Abstract
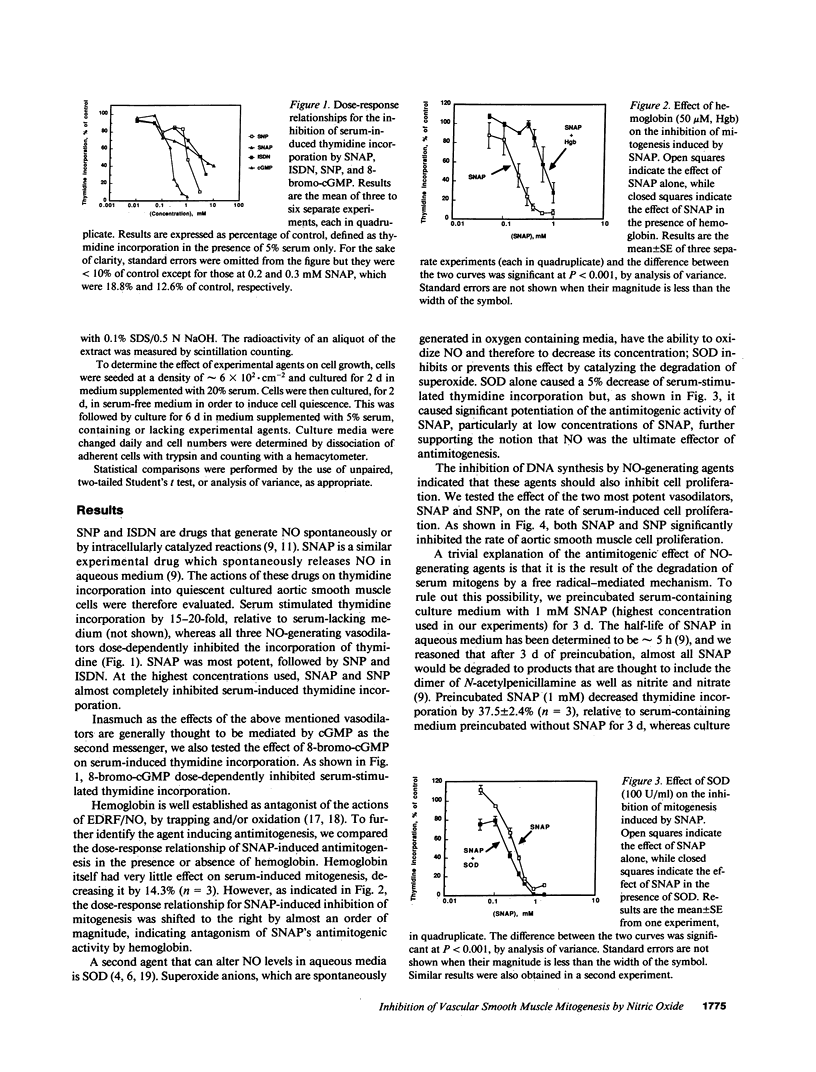
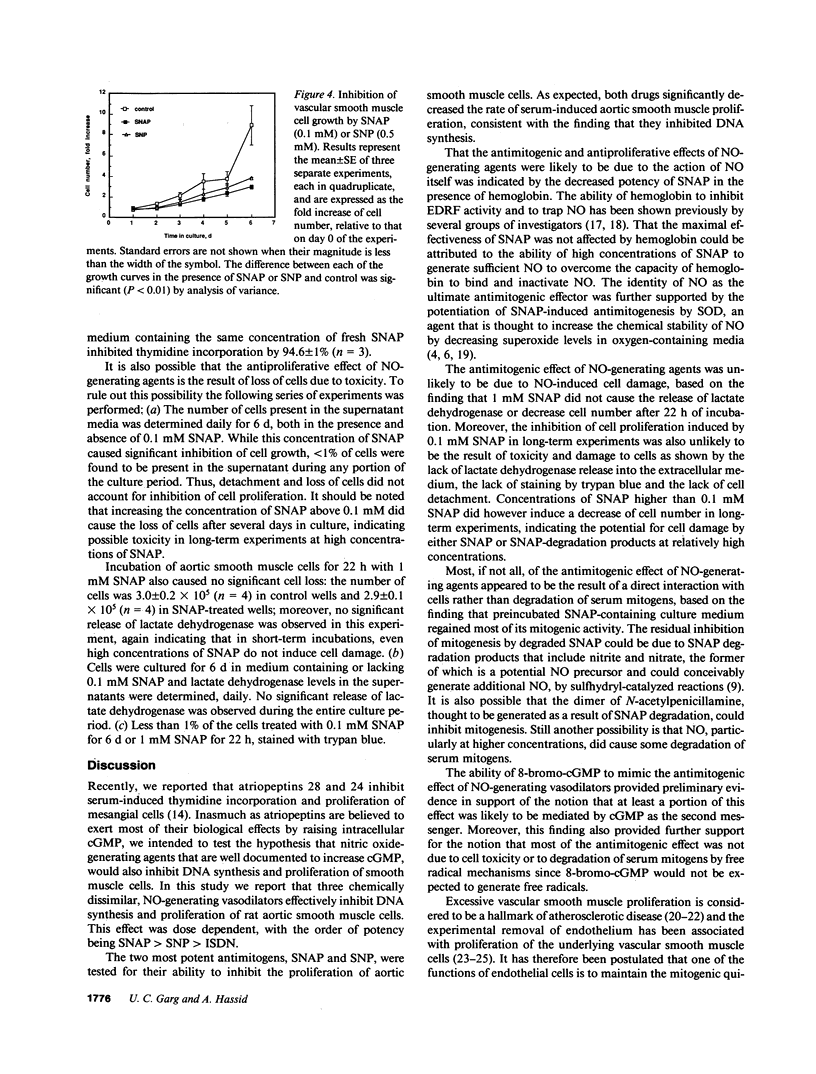
Endothelium-derived relaxing factor has been recently identified as nitric oxide. The purpose of this study was to determine if vasodilator drugs that generate nitric oxide inhibit vascular smooth muscle mitogenesis and proliferation in culture. Three chemically dissimilar vasodilators, sodium nitroprusside, S-nitroso-N-acetylpenicillamine and isosorbide dinitrate, dose-dependently inhibited serum-induced thymidine incorporation by rat aortic smooth muscle cells. Moreover, 8-bromo-cGMP mimicked the antimitogenic effect of the nitric oxide-generating drugs. The antimitogenic effect of S-nitroso-N-acetylpenicillamine was inhibited by hemoglobin and potentiated by superoxide dismutase, supporting the view that nitric oxide was the ultimate effector. Sodium nitroprusside and S-nitroso-N-acetylpenicillamine significantly decreased the proliferation of vascular smooth muscle cells. Moreover, the inhibition of mitogenesis and proliferation was shown to be independent of cell damage, as documented by several criteria of cell viability. These results suggest that endogenous nitric oxide may function as a modulator of vascular smooth muscle cell mitogenesis and proliferation, by a cGMP-mediated mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- Feelisch M., Noack E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur J Pharmacol. 1987 Oct 27;142(3):465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Ransil B. J., Fuhro R. L. In vivo aortic muscle cell growth kinetics. Differences between thoracic and abdominal segments after intimal injury in the rabbit. Circ Res. 1980 Aug;47(2):182–189. doi: 10.1161/01.res.47.2.182. [DOI] [PubMed] [Google Scholar]
- Gordon D., Schwartz S. M. Replication of arterial smooth muscle cells in hypertension and atherosclerosis. Am J Cardiol. 1987 Jan 23;59(2):44A–48A. doi: 10.1016/0002-9149(87)90175-5. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Hassid A. Atriopeptin II decreases cytosolic free Ca in cultured vascular smooth muscle cells. Am J Physiol. 1986 Nov;251(5 Pt 1):C681–C686. doi: 10.1152/ajpcell.1986.251.5.C681. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988 Jan;244(1):181–189. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Johnson A., Lermioglu F., Garg U. C., Morgan-Boyd R., Hassid A. A novel biological effect of atrial natriuretic hormone: inhibition of mesangial cell mitogenesis. Biochem Biophys Res Commun. 1988 Apr 29;152(2):893–897. doi: 10.1016/s0006-291x(88)80123-2. [DOI] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Ross R. Growth factors in the pathogenesis of atherosclerosis. Acta Med Scand Suppl. 1987;715:33–38. doi: 10.1111/j.0954-6820.1987.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schröder H., Noack E., Müller R. Evidence for a correlation between nitric oxide formation by cleavage of organic nitrates and activation of guanylate cyclase. J Mol Cell Cardiol. 1985 Sep;17(9):931–934. doi: 10.1016/s0022-2828(85)80106-1. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Secrest R. J., Chapnick B. M. Endothelial-dependent relaxation induced by leukotrienes C4, D4, and E4 in isolated canine arteries. Circ Res. 1988 May;62(5):983–991. doi: 10.1161/01.res.62.5.983. [DOI] [PubMed] [Google Scholar]



