Abstract
Experimental studies have shown that two main estrogen metabolites hydroxylated by CYP1A1 and CYP1B1 in the breast differentially affect breast cell proliferation and carcinogenesis. Although 16α-hydroxyestrone (16αOHE1) exerts estrogenic activity through covalent estrogen receptor (ER) binding, 2-hydroxyestrone (2OHE1) presumably has antiestrogenic capabilities. The ratio of 2OHE1 to 16αOHE1 represents the relative dominance of one pathway over the other and is believed to be modifiable by diet. It was hypothesized that women with or at high risk of breast cancer have a lower estrogen metabolite ratio (EMR) compared with women without breast cancer. We conducted a systematic review on the EMR as a predictor for breast cancer. A total of nine studies (six prospective and three retrospective) matched our inclusion criteria, comprising 682 premenopausal cases (1027 controls) and 1189 postmenopausal cases (1888 controls). For the highest compared with the lowest quantile of urinary EMR, nonsignificant associations suggested at best a weak protective effect in premenopausal but not in postmenopausal breast cancer (range of odds ratios: 0.50–0.75 for premenopausal and 0.71–1.31 for postmenopausal). Circulating serum/plasma EMR was not associated with breast cancer risk. Associations were inconclusive for receptor subtypes of breast cancer. Uncontrolled factors known to be involved in breast carcinogenesis, such as 4-hydroxyestrone (4OHE1) concentration, may have confounded results for EMR. Results of the prospective studies do not support the hypothesis that EMR can be used as a predictive marker for breast cancer risk. Future research should concentrate on profiles of estrogen metabolites, including 4OHE1, to gain a more complete picture of the relative importance of single metabolites for breast cancer.
Keywords: estrogen metabolite ratio, 2-hydroxyestrone, 16α-hydroxyestrone, breast cancer, predictive marker, review
Introduction
Hypothesis (the extended hormonal risk factor hypothesis)
It is well known that endogenous estrogens have the potential of inducing and promoting cell proliferation and inducing tumor growth in breast tissue. Circulating estrogen levels correlate with breast cancer risk in postmenopausal women.1,2 For premenopausal women, the estrogen–breast cancer association is not as well established. Some evidence for an association was found in one prospective study3 but was not statistically significant in five other studies,4 probably due to the much higher intra- and interindividual variation through menstrual cycle and reproductive lifetime.5,6
Since the early 1980s, a growing number of studies examined whether not only native estrogens but also their hydroxylated estrogen metabolites have potential carcinogenic properties in breast tissue. Experimental studies in breast cancer cells and animal models established the hypothesis of a differential estrogenic activity of the two main estrogen metabolites, 2-hydroxyestrone (2OHE1) and 16α-hydroxyestrone (16αOHE1). Because 2OHE1 has a weak binding capacity to the ER and has been associated with normal cell differentiation and apoptosis,7–9 it is assumed to have antiestrogenic properties.10 In contrast, the other major metabolite, 16αOHE1, can bind covalently to the ER and has been shown to induce abnormal cell proliferation.11,12
Both 2- and 16α-estrogen hydroxylating pathways are mutually exclusive and irreversible. Thus, the metabolite ratio of 2OHE1 to 16αOHE1 reflects the relative dominance of one pathway over the other. Since several relatively small-scale observational studies and human breast tissue studies found lower ratio levels accompanied by higher 16αOHE1 concentrations in breast cancer cases compared with women without breast cancer,13–18 it has been suggested that this ratio may be used as a marker for the assessment of breast cancer risk. Subsequent studies yielded inconsistent results as to whether a lower ratio is associated with a higher risk for breast cancer, both in premenopausal and in postmenopausal women.
A concurrent hypothesis postulates that the catecholestrogens 2OHE1/2OH-estradiol and especially the 4-hydroxylated estrogens 4-hydroxyestrone (4OHE1) and 4-hydroxyestradiol may play an important role in breast carcinogenesis because of their ability to induce DNA depurination independent of ER binding.19,20 Although experimental studies have demonstrated the carcinogenic potential of 4OHE1,21,22 little attention has been given to 4OHE1 in epidemiological studies. Therefore, we focus here on the estrogen metabolite ratio (EMR) of 2OHE1 to 16αOHE1.
Metabolism of endogenous estrogens, genetic polymorphisms
In a first step, native 17β-estradiol (E2) and its oxidized form, estrone (E1), are metabolized to various hydroxylated estrogens by cytochrome P450-dependent pathways. The catecholestrogens 2OHE1/E2 and 4OHE1/E2 are characterized by two neighboring hydroxyl groups, which are rapidly methylated by the enzyme catechol-O-methyltransferase (COMT) to form 2-Meth-O-E1/E2 or 4-Meth-O-E1/E2. Further oxidation of unmethylated hydroxyestrogens may generate semiquinones and quinones, which on one hand can function as substrate for redox cycling processes, leading to reactive oxygen species (ROS), and on the other hand can bind to DNA to form stable or depurinating adducts with adenine or guanine. Subsequently, quinones and hydroxylated estrogens, including 16αOHE1, are sulphatized or glucuronidated before excretion.23 The tissue-specific hydroxylases are encoded by CYP1A1, CYP1B1 (breast), and CYP1A2 (liver) for the 2-hydroxylation of E1 to form 2OHE1/E2 and CYP1A1, CYP3A5 (breast),24 CYP3A4, and CYP3A725 for 16α-hydroxylation. A smaller fraction of estrogens is hydroxylated to 4OHE1/E2 by CYP1B1 (breast) and CYP1A2 (liver) enzymes.22 Tissue concentrations of the CYP enzymes vary considerably, and many of them are genetically polymorph, which implicates differences in activity levels of the hydroxylases.26,27 Polymorphisms have also been described for COMT, leading to slower methylation of catecholestrogens.28,29
Menopausal status
To date, it is not well established whether the menopausal transition is accompanied by a general shift of the EMR to favor 16αOHE1, as has been previously discussed.30 Although levels of estrogen metabolites are several times higher in premenopausal compared with postmenopausal women, a woman’s metabolite ratio seems to be relatively stable throughout her life.31 Most of the earlier studies on the EMR reported no significant mean differences between pre- and postmenopausal control groups without breast cancer.32 Because the limited available data do not provide clear indication of an important influence of menopausal status on the EMR, both pre- and postmenopausal women were considered in this review.
The purpose of the present review was to evaluate the evidence for an inverse relation between EMR and breast cancer risk and whether this ratio can potentially serve as a predictive marker.
Methods
Selection of studies
A PubMed/MEDLINE search on epidemiologic studies of estrogen metabolites and breast cancer was conducted up to February 2010. The search strategy consisted of a combination of MeSH terms (“hydroxyestrones” and “breast neoplasms”) and key words in titles and abstracts (“estrogen metabolites”, “2-hydroxylation”, and “breast cancer”). References from resulting articles were screened for missed studies. If only abstracts of publications were available, they were excluded from the review.
Inclusion criteria
Studies were included in this review if risk estimates and confidence intervals (CIs) of the urinary or circulating EMR (2OHE1/16αOHE1) were reported for subjects not taking oral contraceptives, hormones, or tamoxifen and not pregnant at the time of blood donation or urine collection. Further requirements included frequency or individual matching of controls to cases by age and stratification by or adjustment for menopausal status to assess potentially differential evidence for pre- and postmenopausal women.
Measurement of the 2OHE1/16αOHE1 EMR
All but one study,33 which employed an earlier version, used an improved enzyme immunoassay34 (ELISA; ESTRAMET, Immunacare Corporation, Bethlehem, PA, USA) developed by Klug et al35 for simultaneous urinary metabolites assessment. The assay measures three of at least 15 metabolites and parent estrogens, ie, 2OHE1, 2-hydroxyestradiol (2OHE2), and 16αOHE1. Therefore, the EMR reflects the 2OHE1 + 2OHE2/16αOHE1 ratio. The EMR is independent of urinary creatinine concentration.
Data extraction
To compare the median/mean EMR across studies, the third quintile’s midpoint of EMR in pre- and postmenopausal controls has been derived as an approximate value for Muti et al36 and the numbers in each tertile in the study by Meilahn et al33 were deduced from given percentages. Risk estimates for quantiles and 95% CIs of the EMR were extracted from the tables of the publications and analyzed. To test for overall heterogeneity and heterogeneity by specimen type (urine, serum/plasma), risk estimates were weighted and pooled, and Cochran’s Q statistic37 was calculated.
Results
Overall, 21 studies were identified that dealt with any kind of estrogen metabolites and associated breast cancer risk, of which nine were included in this review (Table 1). One study included only premenopausal women38 and four studies only postmenopausal women,39–42 and the remaining four studies matched for menopausal status or analyzed data stratified by menopausal status.33,36,43,44
Table 1.
Characteristics of included studies on EMR and breast cancer risk estimates
| Authors | Country | Design | Medium | Age (range and/or mean (SD)) | Cases/controls (total) | Cases/controls (in models) | EMR (2OHE1/16αOHE1) | Adjusted OR (95% CI) | Ptrend | Adjustment | Notes (median, mean or gmean in controls) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal | |||||||||||
| Meilahn et al33 | UK | Prospective nested case-control | Spot urine | >34 (40.5 (4.2)) | 60/184 | 21/62 | <1.72 | 1 | NA | Age + menstrual phase matching; parity, others not exactly specified | Median follow-up 9.5 years, median EMR 2.1 |
| 22/61 | 1.72–<2.44 | 0.99 (0.48–2.08) | |||||||||
| 17/61 | ≥2.44 | 0.75 (0.35–1.62) | |||||||||
| Muti et al36 | Italy | Prospective nested case-control | 12-h urine | 35–57 | 67/264 | 19/54 | <1.8 | 1 | NA | Age + menstrual phase matching, BMI, waist-to-hip, reproductive variables | Mean follow-up 5.5 years, median EMR 2.5 |
| 14/51 | 1.8–2.3 | 0.76 (0.34–1.69) | |||||||||
| 11/52 | 2.3–2.72 | 0.60 (0.25–1.44) | |||||||||
| 12/54 | 2.72–3.29 | 0.62 (0.27–1.45) | |||||||||
| 11/53 | ≥3.29 | 0.55 (0.23–1.32) | |||||||||
| Kabat et al43 | USA | Population-based case-control | Spot urine | <50–>60 | 105 (70 is)/129 | 44/46 | ≤1.4 | 1 | 0.05 | Age matching, parity/first birth age, family history, BMI, education, medication (48 h prior urine collection) | In situ Ca separately; smoking, alcohol, diet prior tested; mean EMR (SD) 1.9 (1.0) |
| 35/42 | 1.5–2.2 | 0.63 (0.33–1.23) | |||||||||
| 26/41 | ≥2.3 | 0.50 (0.25–1.01) | |||||||||
| Arslan et al38 | USA | Prospective, nested case-control | Serum | 35–65 | 377/377 | Not given for quartiles | <0.45 | 1 | 0.51 | Age + menstrual phase matching; family history, ever smoking, BMI, menarche age, parity/first birth age | 12–18 years of follow-up, mean EMR (SD) 0.76 (0.49) |
| 44.3 (4.8) | 0.453–0.635 | 0.83 (0.53–1.31) | |||||||||
| 0.636–0.936 | 1.04 (0.65–1.65) | ||||||||||
| >0.936 | 1.13 (0.68–1.87) | ||||||||||
| Mixed menopausal | |||||||||||
| Fowke et al44 (presurgical)a | China | Population-based case-control | Spot urine | 25–65 | 78/78 presurgical | 30/20 | ≤0.69 | 1 | 0.17 | Matched for age and menopause; family history, physical activity, BMI, age at menarche, parity/first birth age, fibroadenoma history | Separate models for presurgical and postsurgical; presurgical included 19/19 postmenopausal; median EMR 1.0 |
| 25/27 | 0.70–1.22 | 0.5 (0.2–1.3) | |||||||||
| 23/31 | ≥1.22 | 0.5 (0.2–1.3) | |||||||||
| Fowke et al44 (postsurgical)a | China | Population-based case-control | Spot urine | 25–65 | 32/32 postsurgical | 5/14 | ≤0.69 | 1 | 0.02 | Matched for age and menopause; family history, physical activity, BMI, age at menarche, parity/first birth age, fibroadenoma history (but not family history, menarche age) | Postsurgical included 18/18 postmenopausal; median EMR 0.6 |
| 10/11 | 0.70–1.22 | 3.1 (0.4–23.6) | |||||||||
| 17/7 | ≥1.22 | 8.1 (1.2–54.6) | |||||||||
| Postmenopausal | |||||||||||
| Meilahn et al33 | UK | Prospective, nested case-control | Spot urine | >50 | 42/139 | 15/47 | <1.39 | 1 | NA | Age matching; Parity, others not exactly specified | Median follow-up 9.5 years; median EMR 1.7 |
| 59 (6.2) | 16/46 | 1.39–<2.09 | 0.99 (0.48–2.08) | ||||||||
| 13/46 | ≥2.09 | 0.75 (0.35–1.62) | |||||||||
| Ursin et al39 | USA | Population-based case-control | Spot urine | 50–70 | 66/76 | 29/26 | ≤1.16 | 1 | 0.96 | Age, family history, SES, education, menarche age, parity, weight | Low stage tumors 3–7 years after diagnosis; gmean EMR 1.76 (95% CI : 1.60–1.93) |
| 11/25 | 1.17–1.73 | 0.34 (0.12–0.98) | |||||||||
| 26/25 | >1.73 | 1.13 (0.46–2.78) | |||||||||
| Muti et al36 | Italy | Prospective, nested case-control | 12-h urine | 43–70 | 71/274 | 12/54 | <1.77 | 1 | NA | Age, BMI, waist-to-hip, reproductive variables | Mean follow-up 5.5 years; median EMR 2.6 |
| 16/55 | 1.77–2.26 | 1.42 (0.60–3.33) | |||||||||
| 17/54 | 2.26–2.80 | 1.41 (0.60–3.33) | |||||||||
| 12/57 | 2.80–3.66 | 1.02 (0.41–2.53) | |||||||||
| 14/54 | >3.66 | 1.31 (0.53–3.18) | |||||||||
| Wellejus et al41 | Denmark | Prospective, nested case-control | Spot urine | 50–64 | 197/197 | 197/197 | log-2-transformed, OR for doubling of EMR | 0.94 (0.71–1.43) | NS | Age matching only; education, parity/first birth age, BMI, alcohol, past HT prior tested | Median follow-up 2.4 years (P5–P95 0.2–4.9 years); median EMR 1.6, P5–P95 0.6–3.5 |
| Kabat et al43 | USA | Population-based case-control | Spot urine | <50–>60 | 164 (88 is)/197 | 70/64 | ≤1.4 | 1 | 0.37 | Age, first birth age, family history, BMI, education, alcohol use and medication (48 h prior to urine collection) | In situ Ca separately; mean EMR (SD) 2.0 (1.0) |
| 62/52 | 1.5–2.2 | 0.97 (0.57–1.64) | |||||||||
| 65/48 | ≥2.3 | 0.78 (0.46–1.33) | |||||||||
| Cauley et al40 | USA | Prospective case-cohort | Serum | 70 (5) | 272/291 | 69/70 | ≤0.576 | 1 | NA | Age, BMI; Family history, past HT, education prior tested | Mean follow-up 8.7 years, 39 in situ Ca included; gmean EMR 0.73 (95% CI 0.70–0.76) |
| >65 | 97/72 | 0.577–0.749 | 0.98 (0.62–1.57) | ||||||||
| 64/75 | 0.750–0.923 | 0.89 (0.55–1.42) | |||||||||
| 72/74 | >0.923 | 1.17 (0.73–1.87) | |||||||||
| Eliassen42 | USA | Prospective, nested case-control | Plasma | 61.5 (4.7) | 340/677 | 74/169 | <0.28 | 1 | 0.35 | Age matching; BMI at age 18, family history, menarche age, parity/age at first birth, menopause age, duration of past HT use | Follow-up 10–11 years, 63 in situ Ca included; median EMR 0.37 |
| 43–69 | 89/168 | 0.28–<0.37 | 1.33 (0.89–1.99) | ||||||||
| 87/170 | 0.37–<0.48 | 1.24 (0.83–1.85) | |||||||||
| 90/168 | ≥0.48 | 1.30 (0.87–1.95) |
Notes:
Fowke et al matched for menopausal status (presurgical: 59 premenopausal and 19 postmenopausal pairs, and postsurgical: 14 pre- and 18 postmenopausal pairs), thus estimates refer to both pre- and postmenopausal women.
Abbreviations: BMI, body mass index; Ca, carcinoma; CI, confidence interval; EMR, estrogen metabolite ratio; gmean, geometric mean; HT, hormone therapy; is, in situ; NA, not available; NS, not significant; P5–P95, 5th percentile to 95th percentile; SD, standard deviation; SE, standard error; SES, socioeconomic status.
Excluded studies
The main reasons for exclusion were nonavailability of risk estimates, including CIs of the association between EMR and breast cancer,13,14,18,45–51 and recruitment of control subjects from convenient samples that were not individually matched to cases by age.13,14,16–18,45,46,48,51 Almost all of these small case-control studies, comprising, in total, 401 cases and 429 controls, found lower mean EMRs in cases compared with controls (Supplementary Table). Kabat et al17 reported for a small hospital-based study an extreme risk reduction for the third versus first tertile of urinary EMR in postmenopausal women (adjusted odds ratio [OR]: 0.03, 95% CI: 0.003–0.29), but not in the combined group of pre- and postmenopausal women (OR: 0.51, 95% CI: 0.17–1.56). Similarly, Ho et al16 who used controls with biopsies for benign breast disease, found at a urinary EMR median cut-point of 0.9 a strong inverse association independent of menopausal status (OR: 0.16, 95% CI: 0.05–0.49).
Table S1.
Characteristics of excluded studies on EMR and breast cancer risk
| Authors | Country | Design | Medium (assay) | Menopausal status | Age (range or mean (SD)) | Cases/controls | EMR (2OHE1/16αOHE1) | Notes |
|---|---|---|---|---|---|---|---|---|
| Excluded studies sorted by medium | ||||||||
| Adlercreutz et al48 | Finland | Case series, controls | 24-h urine (GC–MS) | Premenopausal | <50 | 10/11 vegetarian/12 omnivorous | Means not available | Unmatched, no risk estimate for EMR |
| Kabat et al17 | USA | Case-control (hospital based) | Spot urine (ELISA) | Mixed | <50–>60 | 39/58 (premenopausal 16/30, postmenopausal 23/28) | Third vs first tertile: OR: 0.51, 95% CI: 0.17–1.56, postmenopausal OR: 0.03, 95% CI: 0.003–0.29 | Combined model unadjusted for menopausal status; adjusted for age, race, parity, smoking, alcohol, chronic disease |
| Coker et al45 | USA | Case-control (screening attendees) | Urine (ELISA) | Mixed | Unknown | 74/58 | Means cases/controls: white 1.91 vs 2.52, black 1.27 vs 2.23 | Age, race, menopause adjusted difference, no risk estimate for EMR, only P = 0.008 |
| Ho et al16 | Singapore | Case-control (biopsies for benign disease) | Spot urine (ELISA) | Mixed | 54 (SE 1.2)/54.8 (SE 0.9) | 65/36 (premenopausal 23/12, postmenopausal 41/24) | Mean (SE) cases/controls 0.7 (0.1) vs 2.0 (0.3) Median > 0.9 vs < 0.9; OR: 0.16, 95% CI: 0.05–0.49 |
Unmatched, adjustment for age, menopausal status, parity, OC use |
| Zheng et al18 | Shanghai | Case-control | Spot urine (ELISA) | Unknown | Unknown | 20/20 | Geometric means cases/controls: 1.16 vs 1.52, P = 0.046 | Five-year age-group matching, no risk estimate for EMR |
| Alvarez-Vasquez et al50 | USA | Case series, controls | Urine (ELISA) | Postmenopausal | Unknown | 4/34 | Mean cases/controls 1.35 (0.13)/2.71 (0.84) | Only unadjusted means |
| Gaikwad et al49 | Italy/USA | Case series-high risk-controls | Urine (GC–MS) | Mixed | 34–73 | 12/46/18 high risk | Means not available | 2OHE2, 16αOHE1 presented, age and ethnic differences, no risk estimate for EMR |
| Im et al46 | USA | Cases, high risk and controls | Spot urine (ELISA) | Mixed | 35–70 | 30/41/77 high risk | Means (SD) cases 1.29 (0.80), high risk 1.76 (2.33), controls 2.47 (1.14) | Menopausal status unclear, no risk estimate for EMR |
| Schneider et al14/Fishman et al13 | USA | Case series, controls | Blood (radio-metric, indirect) | Postmenopausal | Ca. 43–74 | 24+9 perimenopausal/10 | Means not available | Design error-prone, %-hydroxylation, no risk estimate for EMR |
| Modugno et al,47 WHI | USA | Prospective nested case-control | Serum (ELISA) | Postmenopausal | 69.9 (6.5) cases | 93/93 (no HT use) | Median cases/controls 0.43 vs 0.46 | No risk estimate for EMR |
| Osborne et al15 | USA | Case series, controls | In vivo tissue (radio-metric, indirect) | Premenopausal | 4/4 | Means not available | 16-hydroxylation higher in cases | |
| Castagnetta et al75 | Italy | Case series, controls | In vivo tissue (HPLC; GC/MS) | Mixed | 35–71 | 17/6 | Means (SD) cases/controls 1.5 (0.11) vs 8.3 (4.6) | 4OHE2 higher in cases |
Abbreviations: EMR, estrogen metabolite ratio; GC–MS, gas chromatography–mass spectrometry; SD, standard deviation.
Included studies
The nine studies included were published between 1998 and 2009. Study characteristics are summarized in Table 1. In total, these studies comprised 682 premenopausal cases and 1027 premenopausal controls (five studies) and 1189 postmenopausal cases and 1888 postmenopausal controls (seven studies). Six studies were nested case-control studies within prospective cohorts,33,36,38,40–42 and three were retrospective population-based case-control studies39,43,44 with determination of the metabolites after disease onset. Studies were conducted in China,44 Italy,36 the UK,33 Denmark,41 and the US.38–40,42,43 The baseline age distribution varied naturally across studies according to menopausal status, with the minimum age ranging from 25 years44 to 65 years.40 All studies were matched for age, and prospective but not retrospective studies in premenopausal women were additionally matched for phase of menstrual cycle at time of specimen sampling (follicular or luteal33,38 and only luteal).36 Median follow-up time between specimen sampling and diagnosis of breast cancer (approximately equal to time between sampling and laboratory analysis) ranged from 2.4 years in the Danish cohort41 to 12–18 years in one of the US cohorts.38 The retrospective studies collected urine prior to any therapy for several months,44 a median of 3 months,43 or several years39 after diagnosis.
Six studies investigated urinary excretion of estrogen metabolites, and three of the more recent studies examined serum/plasma concentrations.38,40,42 Among controls, mean urinary EMR values were consistently above 1, whereas means of circulating EMR were all below 1. In Western countries, urinary mean EMR varied between 1.9 and 2.57 in controls, whereas in the Shanghai study by Fowke et al44 the mean EMR was around 50% lower. A significant difference between pre- and postmenopausal controls in mean urinary EMR has been found only in the UK cohort.33 Studies of serum/plasma EMR examined either pre- or postmenopausal women.
All studies but one41 used percentile distributions of the EMR among controls to assess the association with breast cancer risk by comparing the highest with the lowest group (tertiles, quartiles, or quintiles) with conditional or unconditional logistic regression or Cox’s40 regression analysis. Wellejus et al41 applied log-2-transformed EMR values that reflect a doubling of concentration per unit change. Adjustment for potentially confounding factors differed slightly between studies. Characteristics that were included in most models were age, body mass index (BMI) or waist-to-hip ratio, and reproductive variables known to be risk factors for breast cancer. Wellejus et al41 did not adjust their analysis apart from matching for exact age, and Cauley et al40 adjusted their analysis only for age and BMI. Of the four studies that included both pre- and postmenopausal women, three analyzed pre- and postmenopausal women separately.33,36,43 The Shanghai study reported combined estimates based on analyses stratified by menopausal status and time of urine collection relative to surgery/treatment status.44
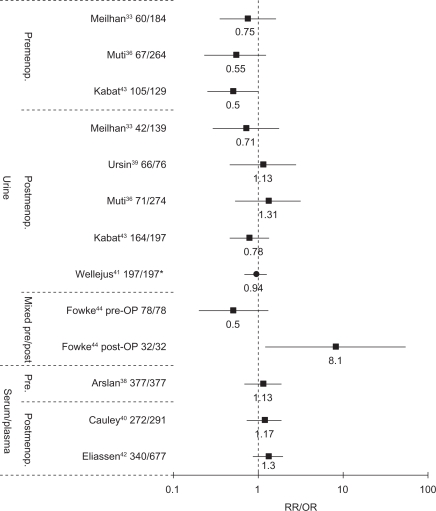
Main study results (risk estimates, 95% CI) are presented by specimen and menopausal status in Table 1 and Figure 1.
Figure 1.
OR/RR for highest versus lowest quantile of EMR in studies on pre- and postmenopausal breast cancer.
Note: Wellejus used log-2-transformed EMR, representing a doubling of EMR per unit increase.
Abbreviations: EMR, estrogen metabolite ratio; OR, odds ratio; RR, risk ratio.
Urinary EMR
Premenopausal breast cancer
In the UK study (60 cases), the EMR was associated with a nonsignificant 25% risk reduction (first vs third tertile: OR = 0.75, 95% CI = 0.35–1.62).33 The Italian study36 (67 cases) reported nonsignificantly decreased ORs for the second to fifth quintile (ORs: Q2–Q5 = 0.76–0.55). A 50% risk reduction (95% CI: 0.25–1.01) for the highest tertile of the EMR was found in the retrospective study by Kabat et al.43 Analysis repeated for cases prior to and after chemotherapy showed no substantial differences in risk estimates. Fowke et al44 reported results for 78 presurgical (the majority being premenopausal) and 32 postsurgical pairs (14 pairs being premenopausal). For presurgical pairs, the tertiles of the EMR were nonsignificantly associated with a 50% risk reduction, whereas a significantly positive association was present postsurgically (OR for third vs first tertile: 8.1, 95% CI: 1.2–54.6). Only Kabat et al43 observed an inverse dose–response relationship over tertiles of EMR (Ptrend = 0.05), whereas other studies found no trends38,44 or did not provide a trend test.33,36
Postmenopausal breast cancer
Meilahn et al33 (42 cases) and Kabat et al43 (164 cases) reported nonstatistically significant inverse associations for the highest versus lowest tertile of the EMR (respective ORs 0.75 and 0.78), whereas Muti et al36 (71 cases) and Ursin et al39 (66 cases) observed nonsignificant risk elevations. Wellejus et al41 found no significant relationship among 197 case-control pairs for a doubling of EMR per increment (OR: 0.94, 95% CI: 0.71–1.43).
In summary, none of the urinary studies reported a statistically significant inverse association between EMR and breast cancer risk, although the premenopausal study by Kabat et al43 showed an upper confidence limit close to 1.
Serum/plasma EMR
Premenopausal breast cancer
Arslan et al38 found no association between the serum EMR and premenopausal breast cancer risk among 377 case-control pairs, the OR for the top versus bottom quartile being 1.13 (95% CI: 0.68–1.87). Repeated analyses according to menstrual cycle phase (follicular pairs and luteal pairs) or after excluding cases diagnosed within 5 or 10 years after enrollment did not yield different results.
Postmenopausal breast cancer
Eliassen et al42 and Cauley et al40 included in situ cases in their analyses of 340 and 272 cases, respectively. Both studies found nonsignificant risk elevations for the highest compared with the lowest quartile of circulating EMR levels. Thus, the serum/plasma EMR was not significantly associated to pre- or postmenopausal breast cancer.
Heterogeneity testing
The test for heterogeneity across the combined pre- and postmenopausal urinary studies (Pheterogeneity = 0.86) and the test for circulating EMR studies (Pheterogeneity = 0.86) were negative. Additionally, the test for heterogeneity across all nine urinary and circulating EMR studies revealed no significant inconsistency between studies (Pheterogeneity = 0.61).
Breast cancer subtypes: receptor status and in situ cancer
Kabat et al found the urinary EMR to be statistically, significantly, and inversely related to ER-positive premenopausal tumors (third vs first tertile OR: 0.32, 95% CI: 0.12–0.84) and nonsignif icantly related to ER-negative tumors in postmenopausal women (OR: 0.38, 95% CI: 0.15–1.01).43 A nonsignificant inverse association in ER-negative cases (OR for doubling of urinary EMR: 0.78, 95% CI: 0.42–1.48) was also reported by Wellejus et al.41 In contrast, serum/plasma levels of EMR were increased in ER-negative postmenopausal cases (OR: 3.7, 95% CI: 1.24–11.09, Ptrend = 0.004, Pheterogeneity = 0.005) in one study42 and in ER-positive premenopausal cases in the other study (OR: 2.15, 95% CI: 0.88–5.27).38 Apart from Eliassen et al42 none of the studies tested for heterogeneity by ER status.
Kabat et al43 separately investigated 70 premenopausal and 88 postmenopausal cases with carcinoma in situ of the breast. No association of EMR was observed in both groups. Likewise, from their overall negative results, Eliassen et al42 reported no differential relationship for postmenopausal in situ breast cancer.
Discussion
Summary of main results
Overall, nine studies that fulfilled our inclusion criteria investigated the association between the EMR of 2- to 16α-hydroxyestrone and breast cancer risk, six of which examined urinary and three circulating EMR. Studies on urinary EMR found ORs between 0.50 and 0.75 (except for the postsurgical OR in Fowke et al)44 in premenopausal women and between 0.71 and 1.31 in postmenopausal women, comparing the highest with the lowest quantiles or, in one study, a doubling of EMR. Although none of the studies reached statistical significance, the premenopausal arm of the retrospective study by Kabat et al was borderline significant, suggesting that an inverse association of urinary EMR might be confined to premenopausal breast cancer. However, there was no differential effect in all urinary studies combined (Pheterogeneity = 0.86), which contradicts a hypothesized modification by menopausal status. Similarly, the circulating EMR was not associated with breast cancer risk in pre- or postmenopausal women (ORs ranging from 1.13 to 1.30, Pheterogeneity = 0.86). There was also no significant heterogeneity across all combined urinary and serum/plasma studies (Pheterogeneity = 0.61).
Receptor status and in situ carcinomas
Limited data on ER status with contradictory results do not allow a firm conclusion regarding heterogeneity in the relationship between EMR and breast cancer risk by ER status. The two studies, which investigated in situ carcinomas separately, did not find the EMR to be inversely associated with risk of in situ breast cancer.
Quality of evidence
Completeness of data
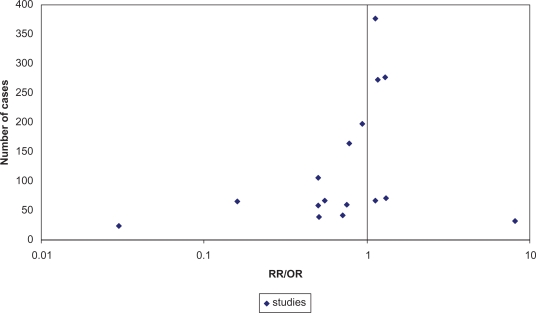
Exclusion of two earlier small studies,16,17 which found strong inverse associations for EMR, may have led to certain bias toward null results, but these studies were not included due to flaws in design or reporting. A funnel plot (Figure 2), including these studies, suggested that there might have been publication bias in this early phase. Another larger excluded study that assessed only single estrogen metabolites in serum of postmenopausal hormone therapy nonusers did not report statistically significant associations with breast cancer risk.47 Though not necessarily, it is likely that the EMR was also not associated with breast cancer in this study.
Figure 2.
Funnel plot of risk estimates of nine included33,36,38–44 and two excluded studies16,17 (separate RR/OR for menopausal status, where available).
Abbreviations: RR, risk ratio; OR, odds ratio.
Study size and power
The earliest publications that reported an inverse association between EMR and breast cancer risk were based on small numbers and thus have a tendency to overestimate the effect. Many of the subsequently published studies also had relatively small study sizes33,36,39,44 and consequently limited statistical power to detect a significant risk reduction of about 30% (likely effect magnitude based on the first cohort studies).33,36 If tertiles of exposure are considered, at least 200 case-control pairs are needed to identify a 50% risk reduction and 400 pairs to detect a relative risk of 0.7 with 80% power at an alpha of 0.05.52
Study design
The studies were heterogeneously designed, including both prospective and retrospective studies, using urinary or circulating EMR measurements for exposure assessment, including diverse populations, and many lacking statistical power. These variations may partly explain the differences in risk estimates for EMR.
The EMR may be influenced by disease status (tumor-driven activity), stage, and treatment.18,43,44,53 Thus, the hypothesized association between the EMR and breast cancer might be subject to reverse causation in retrospective case-control studies. Fowke et al44 found a decreased EMR risk in presurgical cases and an increased EMR risk in postsurgical cases. If pre- and postsurgery subgroups were combined, this would have resulted in a crude OR for the EMR of 1.02 (95% CI: 0.72–1.25). Hence, an existing inverse association may be masked by a potential influence of treatment on EMR. No study actually assessed the intraindividual variability in EMR before and after a breast cancer diagnosis. Therefore, prospective studies of EMR will be required to elucidate a potential association of EMR with breast cancer.
Adjustment for confounders in the individual studies
Only studies that excluded current users of exogenous hormones were included in this review, as oral contraceptives and menopausal hormone therapy have been shown to increase54–57 or decrease EMR.41,47 Many of the known breast cancer risk factors, such as reproductive variables (ie, parity and age at first birth) and BMI, which have been found to be associated with the EMR,42,58 were controlled in most included studies. Menstrual cycle phase in premenopausal women, a family history of breast cancer, and a history of benign breast disease were not consistently adjusted for. Although little evidence indicates that EMR is associated with a family history59 or benign breast disease, circulating EMR has been shown to increase during the menstrual cycle.60 Urinary estrogen metabolite levels vary strongly by ethnicity, showing lowest mean values in healthy African Americans followed by Asians and Caucasians.32,45,61–64 Although ethnic variations in plasma EMR were less pronounced,55 uncontrolled confounding could have masked, diluted, or even pretended a potential EMR effect in studies not adjusted for ethnicity depending on the combination of case-control pairs (even if percentages [other than for Caucasians] were as low as 12%–16%).38,43 However, in some studies, adjusted risk estimates did not differ substantially from crude estimates, and EMR varied only slightly across categories of covariates.33,36,40,41 Therefore, the risk estimates in these studies were considered as appropriate.
Possible modifiers of the association between EMR and breast cancer
Menopausal status did not strongly affect EMR in most studies, which is compatible with the observed overlapping distributions of EMR in pre- and postmenopausal women.32 Genetic makeup and differential activity of metabolizing hydroxylases and other enzymes (eg, COMT) have been estimated to explain most of the variation in EMR.65 In addition, estrogen hydroxylation pathways, particularly 2-hydroxylation, are believed to be modifiable through diet, physical activity, smoking, alcohol consumption, and caffeine intake. Clinical trials and observational studies generated inconsistent results regarding a potential influence of diet (eg, soy products, fat/fiber, and Brassica vegetables) and physical activity habits on EMR. Studies in the present review do not support that EMR may be associated with breast cancer risk in specific subgroups.
Reliability of measurements
Reliability of a single urinary EMR measurement in premenopausal women was rated sufficient in studies on variation in intraindividual EMR during the menstrual cycle.31,66 Intraclass correlation coefficients between two urinary EMR measurements were 0.71 over a 1-year period67 and 0.6730 over a 6-month period, and between two plasma EMR measurements were 0.73 over a 3-year period.42 All included studies, except for the study by Meilahn et al,33 used a refined version of the ESTRAMET enzyme immunoassay to determine the EMR of 2OHE1/16αOHE1 validated against a gas chromatography/mass spectrometry (MS) method. This assay could more sensitively detect estrogen metabolites in the lower postmenopausal range than the first version.68 However, a recent study rated reliability of urinary estrogen metabolite determination of postmenopausal women by this enzyme immunoassay problematic compared with a new technique involving liquid chromatography (LC)–MS/MS.69 Studies of postmenopausal women reported higher between-assay coefficients of variation than those of premenopausal women. Serum and plasma measurements are even more susceptible to errors because of the lower metabolite concentrations. Further, variation within and between studies may have resulted from differing specimens’ sampling patterns, storage length, and time between sampling and diagnoses in prospective studies.
Findings that oppose the postulated hypothesis
Well-established risk factors for breast cancer that are considered to be related to estrogen exposure, eg, family history, age at menarche, and benign breast disease,38 were not substantially or even inversely associated to the EMR, ie, a lower EMR with higher parity,38 lower age at first birth,58 and high breast density,70 respectively. Furthermore, ethnic differences in the EMR do not correspond to breast cancer incidence patterns in Asians and Caucasians. Differences in breast cancer risk between Asians and African Americans were found to be better explained by urinary estradiol, E1, and estriol than by EMR in a cross-sectional study of healthy, nonestrogen-using women.71
Recommendations for future studies
Interrelationship of urinary, circulating, and tissue EMR
Urinary and circulating estrogen metabolites may not be representative of target tissue concentrations of unconjugated metabolites. A recent study of nine patients concluded that the urinary EMR is a good approximation for breast tissue EMR.72 This result needs to be confirmed in women without breast cancer. Moreover, differences in median EMR of serum/plasma and urine samples raise the question of whether risk estimates derived from urinary EMR can be compared with those based on circulating EMR. A single study in young women not using oral contraceptives found fair correlation coefficients between urinary and plasma EMR (rs = 0.60 in Caucasians).60 Hence, further assessment of intraindividual correlations between serum/plasma and urinary EMR both in patients and in healthy individuals is warranted.
Association between EMR and subtypes of breast cancer
There is now ample evidence of etiologic heterogeneity for breast cancer subtypes.73 Therefore, the potential for a differential role of EMR and other estrogen metabolites in subtypes of breast tumors (eg, by ER status, high grade) should be investigated in larger prospective studies with sufficient power.
Measurement of the catecholestrogens 4OHE1/4OHE2
The 4-hydroxylation of estrogens leading to the potentially carcinogenic catecholestrogens 4OHE1/4OHE2 is positively correlated to 2-hydroxylation and might be negatively correlated to 16αOHE1, depending on the extent of cross-reactivity of the CYP1A1/A2 pathway.74 Studies in breast tissue have gained some insight into the relative and absolute amounts of estrogens and their metabolites. Although a higher EMR has been found in normal breast tissues,75 supporting the hypothesized role of 16αOHE1, the most abundant metabolite in cancer tissues was 4OHE2. A study by Rogan et al76 detected significantly higher tissue levels of 4OHE1 but not 16OHE1 in 28 cases compared with 46 controls. Additionally, higher concentrations of quinone conjugates derivatives were found in cancer tissues and interpreted as higher potential for quinones to react with DNA. Finally, in a small study, Gaikwad et al49 focused on depurinating DNA adduct formation in relation to catecholestrogen concentration, expressed as a ratio. Higher ratio levels, particularly for 4OHE conjugates indicating a relatively higher DNA adduct formation, were seen in cases and in a high-risk group than in controls (confirmed in an extended group).77 These preliminary findings may advise the future direction of research to include the detection of 4OHE1 and 4OHE2 in epidemiological studies of 2OHE1/16αOHE1 EMR.
Conclusion
All of nine properly designed epidemiological studies (six prospective case-control studies and three retrospective studies) failed to show a significant relationship between urinary or circulating EMR (2OHE1/16αOHE1) and breast cancer risk. Although premenopausal studies on urinary EMR have suggested a potentially weak inverse relationship, associations were not significantly different compared with postmenopausal or overall combined studies. Thus, at present, there is no evidence that the EMR can predict breast cancer risk. Larger prospective studies are needed to definitely assess a potential association of EMR with risk of breast cancer and risk by subtype (eg, by ER), adjusting for age, menstrual cycle phase, and ethnicity and menopausal status in cases of mixed study populations. A deeper knowledge of interrelationships between urinary, circulating, and tissue levels of estrogen metabolites would help to integrate studies with respect to target tissue values. The measurement of further estrogen metabolites by new LC–MS/MS methods to provide a more complete profile, particularly of 4-hydroxylated and methylated estrogens, may lead to more promising markers for breast cancer.
Supplementary table
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 2.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 3.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 4.Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5(5):239–247. doi: 10.1186/bcr628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WY. Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22(4):573–585. doi: 10.1016/j.beem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97(10):755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 7.Telang NT, Katdare M, Bradlow HL, Osborne MP. Estradiol metabolism: an endocrine biomarker for modulation of human mammary carcinogenesis. Environ Health Perspect. 1997;105(Suppl 3):559–564. doi: 10.1289/ehp.97105s3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta M, McDougal A, Safe S. Estrogenic and antiestrogenic activities of 16alpha- and 2-hydroxy metabolites of 17 beta-estradiol in MCF-7 and T47D human breast cancer cells. J Steroid Biochem Mol Biol. 1998;67(5–6):413–419. doi: 10.1016/s0960-0760(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Sepkovic DW, Bradlow HL, Telang NT, Wong GY. Lycium barbarum inhibits growth of estrogen receptor positive human breast cancer cells by favorably altering estradiol metabolism. Nutr Cancer. 2009;61(3):408–414. doi: 10.1080/01635580802585952. [DOI] [PubMed] [Google Scholar]
- 10.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150(Suppl):S259–S265. [PubMed] [Google Scholar]
- 11.Bradlow HL, Hershcopf RJ, Martucci CP, Fishman J. Estradiol 16 alpha-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proc Natl Acad Sci U S A. 1985;82(18):6295–6299. doi: 10.1073/pnas.82.18.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suto A, Bradlow HL, Wong GY, Osborne MP, Telang NT. Experimental down-regulation of intermediate biomarkers of carcinogenesis in mouse mammary epithelial cells. Breast Cancer Res Treat. 1993;27(3):193–202. doi: 10.1007/BF00665689. [DOI] [PubMed] [Google Scholar]
- 13.Fishman J, Schneider J, Hershcope RJ, Bradlow HL. Increased estrogen-16 alpha-hydroxylase activity in women with breast and endometrial cancer. J Steroid Biochem. 1984;20(4B):1077–1081. doi: 10.1016/0022-4731(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 14.Schneider J, Kinne D, Fracchia A, et al. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci U S A. 1982;79(9):3047–3051. doi: 10.1073/pnas.79.9.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne MP, Bradlow HL, Wong GY, Telang NT. Upregulation of estradiol C16 alpha-hydroxylation in human breast tissue: a potential biomarker of breast cancer risk. J Natl Cancer Inst. 1993;85(23):1917–1920. doi: 10.1093/jnci/85.23.1917. [DOI] [PubMed] [Google Scholar]
- 16.Ho GH, Luo XW, Ji CY, Foo SC, Ng EH. Urinary 2/16 alpha-hydroxyestrone ratio: correlation with serum insulin-like growth factor binding protein-3 and a potential biomarker of breast cancer risk. Ann Acad Med Singapore. 1998;27(2):294–299. [PubMed] [Google Scholar]
- 17.Kabat GC, Chang CJ, Sparano JA, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6(7):505–509. [PubMed] [Google Scholar]
- 18.Zheng W, Dunning L, Jin F, Holtzman J. Correspondence re: Kabat GC, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomark Prev. 1997;6:505–509. Cancer Epidemiol Biomarkers Prev. 1998;7(1):85–86. [PubMed] [Google Scholar]
- 19.Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6(1):75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int J Cancer. 2006;118(8):1862–1868. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 22.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 23.Guillemette C, Bélanger A, Lépine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6(6):246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cribb AE, Knight MJ, Dryer D, et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev. 2006;15(3):551–558. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144(8):3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 26.Paracchini V, Raimondi S, Gram IT, et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432 Leu polymorphism and breast cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165(2):115–125. doi: 10.1093/aje/kwj365. [DOI] [PubMed] [Google Scholar]
- 27.Paracchini V, Pedotti P, Raimondi S, et al. A common CYP1B1 polymorphism is associated with 2-OHE1/16-OHE1 urinary estrone ratio. Clin Chem Lab Med. 2005;43(7):702–706. doi: 10.1515/CCLM.2005.119. [DOI] [PubMed] [Google Scholar]
- 28.Greenlee H, Chen Y, Kabat GC, et al. Variants in estrogen metabolism and biosynthesis genes and urinary estrogen metabolites in women with a family history of breast cancer. Breast Cancer Res Treat. 2007;102(1):111–117. doi: 10.1007/s10549-006-9308-7. [DOI] [PubMed] [Google Scholar]
- 29.Tworoger SS, Chubak J, Aiello EJ, et al. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(1):94–101. doi: 10.1158/1055-9965.epi-03-0026. [DOI] [PubMed] [Google Scholar]
- 30.Pasagian-Macaulay A, Meilahn EN, Bradlow HL, et al. Urinary markers of estrogen metabolism 2- and 16 alpha-hydroxylation in premenopausal women. Steroids. 1996;61(8):461–467. doi: 10.1016/0039-128x(96)00089-x. [DOI] [PubMed] [Google Scholar]
- 31.Westerlind KC, Gibson KJ, Wolfe P. The effect of diurnal and menstrual cyclicity and menopausal status on estrogen metabolites: implications for disease-risk assessment. Steroids. 1999;64(3):233–243. doi: 10.1016/s0039-128x(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 32.Dallal C, Taioli E. Urinary 2/16 estrogen metabolite ratio levels in healthy women: a review of the literature. Mutat Res. 2010;705(2):154–162. doi: 10.1016/j.mrrev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meilahn EN, de Stavola B, Allen DS, et al. Do urinary oestrogen metabolites predict breast cancer? Guernsey III cohort follow-up. Br J Cancer. 1998;78(9):1250–1255. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradlow HL, Sepkovic DW, Klug T, Osborne MP. Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 1998;63(7–8):406–413. doi: 10.1016/s0039-128x(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 35.Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids. 1994;59(11):648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 36.Muti P, Bradlow HL, Micheli A, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11(6):635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Arslan AA, Shore RE, Afanasyeva Y, Koenig KL, Toniolo P, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk for breast cancer in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2273–2279. doi: 10.1158/1055-9965.EPI-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ursin G, London S, Stanczyk FZ, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(12):1067–1072. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Zmuda JM, Danielson ME, et al. Estrogen metabolites and the risk of breast cancer in older women. Epidemiology. 2003;14(6):740–744. doi: 10.1097/01.ede.0000091607.77374.74. [DOI] [PubMed] [Google Scholar]
- 41.Wellejus A, Olsen A, Tjonneland A, Thomsen BL, Overvad K, Loft S. Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2137–2142. doi: 10.1158/1055-9965.EPI-04-0934. [DOI] [PubMed] [Google Scholar]
- 42.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabat GC, O’Leary ES, Gammon MD, et al. Estrogen metabolism and breast cancer. Epidemiology. 2006;17(1):80–88. doi: 10.1097/01.ede.0000190543.40801.75. [DOI] [PubMed] [Google Scholar]
- 44.Fowke JH, Qi D, Bradlow HL, et al. Urinary estrogen metabolites and breast cancer: differential pattern of risk found with pre- versus post-treatment collection. Steroids. 2003;68(1):65–72. doi: 10.1016/s0039-128x(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 45.Coker AL, Crane MM, Sticca RP, Sepkovic DW. Re: Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1997;89(1):89–90. doi: 10.1093/jnci/89.1.89. [DOI] [PubMed] [Google Scholar]
- 46.Im A, Vogel VG, Ahrendt G, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30(9):1532–1535. doi: 10.1093/carcin/bgp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modugno F, Kip KE, Cochrane B, et al. Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int J Cancer. 2006;118(5):1292–1301. doi: 10.1002/ijc.21487. [DOI] [PubMed] [Google Scholar]
- 48.Adlercreutz H, Fotsis T, Höckerstedt K, et al. Diet and urinary estrogen profile in premenopausal omnivorous and vegetarian women and in premenopausal women with breast cancer. J Steroid Biochem. 1989;34(1–6):527–530. doi: 10.1016/0022-4731(89)90138-6. [DOI] [PubMed] [Google Scholar]
- 49.Gaikwad NW, Yang L, Muti P, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008;122(9):1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez-Vasquez RB, Axelrod D, Frenkel K, et al. Influence of postmenopausal hormone replacement therapy on an estrogen metabolite biomarker of risk for breast cancer. Horm Metab Res. 2003;35(6):358–361. doi: 10.1055/s-2003-41357. [DOI] [PubMed] [Google Scholar]
- 51.Bradlow HL, Hershcopf R, Martucci C, Fishman J. 16 alpha-hydroxylation of estradiol: a possible risk marker for breast cancer. Ann N Y Acad Sci. 1986;464:138–151. doi: 10.1111/j.1749-6632.1986.tb16001.x. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Closas M, Hankinson SE, Ho S, et al. Factors critical to the design and execution of epidemiologic studies and description of an innovative technology to follow the progression from normal to cancer tissue. J Natl Cancer Inst Monogr. 2000;2000(27):147–156. doi: 10.1093/oxfordjournals.jncimonographs.a024238. [DOI] [PubMed] [Google Scholar]
- 53.Xu X, Duncan AM, Merz-Demlow BE, Phipps WR, Kurzer MS. Menstrual cycle effects on urinary estrogen metabolites. J Clin Endocrinol Metab. 1999;84(11):3914–3918. doi: 10.1210/jcem.84.11.6134. [DOI] [PubMed] [Google Scholar]
- 54.Fowke JH, Shu XO, Dai Q, et al. Oral contraceptive use and breast cancer risk: modification by NAD(P)H:quinone oxoreductase (NQO1) genetic polymorphisms. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1308–1315. [PubMed] [Google Scholar]
- 55.Jernström H, Klug TL, Sepkovic DW, Bradlow HL, Narod SA. Predictors of the plasma ratio of 2-hydroxyestrone to 16alpha-hydroxye-strone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24(5):991–1005. doi: 10.1093/carcin/bgg047. [DOI] [PubMed] [Google Scholar]
- 56.Konovalova V, Smetnik V. Impact of hormone replacement therapy on endogenous estradiol hydroxymetabolism in Russian postmenopausal women. Maturitas. 2009;63(Suppl 1):S49. [Google Scholar]
- 57.Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer. 1999;34(2):133–139. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- 58.Falk RT, Fears TR, Xu X, et al. Urinary estrogen metabolites and their ratio among Asian American women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):221–226. [PubMed] [Google Scholar]
- 59.Ursin G, London S, Yang D, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and family history of breast cancer in premenopausal women. Breast Cancer Res Treat. 2002;72(2):139–143. doi: 10.1023/a:1014896417653. [DOI] [PubMed] [Google Scholar]
- 60.Bradlow HL, Jernström H, Sepkovic DW, Klug TL, Narod SA. Comparison of plasma and urinary levels of 2-hydroxyestrogen and 16 alpha-hydroxyestrogen metabolites. Mol Genet Metab. 2006;87(2):135–146. doi: 10.1016/j.ymgme.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hämäläinen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994;86(14):1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- 62.Taioli E, Garte SJ, Trachman J, et al. Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1996;88(9):617. doi: 10.1093/jnci/88.9.617. [DOI] [PubMed] [Google Scholar]
- 63.Wacker M, Risendal B, Westerlind K, Lezotte D, Byers T. Ethnicity, body size, and estrogen levels in postmenopausal Hispanic and non-Hispanic white women. J Womens Health (Larchmt) 2009;18(4):487–491. doi: 10.1089/jwh.2008.0835. [DOI] [PubMed] [Google Scholar]
- 64.Sowers MR, Crawford S, McConnell DS, et al. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136(6):1588–1595. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 65.Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat Res. 2003;544(1):9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Zheng W, Dunning LM, Anderson KG, Parrish RS, Holtzman JL. Within-person variability of the ratios of urinary 2-hydroxyestrone to 16alpha-hydroxyestrone in Caucasian women. Steroids. 1999;64(12):856–859. doi: 10.1016/s0039-128x(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 67.Michaud DS, Manson JE, Spiegelman D, et al. Reproducibility of plasma and urinary sex hormone levels in premenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1059–1064. [PubMed] [Google Scholar]
- 68.Ziegler RG, Rossi SC, Fears TR, et al. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ Health Perspect. 1997;105(Suppl 3):607–614. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faupel-Badger JM, Fuhrman BJ, Xu X, et al. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19(1):292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riza E, dos Santos Silva I, de Stavola B, et al. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10(6):627–634. [PubMed] [Google Scholar]
- 71.Ursin G, Wilson M, Henderson BE, et al. Do urinary estrogen metabolites reflect the differences in breast cancer risk between Singapore Chinese and United States African-American and white women? Cancer Res. 2001;61(8):3326–3329. [PubMed] [Google Scholar]
- 72.Taioli E, Im A, Xu X, Veenstra TD, Ahrendt G, Garte S. Comparison of estrogens and estrogen metabolites in human breast tissue and urine. Reprod Biol Endocrinol. 2010;8:93. doi: 10.1186/1477-7827-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 74.Fuhrman BJ, Pfeiffer R, Xu X, et al. Soy intake is associated with increased 2-hydroxylation and decreased 16alpha-hydroxylation of estrogens in Asian-American women. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2751–2760. doi: 10.1158/1055-9965.EPI-09-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castagnetta LA, Granata OM, Traina A, et al. Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin Cancer Res. 2002;8(10):3146–3155. [PubMed] [Google Scholar]
- 76.Rogan EG, Badawi AF, Devanesan PD, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24(4):697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 77.Gaikwad NW, Yang L, Pruthi S, et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer Basic Clin Res. 2009;3(1):1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.