Abstract
The sodium channel blocker, tetrodotoxin (TDT), was conjugated to keyhole limpet hemocyanin (KLH) and used to immunize BALB/c mice. Anti-TDT antibodies were detected in serum by ELISA and reached stable levels 4-5 wk after the first immunization. Spleens from immunized mice were fused with NS-1 mouse myeloma cells and approximately 9,329 resultant hybrids were screened by ELISA for reactivity to TDT. Two stable hybrids were isolated, subcloned, and characterized. These hybrids, termed TD13a1 and TD2C5, secreted specific anti-TDT antibodies that recognized TDT but not the related sodium channel blocker, saxitoxin (STX), as determined by competition ELISA. Both antibodies were of the IgG1k subclass with Ka's approaching 10(7) M-1. The inhibitory ability of these antibodies was tested by a competitive displacement assay for [3H]STX on rat brain membranes. Both antibodies strongly inhibited TDT binding to membranes. A nanomole of TD2C5 was able to bind approximately 1.8 nmol of TDT, whereas a comparable amount of TD13a1 bound half as much. Furthermore, TD2C5 was able to protect against TDT-induced reduction of peripheral nerve action potentials in rat tibial nerve when administered in situ. These antibodies thus represent potentially useful reagents for neurobiologic research, detection of toxin contamination and diagnosis of poisoning, and may provide protection against the toxicity of TDT in vivo.
Full text
PDF

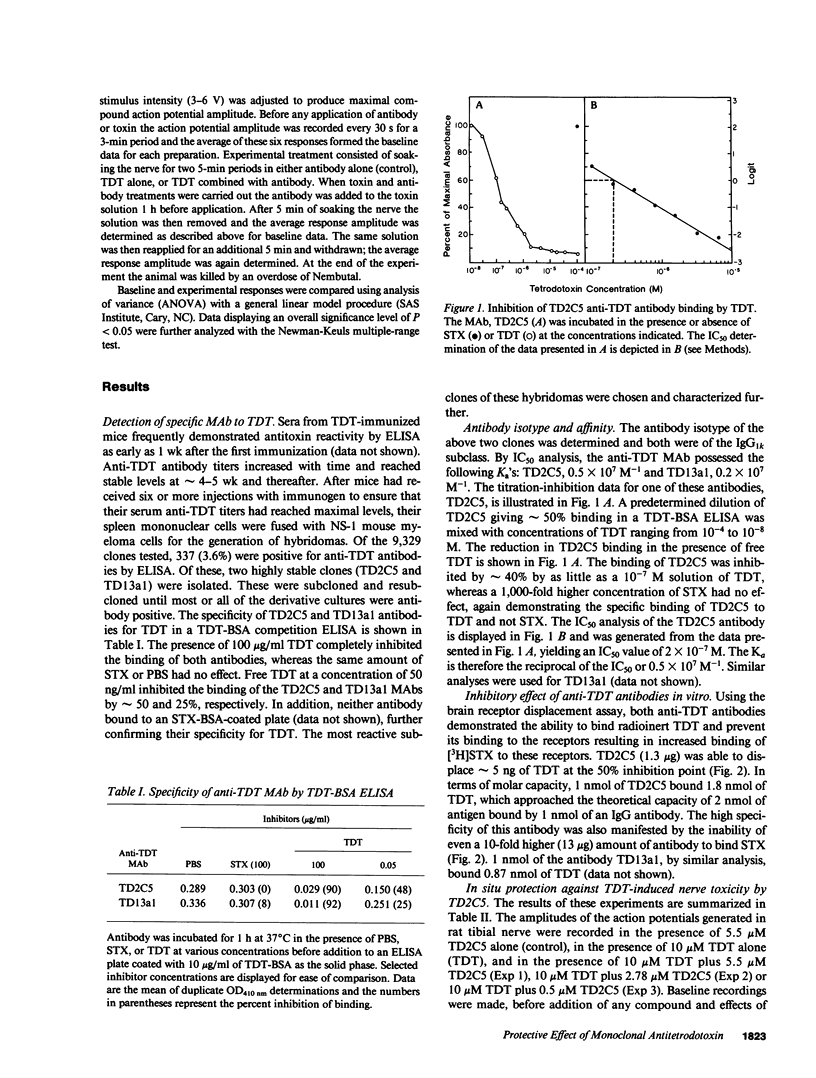
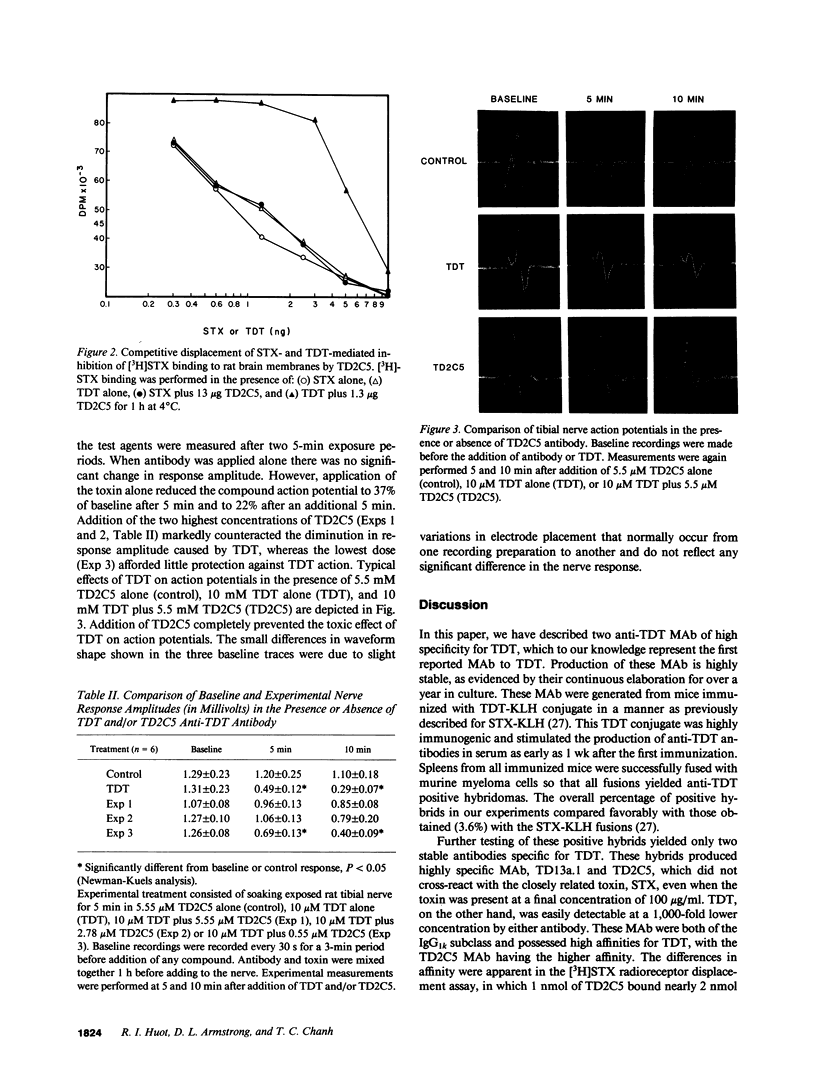


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bower D. J., Hart R. J., Matthews P. A., Howden M. E. Nonprotein neurotoxins. Clin Toxicol. 1981 Jul;18(7):813–863. doi: 10.3109/15563658108990310. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHEYMOL J., BOURILLET F., OGURA Y. [Action of some neuromuscular paralyzants on the liberation of acetylcholine at motor nerve terminations]. Arch Int Pharmacodyn Ther. 1962 Sep 1;139:187–197. [PubMed] [Google Scholar]
- Chu F. S., Fan T. S. Indirect enzyme-linked immunosorbent assay for saxitoxin in shellfish. J Assoc Off Anal Chem. 1985 Jan-Feb;68(1):13–16. [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davio S. R., Fontelo P. A. A competitive displacement assay to detect saxitoxin and tetrodotoxin. Anal Biochem. 1984 Aug 15;141(1):199–204. doi: 10.1016/0003-2697(84)90446-9. [DOI] [PubMed] [Google Scholar]
- Duch D. S., Levinson S. R. Neurotoxin-modulated uptake of sodium by highly purified preparations of the electroplax tetrodotoxin-binding glycopeptide reconstituted into lipid vesicles. J Membr Biol. 1987;98(1):43–55. doi: 10.1007/BF01871044. [DOI] [PubMed] [Google Scholar]
- Evans M. H. Tetrodotoxin, saxitoxin, and related substances: their applications in neurobiology. Int Rev Neurobiol. 1972;15:83–166. doi: 10.1016/s0074-7742(08)60329-3. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Greene M. I. Idiotypic mimicry of biological receptors. Annu Rev Immunol. 1986;4:253–280. doi: 10.1146/annurev.iy.04.040186.001345. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Brimfield A. A., Miller M., Finkelman F. D., Chu S. F. Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl Environ Microbiol. 1985 Jan;49(1):168–172. doi: 10.1128/aem.49.1.168-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON H. M., FREY P. A., ANGELOTTI R., CAMPBELL J. E., LEWIS K. H. HAPTENIC PROPERTIES OF PARALYTIC SHELLFISH POISON CONJUGATED TO PROTEINS BY FORMALDEHYDE TREATMENT. Proc Soc Exp Biol Med. 1964 Nov;117:425–430. doi: 10.3181/00379727-117-29599. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Structure-activity relations of tetrodotoxin, saxitoxin, and analogues. Ann N Y Acad Sci. 1986;479:52–67. doi: 10.1111/j.1749-6632.1986.tb15561.x. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mosher H. S. The chemistry of tetrodotoxin. Ann N Y Acad Sci. 1986;479:32–43. doi: 10.1111/j.1749-6632.1986.tb15559.x. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Haas H. G., Therrien E. F. Saxitoxin and tetrodotoxin: comparison of nerve blocking mechanism. Science. 1967 Sep 22;157(3795):1441–1442. doi: 10.1126/science.157.3795.1441. [DOI] [PubMed] [Google Scholar]
- Onoue Y., Noguchi T., Nagashima Y., Hashimoto K., Kanoh S., Ito M., Tsukada K. Separation of tetrodotoxin and paralytic shellfish poisons by high-performance liquid chromatography with a fluorometric detection using o-phthalaldehyde. J Chromatogr. 1983 Mar 4;257(2):373–379. doi: 10.1016/s0021-9673(01)88193-0. [DOI] [PubMed] [Google Scholar]
- Reik L. M., Maines S. L., Ryan D. E., Levin W., Bandiera S., Thomas P. E. A simple, non-chromatographic purification procedure for monoclonal antibodies. Isolation of monoclonal antibodies against cytochrome P450 isozymes. J Immunol Methods. 1987 Jun 26;100(1-2):123–130. doi: 10.1016/0022-1759(87)90180-3. [DOI] [PubMed] [Google Scholar]
- Strichartz G. Structure of the saxitoxin binding site at sodium channels in nerve membranes. Exchange of tritium from bound toxin molecules. Mol Pharmacol. 1982 Mar;21(2):343–350. [PubMed] [Google Scholar]
- Yotsu M., Yamazaki T., Meguro Y., Endo A., Murata M., Naoki H., Yasumoto T. Production of tetrodotoxin and its derivatives by Pseudomonas sp. isolated from the skin of a pufferfish. Toxicon. 1987;25(2):225–228. doi: 10.1016/0041-0101(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Zettner A. Principles of competitive binding assays (saturation analysis). 1. Equilibrium techniques. Clin Chem. 1973 Jul;19(7):699–705. [PubMed] [Google Scholar]



