Abstract
Background
The relationship between incident congestive heart failure (CHF) and ethnicity as well as racial/ethnic differences in the mechanisms leading to CHF have not been demonstrated in a multiracial, population-based study. Our objective was to evaluate the relationship between race/ethnicity and incident CHF.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a cohort study of 6814 participants of 4 ethnicities: white (38.5%), African American (27.8%), Hispanic (21.9%), and Chinese American (11.8%). Participants with a history of cardiovascular disease at baseline were excluded. Cox proportional hazards models were used for data analysis.
Results
During a median follow-up of 4.0 years, 79 participants developed CHF (incidence rate: 3.1 per 1000 person-years). African Americans had the highest incidence rate of CHF, followed by Hispanic, white, and Chinese American participants (incidence rates: 4.6, 3.5, 2.4, and 1.0 per 1000 person-years, respectively). Although risk of developing CHF was higher among African American compared with white participants (hazard ratio, 1.8; 95% confidence interval, 1.1-3.1), adding hypertension and/or diabetes mellitus to models including ethnicity eliminated statistical ethnic differences in incident CHF. Moreover, African Americans had the highest proportion of incident CHF not preceded by clinical myocardial infarction (75%) compared with other ethnic groups (P = .06).
Conclusions
The higher risk of incident CHF among African Americans was related to differences in the prevalence of hypertension and diabetes mellitus as well as socioeconomic status. The mechanisms of CHF also differed by ethnicity; interim myocardial infarction had the least influence among African Americans, and left ventricular mass increase had the greatest effect among Hispanic and white participants.
Congestive Heart Failure (CHF) is one of the leading causes of mortality and morbidity in the United States,1-7 and its prevalence continues to rise,6 despite the decline in overall cardiovascular disease morbidity and mortality.5 Previous work reporting racial/ethnic disparities in the prevalence of CHF has raised concerns that the incidence of CHF might vary among different racial/ethnic groups. These studies suggest that the relative importance of different genetic and environmental risk factors for CHF as well as the mechanisms or pathways of circulatory impairment leading to symptomatic CHF vary among different racial/ethnic groups.8,9 Given the high incidence of heart failure over the human lifetime10,11 and its increasing prevalence and social burden in this country,1-7 the importance of ascertaining race- or ethnicity-related differences in incident CHF cannot be overestimated.
Although ethnicity has been suggested as an independent risk factor for CHF,8,9 the direct effect of ethnicity on incident CHF has not been demonstrated in a population-based study. Most of the data regarding the incidence of CHF are derived from white populations, and, therefore, it is important to determine the incidence of CHF among other ethnic groups and examine whether the trends in incidence of CHF follow similar patterns to those among whites. Previous studies have shown higher mortality and hospitalization rates due to CHF among African American compared with white populations12,13 but did not elucidate the factors that induce the onset of CHF in a multi-ethnic population. Discrepancies in the prevalence and consequences of CHF between African Americans and whites have been attributed to racial/ethnic differences in the prevalence of coexisting conditions such as hypertension and diabetes mellitus, the quality and availability of medical care, and disparities in socioeconomic factors.14-17 Although crucial as guideposts to the elucidation of factors that determine racial/ethnic differences in incident CHF, these data do not substitute for quantitative knowledge on the relative contribution of etiological factors that underlie such differences. Moreover, the main determinants and pathways of ethnic-related differences in the incidence of CHF as well as racial/ethnic differences in the interrelationship of incident CHF with more proximal risk factors, such as alterations of left ventricular (LV) structure and function, subclinical coronary artery disease (CAD), and incident myocardial infarction (MI) remain unclear.
In this study, differences in the incidence of CHF were evaluated in the 4 racial/ethnic groups included in the Multi-Ethnic Study of Atherosclerosis (MESA).7,18-23 Moreover, we investigated whether established and novel potential risk factors for CHF, as well as LV dysfunction, coronary artery calcification, and the development of an interim clinical MI, can explain racial/ethnic differences in incident as opposed to prevalent CHF. Finally, we examined whether race/ethnicity modifies the associations of subclinical pathological processes such as myocardial hypertrophy, ventricular dysfunction, and subclinical CAD with incident CHF.
Methods
Study Population
The MESA is an ongoing multicenter cohort study of 6 US communities in Maryland, Illinois, North Carolina, California, New York, and Minnesota. Between July 2000 and August 2002, 6814 participants (3601 women; age range, 45-84 years) were recruited for the study. Participants defined themselves as white (38.5%), African American (27.8%), Hispanic (21.9%), or Chinese American (11.8%). The design of the MESA has been previously described in detail.24 More important, the presence or history of any clinical cardiovascular disease at baseline was among the exclusion criteria. The study was approved by the institutional review boards of all participating centers.
Baseline Examination
Information regarding smoking history and medication use for high blood pressure, high cholesterol, or diabetes mellitus was obtained using standardized questionnaires. Obesity was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 or more and diabetes mellitus as a fasting glucose level of 126 mg/dL or more (to convert to millimoles per liter, multiply by 0.0555) and/or use of hypoglycemic medication.25 Hypertension was defined as systolic blood pressure of 140 mm Hg or higher and/or diastolic blood pressure of 90 mm Hg or higher and/or use of any antihypertensive medication. Participant education was categorized as less than high school; completed high school; some college without degree, technical school certificate, or associate's degree; bachelor's degree; and graduate or professional school training. Categories of annual household income were less than $25 000; $25 000 to $49 999; $50 000 to $75 000; and more than $75 000. Intake of trans-fatty acids was estimated using standardized food frequency questionnaires.
Left ventricular mass and LV ejection fraction (LVEF) were determined by magnetic resonance imaging (MRI) at baseline among 5004 MESA participants who agreed to undergo MRI using the previously reported protocol.26 The LV mass was normalized for body surface area. In addition, LV hypertrophy by electrocardiography (ECG) was determined using Novacode criteria27 among all 6814 participants. Moreover, phantom-adjusted Agatston calcium scores were calculated from brightness-adjusted electron-beam computed tomography images and presence of coronary artery calcification (defined as an Agatston score of >0) was used as a measure of subclinical CAD.
Follow-Up and Outcome Variable
Median follow-up time was 4.0 years (interquartile range, 3.1-4.2 years), which resulted in 25 107 person-years of observation. A telephone interviewer contacted each participant every 6 to 9 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Two physicians reviewed each record for independent endpoint classification and assignment of event dates.
The endpoint for this study was symptomatic CHF. End point criteria were (a) CHF diagnosed by a physician and patient receiving medical treatment for CHF; (b) pulmonary edema/congestion seen on a chest radiograph; and (c) dilated ventricle or poor LV systolic function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction by echocardiography. Participants who met only criterion a were considered to meet a “soft” criterion, and participants who met criteria b and c in addition to a physician diagnosis were classified as meeting “hard” criteria for CHF. For this analysis, participants who met either soft or hard criteria were considered as having incident CHF. An MI was diagnosed based on standard criteria consisting of combinations of symptoms, ECG findings, and cardiac biomarker levels.24
Statistical Methods
Data are presented as mean(SD) for normally distributed continuous variables, median (interquartile range) for continuous variables with skewed distributions, and number (percentage) for categorical variables. Analysis of variance, t test, Fisher exact test, and χ2 test were used to compare the baseline distributions of variables among the 4 racial/ethnic groups.
Cox proportional hazards models were used to analyze the association of race/ethnicity with incident CHF. Four sets of models were used: Model 1: unadjusted analysis; Model 2: adjusted for established risk factors of CHF, which included age, sex, hypertension, diabetes mellitus, LV hypertrophy by ECG, obesity, serum total cholesterol level, and current cigarette smoking; Model 3: adjusted for the established risk factors entered in Model 2 plus interim MI as a time-varying covariate; and Model 4: Model 3 plus baseline LVEF.
Results of Cox proportional hazards models are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). All HRs were calculated and reported for 1 SD increase in continuous variables or transfer from one level to another of categorical variables, unless stated otherwise. Proportionality of hazards was checked by visually examining “log-log” plots. We also examined whether the associations of MRI parameters of LV structure and function with CHF are modified by race/ethnicity or sex by adding interaction terms to Model 2. Furthermore, to evaluate how much of the association of race/ethnicity with incident CHF was related to established risk factors, we compared the regression coefficient for race/ethnicity before and after adjusting for these risk factors. Missing values were handled based on our a priori analysis plan, ie, only participants who had missing data on a variable needed for a particular model were excluded from the analysis. This method was used to maximize the statistical power and in view of the negligible percentage of missing data. Cumulative hazards of CHF were illustrated in Nelson-Aalen plots and were compared using the log-rank test. Statistical analyses were performed using Stata statistical software, version 8.2 for Windows (StataCorp, College Station, Texas).
Results
During a median follow-up of 4.0 years, 79 participants developed CHF (incidence rate, 3.1 per 1000 person-years). Baseline characteristics of the study participants are summarized in Table 1. Twenty-six participants (33%) had an MI prior to CHF, and 63% had CHF with preserved LV function (LVEF ≥40%). Of participants with incident CHF, 18% were outpatient cases. The prevalence of obesity was significantly higher among African Americans and Hispanics and significantly lower among Chinese Americans compared with whites. White participants had a significantly lower prevalence of diabetes mellitus compared with other ethnic groups. Moreover, the prevalence of hypertension was significantly higher among African Americans than whites, whereas differences between whites and Chinese Americans or Hispanics were not statistically significant. With regard to subclinical disease markers, whites had the greatest prevalence of coronary calcification, followed by African Americans, Hispanics, and Chinese Americans. Conversely, African Americans and Hispanics had higher prevalences of LV hypertrophy compared with whites, whereas prevalence among Chinese Americans was lower.
Table 1. Participant Characteristics by Race/Ethnicity in the Multi-Ethnic Study of Atherosclerosis, 2006a.
| Characteristic | Racial/Ethnic Group | P Valueb | ||||
|---|---|---|---|---|---|---|
| White (n=2624) | Chinese American (n=803) | African American (n=1895) | Hispanic (n=1492) | |||
| Age, y | 62.6 (10.2)a | 62.3 (10.3) | 62.1 (10.0) | 61.3 (10.3) | .63 | |
| Sex, No. (%) | ||||||
| Male | 1261 (48.1) | 390 (48.6) | 844 (44.5) | 718 (48.1) | .06 | |
| Female | 1363 (51.9) | 413 (51.4) | 1051 (55.5) | 774 (51.9) | ||
| BMI | 27.7 (5.1) | 24.0 (3.3) | 30.2 (5.9) | 29.4 (5.1) | <.01 | |
| Obese, BMI ≥30, No. (%) | 724 (27.6) | 36 (4.5) | 860 (45.4) | 577 (38.7) | <.01 | |
| Cigarette smoking, No. (%) | ||||||
| Never | 1159 (44.4) | 604 (75.3) | 856 (45.6) | 807 (54.2) | <.01 | |
| Former | 1148 (44.0) | 153 (19.1) | 683 (36.4) | 480 (32.3) | ||
| Current | 301 (11.6) | 45 (5.6) | 338 (18.0) | 201 (13.5) | ||
| Diabetes mellitus, No. (%)c | 188 (7.2) | 122 (15.2) | 368 (19.5) | 291 (19.5) | <.01 | |
| Hypertension, No. (%)d | 1130 (43.1) | 323 (40.2) | 1147 (60.5) | 651 (43.6) | <.01 | |
| Blood pressure, mm Hg | ||||||
| Systolic | 123.5 (20.4) | 124.6 (21.6) | 131.6 (21.6) | 126.7 (21.9) | <.01 | |
| Diastolic | 70.2 (10.0) | 72.0 (10.3) | 74.5 (10.2) | 71.5 (10.1) | .54 | |
| Serum total cholesterol level, mg/dL | 195.7 (35.1) | 192 (31.8) | 189.7 (36.3) | 197.9 (37.5) | <.01 | |
| Lack of health insurance | 70 (2.7) | 154 (19.2) | 116 (6.2) | 269 (18.0) | <.01 | |
| MI during follow-up, No. (%) | 35 (1.3) | 8 (1.0) | 16 (0.8) | 19 (1.3) | .44 | |
| Incidence of MI per 1000 person-years | 3.5 | 2.7 | 2.4 | 3.3 | .56 | |
| CHF during follow-up, No. (%) | 25 (1.0) | 3 (0.4) | 32 (1.7) | 19 (1.3) | .02 | |
| Incidence of CHF per 1000 person-years | 2.4 | 1.0 | 4.6 | 3.5 | .02 | |
| Coronary artery calcification by CT, No. (%)e | 1497 (57.1) | 404 (50.3) | 821 (43.3) | 676 (45.3) | <.01 | |
| LVH by electrocardiography | 14 (0.5) | 12 (1.5) | 26 (1.3) | 16 (23.5) | .02 | |
| LV mass index by MRI, g/m2 | 75.8 (15.2) | 73.9 (13.6) | 81.3 (18.0) | 80.4 (16.6) | <.01 | |
| LV ejection fraction by MRI, % | 68.6 (7.3) | 72.1 (6.1) | 68.2 (7.9) | 68.9 (7.3) | <.01 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHF, congestive heart failure; CT, computed tomography; LV, left ventricle; LVH, left ventricular hypertrophy; MI, myocardial infarction; MRI, magnetic resonance image.
SI conversion factors: To convert total cholesterol to millimoles per liter, multiply by 0.0259; to convert glucose to millimoles per liter, multiply by 0.0555.
Data are presented as mean (standard deviation) unless otherwise indicated. Numbers may not sum to total sample size because of missing data.
Overall P values were calculated using χ2 test for categorical variables and analysis of variance for continuous variables.
Diabetes mellitus was defined as a fasting glucose level of 126 mg/dL or higher or use of hypoglycemic medication.
Hypertension was defined as systolic blood pressure of 140 mm Hg or higher and/or diastolic blood pressure of 90 mm Hg or higher and/or use of antihypertensive medications.
Presence of coronary artery calcification was defined as a phantom-adjusted Agatston calcium score of more than 0.
Ethnicity and Risk Factors for Incident CHF
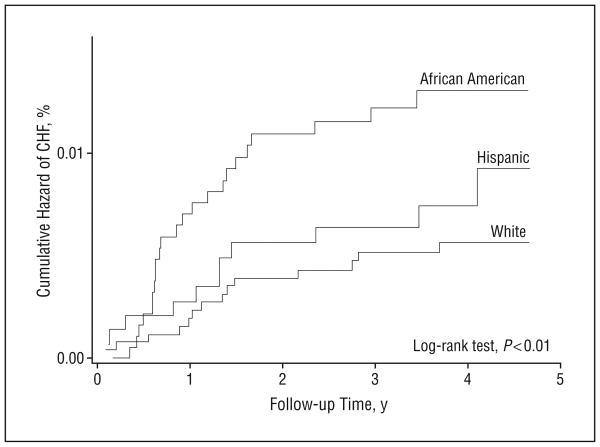
African American participants had the highest incidence rate of CHF, followed by Hispanic, white, and Chinese American participants (incidence rates: 4.6, 3.5, 2.4, and 1.0 in 1000 person-years, respectively). There was a significant difference in the cumulative hazard of CHF among the 4 racial/ethnic groups (log-rank test, P = .01) (Figure 1). In the univariable analysis, African Americans were at a higher risk for developing CHF compared with whites (HR, 1.81; 95% CI, 1.07-3.07; P = .03), but there were no significant differences in the risk for CHF among the other ethnic groups vs whites (Table 2).
Figure 1.
Nelson-Aalen plots of cumulative hazards for congestive heart failure (CHF) by racial/ethnic group in the Multi-Ethnic Study of Atherosclerosis.
Table 2. Association of Race/Ethnicity Among 6814 Participants With Congestive Heart Failure in the Multi-Ethnic Study of Atherosclerosis.
| Racial/Ethnic Group | Hazard Ratio (95% Confidence Interval)a | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Model 1 | Model 2: Established Risk Factorsb | Model 3: Established Risk Factorsb and Interim MI | Model 4: Established Risk Factors,b Interim MI, and LV Function at Baselinec | |
| White | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Chinese American | 0.37 (0.11-1.24) | 0.42 (0.13-1.43) | 0.52 (0.14-1.90) | 0.67 (0.16-2.75) |
| African American | 1.81 (1.07-3.07) | 1.42 (0.81-2.48) | 2.00 (1.11-3.61) | 1.23 (0.59-2.56) |
| Hispanic | 1.36 (0.75-2.47) | 1.22 (0.66-2.25) | 1.51 (0.71-3.23) | 1.14 (0.46-2.80) |
Abbreviations: LV, left ventricle; MI, myocardial infarction.
Boldface type indicates statistical significance at P < .05
Age, sex, hypertension, diabetes mellitus, LV hypertrophy, obesity, serum cholesterol level, and current cigarette smoking.
The LV ejection fraction determined by magnetic resonance imaging at baseline was used as a parameter of LV function.
The addition of hypertension and/or diabetes mellitus to the univariable or multivariable models including ethnicity resulted in a considerable reduction in the relative risk (ie, HR) of CHF in African Americans vs whites, and that association became no longer statistically significant. However, adjusting for age, sex, obesity, cigarette smoking status, hypercholesterolemia, and educational level did not result in any significant changes in the magnitude of the association between race/ethnicity and incident CHF and its statistical significance (Table 3).
Table 3. Changes in the Association of Race/Ethnicity With Incident Congestive Heart Failure After Adjusting for Established Risk Factors and Socioeconomic Status in 6814 Participants in the Multi-Ethnic Study of Atherosclerosis.
| Model | Racial/Ethnic Group, Hazard Ratio (95% Confidence Interval)a | |||
|---|---|---|---|---|
| White | Chinese American | African American | Hispanic | |
| Unadjusted (Model 1) | 1 [Reference] | 0.37 (0.11-1.24) | 1.81 (1.07-3.07) | 1.36 (0.75-2.47) |
| Established Risk Factors | ||||
| Model 1 + age | 1 [Reference] | 0.39 (0.12-1.29) | 1.90 (1.12-3.23) | 1.50 (0.83-2.73) |
| Model 1 + sex | 1 [Reference] | 0.37 (0.11-1.24) | 1.87 (1.10-3.17) | 1.37 (0.75-2.48) |
| Model 1 + hypertension | 1 [Reference] | 0.32 (0.10-1.09) | 1.50 (0.87-2.57) | 1.12 (0.61-2.06) |
| Model 1 + diabetes mellitus | 1 [Reference] | 0.38 (0.12-1.28) | 1.55 (0.91-2.63) | 1.36 (0.75-2.47) |
| Model 1 + obesity (BMI ≥30) | 1 [Reference] | 0.41 (0.12-1.36) | 1.71 (1.00-2.91) | 1.31 (0.72-2.39) |
| Model 1 + cigarette smoking | 1 [Reference] | 0.41 (0.12-1.37) | 1.72 (1.01-2.94) | 1.38 (0.76-2.51) |
| Model 1 + total cholesterol level | 1 [Reference] | 0.37 (0.11-1.24) | 1.82 (1.07-3.09) | 1.36 (0.74-2.46) |
| Model 1 + LV hypertrophy | 1 [Reference] | 0.33 (0.10-1.11) | 1.70 (1.00-2.92) | 1.33 (0.73-2.43) |
| Socioeconomic Factors | ||||
| Model 1 + household income | 1 [Reference] | 0.29 (0.09-0.97) | 1.66 (0.96-2.85) | 1.00 (0.53-1.89) |
| Model 1 + educational level | 1 [Reference] | 0.36 (0.11-1.21) | 1.82 (1.06-3.10) | 1.22 (0.63-2.37) |
| Access to Health Care and Medication | ||||
| Model 1 + health insurance | 1 [Reference] | 0.37 (0.11-1.24) | 1.81 (1.07-3.07) | 1.35 (0.73-2.48) |
| Model 1 + Medicare and Medicaid enrollmentb | 1 [Reference] | 0.36 (0.11-1.19) | 1.79 (1.05-3.05) | 1.32 (0.71-2.46) |
| Model 1 + ACEI use | 1 [Reference] | 0.40 (0.12-1.32) | 1.69 (1.00-2.89) | 1.33 (0.73-2.41) |
| Model 1 + β blocker use | 1 [Reference] | 0.37 (0.11-1.23) | 1.81 (1.07-3.07) | 1.37 (0.75-2.49) |
| Model 1 + CCB use | 1 [Reference] | 0.37 (1.10-1.22) | 1.66 (0.97-2.84) | 1.33 (0.73-2.42) |
| Lifestyle Factors | ||||
| Model 1 + total daily caloric intake | 1 [Reference] | 0.41 (0.12-1.37) | 1.71 (0.98-2.99) | 1.26 (0.68-2.36) |
| Model 1 + total trans-fatty acid intake per day | 1 [Reference] | 0.42 (0.12-1.42) | 1.69 (0.97-2.96) | 1.35 (0.72-2.51) |
| Model 1 + physical activity levelc | 1 [Reference] | 0.35 (0.11-1.17) | 1.89 (1.12-3.21) | 1.37 (0.75-2.49) |
| Proximal Risk Factors | ||||
| Model 1 + LVEF | 1 [Reference] | 0.73 (0.21-2.52) | 1.63 (0.86-3.12) | 1.31 (0.63-2.72) |
| Model 1 + LV mass indexd | 1 [Reference] | 0.53 (0.16-1.79) | 1.09 (0.55-2.15) | 1.00 (0.48-2.07) |
| Model 1 + coronary artery calcificatione | 1 [Reference] | 0.40 (0.12-1.32) | 2.07 (1.22-3.52) | 1.54 (0.84-2.79) |
| Model 1 + interim MI | 1 [Reference] | 0.41 (0.12-1.36) | 2.34 (1.38-3.97) | 1.44 (0.79-2.61) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CCB, calcium channel blocker; LV, left ventricle; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Variables were added to Model 1 one at a time. Boldface type indicates statistical significance at P = .05.
The interaction between Medicare and Medicaid was also included in this model.
Physical activity was determined as the sum of metabolic equivalents of all physical activities.
Left ventricle mass divided by body surface area.
Presence of coronary artery calcification was defined as a phantom-adjusted Agatston calcium score of more than 0.
We also evaluated the effects of socioeconomic and behavioral factors, as well as the influences of access to health care and medication use on racial/ethnic disparities in incident CHF. Adding household income, total daily calorie intake, total daily intake of trans-fatty acid, use of angiotensin-converting enzyme inhibitors, and use of calcium channel blockers to the analytic models yielded very similar results to the effects of adding hypertension or diabetes mellitus. However, adjustment for health insurance status, enrollment in Medicare and/or Medicaid, and physical activity yielded only negligible changes to racial/ethnic disparities in the incidence of CHF (Table 2).
Finally, in multivariable analysis, ethnicity modified the association of LV mass with incident CHF (HR for a 16-g/m2 increase in LV mass indexed by body surface area in Hispanics: 2.5 [95% CI, 1.7-3.7]; P < .01; in whites: 2.4 [1.7-3.5]; P < .01; in Chinese Americans: 1.8 [1.0-3.2]; P = .06; in African Americans: 1.4 [1.1-1.8]; P < .01). Therefore, for any given increase in the LV mass index, a greater increase in CHF risk was seen among whites and Hispanics than among Chinese Americans or African Americans. Conversely, whereas baseline LVEF was only significantly greater among Chinese Americans, there were no interactions among race/ethnicity, incident CHF, and LVEF.
Interim MI and Incident CHF
The incidence rates of MI were not statistically different among men or women from the 4 ethnic groups (Table 1). Moreover, different rates of interim MI among the 4 ethnic groups did not explain racial/ethnic differences in incident CHF rates. The incidence rates of CHF among participants who experienced an MI during follow-up were: 193, 149, 124, and 88 per 1000 person-years among African American, Hispanic, Chinese American, and white participants, respectively.
More important, however, among MESA participants who did not suffer an interim MI, African American participants had the highest incidence of CHF, followed by Hispanic, white, and Chinese American participants (incidence rates: 3.5, 2.1, 1.5, and 0 per 1000 person-years, respectively) (Figure 2). Indeed, 75.0% of incident CHF cases among African Americans, 60.0% among whites, and 58.0% among Hispanics were not preceded by an interim clinical MI (P = .06). In addition, adding interim MI to the models increased the HR of CHF among African Americans vs whites. For example, adding interim MI to Model 1 (univariable analysis) increased the HR associated with the African American vs white comparison from 1.81 (P = .03) to 2.34 (P < .01). Similarly, when interim MI was added to Model 2, this HR increased from 1.42 (P = .22) to 2.0 (P = .02) (Table 3).
Figure 2.
Nelson-Aalen plots of cumulative hazards for congestive heart failure (CHF) among Multi-Ethnic Study of Atherosclerosis participants without an interim myocardial infarction.
Finally, while the association of coronary artery calcification with incident CHF was not modified by race/ethnicity, the substitution of clinical MI by coronary artery calcification as a measure of subclinical CAD yielded similar results. For example, adding coronary artery calcification to Model 1 increased the HR associated with African American vs white participants comparison from 1.81 (P = .03) to 2.07 (P < .01) (Table 3).
Comment
This is the first population-based study to report ethnicity-related differences in the incidence of CHF among 4 major racial/ethnic groups in the United States. The incidence of CHF in our study was 3.1 per 1000 person-years, which is consistent with the estimated annual incidence of 1 to 5 in 1000 in the general population.3,4,10 Our results indicate that the incidence of CHF is greatest among African Americans, intermediate among whites and Hispanics, and lowest among Chinese Americans. We also demonstrate that these differences are in large part determined by higher prevalences of hypertension and diabetes mellitus among African Americans. Moreover, lower socioeconomic status and higher dietary caloric intake were also important factors in explaining racial/ethnic differences in the incidence of CHF. These findings suggest that the important determinants of this condition in the US are mainly environmental in nature. Finally, our study indicates that more than half of the cases of incident CHF among Americans without a history of cardiovascular diseases are not preceded by MI, and adjustment for interim infarction accentuates ethnic differences in the incidence of CHF between African Americans and whites.
As our knowledge of racial/ethnic disparities in cardiovascular diseases augments, there is growing interest in the causes of such disparities and whether they can be diminished by appropriate reduction of contributing factors. Racial/ethnic disparities in the incidence of other medical problems have been attributed to genetic and biological differences,28,29 the effects of various social and environmental factors across the lifespan,30,31 or both.32 In the case of incident CHF, our results indicate that race- and ethnicity-related differences in the prevalences of hypertension and diabetes mellitus represent the main determinants of the greater incidence of CHF among African Americans. These differences are likely related to socioeconomic and behavioral characteristics, including disparities in access to and quality of health care, as documented by previous CHF morbidity and mortality studies12 as well as our own analyses. However, possible racial/ethnic differences in susceptibility to CHF owing to underlying genetic and biological factors cannot be completely excluded.33,34
There are a number of potential mechanisms to explain how racial differences in established risk factors such as hypertension and diabetes mellitus may determine differences in incident CHF. While biological differences in the incidence of hypertension and diabetes mellitus themselves may play a role, less optimal treatment and control of these risk factors (due to less access to health care) or poor compliance with medical treatment targeting these risk factors35,36 likely represent the main contributors. Although our study does not provide a direct and definitive answer to these questions, combining our findings with those of previous studies might be helpful in identifying the most likely determinants of CHF. In our study, lower household income and higher daily caloric intake were significant predictors, even when predisposing conditions such as diabetes mellitus, hypertension, and obesity were taken into account. The lack of health insurance was not a significant contributor to the ethnic disparities of incident CHF, and differences in medication use were attributable to differences in the prevalences of diabetes mellitus and hypertension. Although our study does not support a direct role of access and quality of health care in disparities in incident CHF, it is important to mention that previous studies on the prevalence and consequences of CHF have raised the issue of ethnic disparities in quality of care received for CHF.37-39 Of note, this notion has also been challenged more recently.40-42
Our study also demonstrates racial/ethnic differences in the relationships of incident CHF with more proximal predictors of CHF, ie, interim MI, subclinical CAD, and LV hypertrophy. African American participants had the highest incidence of CHF without prior MI, followed by Hispanic, white, and Chinese American participants. Moreover, adjustment for interim MI and subclinical CAD resulted in greater differences between African Americans and whites in incident CHF risk. These differences may also be at least partially owing to discrepancies in the prevalence and control of hypertension and diabetes mellitus among racial/ethnic groups. In our study, there were significant differences between African Americans and whites not only in the prevalence of hypertension and diabetes mellitus but also in the prevalence and magnitude of LV hypertrophy measured at baseline by both ECG and the gold standard method of MRI (Table 1). In addition, whereas baseline LVEF was significantly greater only among Chinese Americans, racial/ethnic differences in baseline myocardial contractile function measured by MRI tagging have been recently reported among MESA participants.43 Previous studies have also demonstrated that risk factors such as hypertension,44 cigarette smoking, diabetes mellitus,45 and subclinical atherosclerosis,46 among others,47,48 are associated with myocardial dysfunction at study entry, defining what is known from basic studies34 as the typical protoplasm for CHF development.49
In this study, we demonstrate that the relationship between baseline LV mass and incident CHF varies by ethnicity. Moreover, although the incidence of MI was not statistically different among the 4 racial/ethnic groups, after developing an MI, African Americans were more likely to progress to CHF than whites. This observation supports previous research indicating that racial/ethnic differences play an important role in the progression from asymptomatic LV dysfunction to CHF. In the Studies of Left Ventricular Dysfunction (SOLVD), African Americans with mild to moderate LV systolic dysfunction were at higher risk for progression to CHF than whites.14 Our findings are also consistent with the results of the Vasodilator-Heart Failure Trials (V-HeFT) I and II.50 Indeed, previous studies have gone as far as suggesting different treatment strategies for CHF among different ethnic groups.51 In this regard, the accelerated disease progression to CHF from a proximal precursor such as MI among African Americans likely reflects differences in postinfarct remodeling induced by hypertension, diabetes mellitus, and other coexisting subclinical processes that are more prevalent among those who are poorer and have less access to quality medical care. Taken in combination, our findings suggest that incident CHF could be substantially reduced by aggressive control of hypertension and diabetes mellitus particularly among the poor, over and above efforts to reduce the incidence of MI and halt progression to CHF after infarction. Such efforts directed at primary prevention of incident CHF outside the clinical MI pathway should be particularly important to populations of different racial/ethnic backgrounds in the face of declining rates of coronary occlusion events. The efficacy of enhanced control of diabetes mellitus and hypertension in reducing racial/ethnic disparities in incident CHF requires further investigation.
This prospective large population-based study was performed in an ethnically diverse, asymptomatic population. The longitudinal design allowed us to measure the incidence of CHF rather than CHF prevalence, morbidity, or mortality. Nonetheless, there are limitations to this study that deserve discussion. Except for incident CHF and incident MI, all other variables were measured at baseline. Therefore, the study has limitations in making inferences regarding causal or temporal associations among baseline risk factors (eg, between household income and hypertension). Moreover, the median follow-up time was 4 years, and considering the low incidence of CHF, the results regarding relationships between incident MI and incident CHF should be interpreted cautiously. Conversely, the fact that differences between African American and white participants were significant during this relatively short follow-up period might also reflect the importance of these associations. Finally, while the diagnosis of CHF may be more challenging when compared with other cardiovascular events, we required that participants be symptomatic and have a physician-diagnosed CHF. This definition likely limited the inclusion of false-positive cases.
Conclusions
African Americans are at a significantly higher risk for incident CHF compared with other ethnic groups. This increased relative risk is further heightened for incident CHF, which is not induced by clinical MI. Our results also indicate that higher rates of hypertension and diabetes mellitus associated with poverty and other environmental factors, such as high caloric intake, largely explain racial/ethnic differences in the risk of developing CHF. These data reinforce the need for optimal control of hypertension and diabetes mellitus in the United States, particularly among those of lower socioeconomic status and among African Americans, to effectively reduce the increasing social burden of CHF in this country.
Acknowledgments
Funding/Support: This study was supported by grant R01-HL-66075 and contracts N01-HC-95159 through N01-HC-95166, N01-HC-95168, N01-HC-9808, and N01-HC-95168 from the National Heart, Lung, and Blood Institute.
Footnotes
Author Contributions: Drs Bahrami, Liu, Burke, and Lima had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Bahrami, Kronmal, Bluemke, Burke, and Lima. Acquisition of data: Bluemke, Shea, Liu, Burke, and Lima. Analysis and interpretation of data: Bahrami, Kronmal, Bluemke, Olson, Liu, and Lima. Drafting of the manuscript: Bahrami, Kronmal, and Lima. Critical revision of the manuscript for important intellectual content: Kronmal, Bluemke, Olson, Shea, Liu, Burke, and Lima. Statistical analysis: Bahrami and Kronmal. Obtained funding: Kronmal, Bluemke, Shea, Liu, Burke, and Lima. Administrative, technical, and material support: Bahrami, Kronmal, and Lima. Study supervision: Bluemke, Liu, and Lima.
Financial Disclosure: None reported.
Additional Contributions: The other investigators, the staff, and the participants of the MESA study all made valuable contributions to this article.
Additional Information: A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Yusuf S, Thom T, Abbott R. Changes in hypertension treatment and in congestive heart failure mortality in the United States. Hypertension. 1989;13((5)(suppl)):I74–I79. doi: 10.1161/01.hyp.13.5_suppl.i74. [DOI] [PubMed] [Google Scholar]
- 2.Smith WM. Epidemiology of congestive heart failure. Am J Cardiol. 1985;55(2):3A–8A. doi: 10.1016/0002-9149(85)90789-1. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Shah N. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133(6):703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 4.Tavazzi L, Opasich C. Clinical epidemiology of heart failure. Congest Heart Fail. 1999;5(6):260–269. [PubMed] [Google Scholar]
- 5.Chronic disease reports: mortality trends—United States, 1979-1986. MMWR Morb Mortal Wkly Rep. 1989;38(12):189–193. [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute . Morbidity and Mortality: Chartbook on Cardiovascular, Lung, and Blood Disease—1992. Bethesda, MD: US Dept of Health and Human Service; 1992. [Google Scholar]
- 7.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121(3, pt 1):951–957. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 8.Kenchaiah S, Narula J, Vasan RS. Risk factors for heart failure. Med Clin North Am. 2004;88(5):1145–1172. doi: 10.1016/j.mcna.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 9.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Pitt B. A lifetime of prevention: the case of heart failure. Circulation. 2002;106(24):2997–2998. doi: 10.1161/01.cir.0000046804.13847.5d. [DOI] [PubMed] [Google Scholar]
- 12.Alexander M, Grumbach K, Selby J, Brown AF, Washington E. Hospitalization for congestive heart failure: explaining racial differences. JAMA. 1995;274(13):1037–1042. [PubMed] [Google Scholar]
- 13.Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J. 1999;137(5):919–927. doi: 10.1016/s0002-8703(99)70417-5. [DOI] [PubMed] [Google Scholar]
- 14.Dries DL, Exner D, Gersh B, Cooper H, Carson P, Domanski M. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340(8):609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 15.Council of Ethical and Judicial Affairs Black-white disparities in health care. JAMA. 1990;263(17):2344–2346. doi: 10.1001/jama.1990.03440170066038. [DOI] [PubMed] [Google Scholar]
- 16.Mortality from congestive heart failure—United States, 1980-1990. MMWR Morb Mortal Wkly Rep. 1994;43(5):77–81. [PubMed] [Google Scholar]
- 17.Alexander M, Grumbach K, Selby J, Brown A, Washington E. Hospitalization for congestive heart failure: explaining racial differences. JAMA. 1995;274(13):1037–1042. [PubMed] [Google Scholar]
- 18.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–1562. [PubMed] [Google Scholar]
- 19.Eriksson H, Svardsudd K, Larsson B, et al. Risk factors for heart failure in the general population: the study of men born in 1913. Eur Heart J. 1989;10(7):647–656. doi: 10.1093/oxfordjournals.eurheartj.a059542. [DOI] [PubMed] [Google Scholar]
- 20.Ingelsson E, Arnlov J, Sundstrom J, Zethelius B, Vessby B, Lind L. Novel metabolic risk factors for heart failure. J Am Coll Cardiol. 2005;46(11):2054–2060. doi: 10.1016/j.jacc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22((4)(suppl A)):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham Study. Am J Cardiol. 1974;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 23.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multiethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 25.Williams SM, Templeton AR, Swallen KC, Cooper RS, Kaufman JS. Race and genomics. N Engl J Med. 2003;348(25):2581–2582. doi: 10.1056/NEJM200306193482521. [DOI] [PubMed] [Google Scholar]
- 26.Wijeysundera HC, Hansen MS, Stanton E, et al. Neurohormones and oxidative stress in nonischemic cardiomyopathy: relationship to survival and the effect of treatment with amlodipine. Am Heart J. 2003;146(2):291–297. doi: 10.1016/S0002-8703(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 27.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31(3):157–187. [PubMed] [Google Scholar]
- 28.Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 29.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-comment2007. published online ahead of print July 1, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper RS. Race, genes, and health: new wine in old bottles? Int J Epidemiol. 2003;32(1):23–25. doi: 10.1093/ije/dyg036. [DOI] [PubMed] [Google Scholar]
- 31.Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348(12):1166–1170. doi: 10.1056/NEJMsb022863. [DOI] [PubMed] [Google Scholar]
- 32.Karter AJ. Commentary: race, genetics, and disease—in search of a middle ground. Int J Epidemiol. 2003;32(1):26–28. doi: 10.1093/ije/dyg033. [DOI] [PubMed] [Google Scholar]
- 33.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 35.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertension. 2004;17(10):963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119(1):70.e9–70.e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in Medicare managed care. JAMA. 2002;287(10):1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 38.Jha AK, Varosy PD, Kanaya AM, et al. Differences in medical care and disease outcomes among black and white women with heart disease. Circulation. 2003;108(9):1089–1094. doi: 10.1161/01.CIR.0000085994.38132.E5. [DOI] [PubMed] [Google Scholar]
- 39.Ayanian JZ, Weissman JS, Chasan-Taber S, Epstein AM. Quality of care by race and gender for congestive heart failure and pneumonia. Med Care. 1999;37(12):1260–1269. doi: 10.1097/00005650-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J. 2006;152(2):348–354. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Gordon HS, Johnson ML, Ashton CM. Process of care in Hispanic, black, and white VA beneficiaries. Med Care. 2002;40(9):824–833. doi: 10.1097/00005650-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289(19):2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes VR, Agarwal S, Cheng YJ, et al. Race/ethnic relationship with regional myocardial function in an adult asymptomatic population for cardiovascular disease: a tagged MRI study of the MESA cohort. Circulation. 2006;114(18):538–539. [Google Scholar]
- 44.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47(6):1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 45.Bertoni AG, Goff DC, Jr, D'Agostino RB, Jr, et al. Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2006;29(3):588–594. doi: 10.2337/diacare.29.03.06.dc05-1501. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47(12):2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 47.Bahrami H, Bluemke D, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity in the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;51(18):1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 48.Nasir K, Tsai M, Rosen BD, et al. Elevated homocysteine is associated with reduced regional left ventricular function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;115(2):180–187. doi: 10.1161/CIRCULATIONAHA.106.633750. [DOI] [PubMed] [Google Scholar]
- 49.Mann DL. MicroRNAs and the failing heart. N Engl J Med. 2007;356(25):2644–2645. doi: 10.1056/NEJMcibr072068. [DOI] [PubMed] [Google Scholar]
- 50.Carson P, Ziesche S, Johnson G, Cohn JN, for the Vasodilator-Heart Failure Trial Study Group Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5(3):178–187. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- 51.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]