Abstract
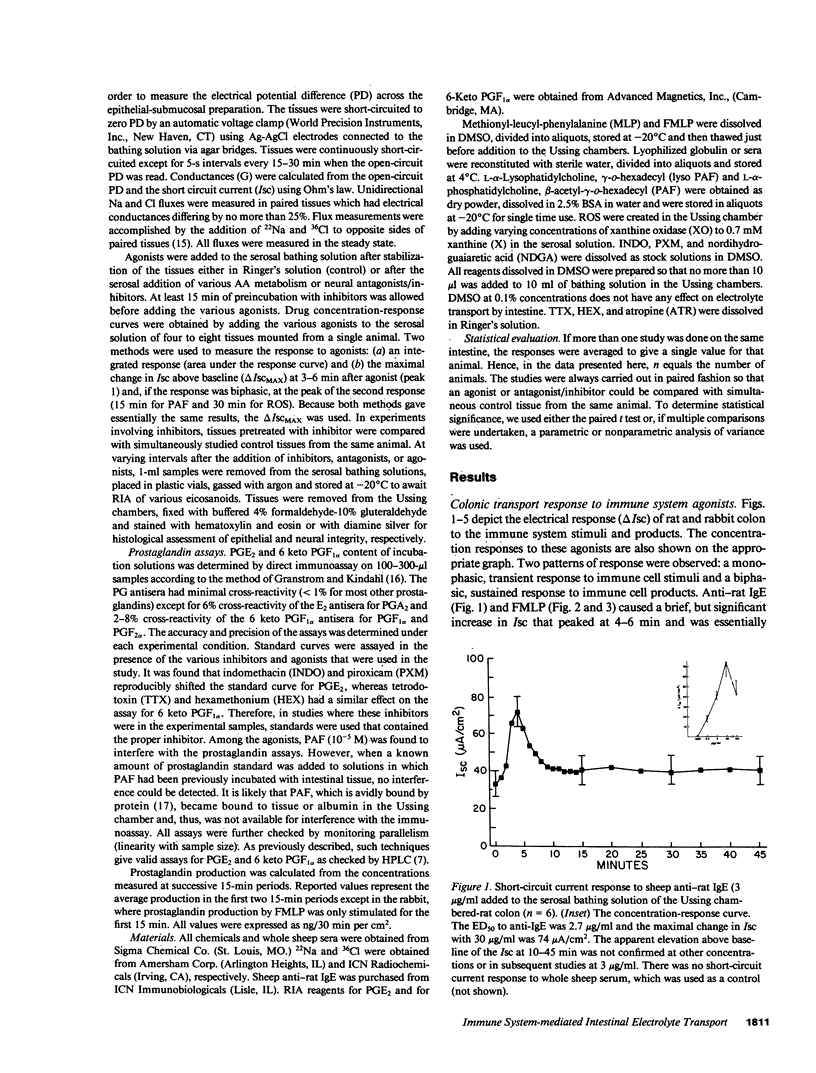
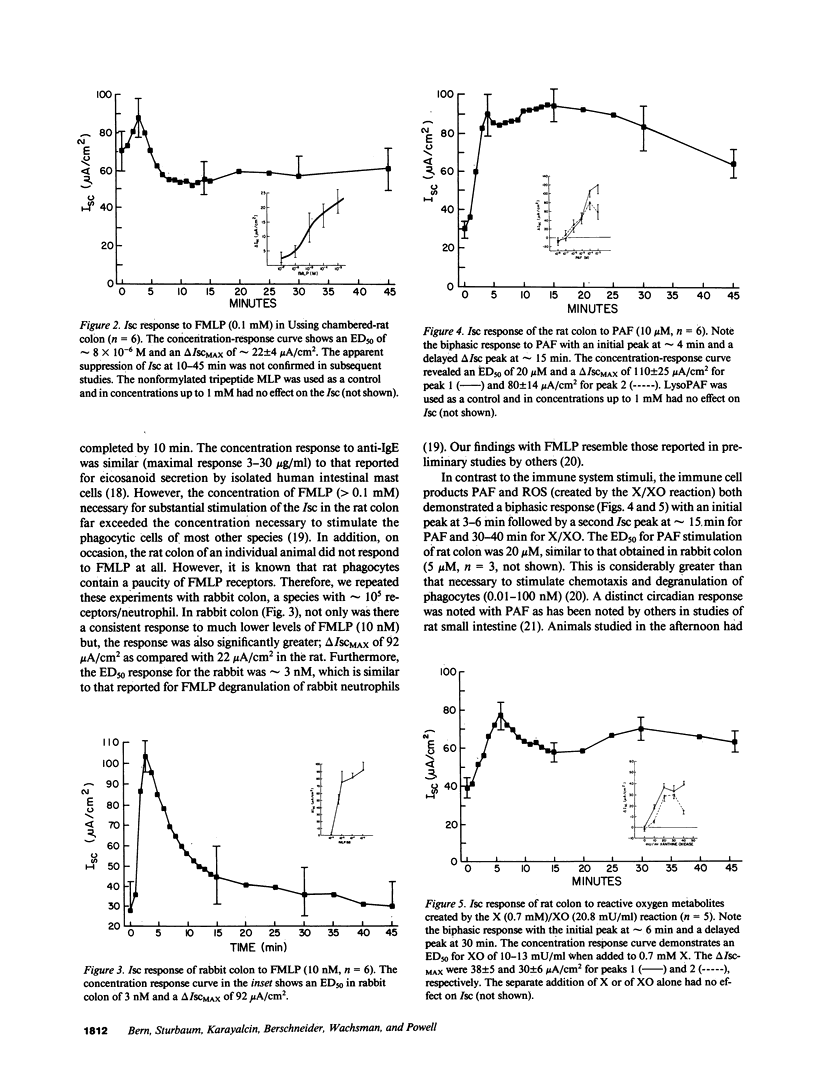
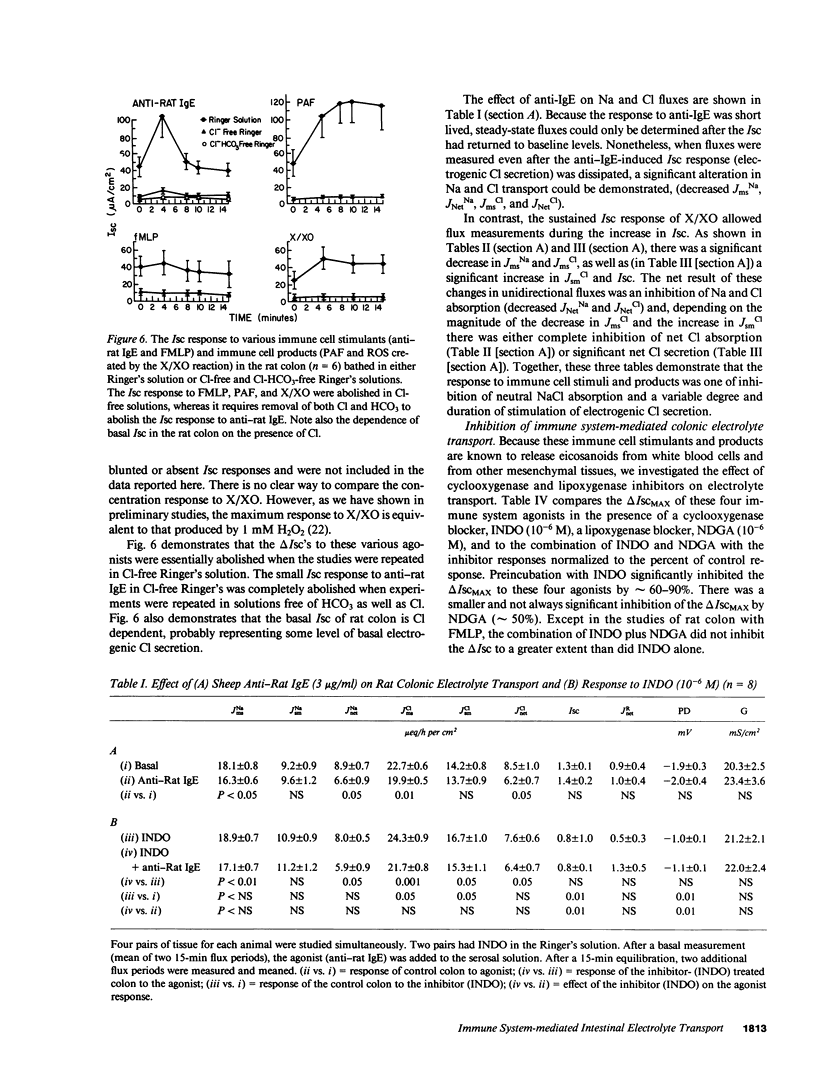
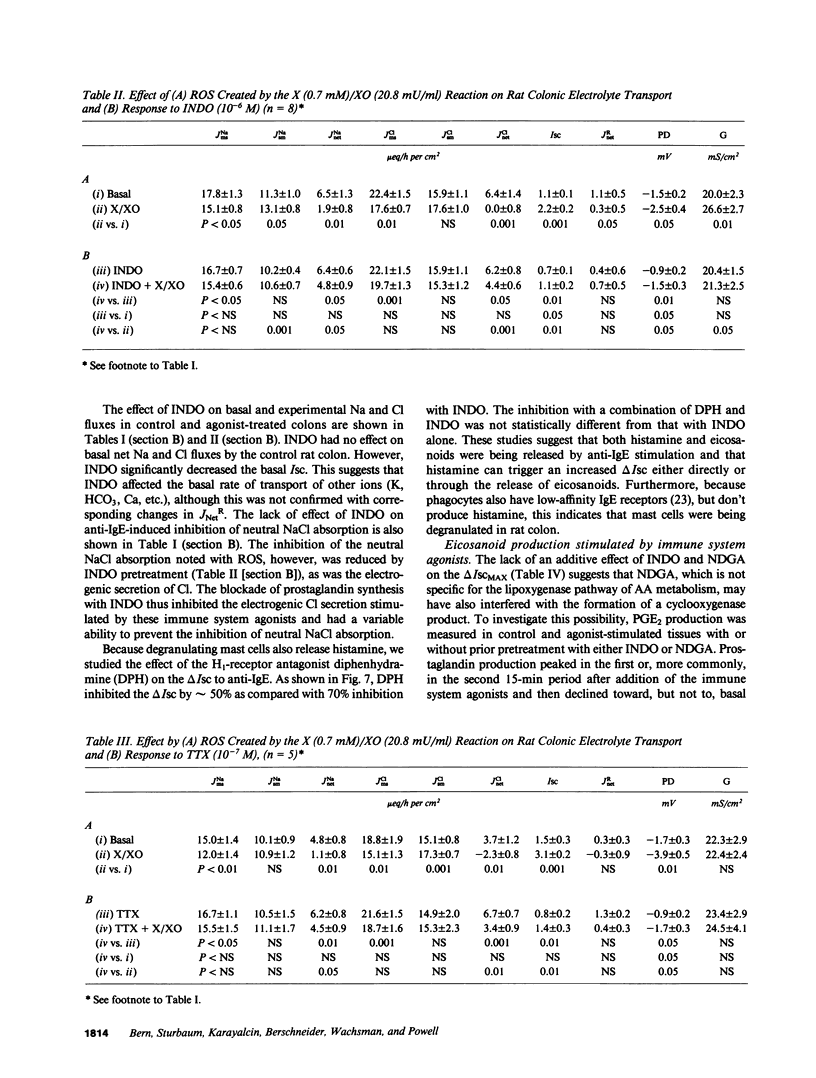
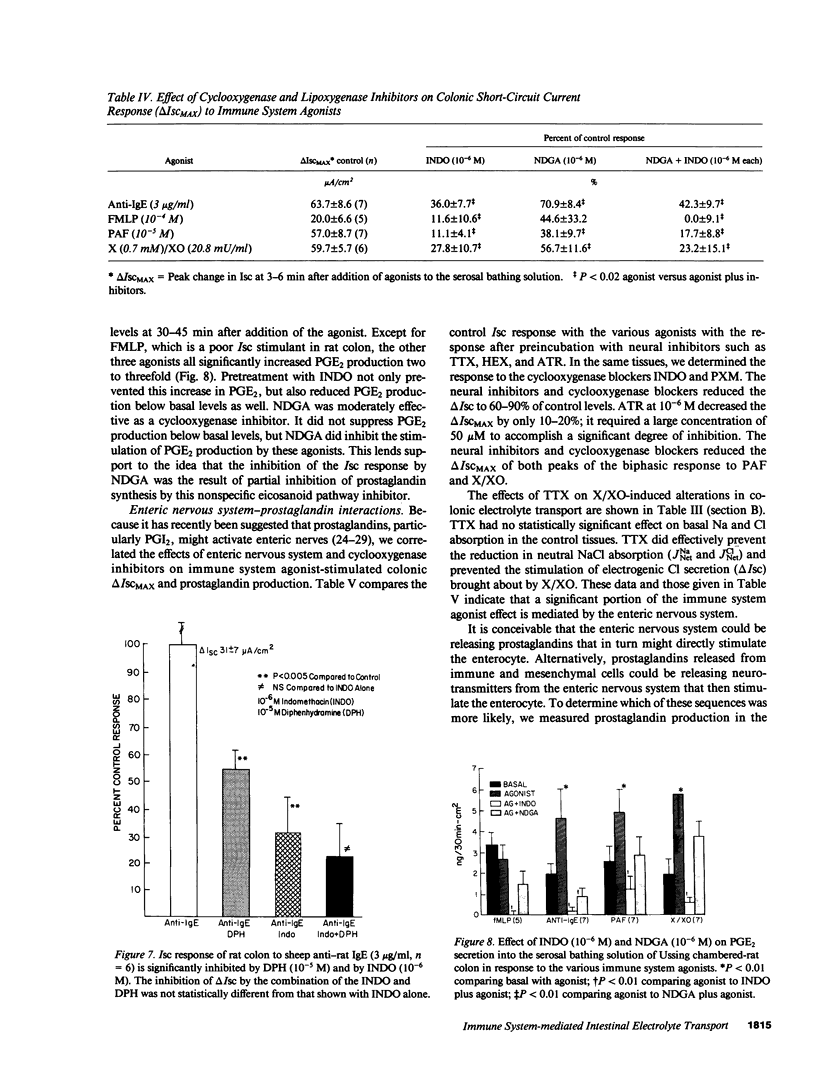
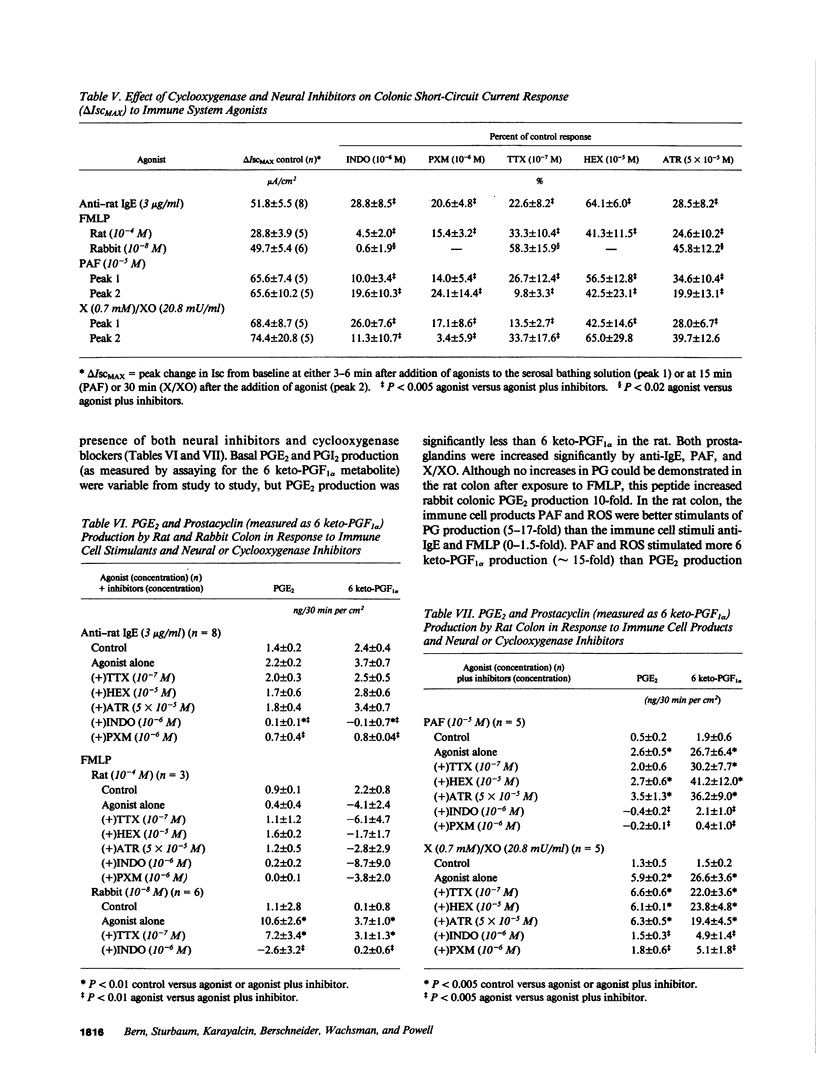
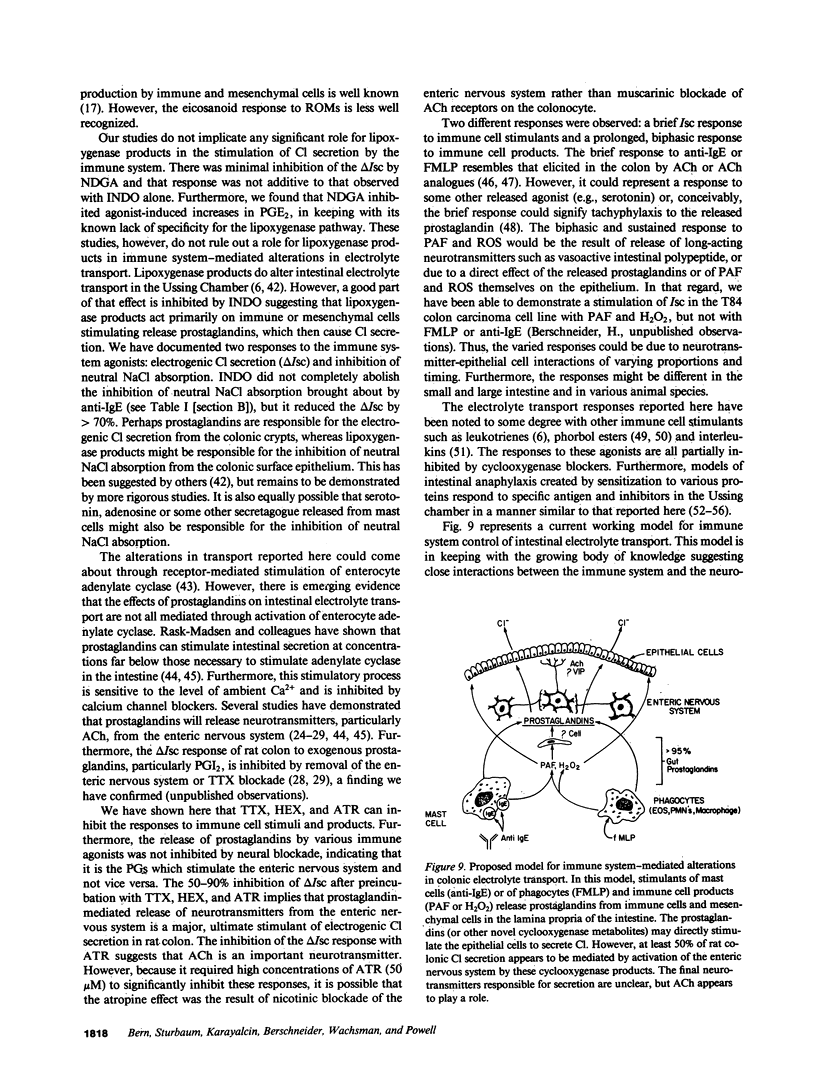
The role of the immune system in controlling intestinal electrolyte transport was studied in rat and rabbit colon in Ussing chambers. A phagocyte stimulus, the chemotactic peptide FMLP, and a mast cell stimulus, sheep anti-rat IgE, caused a brief (less than 10 min) increase in short-circuit current (Isc). Products of immune system activation, platelet-activating factor (PAF) and reactive oxygen species (ROS), caused a sustained, biphasic increase in the Isc. Ion replacement and flux studies indicated that these agonists stimulated electrogenic Cl secretion and inhibited neutral NaCl absorption; responses that were variably inhibited by the cyclooxygenase blockers indomethacin and piroxicam. Lesser degrees of inhibition by nordihydroguaiaretic acid could be accounted for by decreased prostaglandin synthesis rather than by lipoxygenase blockade. Tetrodotoxin, hexamethonium, and atropine also inhibited immune agonist-stimulated Isc, but had no effect on immune agonist-stimulated production of PGE2 or PGI2. These results indicate that immune system agonists alter intestinal epithelial electrolyte transport through release of cyclooxygenase products from cells in the lamina propria with at least 50% of the response being due to cyclooxygenase product activation of the enteric nervous system. The immune system, like the enteric nervous system and the endocrine system, may be a major regulating system for intestinal water and electrolyte transport in health and disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H., Rock R., Bridges R. J., Rummel W., Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985 Jul;364:301–312. doi: 10.1113/jphysiol.1985.sp015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A. W., Coombs R. R., McLaughlan P., Cuthbert A. W. Immediate hypersensitivity reactions to cow milk proteins in isolated epithelium from ileum of milk-drinking guinea-pigs: comparisons with colonic epithelia. Int Arch Allergy Appl Immunol. 1984;75(3):255–263. doi: 10.1159/000233625. [DOI] [PubMed] [Google Scholar]
- Baird A. W., Cuthbert A. W., Pearce F. L. Immediate hypersensitivity reactions in epithelia from rats infected with Nippostrongylus brasiliensis. Br J Pharmacol. 1985 Aug;85(4):787–795. doi: 10.1111/j.1476-5381.1985.tb11077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider H. M., Martens H., Powell D. W. Effect of BW 942C, an enkephalinlike pentapeptide, on sodium and chloride transport in the rabbit ileum. Gastroenterology. 1988 Jan;94(1):127–136. doi: 10.1016/0016-5085(88)90620-8. [DOI] [PubMed] [Google Scholar]
- Beubler E., Bukhave K., Rask-Madsen J. Significance of calcium for the prostaglandin E2-mediated secretory response to 5-hydroxytryptamine in the small intestine of the rat in vivo. Gastroenterology. 1986 Jun;90(6):1972–1977. doi: 10.1016/0016-5085(86)90269-6. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Tomioka M., Matsuda H., Stead R. H., Quinonez G., Simon G. T., Coughlin M. D., Denburg J. A. The role of mast cells in inflammatory processes: evidence for nerve/mast cell interactions. Int Arch Allergy Appl Immunol. 1987;82(3-4):238–243. doi: 10.1159/000234197. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Rawlins C. L. Electrolyte transport across isolated large intestinal mucosa. Am J Physiol. 1973 Nov;225(5):1232–1239. doi: 10.1152/ajplegacy.1973.225.5.1232. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M. A complete regulatory loop between the immune and neuroendocrine systems. Fed Proc. 1985 Jan;44(1 Pt 1):108–111. [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Stimulation of arachidonic acid metabolism in the polymorphonuclear leukocyte by an N-formylated peptide. Comparison with ionophore A23187. J Biol Chem. 1980 Nov 10;255(21):10223–10226. [PubMed] [Google Scholar]
- Browning J. G., Hardcastle J., Hardcastle P. T., Sanford P. A. The role of acetylcholine in the regulation of ion transport by rat colon mucosa. J Physiol. 1977 Nov;272(3):737–754. doi: 10.1113/jphysiol.1977.sp012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsson I., Sjöqvist A., Jodal M., Lundgren O. Mechanisms underlying the small intestinal fluid secretion caused by arachidonic acid, prostaglandin E1 and prostaglandin E2 in the rat in vivo. Acta Physiol Scand. 1987 Aug;130(4):633–642. doi: 10.1111/j.1748-1716.1987.tb08186.x. [DOI] [PubMed] [Google Scholar]
- Bukhave K., Rask-Madsen J. Saturation kinetics applied to in vitro effects of low prostaglandin E2 and F 2 alpha concentrations on ion transport across human jejunal mucosa. Gastroenterology. 1980 Jan;78(1):32–42. [PubMed] [Google Scholar]
- Castro G. A., Harari Y., Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987 Oct;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Castro G. A. Immunological regulation of epithelial function. Am J Physiol. 1982 Nov;243(5):G321–G329. doi: 10.1152/ajpgi.1982.243.5.G321. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Wang N. S., Rao M. C. Phorbol ester stimulation of active anion secretion in intestine. Am J Physiol. 1985 Sep;249(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1985.249.3.C356. [DOI] [PubMed] [Google Scholar]
- Cost H., Gespach C., Abita J. P. Effect of indomethacin on the binding of the chemotactic peptide formyl-Met-Leu-Phe on human polymorphonuclear leukocytes. FEBS Lett. 1981 Sep 14;132(1):85–88. doi: 10.1016/0014-5793(81)80433-4. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Profiles of eicosanoid production by superficial and proliferative colonic epithelial cells and sub-epithelial colonic tissue. Prostaglandins. 1986 Sep;32(3):387–399. doi: 10.1016/0090-6980(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., McLaughlan P., Coombs R. R. Immediate hypersensitivity reaction to beta-lactoglobulin in the epithelium lining the colon of guinea pigs fed cows' milk. Int Arch Allergy Appl Immunol. 1983;72(1):34–40. doi: 10.1159/000234837. [DOI] [PubMed] [Google Scholar]
- Diener M., Bridges R. J., Knobloch S. F., Rummel W. Neuronally mediated and direct effects of prostaglandins on ion transport in rat colon descendens. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jan;337(1):74–78. doi: 10.1007/BF00169480. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cheng H. Y., Sharp G. W. Effects of phorbol esters on sodium and chloride transport in rat colon. Am J Physiol. 1986 Oct;251(4 Pt 1):G509–G517. doi: 10.1152/ajpgi.1986.251.4.G509. [DOI] [PubMed] [Google Scholar]
- Edelson H. S., Kaplan H. B., Korchak H. M., Smolen J. E., Weissmann G. Dissociation by piroxicam of degranulation and superoxide anion generation from decrements in chlortetracycline fluorescence of activated human neutrophils. Biochem Biophys Res Commun. 1982 Jan 15;104(1):247–253. doi: 10.1016/0006-291x(82)91966-0. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Kagnoff M. F., Fiocchi C., Befus A. D., Targan S. Intestinal immunity and inflammation: recent progress. Gastroenterology. 1986 Sep;91(3):746–768. doi: 10.1016/0016-5085(86)90649-9. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Dvorak A. M., Peters S. P., Kagey-Sobotka A., Lichtenstein L. M. Isolation and characterization of human intestinal mucosal mast cells. J Immunol. 1985 Jul;135(1):483–491. [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Gaion R. M., Trento M. The role of prostacyclin in modulating cholinergic neurotransmission in guinea-pig ileum. Br J Pharmacol. 1983 Oct;80(2):279–286. doi: 10.1111/j.1476-5381.1983.tb10031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerg K. J., Roux M., Wanitschke R., Meyer zum Büschenfelde K. H. Die Bedeutung des Meissnerschen Plexus für die sekretorische Funktion der Colonschleimhaut. Z Gastroenterol. 1986 Sep;24 (Suppl 3):70–78. [PubMed] [Google Scholar]
- Goetzl E. J. Oxygenation products of arachidonic acid as mediators of hypersensitivity and inflammation. Med Clin North Am. 1981 Jul;65(4):809–828. doi: 10.1016/s0025-7125(16)31499-7. [DOI] [PubMed] [Google Scholar]
- Granström E., Kindahl H. Radioimmunoassay of prostaglandins and thromboxanes. Adv Prostaglandin Thromboxane Res. 1978;5:119–210. [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T. The secretory actions of histamine in rat small intestine. J Physiol. 1987 Jul;388:521–532. doi: 10.1113/jphysiol.1987.sp016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson L. D., Powell D. W. Bradykinin-stimulated eicosanoid synthesis and secretion by rabbit ileal components. Am J Physiol. 1987 Jun;252(6 Pt 1):G783–G790. doi: 10.1152/ajpgi.1987.252.6.G783. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J. E., Kay N. E., Solomon G. F., Plotnikoff N. P. Neuropeptides: conductors of the immune orchestra. Life Sci. 1987 Aug 3;41(5):527–544. doi: 10.1016/0024-3205(87)90405-x. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Field M., Miller R. J., Stoff J. S. Homologous desensitization to prostaglandins in rabbit ileum. Am J Physiol. 1987 Jan;252(1 Pt 1):G120–G127. doi: 10.1152/ajpgi.1987.252.1.G120. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Miller R. J., Field M., Siegel M. I. Stimulation of colonic secretion by lipoxygenase metabolites of arachidonic acid. Science. 1982 Sep 24;217(4566):1255–1256. doi: 10.1126/science.6810465. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Cuatrecasas P. Formyl peptide chemotactic receptors of leukocytes and macrophages. Curr Top Cell Regul. 1980;17:137–170. doi: 10.1016/b978-0-12-152817-1.50009-2. [DOI] [PubMed] [Google Scholar]
- O'Dorisio M. S. Neuropeptides and gastrointestinal immunity. Am J Med. 1986 Dec 22;81(6B):74–82. doi: 10.1016/0002-9343(86)90587-5. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Intestinal anaphylaxis in the rat: jejunal response to in vitro antigen exposure. Am J Physiol. 1986 Apr;250(4 Pt 1):G427–G431. doi: 10.1152/ajpgi.1986.250.4.G427. [DOI] [PubMed] [Google Scholar]
- Rampton D. S., Hawkey C. J. Prostaglandins and ulcerative colitis. Gut. 1984 Dec;25(12):1399–1413. doi: 10.1136/gut.25.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Serafin W. E., Austen K. F. Mediators of immediate hypersensitivity reactions. N Engl J Med. 1987 Jul 2;317(1):30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984 Mar;86(3):453–460. [PubMed] [Google Scholar]
- Smith G. S., Warhurst G., Turnberg L. A. Synthesis and degradation of prostaglandin E2 in the epithelial and sub-epithelial layers of the rat intestine. Biochim Biophys Acta. 1982 Dec 13;713(3):684–687. doi: 10.1016/0005-2760(82)90331-9. [DOI] [PubMed] [Google Scholar]
- Smith G., Warhurst G., Lees M., Turnberg L. Evidence that PGE2 stimulates intestinal epithelial cell adenylate cyclase by a receptor-mediated mechanism. Dig Dis Sci. 1987 Jan;32(1):71–75. doi: 10.1007/BF01296690. [DOI] [PubMed] [Google Scholar]
- Van Dyke K., Peden D., Van Dyke C., Jones G., Castranova V., Ma J. Inhibition by nonsteroidal antiinflammatory drugs of luminol-dependent human-granulocyte chemiluminescence and [3H]FMLP binding. Effect of sulindac sulfide, indomethacin metabolite, and optical enantiomers (+) and (-) MK830. Inflammation. 1982 Mar;6(1):113–125. doi: 10.1007/BF00910724. [DOI] [PubMed] [Google Scholar]
- Yagasaki O., Takai M., Yanagiya I. Acetylcholine release from the myenteric plexus of guinea-pig ileum by prostaglandin E1. J Pharm Pharmacol. 1981 Aug;33(8):521–525. doi: 10.1111/j.2042-7158.1981.tb13851.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. W., Binder H. J. Effect of tetrodotoxin on cholinergic agonist-mediated colonic electrolyte transport. Am J Physiol. 1983 Apr;244(4):G386–G391. doi: 10.1152/ajpgi.1983.244.4.G386. [DOI] [PubMed] [Google Scholar]