as migration solvent. At the end of the chromatographic runs, the plates were sprayed with a solution of 2',7'-dichlorofluorescein, and viewed under UV light. Free fatty acids (FFA) and TG were directly methylated with boron trifluoride in methanol (14%). Cholesteryl esters (CE) were saponified to remove cholesterol from the methyl esters mixture since cholesterol can damage our GC column if injected with our methyl esters. PL were saponified to assure full fatty acid recovery. Fatty acid recovery from TG is less of a problem than for PL so TG were directly transmethylated.
Data expression and statistics
Six participants in each group was required to have sufficient power analysis with a β = 0.8 which is defined as 80% chance to reject the null hypothesis which state that there is no relationship between the variable of interest. Data are shown as means ± SEM, except as indicated. Sample sizes of less than 12 values simply don't provide enough data to discriminate between Gaussian and non-Gaussian distributions. In this situation non-parametric statistics are appropriate [32]. Fatty acid values and % β-oxidation were compared between young and elderly at each point using the non-parametric Mann-Whitney test (SPSS; Chicago, Illinois, USA). Apparent retroconversion of 13C-DHA was calculated by summing 13C-labelled DPA, EPA and ALA measured in plasma total lipids. The sum of 13C-labelled DPA, EPA and ALA was called 13C-omega-3 PUFA and was expressed as pmol/ml plasma. Statistical significance was set at p ≤ 0.05.
Results
Mean age of the young and the elderly were 26.8 y and 76.5 y, respectively, so that there was a gap of 50 y between the two groups (Table 1). Weight and body mass index was not different between the two groups. At baseline, the elderly had 22% higher plasma cholesterol compared to the young. At baseline, the profile of fatty acids in total lipids of fasting plasma was similar between the young and the elderly with the exception of arachidonic acid and EPA, which were 32% and 100% higher in the elderly, respectively. At baseline, % DHA was not different between the two groups (Table 1).
13C-DHA in plasma
Four h after giving the tracer, 13C-DHA peaked in plasma total lipids of the elderly (Figure 1, upper panel) at a level 3.5 times higher than in the young (p = 0.028). Plasma 13C-DHA concentration decreased 24 h after dosing, at which time it was no longer statistically different in the two groups. Seven days later, 13C-DHA concentration was still declining in both groups but was significantly higher in the elderly (p = 0.045). Twenty eight days post-intake, 13C-DHA returned close to baseline and was not different in the two groups (Figure 1A).
Figure 1.
13C-docosahexaenoic acid (DHA; upper panel), 13C-omega-3 PUFA (13C-alpha-linolenate + 13C-eicosapentaenoate + 13C-docosapentaenoate; middle panel) concentrations and ratio given as a percentage of 13C-omega-3 PUFA/13C-DHA (lower panel) in plasma total lipids of young (full symbols) and elderly (open symbols) over 28d after giving the 13C-DHA. Data are shown for n = 5 for the young and n = 6 for the elderly (*P < 0.05).
Four h post-dose, 13C-DHA was more than 4 fold higher in TG and FFA of the elderly but, in both groups, returned to near baseline after 24 h (Figure 2). 13C-DHA peaked in plasma PL 24 h post-dose but 28d later was still significantly higher in the elderly (p = 0.031; Figure 2). Seven days after dosing, 13C-DHA was 2.5 fold higher in CE of the elderly (p = 0.029) and remained so after 28d (p = 0.015).
Figure 2.
13C-docosahexaenoic acid (DHA) concentration in plasma triglycerides, free fatty acids, phospholipids, and cholesteryl esters in the young (full symbols) and the elderly (open symbols) followed over 28d (N = 6 for the young and the elderly except n = 4 for the elderly at 7d and 28d (* P < 0.05).
Apparent retroconversion (13C-omega-3 PUFA)
In both groups, the summed value for 13C-omega-3 PUFA other than DHA peaked 24 h after the dose of 13C-DHA and was 2.2 times higher in the elderly (p = 0.045; Figure 1, middle panel). 13C-omega-3 PUFA remained 2.1 times higher in the elderly 7d later. 13C-EPA contributed the most to the sum of 13C-omega-3 PUFA after 24 h (p = 0.045), whereas 7d later, 13C-EPA and 13C-ALA jointly contributed the most (data not shown). Percent apparent retroconversion expressed as a ratio of 13C-omega-3 PUFA relative to 13C-DHA concentration in plasma total lipids was not significantly different between the young and the elderly and reached 1.5 ± 1.1% and 2.0 ± 1.6%, respectively after 7d, and 2.5 ± 2.7% and 4.3 ± 6.2% after 28d, respectively (Figure 1, lower panel).
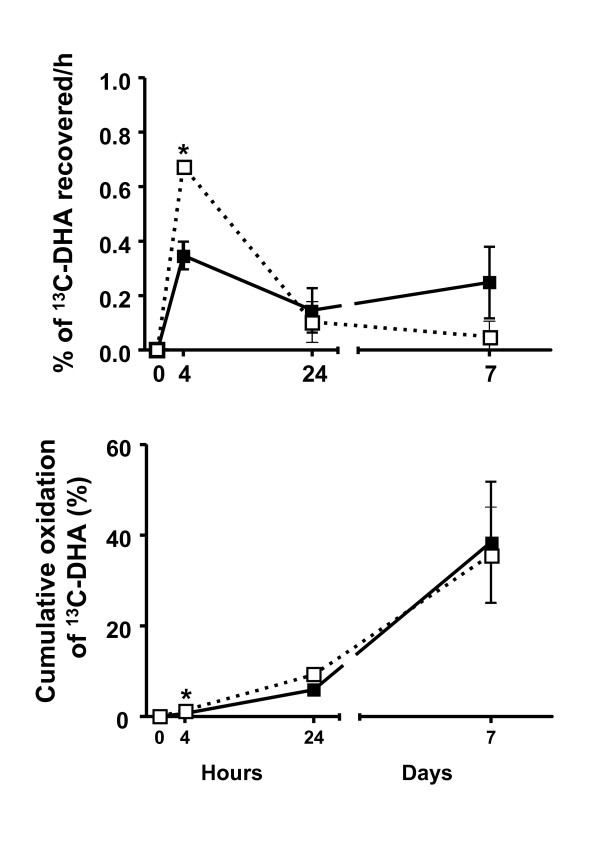
β-Oxidation
Carbon-13 values in breath samples at 28d were very low and not available in 3 young participants and 5 elderly participants. Hence, % dose of 13C-CO2 and cumulative β-oxidation of 13C-DHA was calculated over the first 7d post-dose (Figure 3). As a % of the dose given, 13C-DHA β-oxidation was 1.9 times higher at 4 h post-dose in the elderly (p = 0.004), but was not significantly different between the young and the elderly at 24 h or at 7d (Figure 3; upper panel). Cumulative β-oxidation over 7d reached 35% and 38% in the elderly and the young participants, respectively (not different; Figure 3, lower panel).
Figure 3.
β-oxidation of 13C-docosahexaenoic acid (13C-DHA) shown as % dose recovered over time (upper panel) and as cumulative β-oxidation over time (lower panel) in young (full symbols) and the elderly (open symbols) over 7d (n = 6 for the young and the elderly except n = 4 for the elderly at 24 h; *P < 0.05).
Discussion
The aim of this study was to evaluate whether plasma incorporation, apparent retroconversion, or β-oxidation of 13C-DHA differed in the healthy elderly compared to healthy young adults. We observed that within the post-prandial period (+ 4 h) the elderly had at least 4 fold higher 13C-DHA in plasma TG and FFA, and 1.9 fold higher β-oxidation to 13C-CO2. 13C-DHA was also 2.5 fold higher in plasma CE of the elderly after 7d, and its apparent retroconversion was 2.2 fold higher in the elderly 24 h after the oral dose. Hence, our results indicate that there are significant differences in DHA metabolism during healthy aging which compliment, extend and possibly help explain previous reports showing somewhat higher plasma DHA in the elderly [5-9].
The distribution of 13C-DHA we observed in plasma lipid classes of our young study participants agrees with previous published papers [24,25,31]. We speculate that the higher early rise in 13C-DHA in plasma TG and FFA in the elderly (Figure 2) may potentially be explained by the elderly having both higher postprandial production of very low density lipoproteins which are rich in TG [33], as well as a higher post-prandial FFA response [7]. However, one limitation is that postprandial TG-rich lipoproteins were not measured in this study so this explanation remains to be confirmed. The 4-fold higher 13C-DHA in plasma CE in the elderly after 7d persisted out to 28d and may be due to different lipoprotein metabolism in the elderly as suggested by their (i) higher plasma total cholesterol (Table 1), (ii) higher plasma residence time of low density lipoprotein [34], and/or (iii) lower turnover of low density lipoprotein [33].
The appearance of 13C in other omega-3 PUFA besides DHA (mostly in 13C-EPA) was about 2 times higher in the elderly 24 h to 7d after tracer intake. This measure of apparent retroconversion of 13C-DHA to 13C-EPA could occur by one cycle of β-oxidation and chain shortening [35]. Subsequent chain lengthening of 13C-EPA could explain the appearance of 13C-DPA. Alternatively, recycling of carbon from PUFA through 13C-acetate into newly synthesized fatty acids is a well known phenomenon [35], and could potentially become part of ALA because humans can make small amounts of ALA from 16:3 n-3, or omega-3 PUFA longer than ALA via conventional elongation [36].
The mean total 13C-EPA and 13C-DPA appearing in plasma is well below 10% even at 28d post-dose, so this amount of apparent retroconversion would seem unlikely to fully account for net > 5% rise in plasma phospholipid EPA after DHA supplementation reported elsewhere [37], unless the process were substantially up-regulated using multigram DHA supplements. Raised plasma EPA after a DHA supplement may also be due to reduced EPA turnover by sparing mechanisms with enhanced dietary DHA.
We show for the first time that 13C-CO2 from β-oxidation of 13C-DHA peaked at 4 h post-intake and was 1.9 fold higher in the elderly (Figure 3). These data confirm a preliminary report [29] showing that humans β-oxidize 13C-DHA much more slowly than 13C-ALA [28]. The major regulatory mechanism for the control of β-oxidation is the availability of substrate, mostly as fatty acids in postprandial circulating TG and FFA [38]. The higher β-oxidation of 13C-DHA at 4 h post-dose in our elderly participants is in line with their higher 13C-DHA in plasma TG and FFA 4 h post-dose (Figure 2). After 7d, 13C-CO2 was still above baseline in both groups although plasma 13C-DHA concentration had returned close to baseline in TG and FFA. Cumulative β-oxidation of 13C-DHA was not different in the young and the elderly and reached 35 - 38% after 7d, which is about half that seen for the same dose of 13C-ALA over the same time period [28]. Under the conditions of this study, these results give a rough estimate of about 3 wk to turnover DHA to expired CO2, or a DHA biological half-life of about 10 d. However, overall β-oxidation was not higher in the elderly even though the elderly did have 13C-EPA derived from 13C-DHA. There is no implicit reason why the age-related difference in recovery of 13C as 13C-EPA should be directly related to net loss of 13C-DHA to 13CO2; both processes involve a complex interchange of carbon. Furthermore, we do not yet know how the EPA and other omega-3 fatty acids became labelled with 13C; if it was via ‘direct’ retroconversion, i.e. by chain shortening, there is no implicit reason why this form of retroconversion should be coupled to the β-oxidation of DHA; if it was via oxidation to acetyl-CoA, perhaps one would expect a closer correlation between β-oxidation of DHA and EPA labelling. Analytically, we cannot yet distinguish between these two possibilities. Incidentally, the elderly did have higher β-oxidation, but only at the earliest time point (+ 4 h).
The possible relevance of aging-related changes in DHA metabolism to risk of chronic diseases, particularly cognitive decline, remains to be established. Fish intake seems to decrease the risk of cognitive decline [2] and EPA and DHA in blood are biomarkers of fish intake [4] but, paradoxically, an overview of the literature shows that lower blood DHA is not seen in Alzheimer's disease or other forms of dementia [2,39]. Indeed, the aging-associated changes in DHA metabolism we report here suggest that higher plasma DHA in the elderly could actually mask lower DHA availability to the brain. Another factor that was not considered in this small study is the presence of apolipoprotein E ε4 gene polymorphisms which appear to alter plasma EPA and DHA metabolism [40].
Because of the limited availability of the tracer and its very high cost, we had only six participants per group. However, a tracer is much more sensitive and specific than a fish oil supplement such that low number of participants per group can still generate useful and statistically valid results [23]. Indeed, previous studies using 13C-DHA used only 3 - 4 participants [24,25,29,31].
Conclusions
We conclude from this month long study that in the healthy elderly, 50 mg of 13C-DHA is retained longer in the blood and undergoes more β-oxidation and more apparent retroconversion than in young adults. Given the potential importance of DHA for cardiovascular and brain health and the increasing elderly population, this alteration in DHA metabolism in the elderly may have some bearing on their vulnerability to cognitive decline. Further studies with 13C-DHA should examine -- (i) mechanisms at the root of these aging-associated differences, (ii) whether there is a link to the cognitive health of the elderly, and (iii) whether the dietary need for DHA may be different in the elderly.
Abbreviations
ALA: α-linolenic acid; CE: cholesteryl esters; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; FFA: free fatty acids; TG: triglycerides
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MP, MV and SCC designed the study; MP, RCW and MV conducted the study; YZ, PL and JTB conducted the 13C-analyses; MP, RCW, MV, JTB and SCC analyzed the data, and MP and RCW performed the statistical analysis. MP prepared the first draft and all authors contributed to writing and reviewing the paper, MP had primary responsibility for final content. All authors read and approved the final manuscript.
Contributor Information
Mélanie Plourde, Email: melanie.plourde2@usherbrooke.ca.
Raphaël Chouinard-Watkins, Email: Raphael.chouinard-watkins@usherbrooke.ca.
Milène Vandal, Email: milene.vandal@crchul.ulaval.ca.
Ying Zhang, Email: yz92@cornell.edu.
Peter Lawrence, Email: petegcms@gmail.com.
J Thomas Brenna, Email: jtb4@cornell.edu.
Stephen C Cunnane, Email: Stephen.cunnane@usherbrooke.ca.
Acknowledgements
Funding for this project was provided by the Natural Science and Engineering Research Council of Canada, Canadian Foundation for Innovation, Canada Research Chairs Secretariat (SCC), the Research Center on Aging, and the Department of Medicine and the Fonds de la recherche en santé du Québec for a post-doctoral fellowship to MP. Julie Desgagné and Conrad Filteau provided excellent technical assistance. We thank Dr. Tony Windust (National Research Council, Ottawa) for synthesizing the 13C-DHA.
References
- Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Plourde M, Pifferi F, Begin M, Feart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer's disease. Prog Lipid Res. 2009;48:239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol. 2009;5:140–152. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hanninen O, Uusitupa MI. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids. 1997;32:697–705. doi: 10.1007/s11745-997-0089-x. [DOI] [PubMed] [Google Scholar]
- de Groot RH, van Boxtel MP, Schiepers OJ, Hornstra G, Jolles J. Age dependence of plasma phospholipid fatty acid levels: potential role of linoleic acid in the age-associated increase in docosahexaenoic acid and eicosapentaenoic acid concentrations. Br J Nutr. 2009;102:1058–1064. doi: 10.1017/S0007114509359103. [DOI] [PubMed] [Google Scholar]
- Fortier M, Tremblay-Mercier J, Plourde M, Chouinard-Watkins R, Vandal M, Pifferi F, Freemantle E, Cunnane SC. Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Quebec: higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot Essent Fatty Acids. pp. 277–280. [DOI] [PubMed]
- Plourde M, Tremblay-Mercier J, Fortier M, Pifferi F, Cunnane SC. Eicosapentaenoic acid decreases postprandial beta-hydroxybutyrate and free fatty acid responses in healthy young and elderly. Nutrition. 2009;25:289–294. doi: 10.1016/j.nut.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- Vandal M, Freemantle E, Tremblay-Mercier J, Plourde M, Fortier M, Bruneau J, Gagnon J, Tremblay S, Bégin M, Cunnane SC. Plasma omega-3 fatty acid response to a fish oil supplement in the healthy elderly. Lipids. 2008;43:1085–1089. doi: 10.1007/s11745-008-3232-z. [DOI] [PubMed] [Google Scholar]
- Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA + DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr. 2008;87:449–454. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–1111. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- Dullemeijer C, Durga J, Brouwer IA, van de Rest O, Kok FJ, Brummer RJ, van Boxtel MP, Verhoef P. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86:1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- Samieri C, Feart C, Letenneur L, Dartigues JF, Peres K, Auriacombe S, Peuchant E, Delcourt C, Barberger-Gateau P. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88:714–721. doi: 10.1093/ajcn/88.3.714. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71:430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as suppements. Appl Physiol Nutr Metab. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aid S, Poumes-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev. 2004;44:509–538. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM, Muller M, Afman LA. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- Brenna JT. Use of stable isotopes to study fatty acid and lipoprotein metabolism in man. Prostaglandins Leukot Essent Fatty Acids. 1997;57:467–472. doi: 10.1016/S0952-3278(97)90430-0. [DOI] [PubMed] [Google Scholar]
- Brossard N, Croset M, Pachiaudi C, Riou JP, Tayot JL, Lagarde M. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am J Clin Nutr. 1996;64:577–586. doi: 10.1093/ajcn/64.4.577. [DOI] [PubMed] [Google Scholar]
- Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [(13)C]DHA in phosphatidylcholine. J Lipid Res. 1999;40:1867–1874. [PubMed] [Google Scholar]
- Le PM, Fraser C, Gardner G, Liang WW, Kralovec JA, Cunnane SC, Windust AJ. Biosynthetic production of universally (13)C-labelled polyunsaturated fatty acids as reference materials for natural health product research. Anal Bioanal Chem. 2007;389:241–249. doi: 10.1007/s00216-007-1305-0. [DOI] [PubMed] [Google Scholar]
- Freemantle E, Vandal M, Tremblay-Mercier J, Plourde M, Poirier J, Cunnane SC. Metabolic response to a ketogenic breakfast in the healthy elderly. Journal of Nutrition, Health and Aging. 2008;13(4):293–8. doi: 10.1007/s12603-009-0026-9. [DOI] [PubMed] [Google Scholar]
- McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res. 2004;45:474–485. doi: 10.1194/jlr.M300304-JLR200. [DOI] [PubMed] [Google Scholar]
- Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachere JC, Begin ME, Brenna JT, Windust A, Cunnane SC. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75:213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Goodman KJ, Brenna JT. High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal Chem. 1992;64:1088–1095. doi: 10.1021/ac00034a004. [DOI] [PubMed] [Google Scholar]
- Brossard N, Croset M, Normand S, Pousin J, Lecerf J, Laville M, Tayot JL, Lagarde M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J Lipid Res. 1997;38:1571–1582. [PubMed] [Google Scholar]
- Motulsky HJ. Analyzing Data with GraphPad Prism. San Diego, CA, GraphPad Software Inc; 1999. [Google Scholar]
- Millar JS, Lichtenstein AH, Cuchel M, Dolnikowski GG, Hachey DL, Cohn JS, Schaefer EJ. Impact of age on the metabolism of VLDL, IDL, and LDL apolipoprotein B-100 in men. J Lipid Res. 1995;36:1155–1167. [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991;1081:109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Ryan MA, Craig KS, Brookes S, Koletzko B, Demmelmair H, Singer J, Kyle DJ. Synthesis of linoleate and alpha-linolenate by chain elongation in the rat. Lipids. 1995;30:781–783. doi: 10.1007/BF02537807. [DOI] [PubMed] [Google Scholar]
- Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- Moczulski D, Majak I, Mamczur D. An overview of beta-oxidation disorders. Postepy Hig Med Dosw (Online) 2009;63:266–277. [PubMed] [Google Scholar]
- Plourde M, Fortier M, Vandal M, Tremblay-Mercier J, Freemantle E, Bégin M, Pifferi F, Cunnane SC. Unresolved issues in the link between docosahexaenoic acid and Alzheimer disease. Prostaglandins Leukot Essent Fatty Acids. 2007;77:301–308. doi: 10.1016/j.plefa.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Plourde M, Vohl MC, Vandal M, Couture P, Lemieux S, Cunnane SC. Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by apoE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. Br J Nutr. 2009;102:1121–1124. doi: 10.1017/S000711450938215X. [DOI] [PubMed] [Google Scholar]