Abstract
Oxygen and carbon dioxide levels vary in different environments and locally fluctuate during respiration and photosynthesis. Recent studies in diverse animals have identified sensory neurons that detect these external variations and direct a variety of behaviors. Detection allows animals to stay within a preferred environment as well as identify potential food or dangers. The complexity of sensation is reflected in the fact that neurons compartmentalize detection into increases, decreases, short-range and long-range cues. Animals also adjust their responses to these prevalent signals in context of other cues, allowing for flexible behaviors. In general, the molecular mechanisms for detection suggest that sensory neurons adopted ancient strategies for cellular detection and coupled them to brain activity and behavior. This review highlights the multiple strategies that animals use to extract information about their environment from variations in oxygen and carbon dioxide.
Introduction
Carbon dioxide (CO2) and oxygen (O2) are the substrates and products for maintaining life on earth. Because these two gases are essential, organisms have evolved sophisticated homeostatic mechanisms to ensure that appropriate internal concentrations are maintained. For example, if a jogger runs up a hill, arterial chemoreceptors in the carotid body sense a rapid reduction of oxygen in the bloodstream and elicit panting to increase O2 intake (Gonzalez et al., 1992). In addition to internal monitoring of O2 and CO2, it has become increasingly clear that animals also monitor external concentrations and use this information to direct a variety of behaviors.
In the atmosphere, oxygen levels are 21% and carbon dioxide levels are a trace 0.038%. However, in subterrestrial and aquatic environments, the concentrations of these substances vary enormously. Animals that live in these environments monitor external concentrations as a homeostatic mechanism to stay within a preferred concentration range that meets their metabolic needs. Fish gills have specialized chemoreceptor cells that sense variations in oxygen or carbon dioxide in the environment (Jonz et al., 2004; Qin et al., 2010). Indeed, the size and shape of a school of fish may be a trade-off between access to oxygen-rich water at peripheral edges of the school and safety from predators in the middle (Brierley and Cox, 2010). Soil-dwellers such as the nematode Caenorhabditis elegans also have sensory neurons that detect variations in oxygen and carbon dioxide, allowing them to stay within their preferred environment (Gray et al., 2004; Cheung et al., 2005; Zimmer et al., 2009). Even animals that live in enclosed spaces may monitor ambient concentrations. When CO2 levels in the hive increase by ~1–2%, honeybees exhibit fanning behavior to ventilate the nest in order to maintain a low CO2 environment (Seeley, 1974).
CO2 emitted during respiration may also serve as a secreted chemical signal that other animals detect. In this way, CO2 may act as a chemosensory signal that alerts animals to potential food, predators or danger. Blood-feeding insects such as mosquitoes, black flies and tsetse flies are attracted to CO2 and use this signal to hone in on their human hosts (Gibson and Torr, 1999). The hawkmoth, Manduca Sexta, prefers flowers that emit a high level of CO2, suggesting that CO2 acts as a proximal signal for nectar (Guerenstein et al., 2004; Thom et al., 2004). CO2 increases can also signal avoidance, as CO2 emitted by Drosophila upon stress acts as a signal for other Drosophila to flee (Suh et al., 2004).
How do animals detect and respond to varying concentrations of O2 and CO2 in their environment? Recent studies of the model organisms C. elegans, Drosophila melanogaster and mice have begun to elucidate the neural and molecular bases of detection. In all cases, detection occurs in specialized sensory cells; in Drosophila and mice, subsets of olfactory and gustatory neurons respond specifically to CO2. In most cases, these neurons respond to discrete features in their environment, such as increases or decreases in oxygen, short-range or long-range cues. Detection can lead to attraction or avoidance behavior, and these behaviors are plastic. Plasticity may be especially important to allow animals to interpret the rather non-specific signals of O2 and CO2 in the context of their complex sensory world. The molecular underpinnings of detection are beginning to be elucidated, highlighting similarities across organisms, and commonalities with ancient cellular mechanisms of detection.
Staying within a preferred concentration range: Oxygen sensing in C. elegans and Drosophila
The nematode C. elegans lives in the soil. Oxygen levels in this environment vary from 1–21%, depending on depth from the surface as well as soil properties such as compaction, aeration and drainage (Anderson and Ultsch, 1987). C. elegans show a behavioral preference for 5–10% O2 levels and avoid higher and lower concentrations (Gray et al., 2004). This preferred oxygen setpoint may reflect a compromise between the metabolic needs of the animal (favoring high oxygen) and oxidative stress (favoring low oxygen) (Lee and Atkinson, 1977). The study of C. elegans oxygen sensation has provided a framework for understanding how animals monitor gas levels to select a preferred environment.
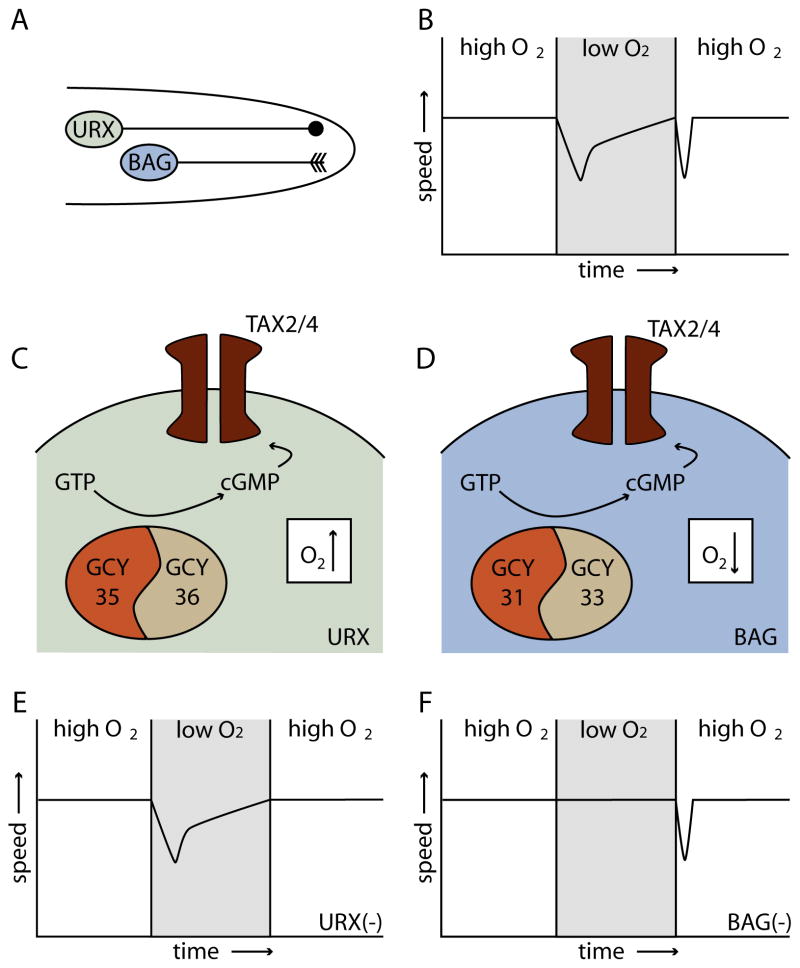
Recent progress has been made elucidating the neural and molecular bases for hyperoxia avoidance. Two pairs of neurons, URX and BAG, play critical roles in sensing oxygen (Zimmer et al., 2009) (Figure 1). URX is a pair of unciliated sensory neurons whose dendrites extend toward the tip of the nose (White et al., 1986). BAG neurons have bag-like dendrites that extend near the lateral lips (Perkins et al., 1986; White et al., 1986). Both URX and BAG neurons respond to changes in oxygen in the environment, but have different response properties and are associated with different behaviors. URX neurons depolarize in response to O2 increases, responding best to upshifts between 10–12% to 15–20% O2 (Zimmer et al., 2009). These neurons are essential for the aggregation behavior that C. elegans displays in response to high O2 and aerotaxis responses to O2 increases (Coates and de Bono, 2002; Gray et al., 2004; Zimmer et al., 2009). The BAG neurons, in contrast, respond to decreases in O2 levels, depolarizing upon downshifts to preferred concentrations (5%) (Zimmer et al., 2009). These neurons mediate aerotaxis response to O2 downshifts (Zimmer et al., 2009).
Figure 1. C. elegans senses increases and decreases in oxygen.
A. The URX and BAG neurons respond to O2.
B. Behavioral response to decreases and increases in O2, measured as a slowing behavior. Changing the oxygen concentration to either higher or lower values causes a temporary slowing.
C. URX neurons sense O2 increases with the guanylate cyclases GCY35 and GCY36, causing an increase in cGMP and opening of cGMP-gated channels (TAX2/4).
D. BAG neurons sense O2 decreases with GCY31 and GCY33, increasing cGMP and opening TAX2/4.
E. Animals lacking URX neurons respond to decreases but do not respond to increases.
F. Animals lacking BAG neurons do not respond to decreases but still detect increases.
Graphs in BEF are schematics based on data in Zimmer et al, 2009.
Soluble guanylate cyclases are expressed in the O2 sensing neurons and mediate recognition. C. elegans have 7 atypical, β-like, soluble GCs (Morton, 2004), four of which have been shown to participate in hyperoxic avoidance. gcy-35 and gcy-36 are expressed in URX and together mediate responses to O2 increases (Cheung et al., 2004; Gray et al., 2004; Cheung et al., 2005; Chang et al., 2006). gcy-31 and gcy-33 are required in BAG neurons for responses to O2 decreases (Zimmer et al., 2009) (Figure 1). Guanylate cyclases are gas sensors that contain a heme-binding domain fused to a cyclase enzymatic domain that converts GTP to cGMP (Boon and Marletta, 2005). For canonical GCs, the heme-binding domain selectively binds the reactive gas nitric oxide and excludes O2; a small change in the binding pocket of GCY-35 alters the ligand selectivity such that the heme binds oxygen (Gray et al., 2004).
How do oxygen increases activate URX while decreases activate BAG? For URX, the model is that GCY-35 and GCY-36 sense an increase in O2, activating the cyclase leading to cGMP production, the opening of CNG ion channels (TAX-2/TAX-4) and cell depolarization (Coates and de Bono, 2002; Cheung et al., 2004; Gray et al., 2004; Zimmer et al., 2009). For BAG, GCY-31 and GCY-33 are activated by a decrease in O2, triggering cyclase activity (Zimmer et al., 2009). Thus, the cyclases themselves are thought to show opposite responses to O2, with GCY-35/36 activated and GCY-31/33 inhibited by oxygen increases. This model predicts that responses to increased and decreased O2 are the property of the cyclase not the neuron. Consistent with this, placing GCY-35 and GCY-36 in BAG neurons (in a gcy-31, gcy-33 double mutant background) causes these neurons to respond to O2 upshifts rather than downshifts (Zimmer et al., 2009).
Interestingly, Drosophila also contains three atypical guanylate cyclases that participate in oxygen-mediated behaviors: Gyc-89Da, Gyc-89Db and Gyc-88E. Gyc88E clusters in a phylogenetic tree with C. elegans GCY-31 and Gyc-89Da/b cluster with GCY-33 (Morton, 2004; Zimmer et al., 2009). Gyc-88E can act as a homodimer or as a heterodimer in conjunction with Gyc-89Da or Gyc-89Db, all of which increase cyclase activity under anoxic conditions (Morton, 2004). Purified Gyc-88E binds O2, and cyclase activity is inhibited as O2 increases (Huang et al., 2007). This argues that these cyclases are activated in the absence of O2, similar to the model for GCY-31 and GCY-33.
Behaviorally, Drosophila larvae avoid hypoxic conditions (Wingrove and O'Farrell, 1999). When there is a decrease in O2 levels, larvae leave the food and wander. Mutants in any of the three Gycs reduce wandering under hypoxic conditions (Vermehren-Schmaedick et al., 2010). When larvae are exposed to hyperoxic or hypoxic environments they decrease stops and turns, suggesting escape behavior. Mutants in gyc-89Da do not show this decrease to hypoxia (11–16% oxygen) and gyc-89Db mutants do not show this decrease to mild hypoxia (18–20%) or hyperoxia (22–30%) (Vermehren-Schmaedick et al., 2010). Thus, different Gycs sense different O2 environments.
A common theme emerging from the studies of oxygen sensation in C. elegans and Drosophila is that sensory cells respond to selective features of O2 in the environment. For C. elegans, one set of oxygen-sensing neurons responds to O2 increases and the other to O2 decreases in hyperoxic environments. For Drosophila, one set is necessary for hyperoxic avoidance; the other for hypoxic avoidance. These animals do not have a single class of O2-sensing neuron that responds best to a preferred concentration; instead they have different sets of neurons to monitor changing concentrations or values above and below the preferred setpoint. The finding that animals use different receptors and cells tuned to different O2 concentrations is reminiscent to what is seen in mammalian thermosensation where different TRP ion channels respond best to different temperature ranges (Jordt et al., 2003). By having some channels tuned for cool environments and others tuned for hot environments, animals can identify their preferred temperature and avoid thermal extremes. A similar strategy in oxygen sensing may allow animals to resolve small variations in their environment and optimize their responses to changing conditions.
Differential detection of long range and short-range cues: CO2 detection in mammals
In addition to monitoring atmospheric gases to maintain favorable environments, animals use long-range and short-range variations to extract information about predators, hosts and food. Carbon dioxide detection may be useful to stay within a low CO2 environment or to detect a specific signal. In many cases the biological relevance of CO2 detection is unknown, as all plants and animals emit CO2 during respiration.
C. elegans show acute avoidance to carbon dioxide, avoiding levels as low as 0.5–1% above ambient concentrations (Bretscher et al., 2008; Hallem and Sternberg, 2008). This avoidance is greatly reduced when BAG neurons are ablated (Hallem and Sternberg, 2008), arguing that the neurons that sense oxygen decreases also sense carbon dioxide increases. Avoidance requires the TAX-4 CNG-channel (Bretscher et al., 2008; Hallem and Sternberg, 2008) but does not require GCY-31/33 (Hallem and Sternberg, 2008). Thus, carbon dioxide sensing and oxygen sensing may be partially mediated by BAG neurons through activation of the same CNG channels but different receptor mechanisms. The molecular sensors for carbon dioxide detection in C. elegans are unknown.
Mammals also sense CO2 in the environment. Recent studies of mammalian CO2 detection have provided insight into cellular and molecular mechanisms of detection. In mammals, CO2 is sensed by both the olfactory system and the gustatory system, demonstrating an unexpected complexity in detection (Figure 2).
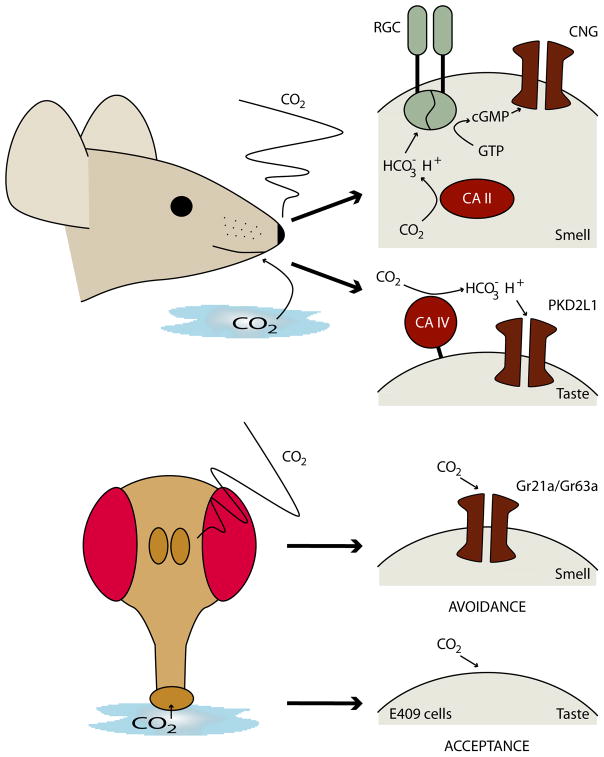
Figure 2. Mice and Drosophila sense CO2 with their olfactory and gustatory systems.
Top panels shows mouse detection of CO2. The top right panel shows the signaling cascade proposed for CO2 detection by the olfactory system. CAII senses CO2, producing HCO3− that activates RGC. RGC produces cGMP, opening CNG channels. The second right panel shows detection by the gustatory system. CAIV produces protons that activate the proton-gated channel, PKD2L1/PKD1L3.
Bottom panels show CO2 detection by Drosophila. Olfactory neurons sense CO2 with Gr21a/Gr63a, leading to avoidance behavior. Gustatory neurons marked by the enhancer trap E409 sense CO2, leading to acceptance behavior.
Although carbon dioxide concentrations up to 30% are odorless to humans (Shusterman and Avila, 2003), mice smell CO2 and show innate avoidance at around 0.2% (Hu et al., 2007). Olfactory neurons have been identified that depolarize in response to CO2, with a detection threshold of 0.1%, consistent with the behavioral threshold (Hu et al., 2007). The olfactory neurons in mouse that respond to CO2 are different from most olfactory neurons. First, whereas most olfactory neurons express members of the odorant receptor family, an olfactory-specific G protein called Golf and adenylate cyclase, the CO2-sensing neurons express a unique complement of signaling molecules involved in CO2 detection (Fulle et al., 1995; Juilfs et al., 1997; Meyer et al., 2000; Hu et al., 2007). Second, these neurons show unusual axonal projection patterns in the first relay the olfactory bulb (Juilfs et al., 1997). In general, olfactory neurons that express the same receptor project to a single glomerulus; CO2-sensing olfactory neurons target a string of caudal glomeruli called necklace glomeruli that are anatomically segregated from other olfactory projections. These differences suggest the CO2 detection system forms a distinct subsystem of the main olfactory system.
The molecules specifically expressed in CO2 neurons provide insight into CO2 detection (Figure 2). A soluble carbonic anhydrase (CAII) and a receptor guanylate cyclase (GC-D) may couple CO2 detection to the production of the second messenger cGMP and cell depolarization (Fulle et al., 1995; Juilfs et al., 1997; Hu et al., 2007; Sun et al., 2009). Carbonic anhydrases are enzymes that catalyze the conversion of CO2 into carbonic acid, bicarbonate ions and protons (Tashian, 1989). Receptor guanylate cyclases (RGC), unlike the soluble guanylate cyclases used in C. elegans oxygen sensation, are single-pass transmembrane proteins with an extracellular ligand binding domain coupled to an intracellular cyclase domain (Wedel and Garbers, 1997). RGCs function as dimers, lack a heme domain, and are activated by binding small peptides. The current model for olfactory sensing is that CO2 diffuses through the membrane and is acted upon by CAII to produce bicarbonate. Bicarbonate then activates GC-D, opening CNGA3 channels and causing cell depolarization (Luo et al., 2009; Han and Luo, 2010). In support of this model, carbonic anhydrase inhibitors block CO2 cellular responses and car2 mutants do not show behavioral responses to CO2 (Hu et al., 2007). In addition, although the biochemical mechanism of activation has not been established, it has been shown that bicarbonate can activate cGMP production when GC-D is expressed in heterologous cells (Guo et al., 2009; Sun et al., 2009). Moreover, cellular and behavioral CO2 responses are absent in animals lacking the CNGA3 channel (Han and Luo, 2010). However, many aspects of this model remain to be tested; for example, the requirement for CAII or GC-D for cellular activation has not been established.
Other studies of GC-D olfactory neurons have shown that they respond to the small peptides guanylin and uroguanylin (Leinders-Zufall et al., 2007) and carbon disulfide (CS2) (Munger et al., 2010). Guanylin and uroguanylin detection requires GC-D but not CAII, whereas CS2 detection is absent in car2 mutants and reduced in gc-d mutants (Munger et al., 2010). The responses to CS2 or peptides were reported to be about 10,000 fold more sensitive than the responses to CO2 (Munger et al., 2010). These results call into question the natural ligand for these cells. One interpretation is that the CO2 sensing neurons may be multimodal neurons that integrate detection of multiple cues. Second-order neurons that synapse onto necklace glomeruli, the sites where GC-D neurons project, also respond to multiple cues. 10% of mitral/tufted cells in proximity of necklace glomeruli respond to CO2 and are activated or inhibited by a small number of other odors (Gao et al., 2010). Together, these findings suggest that CO2 is not processed by a dedicated olfactory channel. Instead, CO2 signals may be integrated with other cues very early on in the olfactory pathway. One way that an animal could glean information from emission of a generic molecule like CO2 would be to couple its detection to that of other odors or peptides.
Whereas the olfactory system mediates long-range detection of volatile CO2, the gustatory system mediates short-range detection. Humans obviously appreciate carbonated beverages but the taste of carbonation does not clearly fall within the classic taste modalities of sweet, bitter, sour, salt or umami. Only recently have there been studies to examine the molecular basis for the taste of carbonation. Taste cells on the mammalian tongue respond to different taste modalities: sugar, bitter, sour and salt-sensing cells have been identified (Yarmolinsky et al., 2009). Sour-sensing cells express a membrane-tethered extracellular carbonic anhydrase (CAR4) (Chandrashekar et al., 2009) in addition to an ion channel PKD2L1/PKD1L3 that can be activated in response to acidic solutions (Huang et al., 2006; Ishimaru et al., 2006; Inada et al., 2008). These cells respond not only to acids but also to carbonation, with a dose sensitive response between 6–30% CO2 (Chandrashekar et al., 2009). Animals that lack car4 or animals in which PKD2L1 cells have been genetically ablated do not show taste responses to carbonation (Chandrashekar et al., 2009). The most parsimonious model for cell activation is that carbonic anhydrase activity produces protons that are sensed by the proton-sensitive channel (Figure 2). How the taste of carbonation differs from sour taste in this model is unclear; however, somatosensory neurons may contribute. It is interesting to speculate that carbonic anhydrase on the tongue may have evolved as a strategy to maintain an appropriate pH environment, similar to its function in blood and other tissues (Tashian, 1989). That specific sensory cells sense the breakdown products suggests that a cellular defense system to maintain acid-base balance may have been co-opted for flavor. Anecdotally, mountain climbers who take the carbonic anhydrase inhibitor acetazolamide to combat altitude sickness report that beer and soda taste flat (“the champagne blues”) (Graber and Kelleher, 1988), hinting that carbonic anhydrases may mediate the taste of carbonation in humans.
For mammals, CO2 may function as a taste or a smell depending on the sensory neurons that detect it. Why use two different senses to detect CO2? The olfactory system is sensitive to levels barely above average atmospheric levels, suggesting that it monitors CO2 in the environment to avoid high concentrations. In contrast, the gustatory system specifically detects high CO2 concentrations on the tongue and acts as a gatekeeper for ingestion. The detection of CO2 by different sensory modalities in mammals allows them to extract additional information about the location of CO2 and use this information to fine-tune behavior.
Generating different behaviors with the same cue: CO2 detection in Drosophila
Like mammals, Drosophila also use specialized olfactory and gustatory neurons to detect changes in CO2 levels (Figure 2). Flies sense olfactory cues with neurons on the third antennal segment and the maxillary palp. Three receptor families are expressed in different subpopulations of olfactory neurons: Drosophila odorant receptors, ionotropic glutamate receptors, and gustatory receptors (Su et al., 2009). Each neuron expresses one or a few members of a single receptor family and responds to a subset of odors. Neurons with the same receptors project to the same glomeruli in the antennal lobe, creating a spatial map of different odors in the first relay. The combinatorial activity of glomeruli in response to different odors provides the potential to encode thousands of different odors.
CO2 is unlike most odors in that it activates only one class of olfactory neurons and is the sole compound that activates them (Suh et al., 2004). The CO2 sensing neurons are ab1c sensilla on the antennae that project to the most ventral glomerulus in the antennal lobe (V) (Suh et al., 2004). Calcium imaging experiments revealed that the V glomerulus is exquisitely sensitive to CO2, with dose-sensitive responses from 0.05- 10% CO2 above atmospheric levels. No other glomeruli respond to CO2. The finding that there is a single olfactory channel for CO2 suggests that this may act as a labeled line transmitting CO2 detection into a stereotyped behavior. Indeed, flies avoid volatile CO2 and this avoidance requires ab1c neurons (Suh et al., 2004; Faucher et al., 2006). Moreover, inducibly activating ab1c neurons elicits avoidance behavior: flies in which channelrhodopsin-2 (a blue-light gated ion channel from Chlamydomonas reinhardtii) (Nagel et al., 2003) is expressed in ab1c neurons avoid blue light (Suh et al., 2007). Thus, unlike mammalian olfactory detection, flies use a dedicated channel for CO2 detection that is tethered to avoidance behavior.
Two members of the gustatory receptor (GR) family, Gr21a and Gr63a, are expressed specifically in the ab1c neurons in the adult as well as single CO2-sensing neurons in larvae (Scott et al., 2001; Jones et al., 2007; Kwon et al., 2007) (Figure 2). Although most members of the GR gene family are expressed in gustatory neurons and mediate taste detection, a few are expressed in the antenna (Scott et al., 2001). Demonstration of their function in CO2 detection came from studies of Gr63a mutants, which do not show cellular or behavioral responses to CO2 (Jones et al., 2007). Moreover, exogenous co-expression of Gr63a and Gr21a confers CO2 responses, arguing that they are the sensors (Jones et al., 2007; Kwon et al., 2007).
CO2 is an important signal for many insects, including blood-feeders and plant-feeders. Orthologues of Gr21a and Gr63a are present in the twelve sequenced Drosophilid species as well as mosquitoes, silk moths and flour beetles, suggesting the conservation of CO2 detection and receptors (Robertson and Kent, 2009). The non-Dipterans have a third gene highly related to Gr21a that is co-expressed with the other two genes in the malaria vector Anopheles gambiae (Lu et al., 2007). Misexpressing the three A. gambiae orthologues in Drosophila olfactory neurons demonstrated that all three genes participate in CO2 detection (Lu et al., 2007). Thus, studies of Drosophila CO2 detection have provided insight into the problem of how disease-carrying insects are attracted to their human hosts. As there are more than 300 million cases of malaria each year, associated with 1–3 million deaths, these studies have important implications for limiting the spread of disease.
In addition to olfactory detection of CO2, recent studies have demonstrated that the gustatory system also detects CO2. Like mammals, Drosophila distinguish a few taste qualities and have modality-specific taste cells, including sugar-, bitter- and water-sensing neurons (Thorne et al., 2004; Wang et al., 2004; Marella et al., 2006; Cameron et al., 2010). Chemosensory bristles on the proboscis, legs, wings and ovipositor and taste pegs on the proboscis labellum contain gustatory neurons (Stocker, 1994). The taste pegs labeled by the enhancer trap E409 house the neurons that selectively respond to CO2 in solution (Fischler et al., 2007). Unlike olfactory CO2 sensing neurons, the gustatory neurons require high CO2 concentrations for detection, with aqueous CO2 activating at 0.2% and volatile CO2 activating at 10%. Behaviorally, flies show a weak preference for CO2 in solution, taste peg CO2 sensors mediate this preference, and artificially activating these neurons also triggers acceptance behavior. The molecules responsible for detection have not been described. Why do flies taste CO2? One possibility is that it acts as a proxy for detecting growing microorganisms like yeast that emit CO2 and are consumed by flies to obtain essential nutrients.
Taken together, these studies highlight the importance of CO2 detection for insects and demonstrate that CO2 acts as a repellent in air and a palatable taste in solution. Like mammals, flies detect CO2 with the gustatory system and the olfactory system. Long-range and short-range, volatile and non-volatile CO2 may be interpreted as different cues triggering different behaviors. The gustatory and olfactory systems compartmentalize the CO2 environment to allow animals to respond differently depending on the CO2 source. It is interesting to speculate that CO2 detection by both the olfactory and gustatory systems may co-operate to determine the value of a food source. Perhaps flies accept rotting fruit with high local concentrations of growing yeast, but avoid it once yeast produce enough CO2 for long-range detection. In this scenario, the taste and smell of CO2 would allow the fly to identify fruit with the right amount of rottenness. Of course, studies of plasticity argue that there are multiple ways to modulate the CO2 response (see below).
The finding that a single compound can act as either a taste or a smell is not unique to CO2. Recent studies of water detection in Drosophila argue that there are olfactory neurons that respond to high or low humidity (Liu et al., 2007) and gustatory neurons that detect water to elicit drinking behavior (Cameron et al., 2010). A general strategy that animals may use to mine additional information about important yet common compounds like water and CO2 is to set up multiple methods of detection that are context-dependent.
Strategies for behavioral adaptability and plasticity
Although oxygen and carbon dioxide are associated with innate behaviors in C. elegans, Drosophila and mammals, these behaviors are also plastic allowing animals to adjust their responses depending on the environment. As both O2 and CO2 are generic signals emitted by numerous organisms, their ability to be interpreted in the context of other sensory cues is essential. Two examples illustrate this plasticity well: one is variation in oxygen sensation in different C. elegans strains, the second is modulation of olfactory CO2 avoidance behavior in Drosophila.
Two commons strains of C. elegans show dramatically different behaviors when placed on a lawn of bacteria (the food supply for C. elegans). Many worm strains including the Hawaiian strain HW move rapidly, prefer the borders of the lawn and aggregate in groups whereas the N2 laboratory strain moves slowly and shows a solitary wandering behavior (de Bono and Bargmann, 1998). Some elements of this behavior are due to variations in oxygen avoidance behavior. Bacterial lawns consume oxygen, creating local oxygen gradients with low O2 at thick borders and high O2 in the center (Gray et al., 2004). Under low oxygen conditions, HW strains show solitary behavior rather than aggregate at the borders. Thus, the aggregation behavior is partially explained as an oxygen avoidance behavior: most strains avoid high O2 in the presence and absence of food; N2 strains avoid high O2 in the absence of food but this avoidance is overridden in the presence of food (Gray et al., 2004; Cheung et al., 2005; Rogers et al., 2006).
Two genetic differences between N2 and HW have been identified that explain much of the behavioral variation (McGrath et al., 2009). First, changes in a globin protein GLB-5 modulate the oxygen sensing behavior (McGrath et al., 2009; Persson et al., 2009). Globin domain proteins are heme proteins important for O2 transport and storage (Weber and Vinogradov, 2001). A partial duplication in glb-5 in N2 strains behaves as a recessive mutation, creating a difference in O2 sensing (McGrath et al., 2009; Persson et al., 2009). GLB-5 acts in URX neurons that sense increased O2 levels and sensitizes these neurons to small changes in O2. For example, URX neurons respond to shifts from 20% O2 to 21% O2 in HW strains but not in N2 strains (McGrath et al., 2009; Persson et al., 2009). Thus, one difference between HW and N2 is that N2 is less sensitive to changes in ambient O2 than HW. However, N2 animals still avoid O2 in the absence of food, consistent with a subtle change in oxygen sensing rather than an inability to detect O2.
A second major difference is in a neuropeptide receptor (NPR) similar to the neuropeptide F receptor involved in feeding in mammals (de Bono and Bargmann, 1998). N2 animals have a polymorphism in npr (215V) making it more active; other strains have a different polymorphism (215F) making it less active. An npr mutant displays bordering and aggregation similar to the 215F variant. Thus, competing forces are thought to produce the solitary versus aggregation behavior: aversive cues (including oxygen) promote aggregation whereas other cues promote solitary behavior (de Bono et al., 2002; Gray et al., 2004; Cheung et al., 2005; Rogers et al., 2006). In the N2 strain, a more active NPR signaling pathway and a less active O2 sensing pathway promote solitary behavior. In HW strains, a less active NPR pathway and a more active O2 sensing pathway promote aggregation. Interestingly, N2 likely arose during selection for survival in a laboratory environment: maintaining C. elegans at atmospheric O2 on agar dishes plated with bacteria likely selected for animals that find high O2 less aversive and move slowly on bacterial lawns (McGrath et al., 2009).
Another example of plasticity in behavior comes from studies of carbon dioxide avoidance in Drosophila. Although olfactory CO2 detection mediates aversive behavior, this behavior can be modulated by context. For example, flies exposed to 5% CO2 for several days showed decreased CO2 avoidance, correlating with changes in activity in the antennal lobe, the first processing station for olfaction (Sachse et al., 2007). The response of sensory neurons did not change, the response of local inhibitory neurons increased and the response of second-order projection neurons decreased. Thus, changes in signal propagation likely allow an animal to adapt to long-term exposure of increased CO2.
Plasticity at the level of the sensory neuron also occurs. In a screen of 46 odorants, ab1c olfactory neurons (Gr21a/Gr63a) were found to be strongly activated by CO2 and inhibited by 1-hexanol and 2,3-butanedione (Turner and Ray, 2009). Intriguingly, 1-hexanol and 2,3-butanedione appear to inhibit the CO2 response directly, as they inhibit the response to CO2 but not other odors when Gr21a/Gr63a are misexpressed in the antenna, under conditions where lateral inhibition is unlikely (Turner and Ray, 2009). Both 1-hexanol and 2,3-butanedione are present in ripe bananas (favorite food of fruitflies) but not unripe ones, increasing several hundred-to several thousand-fold during the ripening process (Mayr et al., 2003; Turner and Ray, 2009). As flies are attracted to odors from ripe bananas that contain CO2, it is possible that emission of other compounds directly inhibits Gr21a/Gr63a and blocks CO2 avoidance responses.
The adaptability of O2 and CO2 detection occurs both on a time scale of generations (C. elegans oxygen sensation) as well rapidly during the life of an animal (Drosophila CO2 olfactory detection). Genetic changes allow altered behavior to long-term changes in environmental conditions, whereas activity-dependent plasticity or modulation by other sensory cues allows more rapid re-adjustments in behavior.
Molecular strategies for CO2 and O2 detection in sensory systems
Although the molecular bases for sensory detection of O2 and CO2 are still being unraveled, some principles of detection are beginning to emerge. For oxygen sensation in C. elegans and Drosophila larvae, soluble guanylate cyclases are essential for detection. sGCs contain a heme binding domain called H-NOX (Heme-Nitric oxide and oxygen binding domain) (Iyer et al., 2003; Karow et al., 2004). This domain is found in bacteria and the animal lineage of eukaryotes, but absent in other eukaryote lineages and archaea. The domain itself can comprise a protein or can be linked to other domains as in the case of guanylate cyclases and some bacterial chemotaxis receptors. Although sGCs have long been known to bind NO and exclude O2, studies over the last ten years have shown that subtle changes in the heme binding domain can reverse the selectivity for O2 and NO (Boon and Marletta, 2005). Studies of sGCs in C. elegans provided critical evidence that these proteins can function as oxygen sensors (Gray et al., 2004). For C. elegans and Drosophila oxygen sensing, ancient heme-based sensors were co-opted by sensory cells to transform detection into a change in neural activity in the brain and animal behavior.
In the case of CO2 detection, sensors have been identified in the mammalian gustatory and olfactory systems and Drosophila olfaction. In mammalian detection, carbonic anhydrases play a central role. These enzymes are found in bacteria and algae, and participate in fundamental processes such as photosynthesis, respiration and acid-base homeostasis (Tashian, 1989). CAs catalyze the reaction of CO2 and water into the intermediate carbonic acid which is instantaneously converted to bicarbonate ions and protons. Different products of CA can act as messengers for signaling: bicarbonate is proposed to activate a receptor guanylate cyclase in mammalian olfactory neurons and protons are proposed to gate a pH-sensitive channel in gustatory neurons. Thus, these cells have also adopted existing strategies for detection and coupled them to brain and behavior. Similarly, chemoreceptors on fish gills and plant stomatal guard cells both sense CO2 in the environment and require carbonic anhydrases for detection (Hu et al., 2010; Qin et al., 2010).
Does sensory detection occur without CA involvement? Drosophila olfactory neurons detect CO2 with two gustatory receptor genes, gr21a and gr63a. GRs are multi-pass transmembrane domain proteins, with most similarity to Drosophila odorant receptors (Robertson et al., 2003). As Drosophila odorant receptors have recently been proposed to function as ligand-gated ion channels with some capacity to activate G proteins (Sato et al., 2008; Wicher et al., 2008), this may also be the case for GRs. CO2 may directly activate GRs, as misexpressing the receptors in heterologous olfactory neurons confers CO2 responses (Jones et al., 2007; Kwon et al., 2007). In this scenario, the function of Gr21a/Gr63a may be akin to Rhesus proteins (Rh), which act as ion channels/transporters directly gated by CO2 (Kustu and Inwood, 2006). Alternatively, it is possible that CAs act upstream of Gr21a/Gr63a and that these receptors detect a reaction product, similar to the mechanism thought to underly mammalian taste. Understanding carbon dioxide detection in additional sensory systems may shed more light on the diversity of CO2 sensors.
Concluding remarks
The ability to extract information about subtle changes in oxygen levels, or carbon dioxide on the tongue or in the air, affords an unanticipated flexibility in behavior toward these essential and prevalent gases. Sensory neurons, for the most part, capitalized on long-standing cellular strategies for detection such as soluble guanylate cyclases and carbonic anhydrases. An elegant solution to sensory detection seems to have been for animals to adopt an existing cellular strategy but use it to control neural activity and behavior rather than cellular behavior. Because O2 and CO2 fluctuations occur in different environments (mountain tops, under the sea, in the ground) at different times (diurnal rhythms, seasonal variation) as well as under different conditions (respiration, photosynthesis), it is remarkable that animals can glean useful information by monitoring external concentrations. The ability to interpret these signals in the context of a variety of other sensory cues is essential to determine whether the appropriate behavior is attraction, avoidance or indifference. How animals evaluate O2, CO2 and other environmental cues is an important problem in neural integration and an exciting avenue of investigation.
Acknowledgments
The author thanks Dr. Henk Roelink for generating the figures for this review and Dr. John Ngai for careful reading of the manuscript. This work was in part supported by a grant from the NIDCD 1R01DC006252 (KS). KS is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JF, Ultsch GR. Respiratory gas concentrations in the microhabitats of some Florida arthropods. Comp Biochem Physiol. 1987;88A:585–588. [Google Scholar]
- Boon EM, Marletta MA. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr Opin Chem Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley AS, Cox MJ. Shapes of Krill Swarms and Fish Schools Emerge as Aggregation Members Avoid Predators and Access Oxygen. Curr Biol. 2010 doi: 10.1016/j.cub.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BH, Arellano-Carbajal F, Rybicki I, de Bono M. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr Biol. 2004;14:1105–1111. doi: 10.1016/j.cub.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Coates JC, de Bono M. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature. 2002;419:925–929. doi: 10.1038/nature01170. [DOI] [PubMed] [Google Scholar]
- de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [see comments] [DOI] [PubMed] [Google Scholar]
- de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Hu J, Zhong C, Luo M. Integration of CO2 and odorant signals in the mouse olfactory bulb. Neuroscience. 2010;170:881–892. doi: 10.1016/j.neuroscience.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992;15:146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Graber M, Kelleher S. Side effects of acetazolamide: the champagne blues. Am J Med. 1988;84:979–980. doi: 10.1016/0002-9343(88)90091-5. [DOI] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Guerenstein PG, E AY, Van Haren J, Williams DG, Hildebrand JG. Floral CO(2) emission may indicate food abundance to nectar-feeding moths. Naturwissenschaften. 2004;91:329–333. doi: 10.1007/s00114-004-0532-x. [DOI] [PubMed] [Google Scholar]
- Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luo M. Loss of CO2 sensing by the olfactory system of CNGA3 knockout mice. Current Zoology. 2010;56:793–799. [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordstrom M, Bohmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol. 2010;12:87–93. doi: 10.1038/ncb2009. sup pp 81-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Rio DC, Marletta MA. Ligand binding and inhibition of an oxygen-sensitive soluble guanylate cyclase, Gyc-88E, from Drosophila. Biochemistry. 2007;46:15115–15122. doi: 10.1021/bi701771r. [DOI] [PubMed] [Google Scholar]
- Inada H, Kawabata F, Ishimaru Y, Fushiki T, Matsunami H, Tominaga M. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 2008;9:690–697. doi: 10.1038/embor.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Anantharaman V, Aravind L. Ancient conserved domains shared by animal soluble guanylyl cyclases and bacterial signaling proteins. BMC Genomics. 2003;4:5. doi: 10.1186/1471-2164-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Fearon IM, Nurse CA. Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol. 2004;560:737–752. doi: 10.1113/jphysiol.2004.069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow DS, Pan D, Tran R, Pellicena P, Presley A, Mathies RA, Marletta MA. Spectroscopic characterization of the soluble guanylate cyclase-like heme domains from Vibrio cholerae and Thermoanaerobacter tengcongensis. Biochemistry. 2004;43:10203–10211. doi: 10.1021/bi049374l. [DOI] [PubMed] [Google Scholar]
- Kustu S, Inwood W. Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol. 2006;13:103–110. doi: 10.1016/j.tracli.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Atkinson HJ. Physiology of nematodes. New York: Columbia University Press; 1977. [Google Scholar]
- Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, Zwiebel LJ. Odor Coding in the Maxillary Palp of the Malaria Vector Mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Sun L, Hu J. Neural detection of gases--carbon dioxide, oxygen--in vertebrates and invertebrates. Curr Opin Neurobiol. 2009;19:354–361. doi: 10.1016/j.conb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Mayr D, Mark T, Lindinger W, Brevard H, Yeretzian C. Breath-by-breath analysis of banana aroma by proton transfer reaction mass spectrometry. International Journal of Mass Spectrometry. 2003;223–224:743–756. [Google Scholar]
- McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, Kruglyak L, Bargmann CI. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DB. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J Biol Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- Morton DB. Invertebrates yield a plethora of atypical guanylyl cyclases. Mol Neurobiol. 2004;29:97–116. doi: 10.1385/MN:29:2:097. [DOI] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Wennemuth G, Biel M, Zufall F, Kelliher KR. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 2010;20:1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Persson A, Gross E, Laurent P, Busch KE, Bretes H, de Bono M. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–1033. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- Qin Z, Lewis JE, Perry SF. Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J Physiol. 2010;588:861–872. doi: 10.1113/jphysiol.2009.184739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr Biol. 2006;16:649–659. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Scott K, Brady RJ, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Seeley TD. Atmospheric carbon dioxide regulation in honey-bee (Apis mellifera) colonies. J Insect Physiol. 1974;20:2301–2305. doi: 10.1016/0022-1910(74)90052-3. [DOI] [PubMed] [Google Scholar]
- Shusterman D, Avila PC. Real-time monitoring of nasal mucosal pH during carbon dioxide stimulation: implications for stimulus dynamics. Chem Senses. 2003;28:595–601. doi: 10.1093/chemse/bjg050. [DOI] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci U S A. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian RE. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989;10:186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Thom C, Guerenstein PG, Mechaber WL, Hildebrand JG. Floral CO2 reveals flower profitability to moths. J Chem Ecol. 2004;30:1285–1288. doi: 10.1023/b:joec.0000030298.77377.7d. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A, Ainsley JA, Johnson WA, Davies SA, Morton DB. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weber RE, Vinogradov SN. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- Wedel BJ, Garbers DL. New insights on the functions of the guanylyl cyclase receptors. FEBS Lett. 1997;410:29–33. doi: 10.1016/s0014-5793(97)00358-x. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond. 1986;B 314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Wingrove JA, O'Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, Marletta MA, Hudson ML, Morton DB, Chronis N, Bargmann CI. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]