Abstract
Differentiation of naïve B cells, including immunoglobulin (Ig) class switch DNA recombination (CSR), is critical for the immune response and depends on the extensive integration of signals from the B cell receptor (BCR), tumor necrosis factor (TNF) receptor family members, Toll-like receptors (TLRs) and cytokine receptors. TLRs and BCR synergize to induce CSR in T cell-dependent and T cell-independent antibody responses to microbial pathogens. BCR triggering together with simultaneous endosomal TLR engagement leads to enhanced B cell differentiation and antibody responses. The requirement of both BCR and TLR engagement would ensure appropriate antigen-specific activation in an infection. Co-stimulation of TLRs and BCR likely plays a significant role in anti-microbial antibody responses to contain pathogen loads until the T cell-dependent antibody responses peak. Furthermore, the temporal sequence of different signals is also critical for optimal B cell responses, as exemplified by the activation of B cells by initial TLR engagement, leading to the upregulation of co-stimulatory CD80 and MHC-II receptors, which, in turn, result in more efficient interactions with T cells, thereby enhancing the germinal center (GC) reaction and antibody affinity maturation. Overall, BCR and TLR stimulation and the integration with signals from the pathogen or immune cells and their products, determine the ensuing B cell antibody response.
Keywords: activation-induced cytidine deaminase (AID), adaptive immunity, adjuvant, antibody, autophagy, B cell receptor (BCR), class-switch DNA recombination (CSR), CD40, CpG, cytokine, innate immunity, LPS, NF-κB, somatic hypermutation (SHM), Toll-like receptor (TLR), vaccine
I. B CELL DIFFERENTIATION VIA INNATE AND ADPAPTIVE IMMUNE RECEPTORS
A. The Innate Immune Response
The protection from a nearly unlimited number of pathogens encountered during the lifetime of an individual is achieved through the integration of innate and adaptive immune responses, which are intrinsically linked by crosstalk through cells and molecules.1–6 The immune system detects and eliminates invading pathogenic microorganisms, as well as infected and neoplastic self-cells, by distinguishing them or associated molecules from “normal” self-cells and self-molecules. While lower organisms have only an innate immune system, higher organisms, beginning with jawed vertebrates, in addition possess a more sophisticated system: the adaptive, or acquired, immune system.7–10 Innate immunity provides an early, though non-specific, initial response to pathogens.7,11–14 In contrast, adaptive immunity requires a lag period for activation, is specific, diverse and highly efficient in targeting of pathogens8,9,15,16 (Table 1).
TABLE 1.
Features of innate and adaptive immunity
| Feature | Innate Immunity | Adaptive Immunity |
|---|---|---|
| Specificity | Low | Very high |
| Receptor clonal distribution | No | Yes |
| Receptor diversity | Encoded in germline | Recombined gene segments |
| Response timeline | No lag | Lag time for induction |
| Efficiency of response | Moderately efficient | Highly efficient |
| Memory | No | Yes |
The innate immune system is a universal and ancient form of host defense against infection.7,10,17 In mammals, it is composed of diverse cell types, including macrophages, dendritic cells (DCs), neutrophils, basophils, mast cells, eosinophils and natural killer (NK) cells, and molecules and molecular systems, such as anti-microbial peptides, acute-phase proteins (collectins, ficolins, and pentraxins) and complement. 8,9,13 Innate immune cells constantly sample the environment for the presence of pathogens through their germline-encoded pattern recognition receptors (PRRs).3,7,9,13,18 PRRs primarily bind to “non-self” molecules that have conserved features known as microbe-associated molecular patterns (MAMPs); an alternative term is pathogen-associated molecular patterns (PAMPs). MAMPs are unique chemical structures found in the microorganism but generally absent in the higher hosts. For example, LPS contains sugars and lipids composed of chemical bond arrangements not found in higher eukaryotes, thereby constituting a unique MAMP. Another MAMP is unmethylated, CG-containing DNA (CpG) from bacteria and viruses.19 LPS and CpG are recognized by an important subset of PRRs known as TLRs.3,20–24 Other PRR classes include NOD-like receptors (NLRs), CARD helicases, C-type lectins, and scavenger receptors.3,10,25 (Table 2). Some of these receptors may be functional in B cells, however their effects on B cell antibody responses are not known, and they will not be considered further here. After sensing pathogens via their PRRs, macrophages and mast cells are activated to phagocytose the pathogen, display peptide fragments on major histocompatibility II (MHC II) receptors, and secrete pro-inflammatory cytokines and lipid mediators. Likewise, stimulation of PRRs on immature DCs leads to their maturation and activation, which in turn activates adaptive immunity.7,10,13,26
TABLE 2.
Pattern recognition receptors
| PRR | Ligand(s) | Immune Cells |
|---|---|---|
| TLRs | Several surface and intracellular MAMPs | Neutrophils; monocytes/Mφ; DCs; B cells |
| NLRs | MDP; anthrax toxin; RNA; flagellin | Monocytes/Mφ; DCs |
| CARD helicases | dsRNA | Neutrophils; monocytes/Mφ; DCs; B cells |
| C-type lectins | Glycosylated microbial ligands | Monocytes/Mφ; DCs |
| Scavenger receptors | Lipid-containing microbial ligands | Monocytes/Mφ; DCs |
Mφ: macrophages
B. The Adaptive Immune Response
Evolutionarily, adaptive immunity emerged after innate immunity by the need to respond to specific and changing antigens through the use of antigen-specific receptors on the surfaces of B and T lymphocytes. 1,4,15,27,28 Even though only jawed vertebrates possess a true adaptive immune system, recent evidence indicates that jawless vertebrates, such as lamprey and hagfish, contain lymphocyte-like immune cells expressing variable leucine-rich receptors (VLRs), which are triggered to rearrange upon infection with pathogen, and display a specificity for antigen similar to antibodies of the jawed vertebrate adaptive immune system.29–36 The adaptive immune response enables higher vertebrate animals to adapt their response to a pathogen to be more specific and effective, and to mount a faster and stronger response during subsequent encounters with the same pathogen (which is referred to as immunological memory).
The mechanisms of generating antigen-specific receptors in the adaptive system involve extensive variability and rearrangement of receptor gene segments. During their development, B and T lymphocytes rearrange those portions of their genomes, which code for BCRs and T cell receptors (TCRs), respectively. Lymphocytes display on the extracellular surface of their plasma membranes multiple (hundreds to thousands) identical copies of their BCR or TCR with unique antigen specificity among the pool of total lymphocytes.37,38 The rearrangement and clonal distribution of antigen receptors is different from the previously described PRRs, which do not rearrange and usually have the same sequence in all cells that express them in an organism (Table 1). When antigen receptors bind an antigen on a bacterium, virus, or parasite, they cluster together, thereby bringing into proximity membrane-associated and intracellular signaling molecules, contributing to lymphocyte differentiation. Such an antigen receptor crosslinking process only occurs in those B and T lymphocytes that express receptors with an above-threshold affinity for antigen to ensure that only antigen-specific lymphocytes are amplified and differentiate into effector and memory cells, which have the same specificity for the antigen that initiated and drove the response.39,40 Antibodies (and BCRs) are further diversified by CSR and SHM.
C. B Cell Differentiation
B lymphocytes play major roles in the immune response by producing antibodies against antigen, as well as by functioning as professional antigen presenting cells (APCs), and by producing cytokines.41–44 B cells produce high-affinity antibodies which can directly inactivate pathogens,45–47 can opsonize pathogens to assist innate immune cells in eliminating pathogens, and, finally, can activate complement.8,9,48 Conversely, B cells are directly and indirectly informed about the presence and nature of pathogens by innate immune elements such as TLRs,10,13,49 and are indirectly informed by complement factor C3d.50–52 Dysregulated and mis-targeted B cell antibody responses could result in autoimmunity, whereas impaired antibody responses during an actual infection could result in an immune deficiency.
In mammals, B cells begin their development in the bone marrow from lymphoid progenitor cells, which in turn derive from pluripotent hematopoietic stem cells. B cells derive their name from the Bursa of Fabricius in chickens, which is the equivalent organ of bone marrow in mammals. Antibody diversification during B cell development occurs by sequential Ig gene recombination where noncontiguous Ig variable (V), diversity, and joining (J) gene segments are recombined into functional V(D)J genes.37,38 The development of mature B cells and the generation of a diversified antigen receptor repertoire are crucial processes, but they are beyond the scope of this review. Here, we will focus on events that occur after B cells have completed their development in the bone marrow and have matured in peripheral organs such as the spleen and lymph nodes, where they are ready to respond to infection.
Upon encountering antigens, naïve mature B cells (IgMlo IgDhi) exit the resting state, increase metabolic activity and enter the cell cycle to initiate proliferation and concomitant differentiation, eventually leading to fully differentiated effector cells which produce high affinity antibodies against pathogenic targets.53–56 Full differentiation of B cells typically results in non-cycling short- or long-lived plasma cells (also referred to as plasmacytes, to include cells at different stages of differentiation), which are specialized to produce large amounts of antibodies, and memory B cells which can be quickly differentiated into plasma cells upon reinfection.
Prolonged and continuous engagement of multiple receptor types is required for B lymphocytes to be activated, to proliferate, to differentiate and to maintain their survival.57,58 B cells begin to proliferate within 24 hours after induction from stimuli, such as BCR crosslinking by antigens, and engagement of other surface receptors, including CD40 and TLRs (Fig. 1); after the initial cell division B cells divide continuously every 6–8 hours.55,59,60 DCs and T cells require prolonged stimulation before differentiation,61,62 whereas innate immune cells, such as macrophages and neutrophils, respond faster upon sensing pathogen and are attracted to pathogens via chemotaxis by following concentration gradients of pathogenic components,63,64 reflecting the differential roles of different types of immune cells in innate and adaptive immunity.
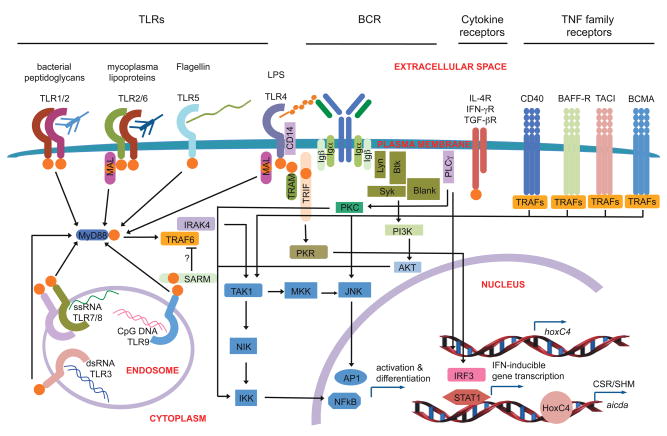
FIGURE 1.
Four main signals for B cell differentiation and antibody responses. During an infection, naïve mature B cells receive several types of stimuli that are then summed up before determining the appropriate response. DCs and TH cells interact with B cells via surface receptors, such as CD40, and by cytokine receptors, such as IL receptors. A pathogen would directly induce signals by crosslinking the BCR (as shown by the proximity of the O-sccharide of LPS) and by activating innate receptors such as the TLRs (as shown by the interaction of TLR4 with the lipid A component of LPS). TLR ligands are sensed by the extracellular, or endosomal, sickle shaped domains, and signals are initially relayed to the nucleus via homotypic TIR-TIR interactions (orange spheres) with TIR adapters. These signals are integrated and initiate a response by inducing NF-κB and AP-1, inflammatory gene transcription, IFN-inducible gene transcription, and induction of AID activity, leading to CSR and SHM
The initial differentiation of naive B cells in secondary lymphoid organs upon antigen recognition can result in their quick differentiation into short-lived plasmacytes, which secrete a burst of mostly IgM antibodies, usually of low-affinity, to limit the spread of pathogens and blunt the infection. Most activated B cells enter the GC reaction to undergo CSR to generate antibody isotypes with different biological effector functions, and SHM to generate antibody mutants as substrates for antigen-mediated positive selection of higher affinity antibodies. Both CSR and SHM are initiated by the enzyme activation-induced cytidine deaminase (AID),65,66 which is preferentially expressed in activated B cells, especially those in GCs.
Activated DCs in the secondary lymphoid organs can process and present peptides to T follicular helper cells (TFH)67 in GCs68–71 of spleen, lymph nodes, Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) in the gut.72 Internalization and processing of pathogenic proteins lead to the display of antigen-derived peptides on the MHC II receptors of antigen-specific B cells, which then make cognate interactions with TFH cells (that are specific for the same antigen) via MHC II-antigenic peptide-TCR interactions. In addition, B cells and T cells further contact each other through the membrane protein receptor pairs CD28:CD80/CD86, and inducible costimulator (ICOS): ICOS ligand (ICOSL), which are located on the surfaces of T cells and B cells, respectively. Activation of TFH by DCs or cognate B cells leads to upregulation of the membrane protein receptor CD154 (CD40L) on T cells. The engagement of CD40, a member of the TNF family of transmembrane receptors located on the surface of B cells,73,74 by CD154 results in vigorous B cell proliferation and differentiation, as will be detailed in section II.C. In addition to membrane protein receptor interactions, several types of interleukin (IL) molecules greatly influence the nature of the differentiation program for T and B cells.
The clonal expansion of a few parental B lymphocytes that are activated, and their concomitant differentiation to plasmacytes, which secrete thousands of antibodies per second,75–78 leads to the secretion of millions of affinity-matured and class-switched antibody molecules targeting specific antigens. Following antigen clearance, the expanded B cell pool contracts, likely due to engagement of inhibitory Fc receptors (FcRs) on B cells by excess antibodies,79–84 and by competition for homing in the bone marrow stromal environment,85,86 thereby ensuring that optimal titers of high-affinity antibodies are produced.
D. CSR in Humoral Immune Responses
The prominence of antibodies in immunity is illustrated by the fact that all current clinical vaccines function at least in part by eliciting protective antibodies,87,88 which are usually class-switched and of high affinity, similar to those generated during natural infections. Naïve B cells display IgM and IgD on their surface, and CSR substitutes the original IgM isotype with IgG, IgA and IgE by replacing the constant region of heavy chain (CH) of antibodies65,89–97 (Fig. 2).
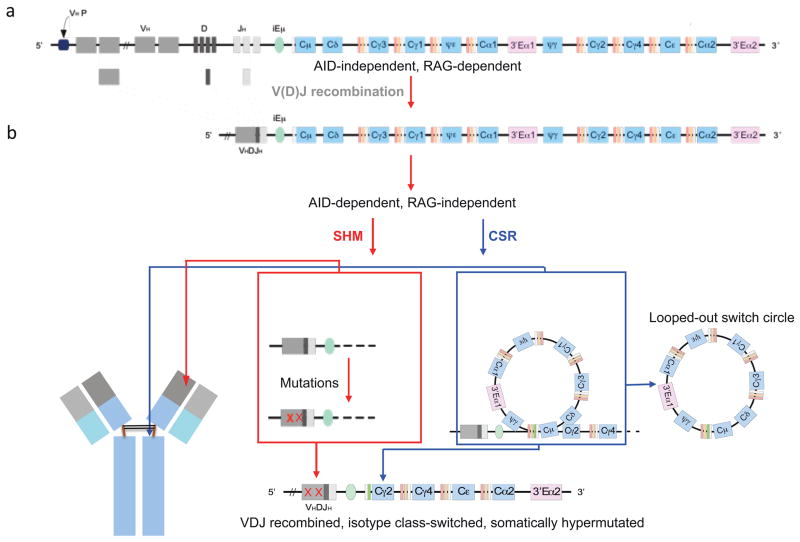
FIGURE 2.
Overview of CSR and SHM. a. Human B cells diversify the variable portion of their antigen receptors during development in the bone marrow through V(D)J recombination, which is mediated by recombination activating gene 1 (RAG1) and RAG2 recombinases. b. During an infection, naïve mature B cells in the spleen and lymph nodes undergo rearrangement of the constant portion of the IgH (i.e., CSR) to endow it with new biological effector functions, as well point mutations in the variable regions (i.e., SHM) to further increase its affinity, both of which depend on AID. Each CH region is indicated in blue color, beginning with Cμ on the left. The intronic IgH enhancer (iEμ, light green color), which is essential for optimal IgH gene expression, is located upstream of Cμ. The different orange colored thin segments just upstream of each CH region are the IH promoters followed by the S regions. IH promoters are activated in response to particular cytokines, and serve to drive germline transcription through S and CH regions, possibly opening up local chromatin structure for AID activity, or delivering AID to the S regions by RNA pol II or other trans-acting factors.
The first antibodies to be produced are of IgM class, and are likely encoded in the germline, as the B cells that produce them have not yet completed the GC reaction and thus have not undergone SHM. The intrinsic affinity of the initial IgM for antigen is relatively low, however the overall avidity of IgM for antigen is relatively high, as its pentameric structure containing a total of ten identical Fab arms results in a gain in local entropy when antigen binding has been initiated in any one of the ten arms, enabling these early IgMs to efficiently coat bacterial and viral antigens during the early stages of infection.98 99 The large size of the initially produced pentameric IgM restricts its distribution mainly to the blood and prevents it from crossing efficiently into extravascular spaces and binding pathogens systemically.
In contrast to IgM, all other antibody isotypes diffuse efficiently to extravascular sites, and, therefore, their generation is critical during an infection. Class-switched antibodies possess diverse biological effector functions, including direct pathogen or toxin/virulence factor neutralization, opsonization, complement activation, sensitization of mast and NK cells, extravascular diffusion, improved transport properties and longer half-lives.9,92,96,97,100,101 A deficiency in CSR, whether due to B cell intrinsic or extrinsic causes, would lead to an impaired antibody response during an infection, as occurs in humans with the Hyper IgM (HIGM) syndrome.97,100–102 These individuals are highly susceptible to infection and require injections of IgG fractions from pooled sera of many healthy donors to provide passive antibody-mediated protection against potential pathogens.
B cells express both the exquisite receptor for adaptive immunity, i.e. BCR, and receptors for innate immunity, such as TLRs. In addition to being stimulated by T-dependent signals, B cells can be directly stimulated by engagement of their TLRs by the respective ligands. Innate and adaptive immune signals can therefore be integrated in the same B cell.17,54
II. THE ROLES OF BCR, CD40, TLRS AND CYTOKINE RECEPTORS IN CSR
Signaling pathways from BCR, CD40, TLRs, and cytokine receptors play dominant roles in B cell antibody responses. While T cell help is critical for optimal B cell differentiation, including CSR and SHM, recent evidence indicates that T-independent signals from BCR and TLRs, also significantly contribute to the antibody responses against microbial pathogens. Mutations that interfere with BCR signaling, such as those in genes encoding several tyrosine kinases, phosphoinositide 3 kinase (PI3K), and phospholipase Cγ2 (PLCγ2) and their several signaling adapters, while they result in varying deficiencies in B cell development, compromise antigen recognition and subsequent signaling, resulting in severe impairment of antibody production and CSR.16,103–105 Humans106–110 and mice111–114 carrying mutations in TLR genes or in genes encoding TIR-domain adapters (such as MyD88115–118, TRIF119) and TLR regulatory molecules (such as CD14,120,121 which is a co-receptor for TLR4, and Unc93b1,113,122,123 which is involved in correct endosomal TLR trafficking) display varying impairments of innate and adaptive immune responses, as reviewed recently.124–127 However, the extent to which the defect directly impairs B cell responses remains unclear.20,49,128,129 While one signal alone can directly activate B cells, the response is limited in terms of the antibody isotypes produced, and combining multiple signals together broadens the scope of the antibody response by leading to increased B cell differentiation. It is likely that B cells are optimally differentiated by more than one signal,17,54 with BCR and TLR signaling contributing to the generation of optimal antibody responses.
A. BCR Signals
One of the primary B cell activating signals is provided by BCR crosslinking, where several BCR units composed of the membrane Ab, Igα, Igβ, CD19, CD21, CD81 and associated adapters (Fig. 1) are brought together into close proximity to initiate downstream signaling. 104,130,131 BCR crosslinking leads to phosphorylation of immunotyrosine-based activation motif (ITAM) receptors, which in turn activate several enzymes, in particular PI3K and PLCγ2. These two enzymes generate second messenger molecules, such as phosphorylated phosphotidylinositols (PtdIns) by PI3K, and inositol triphosphate (IP3) and diacylglycerol (DAG) by PLCγ2, leading to the release of free intracellular calcium ions (Ca2+). Subsequent nuclear factor bound to κB (NF-κB) activation results in an increase in the levels of transcription factors required for entering the cell cycle and differentiation pathways. However, in the absence of other stimulating signals, B cell proliferation is followed by activation-induced cell death (AICD),56,132–135 a process probably involved in the elimination of B cells activated by self-antigens in the absence of an infection. Thus, BCR signaling per se is insufficient for full B cell differentiation, including CSR, but it synergizes with strong signals from different receptor families, such as CD40 or TLRs, to induce B cell terminal differentiation, including CSR.
B. Cytokine Receptor Signals
Cytokines, such as IL-4, IFN-γ and TGF-β, influence B cell differentiation by combining with other signals to direct the B cell response, such as CSR to specific isotypes.67,136–138 Cytokine receptor signaling in B cells acts in part by inducing IgH germline transcription (IH-CH) of heavy chain (CH) regions participating in CSR. 91,92,96,136,138 Each CH gene cluster consists of three distinct and sequential components: the IH promoters for germline transcript initiation, the switch (S) regions where DNA breaks for CSR occur, and finally, the CH genes themselves (Fig. 2B).
In the presence of CD40 or LPS engagement, IL-4 induces the activation of the transcription factors NF-κB and STAT-6, whose DNA binding sites include those located in Iγ1 and Iε promoters. After NF-κB and STAT-6 bind to these promoters, germline Iγ1-Cγ1 and Iε-Cε transcription for IgG1 and IgE, respectively, is initiated. Likewise, Iγ2b and Iα promoters contain binding sites for the transcription factors Smud and Runx, which are induced upon stimulation with TGF-β to mediate CSR to these two classes.91,92,96,139 As cytokines are mainly, although not exclusively, secreted by TH1 and TH2 cells, and can be classified into TH1 (IFN-γ and TGF-β) or TH2 (IL-4) classes138, the antibody isotypes directed by cytokines can also be classified, in the mouse, into TH1 (IgG2a, IgG2b, IgG3) and TH2 (IgG1, IgE) isotypes, with IgM and IgA not falling exactly into either category.
Germline IH-CH transcription plays a critical role in CSR, likely by either opening up DNA, and/or by routing RNA polymerase II to deliver AID to S regions. S regions contain an unusual abundance of the ‘5-WRCY-3’ hotspot,140,141 and particularly the ‘5-AGCT-3’ iteration. These sequences could play important roles in recruiting CSR factors, including AID, and could also serve as the preferred substrate for AID142. In S regions, AID deaminates cytosine (dC) to uracil (dU).96,142 dU can be excised by apurinic/apyramidinic endonuclease (APE). Excision of nearby dUs on opposite DNA strands results in dsDNA breaks, which are then thought to be re-ligated mostly by components of the nonhomologous end-joining (NHEJ) machinery.96,143,144 The entire process replaces one CH gene with a downstream CH gene, e.g. it replaces the Cμ gene with the Cγ1, in the case of CSR to IgG195,96,141,145 (Fig. 2B).
C. TNFR Superfamily Signals
The TNF receptor (TNFR) superfamily consists of members such as CD40, B cell activating factor of the TNF family receptor (BAFFR), B cell maturation antigen (BCMA), and transmembrane activator and calcium signaling modulating and cyclophilin ligand [CAML] interactor (TACI).146 Each member is a homotrimeric type II membrane protein consisting of an extracellular ligand-binding domain, a membrane-spanning domain, and an intracellular signaling domain. There is a difference in the ligands that these members bind, with CD40 engaging CD154 on the surface of T cells, and BAFFR, BCMA and TACI binding one or both of the soluble proteins BAFF and a proliferation-inducing ligand (APRIL).
BAFF and APRIL are produced mainly by innate immune cells as well as by epithelial cells. BAFFR binds BAFF, BCMA binds APRIL, and TACI binds both BAFF and APRIL.147,148 BAFF and APRIL function to promote the maturation of T1 immature transitional B cells to mature B cells to maintain appropriate levels of mature B cells in a negative feedback fashion, to help with the establishment of plasma cells to their niches in the bone marrow,147–150 and, finally, to induce or enhance B cell differentiation, including CSR.151–155 One report suggested the direct induction of CSR by engagement of TACI or BAFFR,152 however, in our experiments, BAFF did not induce CSR, although BAFF enhanced LPS- or CD40-driven CSR, in mouse B cells stimulated in vitro. Thus, BAFF may enhance CSR when B cells are activated by an additional stimulus, as evidence suggests in the case of B cell activation by viral components151,153,155 or epithelial cell-secreted BAFF and APRIL156–159 as will be described in section D1 below. Finally, BAFF can be produced by IgD-armed basophils upon binding bacteria, thus establishing a link between initial IgD production and sustained, local B cell differentiation, including CSR.160
In B cells, the main stimulatory surface receptor belonging to the TNFR superfamily that induces robust B cell differentiation is CD40. CD40 is found on the surfaces of other APCs, and is generally required for their activation. This receptor has been found to be essential in mediating a broad variety of immune responses, including T-dependent CSR, SHM, GC reaction, and plasma and memory B cell formation.74,161–163 CD40 signals are transmitted by interaction of CD40 cytoplasmic tail with TNF receptor-associated factor (TRAF) adapter proteins, including TRAF2, TRAF3, TRAF5, TRAF6, and TTRAP,164,165 which ultimately results in the activation of transcription factors such as NF-κB and activator protein 1 (AP-1).163 The most important TRAFs that transduce CD40 signals are TRAF2 and TRAF6. These subsequently interact with germinal center kinase (GCK), which then activates the c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) pathways.165 TRAFs mediate NF-κB induction by activating the inhibitor of NF-κB kinase (IKK) complex to phosphorylate the inhibitor of κB (IκB) proteins, thereby releasing NF-κB for nuclear translocation and binding to its target DNA166 (Fig. 1). NF-κB then orchestrates a transcriptional program, which includes the induction of molecules necessary for B cell differentiation, including induction of AID for initiation of CSR and SHM.164 As the T-dependent B cell response in vivo is MHC-II restricted, in vitro studies of B cell differentiation by CD40 should perhaps also involve simultaneous MHC-II engagement.167
D. TLR Signals
1. TLRs in Immune Cells
The involvement of TLRs in mammalian immunity was first discovered based on their homology to the Drosophila Toll receptor, where Toll plays a dual role in the dorso-ventral segmentation during development and in the production of antifungal peptides during an infection.54,168,169 Most mammalian species contain ten to fifteen different TLRs, each of which senses one or a limited number of MAMPs (Table 3), and, in a few cases, host-derived molecules.21,170–172 TLRs are expressed in innate immune cells, B cells and in some non-immune cell types, including epithelial cells. In particular, epithelial cells sense bacteria via TLRs, and then secrete BAFF or APRIL, which activates B cells residing in the periphery and promotes their CSR, thereby containing the bacteria.156–159 Non-immune functions of TLRs, as in development, cancer or tissue injury173 are outside the scope of this review.
TABLE 3.
TLRs and their ligands
| TLR | Ligand(s) | Microorganism | TIR Adapters | Immune Cells |
|---|---|---|---|---|
| TLR1 | Triacyl lipopeptides, e.g. Pam3CSK4 | Bacteria, mycobacteria | MyD88; TIRAP | Neutrophils; monocytes/Mφ; mDC; B cells |
| TLR2 | Diacyl lipopeptides, e.g. Pam2CSK4; Lipotechoic acid; Zymosan | Bacteria, mycobacteria, yeasts | MyD88; TIRAP | Neutrophils; monocytes/Mφ; mDCs; B cells |
| TLR3 | dsRNA | Viruses | TRIF | mDCs; Mφ; B cells |
| TLR4 | LPS | Gram-negative acteria | MyD88; TIRAP; TRAM; TRIF | Neutrophils; monocytes/Mφ; mDCs; B cells |
| TLR5 | Flagellin | Bacteria | MyD88 | Monocytes/Mφ; mDCs; B cells |
| TLR6 | Diacyl lipopeptides | Mycoplasma | MyD88; TIRAP | Neutrophils; monocytes/Mφ; mDCs; B cells |
| TLR7 | ssRNA | Bacteria and viruses | MyD88 | Neutrophils; monocytes/Mφ; pDCs; B cells |
| TLR8 | ssRNA | Bacteria and viruses | MyD88 | Neutrophils; monocytes/Mφ; mast cells; B cells |
| TLR9 | Unmethylated CpG containing DNA | Bacteria and viruses | MyD88 | Neutrophils; monocytes/Mφ; pDCs; B cells |
| TLR11 | Profilin | Toxoplasma | MyD88 | Monocytes/Mφ; B cells |
Mφ: macrophages; mDCs: myeloid DCs; pDCs: plasmacytoid DCs.
TLRs consist of an extracellular or endosomal sensing domain that contains leucine-rich repeats (LRRs), a transmembrane region, and a cytoplasmic Toll/interleukin I/resistance protein (TIR) domain.23,174,175 LRRs fold into a sickle-shaped β-coil domain and confer specificity in sensing a particular class of ligands, mainly through their concave, leucine-rich surface.22 The intracellular and variable TIR domain confers signaling specificities to different TLRs, enabling each of them to interact with a limited number of TIR-domain signaling adaptor proteins via homotypic TIR-TIR interactions.21,22,24,174,176,177 Comparative sequence analysis of the human TLRs have revealed that the members of the TLR family can be divided into five subfamilies, as represented by TLR2, TLR3, TLR4, TLR5, and TLR9. The TLR2 family consists of TLR1, TLR2, TLR6, and TLR10; the TLR9 subfamily consists of TLR7, TLR8, and TLR9; the other three subfamilies, TLR3, TLR4 and TLR5, contain only the one indicated member.178
Upon engagement by their ligands, TLRs are thought to dimerize and signal as homodimers (or possibly homomultimers), with the exception of TLR1/TLR2 and TLR2/TLR6, which signal as heterodimers. Signaling pathways emanating from different TLRs are complex and often redundant (Fig. 1). It is generally accepted that there are two main transmission pathways in the propagation of TLR signals, mediated by two TIR domain-containing adaptors: the myeloid differentiation factor 88 (MyD88) pathway and the TIR domain-containing adaptor inducing interferon-β (TRIF) pathway, respectively. The other three known TIR domain-containing adapters are MyD88-adaptor-like (MAL, also known as Toll/interleukin 1 receptor domain containing adaptor protein, TIRAP), TRIF-related adaptor molecule (TRAM), and the recently discovered sterile and armadillo motifs (SARM). TLR4 signals through both the MyD88 pathway, as bridged by TIRAP, and the TRIF pathway, as bridged by TRAM; TLR9 signals directly and only through the MyD88 pathway. With the exception of SARM, the other four adaptors utilize IL-1 receptor associated kinases (IRAKs) to transmit signals to TRAFs, which ultimately leads to the nuclear translocation and target gene promoter binding of transcription factors such as NF-κB and AP-1, thereby initiating gene transcription of IRF-3 and STAT-1, and to induction of AID expression to mediate CSR and SHM in activated B cells.176,177 SARM has been suggested to negatively modulate signaling,174 possibly to restore the pre-activation non-signaling state or to dampen the response.
In monocytes, in which TLRs are highly expressed, TLR engagement induces their differentiation into macrophages or DCs, and further TLR stimulation can modulate immune functions of these cells, including enhancement of phagocytosis of pathogens.179,180 In DCs, TLRs appear to be expressed in a cell subset-dependent manner: myeloid DCs in peripheral tissues express most TLRs except TLR9, while plasmacytoid DCs in blood express mostly TLR1, TLR6, TLR7, and TLR9.26,181,182 Binding of the appropriate ligand induces DC maturation, including enhanced antigen presentation and cytokine secretion, and upregulation of the stimulatory receptor CD86, all of which greatly influence the adaptive immune response.181 The presence and potential function of TLRs in T lymphocytes, which generally sense antigen presented by DCs and B cells, is currently unclear, though there is some evidence indicating that TLR2 engagement may remove the Treg suppressive functions during ongoing infection.183,184
2. TLR Signaling in B Cell Antibody Responses
B cells express high levels of most TLRs and respond to stimulation by TLR ligands associated with pathogens, likely those present in pathogens phagocytosed by B cells, as well as released, soluble TLR ligands. The details of the TLR signal transduction pathways in cells such as macrophages and DCs21,22,185 described in the previous section are likely relevant to B cells as well (Fig. 1). TLR engagement by MAMPs such as LPS or CpG induces potent B cell differentiation,19 similar in magnitude to that induced by CD40, and much stronger than that induced by BCR crosslinking alone. Even though direct B cell differentiation and antibody production in response to TLR ligands, such as LPS or flagellin, was reported since the 1960s,17,54,186–189 only recently has the significance and scope of these data received due attention.10,17,20,49,54,177 While TLRs are clearly functional in B cells, relatively little is known about how TLRs influence B cell differentiation, including CSR and SHM.176,177,190
The role of TLRs in B cell antibody responses has been somewhat controversial, at least in part due to different outcomes from different experimental settings. In one study, TLR signaling in B cells was found to be indispensable for optimal B cell antibody responses against native proteins, using LPS or flagellin as TLR ligands in immunizations employing wild-type, TLR4- or MyD88-deficient B cells adoptively transferred into B cell-deficient mice.49 However, in other studies, antibody responses to haptenated proteins mixed with different adjuvants, many of which contain TLR agonists, was not significantly impaired in mice doubly deficient in MyD88 and TRIF.128,175,191 One explanation for these data is that antibody responses to haptenated proteins may be different from those against natural and unhaptenated proteins, perhaps due to the alternation of the protein structures by the haptenation process.175,191 Alternatively, it has been proposed that the repetitive arrays of haptens cross-link BCR with high efficiency such that TLR signaling may not be essential for the B cell responses, whereas soluble protein antigens that cannot efficiently crosslink the BCR may require TLR stimulation to elicit a robust response.192 The fact that B cells highly express most TLRs and are efficiently activated by engagement of their ligands points to a role for TLRs in optimal B cell differentiation, as will be detailed in the subsequent sections.
TLR stimulation of B cells in combination with cytokines has been used to drive CSR to specific isotypes. In fact, well before the discovery of CD154 as one of the main TH cell receptors, LPS was used to activate mouse B cells,187 and was subsequently found to do so by engaging TLR4.111,193 LPS stimulation of B cells also led to the historical discovery of NF-κB.194–196 Since LPS stimulation of B cells induces NF-κB and AID, and results in CSR to IgG3, the specificity of CSR could be partially influenced by TLR ligands such as LPS, but is greatly influenced by cytokines, as was mentioned earlier.
III. TLR AND BCR SIGNALING SYNERGIZE TO INDUCE CSR
A. Surface TLRs in the Induction of CSR
The surface TLR1, TLR2, TLR4, TLR5, and TLR6 bind MAMPs that are expressed on the surface of microorganisms, while the endosomal TLR3, TLR7, TLR8, and TLR9 bind nucleic acid MAMPs located inside microorganisms. TLR4, which is expressed on the plasma membrane, binds LPS present on the surface of bacteria, and is internalized together with LPS,197,198 which could be a general feature of TLR recognition and downstream trafficking and signaling events.199–202 TLR agonists are potent drivers of B cell differentiation, including NF-κB and AID expression, which coupled with cytokine-directed germline IH-CH transcription, induces CSR to specific isotypes. Experimentally, LPS stimulation of mouse B cells induces CSR to IgG3; LPS in combination with IL-4 induces CSR to IgG1 and IgE; LPS in combination with IFN-γ induces CSR to IgG2a; finally, LPS in combination with TGF-β induces CSR to IgG2b and IgA.91,92,96 Likewise, we have recently found that stimulation of TLR1/6 by Pam3CSK4 results in CSR to the isotypes specified by cytokines.203 Therefore, TLR signaling results in context-dependent modulation of both the magnitude and the fine structure of the B cell antibody responses.
TLR ligands may stimulate not only their cognate TLRs, but they may also happen to bind and crosslink the BCR in a small fraction of B cells, thereby activating an entirely different stimulation pathway.204–206 In particular, TLR ligands are thought to behave in two different ways: a T-independent type I (TI-I) and a T-independent type II (TI-II) way.8,9,54,189,204,207 At saturating doses, TI-I antigens, including LPS, will bind all B cells via their TLR4 and, therefore, virtually all B cells will be (polyclonally) activated (mitogenic stimulation). At much lower LPS concentrations, those fewer B cells whose BCR also happens to bind the O-saccharide component of LPS with high affinity, are thought to receive enough signals from both TLR4 (engaged by the lipid A component of LPS) and BCR, hence the name TI-II. Even though TLR4 engagement by LPS does not require BCR crosslinking to induce B cell differentiation, BCR crosslinking can further enhance TLR4-induced B cell differentiation198,208 and CSR;203 the same also holds for TLR1/6-induced CSR.203 Furthermore, while in vitro stimulation of B cells with high (mitogenic) doses of LPS, in the presence of cytokines can induce CSR to specific isotypes, the contribution of BCR crosslinking by LPS under these in vitro conditions may skew the response to a TI-II response (TLR + BCR), which engages the BCR and thus results in the amplification of antigen-specific B cells, rather than a genuine TI-I (TLR alone) response.
B. Endosomal TLRs in the Induction of CSR
The endosomal location of TLR3, TLR7, TLR8, and TLR9 may be optimal in allowing their sensing of internalized nucleic acid MAMPs from viruses and bacteria bacteria, while likely avoiding sensing host nucleic acids.202,209 While the dynamics of distribution and maturation of endosomal TLRs is still a matter of active research,210 several reports indicate that each endosomal TLR may not be located in exclusive endosomes, but rather they may be located to common endosomes hosting several different TLRs, where each TLR senses its respective ligand as it is gradually exposed in a disintegrating microorganism.211,212
Stimulation of the endosomal TLR9 by unmethylated CpG DNA has been thought to suppress class switching to IgG1 and IgE isotypes but promote class switching to IgG2a in B cells activated by LPS or CD154 and IL-4, likely by regulating germline transcription.213 However, there has been a great variability in the data of different research groups, particularly in the magnitude and the identity of the TLR9-enhanced or -suppressed isotypes,213–218 and the controversial involvement of T box expressed in T cells (T-bet),213,219–221 a transcription factor important for TH1 cell polarization. CpG stimulation was reported to suppress CSR to IgG1 and IgE induced by LPS or CD40 and the cytokine IL-4, and at the same time promote CSR to the TH1 isotype IgG2a under these TH2 conditions,213 suggesting that CpG stimulation could somehow divert the CSR machinery to other target S regions, which is a role usually played by cytokines.
CpG can also modulate B cell differentiation to plasmablasts/plasmacytes,86,222 thereby resulting in complex changes in the titer of isotype-switched antibodies in culture supernatants, and therefore complicating the monitoring of CSR. Furthermore, IFN-γ and TGF-β, two cytokines critical for CSR to IgG2a and IgG2b, respectively, are known to decrease B cell proliferation and survival, whereas CpG is a potent proliferation and survival stimulus, which adds additional complexity to the overall antibody response.203
C. BCR signaling synergizes with TLR signaling
TLR-induced CSR depends on the correct cellular trafficking and localization of the engaged TLRs, as well as whether other signals, particularly those from the BCR, are also activated.6,199–202,223,224 TLR ligands such as LPS or flagellin, can elicit antibody responses directed against them, complicating the delineation of the respective roles of BCR and TLRs in anti-microbial antibody responses. B cells phagocytose antigen in a FcR- or BCR-dependent way,225–228 efficiently process internalized antigenic proteins, as well as sense various MAMPs present in the phagocytosed pathogen. BCR and TLR9 signaling synergize in the induction of NF-κB in B cells,199,223,229 and BCR signaling recruits TLR9-containing endosomes to autophagosomes,199,223 possibly as a way of efficiently coordinating the sensing of nucleic acid TLR ligands present when a B cell internalizes pathogens.
Signaling cross-talk between ITAM receptors and TLRs could modulate responses in immune cells.6,224 For example, DCs responded to ssRNA by producing IFN-γ only if the viruses had been previously opsonized with antibodies, which crosslink Fc receptors on the DC membrane;230 crosslinking of activating FcRs in DCs and other cells can serve the same function as the crosslinking of BCR on B cells, as both are ITAM-bearing receptors. In another study, vesicular stomatitis virus (VSV) induced IFN-α in DCs upon its internalization to autophagosomes and simultaneous engagement of TLR7 by viral RNA.231
In our studies, we have found that CpG stimulation alone results in robust B cell proliferation, but not in significant CSR, as indicated by the low frequency of B cells expressing surface IgG, IgE, or IgA measured by flow cytometry.203 However, in the presence of BCR crosslinking (and appropriate cytokines), CpG stimulation could lead to robust CSR to all respective isotypes, as well as to plasma cell formation and antibody secretion.203 Furthermore, contrary to previous reports,213,217,232 our data indicate that TLR9 stimulation in the absence of BCR crosslinking suppresses LPS- (or CD154-) and cytokine-induced CSR not only to TH1 but also to TH2 isotypes, and suppresses plasma cell formation.203 However, in the presence of BCR crosslinking, LPS and CpG, as well as LPS and Pam3CKS4, synergized in inducing CSR in a dose-dependent way.203 Similar synergistic and antagonistic effects of have been reported for cytokine production by B cells43 and PBMCs233 stimulated with specific pairwise combinations of TLR ligands.
CpG and other surface and endosomal TLR ligands, such as Pam3CSK4 and R848, might not display a TH1 vs TH2 bias in inducing B cells to undergo CSR to specific Ig isotypes (like cytokines do), but like LPS, these may act as inducers of NF-κB and AID, with the latter initiating CSR to isotypes specified by cytokine-induced germline transcription. The mechanism by which CpG antagonizes LPS- and CD40-induced CSR in the absence of BCR crosslinking could be due to sequestration of signaling adapters, or due to signal paralysis. Another possibility could be that in the absence of strong BCR crosslinking, CpG may enter the cell via BCR-mediated uptake, setting off weak BCR signaling (low ITAM signaling), which has been proposed to negatively regulate TLR and cytokine signaling in macrophages.6,224 Conversely, during dual stimulation with BCR crosslinking and CpG (mimicking the internalization of microorganisms by BCRs that have high-affinity for their external epitopes), the strong ITAM signaling synergizes with TLR9 signaling, and results in differentiation, as hallmarked by CSR (Fig. 3).
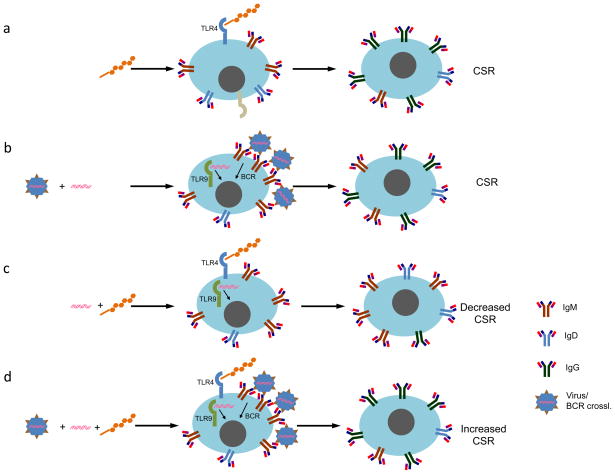
FIGURE 3.
The interplay of surface TLR4 and endosomal TLR9 in CSR. a. LPS induces CSR to isotypes, as directed by cytokines. b. CpG by itself does not induce significant CSR; however, in the presence of BCR crosslinking (indicated by a virus which is bound by BCR; experimentally it can be mimicked by reagents that crosslink the BCR polyclonally), it behaves similar to LPS in inducing CSR to the isotypes specified by cytokines. c. CpG suppresses LPS-induced CSR; however CpG synergizes with LPS in a dose-dependent way in the presence of BCR crosslinking (d).
Considering CpG as a general inducer of B cells to undergo CSR to isotypes directed by the different cytokines, without itself displaying an intrinsic TH1 vs TH2 isotype bias, does not contradict the reported TH1 vs TH2 polarizing effect of CpG in other innate immune cell types, and its use as a predominantly TH1 vaccine adjuvant.234–238 It is likely that cells such as DCs and macrophages sense the pathogen type present in an infection by engagement of various PRRs (including TLRs) by corresponding pathogenic MAMPs, and then produce TH1 or TH2 cytokines that initiate the induction of TH1 or TH2 cells, which then amplify the polarization and induce B cell differentiation and CSR to specific TH1 or TH2 isotypes.13,26,239–241 Whether B cells, after directly sensing the type of pathogen via engagement of their TLRs by the corresponding pathogenic MAMPs, can undergo CSR to the most appropriate isotypes, as is the case for LPS-induced CSR to IgG3, remains to be determined.
While the role of TLRs in autoimmunity is beyond the scope of this review, the reader is referred to several recent reviews on the subject.172,242–249 Briefly, it appears that under conditions where TLRs sense host ligands that resemble MAMPs, such as phospholipids that resemble LPS and could engage TLR4, or self RNA or DNA that could engage TLR3, TLR7/8 and TLR9, B cells are activated to produce autoantibodies.250–253 This could be particularly important in the generation of anti-DNA class-switched autoantibodies, which are the main cause of systemic lupus erythrematosus (SLE).
IV. TLR- AND BCR-INDUCED B CELL ANTIBODY RESPONSES AGAINST MICROBIAL PATHOGENS
A. TLR Signaling in T-independent B Cell Differentiation
1. TLR Signaling During the Initial Phase of B Cell Differentiation
TLRs were likely one of the first immune receptors that recognized conserved molecular patterns of pathogens.4 A recent report described the rearrangement of lamprey antigen receptors, where an APOBEC-related enzyme was found to deaminate the gene segments encoding these receptors, leading to their rearrangement and thus diversification to a limited number of configurations.32 Another report on lamprey immunity revealed the presence of B- and T-like cells, which sense pathogens via membrane-expressed VLRs, with the B-like cells progressing through blast formation and secreting soluble antigen-specific VLRs.36 Thus, it is possible that TLRs, VLRs or related primordial PRRs were the original antigen receptors, and during evolution were replaced by the newer and more and versatile BCRs, which are based on the Ig domain fold. TLRs continued to provide information regarding the presence and type of pathogen to innate immune cells and B cells.
TLR-mediated differentiation of B cells may be a critical component of T-independent, but early and effective B cell clonal amplification and differentiation leading to the production of class switched, potentially hypermutated, protective antibodies during early infection when pathogen loads are relatively low and T cell help is not yet fully available. LPS stimulation of mouse B cells in vitro results in IgM and IgG3 antibody production. The first antibodies to be produced in vivo against an antigen encountered for the first time are IgM, which can contain the pathogen in the first hours to days of infection. Studies in mice selectively deficient in secreted IgM,254 suggest that the initial T-independent production of IgM serves not only to contain pathogens, but by forming complexes with pathogens, serves as an autocrine factor for continued local B cell proliferation via IgM-mediated multimerization of soluble antigen that can now efficiently crosslink the BCR. In the absence of timely T cell help, other cell types, such as follicular dendritic cells (FDCs), may provide a level of stimulation sufficient for B cell activation and early IgM secretion.255
2. TLR Signaling During B Cell Differentiation in the Absence of T Cell Help
Even though optimal CSR requires T cell help, some CSR does occur without T cell help following challenge with viral, bacterial and fungal pathogens (Table 4). While the titers of class switched antibodies in the absence of T cell help are usually much lower than the optimal titers produced in the presence of normal T cell help, in several cases the titers are enough to confer some protection against infection. Class-switched antibodies can be generated against invading pathogens without T cell help because the enzyme AID can be induced in part by the conserved homeodomain transcription factor HoxC4145,256,257 in a T-independent fashion.17,54,176,177,189,192,258–260
TABLE 4.
T cell-independent anti-microbial class switched antibody responses
| Pathogen/Antigen | Mouse Model | Ig produced | Refs. |
|---|---|---|---|
| Polyoma virus | TCRβ−/− x δ−/− | IgM, IgG | 316 |
| B cell transfer in SCID | IgM, IgG | 317 | |
| CR2−/− B cell transfer in SCID | IgM, IgG | 318 | |
| VSV | TCRα−/−; TCRβ−/− | IgM, IgG2a | 319 |
| Rotavirus | TCRαβ−/− x γδ−/− | IgM, IgG, IgA | 320 |
| TCRαβ−/− | IgA | 321 | |
| LCMV, VSV, Pichinde virus | CD154−/− | IgM, IgG2a | 322 |
| Influenza virus | CD4−/− | IgM, IgG1, IgG2b, IgG3 | 323 |
| CD40−/−; MHCII−/− | IgM, IgG | 324 | |
| Ehrlichia muris | CD4−/−; CD8−/−; MHCII−/− | IgM, IgG1, IgG2c, IgG2b, IgG3 | 325 |
| Ehrlichia chafeensis | CD4−/− | IgG1, IgG2a, IgG2b, IgG3 | 326 |
| Borrelia burgdorferi | TCRβ−/−; TCRβ−/− x δ−/− | IgM, IgG3 | 327 |
| Bacillus anthracis | TCRα−/− | IgG | 328 |
| Yersinia enterocolitica | nude | IgG | 329 |
| Citrobacter rodetium | CD4−/− | IgM, IgG1, IgG2c, IgG2b, IgG3, IgA | 330 |
| Porphyromonas gingivalis | TCRα−/− | IgG, IgA | 331 |
| Melanin (C. neoformans) | nude | IgM, IgG1, IgG2b, IgG3 | 332 |
| Cryptosporidium parvum | nude | IgM, IgA | 333 |
| Protoscolex (E. granulosus) | CD4−/− | IgG1, IgG2a, IgG2b, IgG3 | 334 |
| Echinococcus multilocularis | nude; TCRβ−/−; MHCI−/−; MHCII−/− | IgM, IgG1, IgG2a, IgG2b, IgG3 | 335 |
CSR that occurs in the absence of TH cells261 is particularly applicable to antiviral antibody responses.258,262 Immunization of T cell-deficient mice with polyoma virus resulted in the production of virus-specific class switched IgG, although in lower titers compared to wild type mice, whereas immunization with either a viral antigen or virus-like particles (VLPs) did not result in IgG production, possibly due to the presence of dual TLR and BCR engagement in the case of whole virus and lack of TLR engagement in the case of antigen alone or VLP immunization.263 In other studies, natural IgA antibodies were produced in response to TLR ligands of commensal gut bacteria,264 natural IgE antibodies were generated to activate innate immune mast cells without T cell help,265 and IgG2a antibodies were produced outside GC follicles three days after immunization.266 Considering the low level CSR that occurs in the absence of T cell help, it is possible that this can have functional significance in helping to contain the low pathogen loads during the early stages of infection when T cell help has not yet peaked198,267 (Fig. 4).
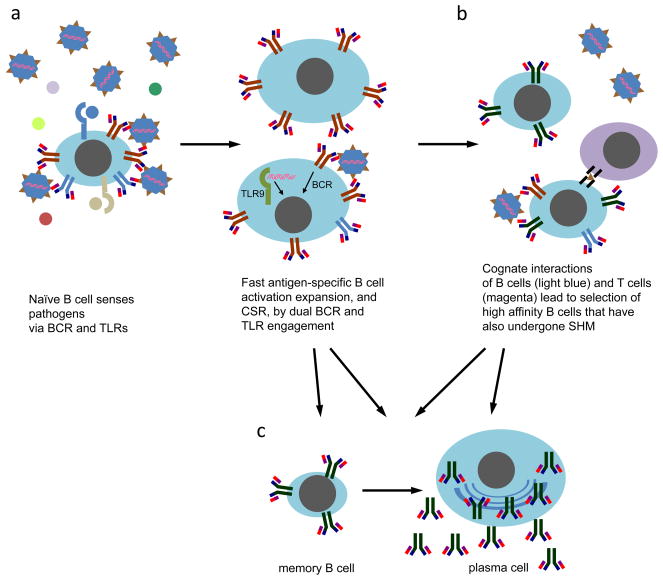
FIGURE 4.
Model on the role of endosomal TLRs in B cell antibody responses. a. B cells in the secondary lymphoid organs can be directly activated by viruses which crosslink the BCR. This then leads to their phagocytosis, double membrane autophagosome formation, and trafficking of the autophagosomes to TLR-containing endosomes. If any present viral TLR ligands are sensed by TLR3, TLR7/8, or TLR9, an appropriate response is then mounted by the B cells, which includes their differentiation (and in some cells, T-independent CSR), the upregulation of receptors that interact with TH cells and DCs, thus enhancing the GC reaction (b). The result of T-independent (a) and T-dependent (b) B cell differentiation is the formation of antigen-specific plasma cells, and memory B cells, which can be quickly differentiated to plasma cells upon subsequent re-infection (c).
B. TLR Signaling may Play Roles During the T-Dependent Phase of B cell Differentiation
Initial IgM production by B cells is likely due to their activation by various TLRs and the BCR, since antigen-specific T helper cell numbers do not peak until several days after their initial activation. IgM production, in addition to providing some early and direct protection by targeting pathogenic epitopes, also serves in the activation of complement, which then results in microbe coating with C3d, which binds the CD21/CD35 receptors on the B cell surface and enhances B cell activation. 267 The antibody-pathogen complexes are drained in secondary lymphoid organs, where they are destroyed by professional phagocytes such as macrophages, and in the process stimulate the T-dependent GC reaction.99
Direct TLR stimulation of B cells during the GC reaction, may help to sustain and shape the processes of antibody affinity maturation, by acting as a reporter of pathogen load and infection conditions.10,20,49,175,191 For example, initial TLR-mediated activation of B cells leads to the upregulation of co-stimulatory CD80/CD86 and MHC-II receptors, thereby priming B cells for more abundant and efficient interactions with T cells and DCs.268 T cell help alone may not be sufficient for optimal antibody responses, with direct TLR stimulation of B cells contributing to optimal antibody titers and CSR49, though this issue remains unclear128,129,175,191. During the GC reaction, TLR signals in B cells are likely integrated with signals from the BCR, T cells and DCs, eventually giving rise to plasma and memory B cells, whose Ig genes encode antigen-specific, high-affinity and class-switched antibodies.199,223,269
Immunization with CpG and hepatitis antigen resulted in higher antibody affinities than immunization with antigen alone.270 When TLR ligands in a respiratory syncytial virus (RSV) vaccine were inactive, the resulting antibodies were low-affinity due to impaired SHM and were not protective.271 These reports suggest that TLRs play a role in SHM, since their engagement contributes to the generation of high-affinity antibodies, though the molecular and cellular mechanisms responsible have not yet been elucidated. The immune system, including B cells, need to continually recognize and bind pathogens in order to ultimately clear them. In particular, nucleic acid TLR ligands may be important signals for SHM, since they would signal the presence of replicating, and potentially hypermutating, microorganisms.272
C. TLR and BCR Signaling in Vaccine Research
1. Vaccine Adjuvants
Well before the discovery of the TLRs, vaccinologists empirically used adjuvants such as Freund’s complete adjuvant (FCA), Freund’s incomplete adjuvant (FIA) and Ribbi’s adjuvant, which, in addition to containing oils for forming depots upon injection, also contain mycobacterial components (which include various TLR ligands) in order to elicit robust immune responses to soluble protein antigens.7,10,175,273,274 Since the discovery of the crucial adjuvanting properties of TLR and other PRR ligands, there has justifiably been an explosion in the application of defined adjuvants for use in vaccine development.274–297
All current clinical vaccines elicit protective antibodies,87,88 frequently also eliciting the mobilization of other immune cells, including NK cells, cytotoxic T cells, TH cells, DCs, macrophages, etc. 274 As mentioned previously, several studies have reported that CpG or antigen-CpG fusion immunization in humans and mice result in higher antibody titers, and/or antibody affinities for antigen and/or longer-lasting protection,19,234,235,269,270,298–303 possibly due to enhanced CSR and SHM and expansion of high-affinity memory B cells. The reader may refer to a recent review for an extensive list of examples where prior CpG administration has resulted in some protection before challenge with pathogens.304 A comprehensive list of current clinical and experimental vaccines that target T-independent bacterial antigens has also been recently reviewed.305
2. New Generation Modular Vaccines
Based on the promise of defined TLR agonists for use in vaccines, a new generation of vaccines is currently being developed. These vaccines employ particles made of biodegradable materials, lipid vesicles, or self-assembling protein subunits, that in addition to containing protein antigen, are infused with internal or external TLR ligands192,306–312 (Fig. 5). This approach allows for modular, systematic and controlled testing of the best combinations of protein antigens from the pathogens and natural or synthetic TLR agonists, taking into account the dual TLR and BCR nature of B cell differentiation that was described in the previous section. Generally, a rigid array of a minimum of 15–20 repeating antigens induces optimal BCR signaling.192
FIGURE 5.
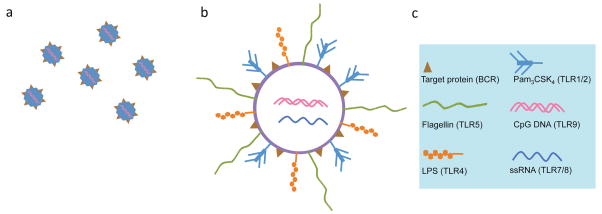
The design features of modular vaccines. a. Virus-mimicking biodegradable particles with favorable pharmacokinetics, which by themselves are not immunogenic, are used as scaffolds for the incorporation of protein antigens from one or several pathogens. Initial screens determine the most immunogenically suitable protein antigens (brown triangles) to use. In addition, combinations of one or more natural or synthetic TLR ligands are included, usually mimicking their occurrence in the actual pathogen(s). b. Particles mimicking bacteria are coated with bacterial protein antigens as well as TLR ligands characteristic of each bacterium. These composite vaccines (a, b) activate both innate immune cells as well as B lymphocytes, which will differentiate to plasma cells that secrete antigen-specific antibodies, as well as to memory B cells which can be activated upon potential future re-infection. c. Legend indicates the protein antigens and the various TLR ligands used in modular vaccines.
While modular vaccine design is still under development, as a general approach, the particle and the TLR ligand combinations used should mimic the pathogen as closely as possible, e.g. it may not be beneficial to use flagellin to develop modular viral vaccines since it does not naturally occur in viruses. The TLR ligands can be LPS, Pam3CSK4, flagellin etc, which should be incorporated on the outside of the vaccine particle, and CpG, ssRNA, dsRNA or agonists such as imiquimod or R848, which should be best incorporated inside the particle. Future studies may, however, report beneficial, synergistic combinations of TLR ligands that are not normally found in the same pathogen, which may apply especially in the cases of co-infection by two or more pathogens.
Taking this approach one step further, recent studies have explored the feasibility of developing broad-spectrum vaccines, where immunity is provided against more than one pathogenic strain, or against several related or even unrelated pathogens.313,314 In this case, antigens from several different pathogens are incorporated in the same particle or separately in mixtures of different particles, with TLR ligands typical of each pathogen also incorporated in the particles. In principle, this approach should allow for mobilization of B cells that produce antibodies specific for each pathogen. Issues remaining to be resolved relate to the safety and efficacy of CpG and other TLR agonists in eliciting broad-spectrum immunity to emerging and re-emerging pathogens.313,315
V. CONCLUSIONS
The synergy of BCR and TLR signaling critically regulates B cell antibody responses, and in particular CSR. BCR crosslinking facilitates the encounter of endosomes containing TLR9, and likely other TLRs, to autophagosomes as a way of surveilling the presence of nucleic acid TLR ligands during an infection. BCR stimulation provides information on the affinity of antigen for antibody, and the spacing and topology of antigenic arrays on the pathogen, whereas TLR stimulation may instruct B cells on the nature of the pathogen, e.g., the presence of a Gram+ve or Gram−ve bacterium or of an RNA or DNA virus. It would be particularly useful for B cells to sense multiple TLR signals from a pathogen in a combinatorial fashion, thereby leading to a more appropriate and specific response.
The dual presence of BCR and TLRs on B cells allows them to respond to a greater variety and combination of signals than previously thought, including traditionally innate signals, thereby allowing for fine-tuned responses that best deal with the pathogenic threat. The interplay and synergy between BCR, TLRs, and T-dependent signals is also proving to be both challenging and rewarding. The ultimate task remains to predict B cell behavior during an infection in vivo based on studies of responses to defined combinations of stimuli under different conditions in vitro and in vivo. Knowledge of fundamental B cell signals and typical responses would be beneficial for the design of therapies for immune deficiency or autoimmunity, and for the development of effective vaccines.
Acknowledgments
Owing to space limitations, we could cite only a fraction of the papers relevant to the topics discussed in this review article. We apologize to the other authors of publications that were not cited. We would like to thank members of the Casali laboratory for invaluable discussions and critical reading of this manuscript. This work was supported by NIH grants AI 045011, AI 079705 and AI 060573 to P.C.
References
- 1.Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25:640–644. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Malissen B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat Immunol. 2005;6:17–21. doi: 10.1038/ni1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 4.Litman GW, Cooper MD. Why study the evolution of immunity? Nat Immunol. 2007;8:547–548. doi: 10.1038/ni0607-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009;10:333–339. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 6.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 8.DeFranco AL, Locksley RM, Robertson RM. Immunity: the immune response in infectous and inflammatory disease. Sunderland, MA: New Science Press; 2007. [Google Scholar]
- 9.Murphy KM, Travers P, Walport M. Janeway's Immunobiology. New York: Garland Science; 2007. [Google Scholar]
- 10.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey PW, Vaidya SA, Cheng G. The art of war: Innate and adaptive immune responses. Cell Mol Life Sci. 2003;60:2604–2621. doi: 10.1007/s00018-003-3180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol. 2005;116:241–249. doi: 10.1016/j.jaci.2005.05.036. quiz 250. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 14.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 16.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, Coustan-Smith E, Howard V, Campana D. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho A, Poltorack A. Innate immunity: from lymphocyte mitogens to Toll-like receptors and back. Curr Opin Immunol. 2003;15:599–602. doi: 10.1016/j.coi.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 19.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 20.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 21.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 22.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 23.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 27.Rolff J. Why did the acquired immune system of vertebrates evolve? Dev Comp Immunol. 2007;31:476–482. doi: 10.1016/j.dci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Zakharova LA. Evolution of adaptive immunity. Biol Bull. 2009;36:107–116. [PubMed] [Google Scholar]
- 29.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 30.Pancer Z, Saha NR, Kasamatsu J, Suzuki T, Amemiya CT, Kasahara M, Cooper MD. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci U S A. 2005;102:9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 32.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 33.Nagawa F, Kishishita N, Shimizu K, Hirose S, Miyoshi M, Nezu J, Nishimura T, Nishizumi H, Takahashi Y, Hashimoto S, Takeuchi M, Miyajima A, Takemori T, Otsuka AJ, Sakano H. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]
- 34.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, Boydston JA, Turnbough CL, Jr, Cooper MD. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci U S A. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 38.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 39.Hodgkin PD, Heath WR, Baxter AG. The clonal selection theory: 50 years since the revolution. Nat Immunol. 2007;8:1019–1026. doi: 10.1038/ni1007-1019. [DOI] [PubMed] [Google Scholar]
- 40.Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol. 2008;86:133–138. doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- 41.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 42.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 43.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casadevall A, Pirofski LA. New concepts in antibody-mediated immunity. Infect Immun. 2004;72:6191–6196. doi: 10.1128/IAI.72.11.6191-6196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bebbington C, Yarranton G. Antibodies for the treatment of bacterial infections: current experience and future prospects. Curr Opin Biotechnol. 2008;19:613–619. doi: 10.1016/j.copbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Law M, Hangartner L. Antibodies against viruses: passive and active immunization. Curr Opin Immunol. 2008;20:486–492. doi: 10.1016/j.coi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 49.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 50.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 51.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005;17:237–243. doi: 10.1016/j.coi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 53.Pike BL, Alderson MR, Nossal GJ. T-independent activation of single B cells: an orderly analysis of overlapping stages in the activation pathway. Immunol Rev. 1987;99:119–152. doi: 10.1111/j.1600-065x.1987.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 54.Moller G. Receptors for innate pathogen defence in insects are normal activation receptors for specific immune responses in mammals. Scand J Immunol. 1999;50:341–347. doi: 10.1046/j.1365-3083.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 55.Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004;112:509–520. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards S, Watanabe C, Santos L, Craxton A, Clark EA. Regulation of B-cell entry into the cell cycle. Immunol Rev. 2008;224:183–200. doi: 10.1111/j.1600-065X.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rush JS, Hodgkin PD. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. Eur J Immunol. 2001;31:1150–1159. doi: 10.1002/1521-4141(200104)31:4<1150::aid-immu1150>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 58.Donahue AC, Fruman DA. Proliferation and survival of activated B cells requires sustained antigen receptor engagement and phosphoinositide 3-kinase activation. J Immunol. 2003;170:5851–5860. doi: 10.4049/jimmunol.170.12.5851. [DOI] [PubMed] [Google Scholar]
- 59.Hodgkin PD, Chin SH, Bartell G, Mamchak A, Doherty K, Lyons AB, Hasbold J. The importance of efficacy and partial agonism in evaluating models of B lymphocyte activation. Int Rev Immunol. 1997;15:101–127. doi: 10.3109/08830189709068173. [DOI] [PubMed] [Google Scholar]
- 60.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc. 2007;2:2057–2067. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 61.Rachmilewitz J, Lanzavecchia A. A temporal and spatial summation model for T-cell activation: signal integration and antigen decoding. Trends Immunol. 2002;23:592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
- 62.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 64.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41 (Suppl 7):S421–426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- 65.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 66.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 67.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 69.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24:39–65. doi: 10.1615/critrevimmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 70.Cozine CL, Wolniak KL, Waldschmidt TJ. The primary germinal center response in mice. Curr Opin Immunol. 2005;17:298–302. doi: 10.1016/j.coi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki K, Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–531. doi: 10.1016/j.it.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 74.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 75.Ma Y, Hendershot LM. The stressful road to antibody secretion. Nat Immunol. 2003;4:310–311. doi: 10.1038/ni0403-310. [DOI] [PubMed] [Google Scholar]
- 76.Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–29. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- 77.Cenci S, Sitia R. Managing and exploiting stress in the antibody factory. FEBS Lett. 2007;581:3652–3657. doi: 10.1016/j.febslet.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 78.Masciarelli S, Sitia R. Building and operating an antibody factory: redox control during B to plasma cell terminal differentiation. Biochim Biophys Acta. 2008;1783:578–588. doi: 10.1016/j.bbamcr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24:474–478. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 80.Brady LJ. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect Immun. 2005;73:671–678. doi: 10.1128/IAI.73.2.671-678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hjelm F, Carlsson F, Getahun A, Heyman B. Antibody-mediated regulation of the immune response. Scand J Immunol. 2006;64:177–184. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 82.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 83.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 84.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008;20:162–169. doi: 10.1016/j.coi.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Casadevall A. The methodology for determining the efficacy of antibody-mediated immunity. J Immunol Methods. 2004;291:1–10. doi: 10.1016/j.jim.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 88.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 89.Radbruch A, Burger C, Klein S, Muller W. Control of immunoglobulin class switch recombination. Immunol Rev. 1986;89:69–83. doi: 10.1111/j.1600-065x.1986.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 90.Iwasato T, Shimizu A, Honjo T, Yamagishi H. Circular DNA is excised by immunoglobulin class switch recombination. Cell. 1990;62:143–149. doi: 10.1016/0092-8674(90)90248-d. [DOI] [PubMed] [Google Scholar]
- 91.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 92.Stavnezer J. Molecular processes that regulate class switching. Curr Top Microbiol Immunol. 2000;245:127–168. doi: 10.1007/978-3-642-59641-4_6. [DOI] [PubMed] [Google Scholar]
- 93.Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 94.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kenter AL. Class switch recombination: an emerging mechanism. Curr Top Microbiol Immunol. 2005;290:171–199. doi: 10.1007/3-540-26363-2_8. [DOI] [PubMed] [Google Scholar]
- 96.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Durandy A. Immunoglobulin class switch recombination: study through human natural mutants. Philos Trans R Soc Lond B Biol Sci. 2009;364:577–582. doi: 10.1098/rstb.2008.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casali P, Immunoglobulin M. In: Encyclopedia of Immunology. 2. Roitt IM, Delves PJ, editors. London: Academic Press Ltd; 1998. pp. 1212–1217. [Google Scholar]
- 99.Matter MS, Ochsenbein AF. Natural antibodies target virus-antibody complexes to organized lymphoid tissue. Autoimmun Rev. 2008;7:480–486. doi: 10.1016/j.autrev.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 100.Durandy A, Peron S, Fischer A. Hyper-IgM syndromes. Curr Opin Rheumatol. 2006;18:369–376. doi: 10.1097/01.bor.0000231905.12172.b5. [DOI] [PubMed] [Google Scholar]
- 101.Durandy A, Taubenheim N, Peron S, Fischer A. Pathophysiology of B-cell intrinsic immunoglobulin class switch recombination deficiencies. Adv Immunol. 2007;94:275–306. doi: 10.1016/S0065-2776(06)94009-7. [DOI] [PubMed] [Google Scholar]
- 102.Ta VT, Nagaoka H, Catalan N, Durandy A, Fischer A, Imai K, Nonoyama S, Tashiro J, Ikegawa M, Ito S, Kinoshita K, Muramatsu M, Honjo T. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 103.Valiaho J, Smith CI, Vihinen M. BTKbase: the mutation database for X-linked agammaglobulinemia. Hum Mutat. 2006;27:1209–1217. doi: 10.1002/humu.20410. [DOI] [PubMed] [Google Scholar]
- 104.Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 105.Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 106.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 107.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kesh S, Mensah NY, Peterlongo P, Jaffe D, Hsu K, MVDB, O’Reilly R, Pamer E, Satagopan J, Papanicolaou GA. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci. 2005;1062:95–103. doi: 10.1196/annals.1358.012. [DOI] [PubMed] [Google Scholar]
- 109.Thuong NT, Hawn TR, Thwaites GE, Chau TT, Lan NT, Quy HT, Hieu NT, Aderem A, Hien TT, Farrar JJ, Dunstan SJ. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8:422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 110.Bochud PY, Hersberger M, Taffe P, Bochud M, Stein CM, Rodrigues SD, Calandra T, Francioli P, Telenti A, Speck RF, Aderem A. Polymorphisms in Toll-like receptor 9 influence the clinical course of HIV-1 infection. AIDS. 2007;21:441–446. doi: 10.1097/QAD.0b013e328012b8ac. [DOI] [PubMed] [Google Scholar]
- 111.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 112.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 113.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 114.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 115.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 116.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 117.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 118.Ryffel B, Fremond C, Jacobs M, Parida S, Botha T, Schnyder B, Quesniaux V. Innate immunity to mycobacterial infection in mice: critical role for toll-like receptors. Tuberculosis (Edinb) 2005;85:395–405. doi: 10.1016/j.tube.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 119.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 120.Sutherland AM, Walley KR, Russell JA. Polymorphisms in CD14, mannose-binding lectin, and Toll-like receptor-2 are associated with increased prevalence of infection in critically ill adults. Crit Care Med. 2005;33:638–644. doi: 10.1097/01.ccm.0000156242.44356.c5. [DOI] [PubMed] [Google Scholar]
- 121.Yuan FF, Marks K, Wong M, Watson S, de Leon E, McIntyre PB, Sullivan JS. Clinical relevance of TLR2, TLR4, CD14 and FcgammaRIIA gene polymorphisms in Streptococcus pneumoniae infection. Immunol Cell Biol. 2008;86:268–270. doi: 10.1038/sj.icb.7100155. [DOI] [PubMed] [Google Scholar]
- 122.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 123.Saitoh S, Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev. 2009;227:32–43. doi: 10.1111/j.1600-065X.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 124.Carpenter S, O’Neill LA. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 125.Beutler B, Moresco EM. The forward genetic dissection of afferent innate immunity. Curr Top Microbiol Immunol. 2008;321:3–26. doi: 10.1007/978-3-540-75203-5_1. [DOI] [PubMed] [Google Scholar]
- 126.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci. 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 127.Garantziotis S, Hollingsworth JW, Zaas AK, Schwartz DA. The effect of toll-like receptors and toll-like receptor genetics in human disease. Annu Rev Med. 2008;59:343–359. doi: 10.1146/annurev.med.59.061206.112455. [DOI] [PubMed] [Google Scholar]
- 128.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 131.Bishop GA, Haxhinasto SA, Stunz LL, Hostager BS. Antigen-specific B-lymphocyte activation. Crit Rev Immunol. 2003;23:149–197. doi: 10.1615/critrevimmunol.v23.i3.10. [DOI] [PubMed] [Google Scholar]
- 132.Fulcher DA, Basten A. B-cell activation versus tolerance--the central role of immunoglobulin receptor engagement and T-cell help. Int Rev Immunol. 1997;15:33–52. doi: 10.3109/08830189709068170. [DOI] [PubMed] [Google Scholar]
- 133.Tarlinton DM, Smith KG. Apoptosis and the B cell response to antigen. Int Rev Immunol. 1997;15:53–71. doi: 10.3109/08830189709068171. [DOI] [PubMed] [Google Scholar]
- 134.Donjerkovic D, Scott DW. Activation-induced cell death in B lymphocytes. Cell Res. 2000;10:179–192. doi: 10.1038/sj.cr.7290047. [DOI] [PubMed] [Google Scholar]
- 135.Silverman GJ, Goodyear CS. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat Rev Immunol. 2006;6:465–475. doi: 10.1038/nri1853. [DOI] [PubMed] [Google Scholar]
- 136.Acosta-Rodriguez EV, Merino MC, Montes CL, Motran CC, Gruppi A. Cytokines and chemokines shaping the B-cell compartment. Cytokine Growth Factor Rev. 2007;18:73–83. doi: 10.1016/j.cytogfr.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 137.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 138.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stavnezer J, Amemiya CT. Evolution of isotype switching. Semin Immunol. 2004;16:257–275. doi: 10.1016/j.smim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Zarrin AA, Alt FW, Chaudhuri J, Stokes N, Kaushal D, Du Pasquier L, Tian M. An evolutionarily conserved target motif for immunoglobulin class-switch recombination. Nat Immunol. 2004;5:1275–1281. doi: 10.1038/ni1137. [DOI] [PubMed] [Google Scholar]
- 141.Chaudhuri J, Basu U, Zarrin A, Yan C, Franco S, Perlot T, Vuong B, Wang J, Phan RT, Datta A, Manis J, Alt FW. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv Immunol. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 142.Delker RK, Fugmann SD, Papavasiliou FN. A coming-of-age story: activation-induced cytidine deaminase turns 10. Nat Immunol. 2009;10:1147–1153. doi: 10.1038/ni.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lieber MR, Yu K, Raghavan SC. Roles of nonhomologous DNA end joining, V(D)J recombination, and class switch recombination in chromosomal translocations. DNA Repair. 2006;5:1234–1245. doi: 10.1016/j.dnarep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 144.Jolly CJ, Cook AJ, Manis JP. Fixing DNA breaks during class switch recombination. J Exp Med. 2008;205:509–513. doi: 10.1084/jem.20080356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu Z, Pone EJ, Al-Qahtani A, Park SR, Zan H, Casali P. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol. 2007;27:367–397. doi: 10.1615/critrevimmunol.v27.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 147.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 148.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009;53:1–16. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 150.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–276. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 151.He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 154.Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007;109:2961–2967. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- 155.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 157.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 158.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, Cattalini M, Litzman J, JBB, Huang B, Meini A, Riesbeck K, Cunningham-Rundles C, Plebani A, Cerutti A. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klaus GG, Choi MS, Lam EW, Johnson-Leger C, Cliff J. CD40: a pivotal receptor in the determination of life/death decisions in B lymphocytes. Int Rev Immunol. 1997;15:5–31. doi: 10.3109/08830189709068169. [DOI] [PubMed] [Google Scholar]
- 162.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–2157. [PMC free article] [PubMed] [Google Scholar]
- 163.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 165.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 166.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nashar TO, Drake JR. Dynamics of MHC class II-activating signals in murine resting B cells. J Immunol. 2006;176:827–838. doi: 10.4049/jimmunol.176.2.827. [DOI] [PubMed] [Google Scholar]
- 168.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 169.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 170.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 171.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 172.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 173.Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 174.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 175.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 176.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 177.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 178.Werling D, Jann OC, Offord V, Glass EJ, Coffey TJ. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009;30:124–130. doi: 10.1016/j.it.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 179.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 180.McCoy CE, O’Neill LA. The role of toll-like receptors in macrophages. Front Biosci. 2008;13:62–70. doi: 10.2741/2660. [DOI] [PubMed] [Google Scholar]
- 181.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–4254. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 182.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 183.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 184.van Maren WW, Jacobs JF, de Vries IJ, Nierkens S, Adema GJ. Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology. 2008;124:445–452. doi: 10.1111/j.1365-2567.2008.02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 186.Nossal GJ, Szenberg A, Ada GL, Austin CM. Single Cell Studies on 19s Antibody Production. J Exp Med. 1964;119:485–502. doi: 10.1084/jem.119.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moller G. 19S antibody production against soluble lipopolysaccharide antigens by individual lymphoid cells in vitro. Nature. 1965;207:1166–1168. doi: 10.1038/2071166a0. [DOI] [PubMed] [Google Scholar]
- 188.Kearney JF, Lawton AR. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975;115:671–676. [PubMed] [Google Scholar]
- 189.Coutinho A, Moller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- 190.Gerondakis S, Grumont RJ, Banerjee A. Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol. 2007;85:471–475. doi: 10.1038/sj.icb.7100097. [DOI] [PubMed] [Google Scholar]
- 191.Palm NW, Medzhitov R. Immunostimulatory activity of haptenated proteins. Proc Natl Acad Sci U S A. 2009;106:4782–4787. doi: 10.1073/pnas.0809403105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hinton HJ, Jegerlehner A, Bachmann MF. Pattern recognition by B cells: the role of antigen repetitiveness versus Toll-like receptors. Curr Top Microbiol Immunol. 2008;319:1–15. doi: 10.1007/978-3-540-73900-5_1. [DOI] [PubMed] [Google Scholar]
- 193.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 194.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 195.May MJ. A nuclear factor in B cells and beyond. J Immunol. 2006;177:7483–7484. doi: 10.4049/jimmunol.177.11.7483. [DOI] [PubMed] [Google Scholar]
- 196.Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 197.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Quintana FJ, Solomon A, Cohen IR, Nussbaum G. Induction of IgG3 to LPS via Toll-like receptor 4 co-stimulation. PLoS ONE. 2008;3:e3509. doi: 10.1371/journal.pone.0003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chaturvedi A, Pierce SK. How Location Governs Toll-Like Receptor Signaling. Traffic. 2009;10:621–628. doi: 10.1111/j.1600-0854.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203–220. doi: 10.1111/j.1600-065X.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 201.Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol Rev. 2009;227:189–202. doi: 10.1111/j.1600-065X.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang J, Pone EJ, Xu Z, Al-Qahtani A, Zan H, Casali P. Engagement of endosomal TLRs synergizes with BCR-mediated and PI3K-dependent signaling to induce immunoglobulin class-switch DNA recombination. 2009. In preparation. [Google Scholar]
- 204.Hodgkin PD. An antigen valence theory to explain the evolution and organization of the humoral immune response. Immunol Cell Biol. 1997;75:604–618. doi: 10.1038/icb.1997.95. [DOI] [PubMed] [Google Scholar]
- 205.Bone H, Williams NA. Antigen-receptor cross-linking and lipopolysaccharide trigger distinct phosphoinositide 3-kinase-dependent pathways to NF-κB activation in primary B cells. Int Immunol. 2001;13:807–816. doi: 10.1093/intimm/13.6.807. [DOI] [PubMed] [Google Scholar]
- 206.Dye JR, Palvanov A, Guo B, Rothstein TL. B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-κB activation. J Immunol. 2007;179:229–235. doi: 10.4049/jimmunol.179.1.229. [DOI] [PubMed] [Google Scholar]
- 207.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 208.Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- 209.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 210.Halaas O, Husebye H, Espevik T. The journey of Toll-like receptors in the cell. Adv Exp Med Biol. 2007;598:35–48. doi: 10.1007/978-0-387-71767-8_4. [DOI] [PubMed] [Google Scholar]
- 211.Nishiya T, Kajita E, Miwa S, Defranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem. 2005;280:37107–37117. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
- 212.Kajita E, Nishiya T, Miwa S. The transmembrane domain directs TLR9 to intracellular compartments that contain TLR3. Biochem Biophys Res Commun. 2006;343:578–584. doi: 10.1016/j.bbrc.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 213.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 214.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 215.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 216.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 218.Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, Milner EC, Bernstein SH, Sanz I, Zand MS. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Peng SL, Li J, Lin L, Gerth A. The role of T-bet in B cells. Nat Immunol. 2003;4:1041. doi: 10.1038/ni1103-1041a. author reply 1041. [DOI] [PubMed] [Google Scholar]
- 220.Liu N. Response to ‘The role of T-bet in B cells’. Nat Immunol. 2003;4:1041. doi: 10.1038/ni1103-1041a. [DOI] [PubMed] [Google Scholar]
- 221.Rifkin IR, Marshak-Rothstein A. T-bet: the Toll-bridge to class-switch recombination? Nat Immunol. 2003;4:650–652. doi: 10.1038/ni0703-650. [DOI] [PubMed] [Google Scholar]
- 222.Fairfax KA, Corcoran LM, Pridans C, Huntington ND, Kallies A, Nutt SL, Tarlinton DM. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 223.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Luxembourg AT, Cooper NR. T cell-dependent, B cell-activating properties of antibody-coated small latex beads. A new model for B cell activation. J Immunol. 1994;153:604–614. [PubMed] [Google Scholar]
- 226.Jendholm J, Morgelin M, Perez Vidakovics ML, Carlsson M, Leffler H, Cardell LO, Riesbeck K. Superantigen- and TLR-dependent activation of tonsillar B cells after receptor-mediated endocytosis. J Immunol. 2009;182:4713–4720. doi: 10.4049/jimmunol.0803032. [DOI] [PubMed] [Google Scholar]
- 227.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre HJ, Sutherland GR, Endo Y, Fujita T, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Phillips JH, Lanier LL, Nakauchi H. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 228.Souwer Y, Griekspoor A, Jorritsma T, de Wit J, Janssen H, Neefjes J, van Ham SM. B cell receptor-mediated internalization of salmonella: a novel pathway for autonomous B cell activation and antibody production. J Immunol. 2009;182:7473–7481. doi: 10.4049/jimmunol.0802831. [DOI] [PubMed] [Google Scholar]
- 229.Yi AK, Yoon JG, Krieg AM. Convergence of CpG DNA- and BCR-mediated signals at the c-Jun N-terminal kinase and NF-κB activation pathways: regulation by mitogen-activated protein kinases. Int Immunol. 2003;15:577–591. doi: 10.1093/intimm/dxg058. [DOI] [PubMed] [Google Scholar]
- 230.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 231.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 232.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 233.Ghosh TK, Mickelson DJ, Solberg JC, Lipson KE, Inglefield JR, Alkan SS. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol. 2007;7:1111–1121. doi: 10.1016/j.intimp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 234.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- 235.Wagner H. The immunogenicity of CpG-antigen conjugates. Adv Drug Deliv Rev. 2009;61:243–247. doi: 10.1016/j.addr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 236.Mutwiri G, van Drunen Littel-van den Hurk S, Babiuk LA. Approaches to enhancing immune responses stimulated by CpG oligodeoxynucleotides. Adv Drug Deliv Rev. 2009;61:226–232. doi: 10.1016/j.addr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 237.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 238.Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009;61:248–255. doi: 10.1016/j.addr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 239.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother. 2005;49:3991–3996. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Corthay A. A three-cell model for activation of naive T helper cells. Scand J Immunol. 2006;64:93–96. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 241.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 244.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–269. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 245.Christensen SR, Shlomchik MJ. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin Immunol. 2007;19:11–23. doi: 10.1016/j.smim.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Krug A. Nucleic acid recognition receptors in autoimmunity. Handb Exp Pharmacol. 2008:129–151. doi: 10.1007/978-3-540-72167-3_7. [DOI] [PubMed] [Google Scholar]
- 247.Raschi E, Borghi MO, Grossi C, Broggini V, Pierangeli S, Meroni PL. Toll-like receptors: another player in the pathogenesis of the anti-phospholipid syndrome. Lupus. 2008;17:937–942. doi: 10.1177/0961203308095140. [DOI] [PubMed] [Google Scholar]
- 248.Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21–34. doi: 10.1196/annals.1443.012. [DOI] [PubMed] [Google Scholar]
- 249.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 251.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 252.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 253.Busconi L, Bauer JW, Tumang JR, Laws A, Perkins-Mesires K, Tabor AS, Lau C, Corley RB, Rothstein TL, Lund FE, Behrens TW, Marshak-Rothstein A. Functional outcome of B cell activation by chromatin immune complex engagement of the B cell receptor and TLR9. J Immunol. 2007;179:7397–7405. doi: 10.4049/jimmunol.179.11.7397. [DOI] [PubMed] [Google Scholar]
- 254.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. 2009;182:3482–3491. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 256.Zan H, Wu X, Komori A, Holloman WK, Casali P. AID-dependent generation of resected double-strand DNA breaks and recruitment of Rad52/Rad51 in somatic hypermutation. Immunity. 2003;18:727–738. doi: 10.1016/s1074-7613(03)00151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Park SR, Zan H, Pal Z, Zhang J, Al-Qahtani A, Pone EJ, Xu Z, Mai T, Casali P. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10:540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Szomolanyi-Tsuda E, Welsh RM. T-cell-independent antiviral antibody responses. Curr Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 259.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 260.Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–3975. doi: 10.1182/blood-2005-10-4170. [DOI] [PubMed] [Google Scholar]
- 261.Zhan Y, Brown LE, Deliyannis G, Seah S, Wijburg OL, Price J, Strugnell RA, O’Connell PJ, Lew AM. Responses against complex antigens in various models of CD4 T-cell deficiency: surprises from an anti-CD4 antibody transgenic mouse. Immunol Res. 2004;30:1–14. doi: 10.1385/IR:30:1:001. [DOI] [PubMed] [Google Scholar]
- 262.Swanson PA, 2nd, Lukacher AE, Szomolanyi-Tsuda E. Immunity to polyomavirus infection: the polyomavirus-mouse model. Semin Cancer Biol. 2009;19:244–251. doi: 10.1016/j.semcancer.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Szomolanyi-Tsuda E, Le QP, Garcea RL, Welsh RM. T-Cell-independent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J Virol. 1998;72:6665–6670. doi: 10.1128/jvi.72.8.6665-6670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Casola S, Rajewsky K. B cell recruitment and selection in mouse GALT germinal centers. Curr Top Microbiol Immunol. 2006;308:155–171. doi: 10.1007/3-540-30657-9_7. [DOI] [PubMed] [Google Scholar]
- 265.McCoy KD, Harris NL, Diener P, Hatak S, Odermatt B, Hangartner L, Senn BM, Marsland BJ, Geuking MB, Hengartner H, Macpherson AJ, Zinkernagel RM. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24:329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 266.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 267.Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 268.Meyer-Bahlburg A, Rawlings DJ. B cell autonomous TLR signaling and autoimmunity. Autoimmun Rev. 2008;7:313–316. doi: 10.1016/j.autrev.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9-ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009 doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 270.Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, Cameron DW, Cooper CL, Heathcote J, Davis HL, Lambert PH. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23:615–622. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 271.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Longo NS, Lipsky PE. Why do B cells mutate their immunoglobulin receptors? Trends Immunol. 2006;27:374–380. doi: 10.1016/j.it.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 273.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 274.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 275.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 276.Kornbluth RS, Stone GW. Immunostimulatory combinations: designing the next generation of vaccine adjuvants. J Leukoc Biol. 2006;80:1084–1102. doi: 10.1189/jlb.0306147. [DOI] [PubMed] [Google Scholar]
- 277.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6:835–847. doi: 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- 278.Schmidt CS, Morrow WJ, Sheikh NA. Smart adjuvants. Expert Rev Vaccines. 2007;6:391–400. doi: 10.1586/14760584.6.3.391. [DOI] [PubMed] [Google Scholar]
- 279.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25:3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 280.Mutwiri G, Gerdts V, Lopez M, Babiuk LA. Innate immunity and new adjuvants. Rev Sci Tech. 2007;26:147–156. [PubMed] [Google Scholar]
- 281.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27:687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 282.Chiarella P, Massi E, De Robertis M, Signori E, Fazio VM. Adjuvants in vaccines and for immunisation: current trends. Expert Opin Biol Ther. 2007;7:1551–1562. doi: 10.1517/14712598.7.10.1551. [DOI] [PubMed] [Google Scholar]
- 283.Wales J, Andreakos E, Feldmann M, Foxwell B. Targeting intracellular mediators of pattern-recognition receptor signalling to adjuvant vaccination. Biochem Soc Trans. 2007;35:1501–1503. doi: 10.1042/BST0351501. [DOI] [PubMed] [Google Scholar]
- 284.Schijns VE, Degen WG. Vaccine immunopotentiators of the future. Clin Pharmacol Ther. 2007;82:750–755. doi: 10.1038/sj.clpt.6100394. [DOI] [PubMed] [Google Scholar]
- 285.Fraser CK, Diener KR, Brown MP, Hayball JD. Improving vaccines by incorporating immunological coadjuvants. Expert Rev Vaccines. 2007;6:559–578. doi: 10.1586/14760584.6.4.559. [DOI] [PubMed] [Google Scholar]
- 286.Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–371. doi: 10.1007/s10875-007-9087-x. [DOI] [PubMed] [Google Scholar]
- 287.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 288.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 289.Higgins D, Marshall JD, Traquina P, Van Nest G, Livingston BD. Immunostimulatory DNA as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:747–759. doi: 10.1586/14760584.6.5.747. [DOI] [PubMed] [Google Scholar]
- 290.Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, Perrie Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J Drug Target. 2008;16:543–554. doi: 10.1080/10611860802228558. [DOI] [PubMed] [Google Scholar]
- 291.Parkinson T. The future of toll-like receptor therapeutics. Curr Opin Mol Ther. 2008;10:21–31. [PubMed] [Google Scholar]
- 292.Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine. 2008;26:6777–6783. doi: 10.1016/j.vaccine.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 293.Hauguel TM, Hackett CJ. Rationally-designed vaccine adjuvants: separating efficacy from toxicity. Front Biosci. 2008;13:2806–2813. doi: 10.2741/2887. [DOI] [PubMed] [Google Scholar]
- 294.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ebensen T, Guzman CA. Immune modulators with defined molecular targets: cornerstone to optimize rational vaccine design. Hum Vaccin. 2008;4:13–22. doi: 10.4161/hv.4.1.5560. [DOI] [PubMed] [Google Scholar]
- 296.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 297.Harandi AM, Davies G, Olesen OF. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev Vaccines. 2009;8:293–298. doi: 10.1586/14760584.8.3.293. [DOI] [PubMed] [Google Scholar]
- 298.Coban C, Ishii KJ, Stowers AW, Keister DB, Klinman DM, Kumar N. Effect of CpG oligodeoxynucleotides on the immunogenicity of Pfs25, a Plasmodium falciparum transmission-blocking vaccine antigen. Infect Immun. 2004;72:584–588. doi: 10.1128/IAI.72.1.584-588.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Semenov B, Zverev V. Vaccine containing natural TLR ligands protects from Salmonella typhimurium infection in mice and acute respiratory infections in children. Adv Exp Med Biol. 2007;601:377–380. doi: 10.1007/978-0-387-72005-0_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Higgins D, Marshall JD, Traquina P, Van Nest G, Livingston BD. Immunostimulatory DNA as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:747–759. doi: 10.1586/14760584.6.5.747. [DOI] [PubMed] [Google Scholar]
- 301.Averett DR, Fletcher SP, Li W, Webber SE, Appleman JR. The pharmacology of endosomal TLR agonists in viral disease. Biochem Soc Trans. 2007;35:1468–1472. doi: 10.1042/BST0351468. [DOI] [PubMed] [Google Scholar]
- 302.Jurk M, Vollmer J. Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs. 2007;21:387–401. doi: 10.2165/00063030-200721060-00006. [DOI] [PubMed] [Google Scholar]
- 303.Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J Immunol. 2008;181:5785–5790. doi: 10.4049/jimmunol.181.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Krieg AM. Antiinfective applications of toll-like receptor 9 agonists. Proc Am Thorac Soc. 2007;4:289–294. doi: 10.1513/pats.200701-021AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mond JJ, Kokai-Kun JF. The multifunctional role of antibodies in the protective response to bacterial T cell-independent antigens. Curr Top Microbiol Immunol. 2008;319:17–40. doi: 10.1007/978-3-540-73900-5_2. [DOI] [PubMed] [Google Scholar]
- 306.Jennings GT, Bachmann MF. Designing recombinant vaccines with viral properties: a rational approach to more effective vaccines. Curr Mol Med. 2007;7:143–155. doi: 10.2174/156652407780059140. [DOI] [PubMed] [Google Scholar]
- 307.Heit A, Busch DH, Wagner H, Schmitz F. Vaccine protocols for enhanced immunogenicity of exogenous antigens. Int J Med Microbiol. 2008;298:27–32. doi: 10.1016/j.ijmm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 308.Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4:5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- 309.Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol. 2009;49:303–326. doi: 10.1146/annurev-pharmtox-061008-103129. [DOI] [PubMed] [Google Scholar]
- 310.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv Drug Deliv Rev. 2009;61:205–217. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Malyala P, O’Hagan DT, Singh M. Enhancing the therapeutic efficacy of CpG oligonucleotides using biodegradable microparticles. Adv Drug Deliv Rev. 2009;61:218–225. doi: 10.1016/j.addr.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 312.Wilson KD, de Jong SD, Tam YK. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev. 2009;61:233–242. doi: 10.1016/j.addr.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 313.Hackett CJ. Innate immune activation as a broad-spectrum biodefense strategy: prospects and research challenges. J Allergy Clin Immunol. 2003;112:686–694. doi: 10.1016/S0091-6749(03)02025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nagy G, Emody L, Pal T. Strategies for the development of vaccines conferring broad-spectrum protection. Int J Med Microbiol. 2008;298:379–395. doi: 10.1016/j.ijmm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 315.Amlie-Lefond C, Paz DA, Connelly MP, Huffnagle GB, Whelan NT, Whelan HT. Innate immunity for biodefense: a strategy whose time has come. J Allergy Clin Immunol. 2005;116:1334–1342. doi: 10.1016/j.jaci.2005.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Szomolanyi-Tsuda E, Brien JD, Dorgan JE, Garcea RL, Woodland RT, Welsh RM. Antiviral T-cell-independent type 2 antibody responses induced in vivo in the absence of T and NK cells. Virology. 2001;280:160–168. doi: 10.1006/viro.2000.0766. [DOI] [PubMed] [Google Scholar]
- 317.Guay HM, Mishra R, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. Generation of protective T cell-independent antiviral antibody responses in SCID mice reconstituted with follicular or marginal zone B cells. J Immunol. 2009;183:518–523. doi: 10.4049/jimmunol.0900068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Szomolanyi-Tsuda E, Seedhom MO, Carroll MC, Garcea RL. T cell-independent and T cell-dependent immunoglobulin G responses to polyomavirus infection are impaired in complement receptor 2-deficient mice. Virology. 2006;352:52–60. doi: 10.1016/j.virol.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci U S A. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 321.VanCott JL, McNeal MM, Flint J, Bailey SA, Choi AH, Ward RL. Role for T cell-independent B cell activity in the resolution of primary rotavirus infection in mice. Eur J Immunol. 2001;31:3380–3387. doi: 10.1002/1521-4141(200111)31:11<3380::aid-immu3380>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 322.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175:5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 325.Bitsaktsis C, Nandi B, Racine R, MacNamara KC, Winslow G. T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect Immun. 2007;75:4933–4941. doi: 10.1128/IAI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ganta RR, Cheng C, Wilkerson MJ, Chapes SK. Delayed clearance of Ehrlichia chaffeensis infection in CD4+ T-cell knockout mice. Infect Immun. 2004;72:159–167. doi: 10.1128/IAI.72.1.159-167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.McKisic MD, Barthold SW. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of lyme disease. Infect Immun. 2000;68:5190–5197. doi: 10.1128/iai.68.9.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Harvill ET, Osorio M, Loving CL, Lee GM, Kelly VK, Merkel TJ. Anamnestic protective immunity to Bacillus anthracis is antibody mediated but independent of complement and Fc receptors. Infect Immun. 2008;76:2177–2182. doi: 10.1128/IAI.00647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Autenrieth IB, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J Immunol. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- 331.Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2002;34:45–50. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 332.Nosanchuk JD, Rosas AL, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 333.Adjei AA, Jones JT, Riggs MW, Enriquez FJ. Evidence of thymus-independent local and systemic antibody responses to Cryptosporidium parvum infection in nude mice. Infect Immun. 1999;67:3947–3951. doi: 10.1128/iai.67.8.3947-3951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Baz A, Carol H, Fernandez V, Mourglia-Ettlin G, Nieto A, Orn A, Dematteis S. Echinococcus granulosus: induction of T-independent antibody response against protoscolex glycoconjugates in early experimental infection. Exp Parasitol. 2008;119:460–466. doi: 10.1016/j.exppara.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 335.Dai WJ, Hemphill A, Waldvogel A, Ingold K, Deplazes P, Mossmann H, Gottstein B. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of alphabeta+ CD4+ T cells. Infect Immun. 2001;69:6074–6083. doi: 10.1128/IAI.69.10.6074-6083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]