Abstract
UNC-45 is a chaperone that facilitates folding of myosin motor domains. We have used Drosophila melanogaster to investigate the role of UNC-45 in muscle development and function. Drosophila UNC-45 (dUNC-45) is expressed at all developmental stages. It colocalizes with non-muscle myosin in embryonic blastoderm of 2-hour-old embryos. At 14 hours, it accumulates most strongly in embryonic striated muscles, similarly to muscle myosin. dUNC-45 localizes to the Z-discs of sarcomeres in third instar larval body-wall muscles. We produced a dunc-45 mutant in which zygotic expression is disrupted. This results in nearly undetectable dUNC-45 levels in maturing embryos as well as late embryonic lethality. Muscle myosin accumulation is robust in dunc-45 mutant embryos at 14 hours. However, myosin is dramatically decreased in the body-wall muscles of 22-hour-old mutant embryos. Furthermore, electron microscopy showed only a few thick filaments and irregular thick–thin filament lattice spacing. The lethality, defective protein accumulation, and ultrastructural abnormalities are rescued with a wild-type dunc-45 transgene, indicating that the mutant phenotypes arise from the dUNC-45 deficiency. Overall, our data indicate that dUNC-45 is important for myosin accumulation and muscle function. Furthermore, our results suggest that dUNC-45 acts post-translationally for proper myosin folding and maturation.
Keywords: Chaperone, Drosophila, Muscle, Myofibril, UNC-45
Introduction
Myosins are molecular motors that function in cellular processes from cytokinesis and vesicle transport to cell motility. Currently, there are at least 24 classes of myosin (Foth et al., 2006), each with its own unique characteristics. Structurally, myosin molecules have globular heads and rod-like tails. The globular head contains the motor domain, including the ATPase and actin binding sites. The tail can be used to form coiled-coil dimers or attachments to substrates. Even though the different types of myosin are involved in different cellular functions, they have similar overall structure in the motor domain.
Myosin II is a major component of muscle thick filaments and is indispensable in muscle contraction. To facilitate the investigation of its function, researchers have attempted to synthesize muscle myosin in vitro. Functional cardiac (Sweeney et al., 1994) and smooth muscle (Trybus, 1994) myosin isoforms have been produced using insect cells, but in vitro expression of skeletal muscle isoforms has not been routinely performed without using a myogenic cell line (Chow et al., 2002) or its lysate (Srikakulam and Winkelmann, 1999). Because functional α-helical tails of rabbit skeletal muscle myosin can be synthesized in Escherichia coli (Atkinson and Stewart, 1991), it appears that some factor(s) in the myogenic cell line facilitates the folding of skeletal muscle myosin globular heads into the correct conformation. To gain an understanding of myosin folding, we have investigated the function of a recently characterized myosin chaperone UNC-45.
From its first description in a temperature-sensitive Caernorhabditis elegans mutant (Epstein and Thomson, 1974), to recent data supporting its role in facilitating myosin degradation (Landsverk et al., 2007), UNC-45 has been shown to be important for myosin maturation, thick filament assembly and muscle function. The discovery of a muscle-specific isoform of UNC-45 in vertebrates (Price et al., 2002) further underscores the importance of UNC-45 in muscle. C. elegans mutants of UNC-45 show movement defects and decreased thick filament formation (Barral et al., 1998), and morpholino knockdown of UNC-45 in zebrafish results in paralysis and cardiac dysfunction (Wohlgemuth et al., 2007). RNA interference (RNAi) knockdown of UNC-45 in Drosophila embryos results in wild-type body-wall muscle patterning, yet these muscles do not contract (Estrada et al., 2006).
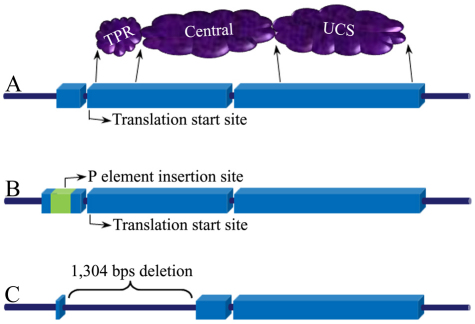
UNC-45 is composed of three domains: an N-terminal tetratricopeptide repeat (TPR) motif, a central domain, and a C-terminal UCS domain (Fig. 1A). The UCS domain is named after the three proteins (UNC-45, Cro1 and She4p) discovered to contain the homologous domain that was subsequently found to interact with myosin (Barral et al., 1998; Barral et al., 2002; Toi et al., 2003). The central domain of UNC-45 has an unknown function but its sequence is approximately 40% conserved between C. elegans and humans. The TPR domain has been found to interact with heat shock protein 90 (Barral et al., 2002; Mishra et al., 2005; Etard et al., 2007; Liu et al., 2008), which led to the notion that UNC-45 is a co-chaperone for heat shock protein 90. In-depth reviews of UNC-45 function have been published previously (Hutagalung et al., 2002; Yu and Bernstein, 2003; Kachur and Pilgrim, 2008; Kim et al., 2008; Willis et al., 2009).
Fig. 1.
Drosophila unc-45 genomic region in three fly lines. (A) The wild-type gene consists of three exons and two introns, with the translation start site located 14 bp downstream from the beginning of the second exon. The gene encodes a three-domain protein of approximately 105 kDa with an N-terminal TPR domain, a central domain and a C-terminal UCS domain. (B) The dunc-45 mutant Tom34EY03034 contains a P element insertion in the first exon, upstream of the translation start site. (C) The T-33 dunc-45 knockout mutant contains a 1304 bp deletion that removes the majority of both the first and second exons.
Drosophila is an excellent model organism for muscle research due to its well-developed genetics and the availability of techniques to study its muscle structure and physiology (Bernstein et al., 1993; Maughan and Vigoreaux, 1999; Vigoreaux, 2006). Its genome is composed of four chromosomes, which have been completely sequenced (Adams et al., 2000). Genetic and transgenic analyses have provided insights into the mechanisms of muscle development, myofibril assembly and muscle contraction. Here, we present cell biological and genetic analyses of UNC-45 function in Drosophila. Our data show that Drosophila UNC-45 (dUNC-45) is expressed during the entire life cycle, that it is enriched in muscle as embryogenesis proceeds, and that it is essential for thick filament accumulation and embryo viability.
Results
Developmental expression of UNC-45 in Drosophila
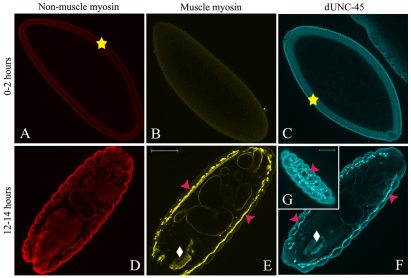
We determined dUNC-45 expression in the yellow white (yw) genotype (wild-type background) using western blotting analysis for developmental expression and immunofluorescence confocal microscopy for localization. A polyclonal antibody was generated in rabbits using bacterially expressed full-length, His-tagged dUNC-45 as the immunizing agent. The antibodies were highly specific on western blots (Fig. 2A) when probed against whole fly lysates, recognizing a single band of 105 kD. The western blot analyses showed that dUNC-45 is expressed at all developmental stages (Fig. 2B–D). To determine the correlation between dUNC-45 localization and myosin accumulation, we used immunofluorescence confocal microscopy to visualize yw embryos labeled with antibodies to dUNC-45, muscle myosin or non-muscle myosin II. At 2 hours after egg laying (AEL, stage 5) (Fig. 3A–C), dUNC-45 localized to the embryonic blastoderm and colocalized with non-muscle myosin II. Muscle myosin was not expressed at this time. At 14 hours AEL (stage 12) (Fig. 3D–G), dUNC-45 localization was similar to that of muscle myosin, which is expressed strongly in skeletal muscles such as body-wall muscle and pharyngeal muscle. Non-muscle myosin localized to non-muscle tissues, as did some dUNC-45 (particularly to ectoderm). UNC-45 was also found in muscle-containing gut. However, dUNC-45 staining intensity was much brighter in the skeletal muscles. Lateral views of 14-hour-old embryos showed intense dUNC-45 fluorescence in the body-wall muscle, similar to that of skeletal muscle myosin (Fig. 3G). Furthermore, dUNC-45 localized to other skeletal muscle examined, including embryonic dorsal tube, adult body-wall muscle, adult dorsal tube and adult indirect flight muscle (C.F.L., unpublished results).
Fig. 2.

Western blot analysis showing dUNC-45 antibody specificity and dUNC-45 expression at all stages of development in wild-type Drosophila. (A) Western blot of 2-hour-old adult (2hA) and 1-day-old adult (1dA) whole fly lysates. The third lane is bacterially expressed recombinant dUNC-45. It contains a His tag, which accounts for its slightly larger size. The protein size ladder is given on the right. (B–D) Developmental expression of dUNC-45. (B) Early (EE) and late stage embryos (LE). (C) First instar (1L), second instar (2L), and third instar larvae (3L). (D) Early pupae (EP), late pupae (LP), 2-hour-old adult (2hA), and 1-day-old adult (1dA). Equivalent amounts of protein were loaded in each lane.
Fig. 3.
Immunofluorescence confocal micrographs showing protein localization in wild-type yw embryos. (A–G) Embryos were probed with non-muscle myosin (A,D), muscle myosin (B,E), and dUNC-45 (C,F,G) antibodies. (A–C) 2-hour-old embryos. At this stage, both non-muscle myosin and dUNC-45 localized to the embryonic blastoderm (yellow stars). Furthermore, UNC-45 can be seen in the pole cells (upper left of C). Muscle myosin is not detected in 2-hour-old embryos (B). (D–F) Ventral views of 14-hour-old embryos. At 14 hours, dUNC-45 localization is more similar to that of muscle myosin, which can be found in the body wall (pink arrowheads) and the pharyngeal muscles (white diamonds). G gives a lateral view of an embryo, confirming dUNC-45 localization in the body-wall muscle. Scale bars: 75 μm.
dUNC-45 subcellular localization was also investigated using immunofluorescence confocal microscopy. Third instar larval body-wall muscle was used to examine dUNC-45 sarcomeric localization because embryonic body-wall muscles do not show a distinct sarcomeric structure at 14 hours of age, and antibodies do not penetrate older embryos due to cuticle formation. Because both muscle myosin and dUNC-45 antibodies were generated using rabbits as host species, co-staining was not feasible. Instead, dissected third instar yw larvae were co-stained with dUNC-45 antibodies, α-actinin antibodies (Z-disc-specific) and phalloidin (for actin and I band visualization). The result of the triple staining indicated that dUNC-45 is localized to the Z-discs (Fig. 4). It colocalized with α-actinin staining and bisected phalloidin staining.
Fig. 4.
Immunofluorescence confocal micrographs showing subcellular dUNC-45 localization in the body-wall muscle of third instar wild-type larvae. (A) α-Actinin staining highlights the location of the Z-discs. (B) dUNC-45 is discretely localized in the sarcomere. (C) Actin staining with phalloidin shows the location of I bands in the sarcomeres. (D) dUNC-45 colocalizes with α-actinin (bright pink in the overlay panel) and bisects the actin-containing I bands, which supports dUNC-45 location in the Z-discs of the sarcomeres.
Mutant analysis
To discern the effects of mutating unc-45 in Drosophila, a mutant line (Tom34EY03034) from the Bloomington Stock Center was examined. Tom34EY03034 is an unc-45 allele created by insertion of a P element in the promoter region, upstream of the translation start site (Fig. 1B). Tom34EY03034 allele homozygotes display third larval instar lethality. Because the coding region of Drosophila unc-45 (dunc-45) is not disrupted, it is conceivable that functional protein could still be synthesized, albeit in lower amounts than normal, which could impede our mutant phenotype analysis. Western blotting of 22-hour-old homozygous Tom34EY03034 embryos confirmed the presence of the full-length protein (Fig. 5A, lane 2).
Fig. 5.
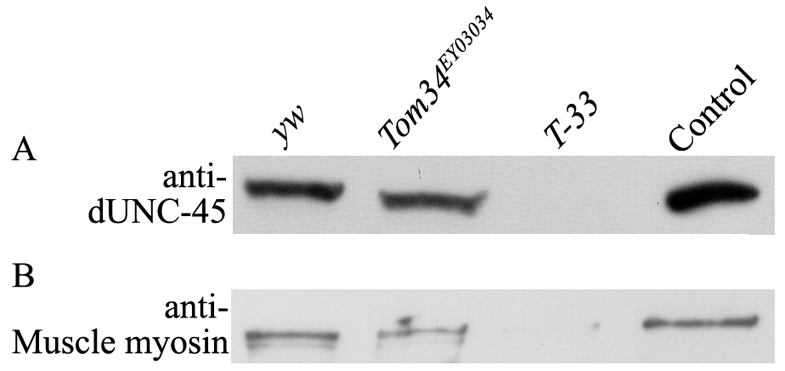
Western blot analysis of dUNC-45 and myosin expression in wild-type (yw), Tom34EY03034 and T-33 22-hour-old embryos. (A) Western blot analysis of dUNC-45 expression. Control is bacterially expressed recombinant dUNC-45. (B) Western blot analysis of myosin expression. Control is indirect flight muscle lysate. Results of the analysis show that dUNC-45 can still be detected in the Tom34EY03034 line but not in the T-33 line. Muscle myosin heavy chain is somewhat reduced in Tom34EY03034 homozygotes, but is nearly absent from T-33 embryos. 10 μg of protein were loaded for each sample.
To create an unc-45 null allele, which might have a more severe phenotype, a P element mobilization procedure was performed. Potential dUNC-45 null lines were identified by the absence of specific genetic marker phenotypes (see Materials and Methods) and confirmed by western blotting and DNA sequencing. Analysis of 22-hour-old homozygous embryos showed that line 33 (Tom34EY03043–33 or T-33) was dUNC-45 null (Fig. 5A, lane 3).
T-33 homozygotes display embryonic lethality, which could be rescued by a wild-type dunc-45 transgene but not by the Tom34EY03034 allele in the heterozygous complementation test (see Materials and Methods). Together, the data confirm that the lethal phenotype was specifically due to the dunc-45 knockout. DNA sequencing showed that T-33 contains a 1304 base pair (bp) deletion of the dunc-45 DNA from the promoter to the second exon (Fig. 1C), which includes the translation start site (details of the deleted region are presented in Materials and Methods); therefore, subsequent mutant characterization was carried out using this line.
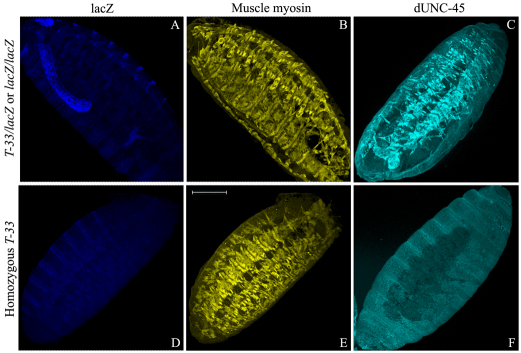
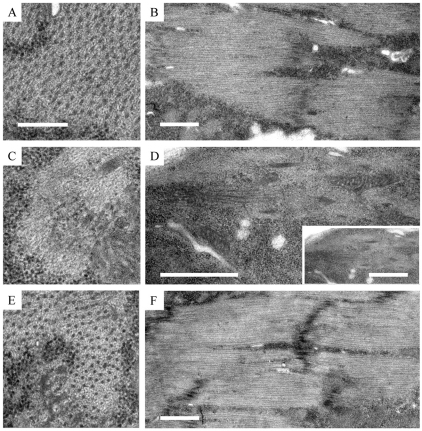
To determine whether the T-33 allele disrupts dUNC-45 localization, we performed immunofluorescence microscopy of 14-hour-old embryos. Fig. 6A–C shows that embryos carrying at least one wild-type dunc-45 allele, identified by lacZ expression in the hindgut, had intense UNC-45 expression in the body-wall muscles, similar to the yw wild-type. Homozygous T-33 embryos showed barely detectable dUNC-45 staining in the body-wall muscles (Fig. 6F). Interestingly, the lack of dUNC-45 did not dramatically affect skeletal muscle myosin translation (Fig. 6E). The muscle myosin expression in the confocal images was assessed by comparing the staining levels of homozygous T-33 to other embryos within the same batch. Because each batch of embryos was stained in the same tube and imaged in parallel, qualitative assessment of fluorescence intensities suggested similar expression levels of muscle myosin in all the embryos. However, myosin did not remain stable during the normal period of myofibril assembly, in that myosin levels were greatly reduced in 22-hour-old homozygous T-33 embryos (Fig. 5B, lane 2). We examined the body-wall muscle ultrastructure of such embryos using transmission electron microscopy (TEM). Fig. 7 shows that 22-hour-old yw wild-type embryos exhibited regular thick and thin filament packing in transverse section (Fig. 7A) and regularly spaced thick filaments in longitudinal section (Fig. 7B). Homozygous T-33 embryos of the same age showed disrupted filament packing and a near absence of thick filaments (Fig. 7C,D). The muscle defects in the T-33 homozygous embryos were rescued by a wild-type dunc-45 transgene (Fig. 7E,F).
Fig. 6.
Immunofluorescence confocal micrographs of 14-hour-old T-33/lacZ or lacZ/lacZ embryos and homozygous T-33 embryos. (A–F) Embryos were probed with antibodies to lacZ (A,D), muscle myosin (B,E) and dUNC-45 (C,F). T-33/lacZ or lacZ/lacZ embryos were identified by lacZ expression in the hind-gut (A). Homozygous T-33 embryos do not show a detectable expression of lacZ (D). dUNC-45 cannot be detected in the body-wall muscle of the homozygous T-33 embryos (F). Muscle patterning and muscle myosin expression are not affected in the absence of dUNC-45 at this stage of embryogenesis (E). Scale bar: 75 μm.
Fig. 7.
Electron micrographs showing body-wall muscle ultrastructure in 22-hour-old embryos. (A,B) yw control line, (C,D) T-33 dUNC-45 knockout embryos, and (E,F) T-33 rescued embryos with a wild-type transgene. Electron micrographs show that T-33 embryos exhibit a reduced level of thick filaments and a loss of the thick–thin filament lattice spacing (C,D). This phenotype can be rescued with a wild-type dunc-45 transgene, which confirms the effects of dUNC-45 knockout in the muscles. Transverse orientation is shown in A, C and E. Longitudinal orientation is shown in B, D and F. Scale bars: 0.25 μm (A,C,E); 1.0 μm (B,D inset,F); 1.0 μm (D).
Discussion
Using Drosophila melanogaster wild-type and mutant flies, we examined UNC-45 localization and function. We found that dUNC-45 is expressed throughout development and its expression is maintained in adulthood (Fig. 2B–D), suggesting that it functions not only in myosin assembly and folding but possibly in muscle maintenance and/or repair.
dUNC-45 colocalized with non-muscle myosin in 2-hour-old embryos, prior to the expression of muscle myosin (Fig. 3, upper panels). At this cellularization stage, non-muscle myosin displays a cytosolic and peri-nuclear localization pattern (Young et al., 1991), as does dUNC-45. It is possible that at this early embryonic stage, both non-muscle myosin and dUNC-45 are distributed diffusely throughout the cytoplasm and that the immunofluorescence staining results do not indicate direct interaction. However, it is more likely that dUNC-45 interacts with non-muscle myosins, because this is consistent with findings in C. elegans, where UNC-45 and non-muscle myosin II colocalized at the cell cortex and were both needed for cytokinesis (Kachur et al., 2004; Kachur et al., 2008). Similarly, other UNC-45-like proteins such as She4p and Rng3 interact with myosin V and non-muscle myosin II, respectively as shown using genetic interaction, yeast two-hybrid, in vitro protein pull-down and in vivo mutant characterization experiments (Wong et al., 2000; Wesche et al., 2003). Furthermore, UNC-45 is maternally inherited in C. elegans (Venolia and Waterston, 1990). It is plausible that the Drosophila UNC-45 present at the blastoderm stage is maternally inherited as well.
In 14-hour-old embryos, dUNC-45 colocalized with muscle myosin (Fig. 3, lower panels). Muscle myosin expression starts at approximately 10 hours AEL, correlating with the onset of muscle fiber formation and patterning (C.F.L., unpublished results), where muscle fibers are present in the correct pattern but muscle striation is still not apparent. Because skeletal muscle myosin and dUNC-45 showed intense staining in the embryonic body-wall muscle, it appears that the major zygotic expression of dUNC-45 coincides with muscle myosin expression both spatially and temporally. Subcellularly, dUNC-45 localized to the Z-discs of the sarcomeres in the body-wall muscle of wild-type yw third instar larvae (Fig. 4). This result agrees with the observations of Etard and colleagues (Etard et al. 2008), who localized UNC-45 at the Z-discs of zebrafish under normal developmental conditions. We used a polyclonal antibody against wild-type dUNC-45, whereas an UNC-45-GFP chimera was used in the zebrafish study. It is possible that our polyclonal dUNC-45 antibodies recognize targets other than UNC-45. However, this is unlikely because immunofluorescence confocal microscopy staining using pre-immunized rabbit serum did not yield a specific staining pattern above background (C.F.L., unpublished results) and western blot analysis showed a single band of the correct protein size when using total adult fly lysate (Fig. 2A). Because UNC-45 contributes to myosin degradation when overexpressed in C. elegans (Landsverk et al., 2007), it might be important to regulate UNC-45 localization. Consequently, Z-disc localization might be required to prevent the degradation of normal, functional myosin. The Z-disc could sequester UNC-45, which is then released in response to muscle stress and/or damage, as has been shown to occur in zebrafish (Etard et al., 2008). The timeliness of Z-disc formation appears to be dependent upon UNC-45 function in Xenopus tropicalis (Geach and Zimmerman, 2010). Although dUNC-45 localization to the Z-disc is similar to that reported for zebrafish muscle, it is different to the localization observed in C. elegans. In the nematode muscles, UNC-45 is localized to the two ends of the A band, corresponding to the location of the myosin heavy chain B myosin isoform (Ao and Pilgrim, 2000). The reason for this discrepancy is unclear, but Kachur and Pilgrim speculate that C. elegans might employ an alternative strategy in keeping UNC-45 close to myosin by binding to the neck region of myosin (Kachur and Pilgrim, 2008). Interestingly, dUNC-45 staining in the Drosophila sarcomere appears to be wider and more diffuse than that of α-actinin (Fig. 4), which could suggest dUNC-45 mobility in and out of the Z-disc or the presence of peri-Z-disc components that anchor dUNC-45.
Analysis of T-33 dunc-45 mutant flies provides important insights into the function of UNC-45 during muscle formation. Western blot analysis showed a drastic decrease of dUNC-45 in the 22-hour-old homozygous embryos compared to wild-type yw embryos (Fig. 5A). Occasionally, a small amount of dUNC-45 could be seen in the T-33 line, which is probably the remnant of maternally inherited protein. Immunofluorescence confocal microscopy showed that 14-hour-old T-33 homozygous embryos barely stained for dUNC-45 in their body-wall muscles (Fig. 6), which contrasts with intense staining in the heterozygous T-33 and wild-type embryos. This confirms the zygotic expression of dUNC-45 at this stage, which coincides with muscle myosin expression. Interestingly, in the absence of zygotically expressed dUNC-45, skeletal muscle myosin was still expressed and the muscle patterning appeared normal (Fig. 6E). The muscle myosin produced at this time might be folded by the remnants of maternally inherited dUNC-45 or not folded appropriately. By contrast, western blot analysis of 22-hour-old, only 8 hours older, homozygous T-33 embryos showed a drastic decrease in muscle myosin (Fig. 5B). The body-wall muscles of 22-hour-old homozygous T-33 embryos exhibited a lack of sarcomeric structure, with only a few thick filaments visible in electron micrographs (Fig. 7C,D). Together, the data support the idea that the lack of dUNC-45 does not affect muscle myosin translation, but results in the accumulation of non-functional myosin that is later degraded, leading to embryonic lethality.
If dUNC-45 is not needed during myosin translation, then it must be needed post-translationally for proper myosin folding and/or stabilization. In vitro translation of chicken smooth muscle myosin in the presence of mouse UNC-45 also supports a post-translational association of the two proteins (Liu et al., 2008). However, a yeast UCS protein, Rng3, associates with myosin-encoding mRNA in polysomes in ribonucleoprotein immunoprecipitation–microarray experiments, which implies a co-translational involvement (Amorim and Mata, 2009).
Overall, we have demonstrated that dUNC-45 is essential for Drosophila skeletal muscle myosin stability, muscle function and viability. However, as a chaperone, UNC-45 could also function in muscle maintenance and protection during stress. Structural information (Lee et al., 2011) will greatly advance our knowledge about this protein by facilitating in vivo studies of specific UNC-45 site-directed mutants in Drosophila and other model organisms. Once the mechanism of action of UNC-45 is unraveled, it could serve as a therapeutic target for muscle and heart disease.
Materials and Methods
Fly lines
Drosophila yellow white (y, w) flies were used as a wild-type control line. The Tom34EY03034/TM3, Sb UNC-45 mutant was obtained from the Bloomington Stock Center. It was crossed to a balancer line containing MKRS, Sb/TM3, y+, Ser to take advantage of the y+ and the Serrate markers, which resulted in the generation of Tom34EY03034/TM3, y+, Ser. This line was used to create the dUNC-45 knockout T-33/TM3, y+, Ser line through P element mobilization by selecting for imperfect excision mutants with deletions in the genomic DNA regions of dunc-45 (Robertson et al., 1988). Briefly, virgin females of Tom34EY03034/TM3, y+, Ser were crossed with males of the balancer line w*; CyO/Sp; TM2, Ubx/Δ2–3, Sb containing the Δ2–3 transposase. The F1 male individuals with blotchy eye color, stubble bristles and curly wings (y, w; CyO/+; Tom34EY03034/Δ2–3, Sb) were selected and crossed with the yw; +; MKRS, Sb/TM3, y+, Ser balancer line to remove the transposase. The F2 male individuals with white eyes, wild-type cuticle coloration and serrated wings (y, w; +; Tom34EY03034Δ/TM3, y+, Ser) were selected and crossed with the MKRS, Sb/TM3, y+, Ser balancer line again to stabilize the line. A total of 23 potential hypomorphic dunc-45 jump-out lines were created and each was assessed by western blot analysis for dUNC-45 expression. Each line was placed in a Plexiglas box containing apple agar plates at 25°C to obtain fertilized eggs that produced homozygous 22-hour-old embryos, identified by yellow mouth hooks (y, w; +; Tom34EY03034Δ/Tom34EY03034Δ). Western blotting analysis identified the T-33 line as a dUNC-45 knockout line. PCR and DNA sequencing of T-33 genomic DNA showed a 1304 bp deletion starting from 5′-CTTCTCAATTTTTATTTATA-3′ to 5′-ACGAGAGTGCAATGGATATC-3′. Furthermore, a piece of the P element (5′-CATGATGAAATAACATCATGTTAGCCACAG-3′) was left behind.
To show that T-33 is an allele of Tom34EY03034, the T-33 line (y, w; T-33/TM3, y+, Ser) was crossed with the original Tom34EY03034 mutant line (Tom34EY03034/TM3, y+, Ser). All of the 100 offspring examined showed the balancer markers black body (y+) and serrated wings (Ser), indicating that T-33/Tom34EY03034 is not viable and the mutations are allelic.
To identify homozygous 14-hour-old T-33 embryos, the T-33/TM3, y+, Ser line was crossed with the TM3, lacZ-hg, Sb balancer (Gorman and Kaufman, 1995). The resulting line, T-33/TM3, lacZ-hg, Sb, with stubble bristles and normal wings was used to obtain fertilized eggs for immunofluorescence confocal microscopy. Homozygous T-33 embryos showed an absence of lacZ staining in the hind-gut.
Antibodies, sera, and fluorescent probes
Polyclonal dUNC-45 antibody was obtained through Sigma-Genosys (St Louis, MO) using the standard antibody service without affinity purification. Bacterially expressed recombinant His-tagged full-length dUNC-45 (Melkani et al., 2010) was used as the immunizing agent in two New Zealand white rabbits (GN-15577 and GN-15578). The pre-immune serum and third bleed serum from rabbit GN-15578 were used in this study. Rabbit anti-non-muscle myosin and rabbit anti-muscle myosin antibodies were kindly provided by Dan Kiehart, Duke University, Durham, NC (Kiehart and Feghali, 1986). Mouse anti-Drosophila-α-actinin antibody was a generous gift from Judith Saide, Boston University School of Medicine, Boston, MA. Other antibodies, sera and fluorescent probes were purchased from commercial sources: goat-anti-rabbit-HRP (Bio-Rad, Hercules, CA), goat-anti-rabbit-Cy5 (Chemicon, Temecula, CA), goat-anti-mouse-FITC (Sigma, St Louis, MO), mouse-anti-LacZ (Promega, Madison, WI), normal goat serum (Thermo Fisher, Waltham, MA) and phalloidin-TRITC (Sigma).
Protein electrophoresis and western blot analysis
Protein electrophoresis of Drosophila lysates and western blotting were performed as described (Laemmli, 1970; Sambrook and Russell, 2001) using a Bio-Rad Mini-Protean II minigel apparatus and precast 4% stacking, 10% separating acrylamide gels. For detection of dUNC-45 and/or muscle myosin in the various developmental stages and fly lines, specimens were collected and protein was extracted with Laemmli sample buffer (Bio-Rad). Insoluble debris was removed by centrifugation. Protein quantification was done using advanced protein assay reagent (Cytoskeleton, Denver, CO). Aliquots of 10 μg of protein were loaded for each sample. dUNC-45 antibody was diluted 1:1000 and myosin antibody was diluted 1:20,000. Each primary antibody was incubated with the membrane for at least 4 hours (maximum overnight) at 4°C. Secondary goat-anti-rabbit-horseradish peroxidase antibody was diluted 1:2000 and incubated with the membrane for 2 hours at room temperature.
Wild-type dunc-45 transgene construction and rescue
The wild-type genomic DNA sequence of dunc-45 was cloned into the P element vector pCaSpeR4 (Thummel and Pirrotta, 1992). It contains the mini-white gene, w+, as a selectable eye color marker to identify transformants. The dunc-45 transgene contains the sequence from 5′-CGTTATATCACATGAAAATT-3′ to 5′-AGAAACTTTCGGTTTCGGTT-3′, which includes 654 bp upstream of the transcription initiation site and 1154 bp downstream of the translation stop codon. The final plasmid construct pW-UNC was verified by restriction enzyme digest and DNA sequencing. DNA for injection was purified using a QIAfilter Plasmid Maxi Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The pW-UNC construct was inserted into the yw Drosophila background via P element-mediated germline transformation using previously established methods (Rubin and Spradling, 1982; Cripps et al., 1994). Red or orange eye color was used as an indication of the presence of both the w+ marker gene and the P[w+, dunc-45] transgene in the G1 generation.
To determine the chromosomal location of the inserted transgene, transformants were crossed with w1118; CyO/Bl1; TM2, Ubx/TM6B, Tb flies. We obtained five transgenic lines. Two transgenes were inserted into the second chromosome, two in the third chromosome and one in the fourth chromosome. Transgenic lines on the second and fourth chromosomes were used to rescue the dunc-45 mutant.
For rescue, adults heterozygous for the T-33 allele (y, w; +/+; T-33/TM3, y+, Ser) were crossed with flies homozygous for the wild-type dunc-45 transgene on the second chromosome (y, w; P[w+, dunc-45]/P[w+, dunc-45]; TM2/TM6). Offspring heterozygous for the mutant dunc-45 allele and for the transgene (y, w; P[w+, dunc-45]/+; T-33/TM6) were self-crossed. Adult offspring from this cross were screened for the presence of two copies of T-33 (absence of the balancer chromosome) in the presence of two copies of the dunc-45 transgene (y, w; P[w+, dunc-45]/P[w+, dunc-45]; T-33/T-33 with dark orange eye color). y, w; P[w+, dunc-45]/P[w+, dunc-45]; T-33/T-33 can be maintained as a stable line, indicating rescue of the T-33 allele using a wild-type dunc-45 transgene.
Immunofluorescence confocal microscopy
Whole mount embryo immunofluorescence confocal microscopy was carried out using the fixation protocol previously described (Kosman et al., 2004), but with 4% paraformaldehyde. After fixation, embryos were rehydrated using a series of brief washes in methanol–PBST (phosphate-buffered saline containing 0.1% Tween) solutions with increasing PBST concentration (5%, 15%, 25%, 50%, 70%) until 100% PBST was reached. The subsequent washes and incubations were all performed using PBST. Blocking of embryos was done using 5% normal goat serum in PBST at room temperature for 30 minutes. Primary antibodies were used at the following dilutions: anti-dUNC-45 1:500, anti-skeletal muscle myosin 1:1000, anti-non-muscle myosin 1:1000, anti-LacZ 1:200. Each primary antibody was incubated with the embryos for at least 4 hours (maximum overnight) at 4°C. Secondary goat-anti-rabbit-Cy5 antibody was diluted 1:500 and goat-anti-mouse-FITC 1:500, and incubated with the embryos for 2 hours at room temperature. Stained embryos were stored in PBS with 50% glycerol. Vectashield (Vector Laboratories, Burlingame, CA) was added before imaging using a Leica DM IRBE confocal microscope. Phalloidin-TRITC (1:500) was added with the secondary antibodies to stain filamentous actin.
Drosophila yw third instar larvae were dissected on Sylgard 184 Silicone elastomer (K. R. Anderson, Morgan Hill, CA). Fixation and staining were performed as previously described (Molina and Cripps, 2001). Primary antibodies were used at the following dilutions: anti-dUNC-45 1:500, anti-skeletal muscle myosin 1:1000, anti-α-actinin 1:500. Each primary antibody was incubated with the larvae for at least 4 hours (maximum overnight) at 4°C. Secondary goat-anti-rabbit-Cy5 or goat-anti-mouse-FITC antibodies were diluted 1:500 and incubated with the larvae for 2 hours at room temperature. Stained larvae were then visualized as for embryos.
Electron microscopy
22-hour-old embryos of yw, T-33 mutant, and T-33 mutant rescued with a dunc-45 transgene, were isolated and prepared for TEM as described (Cripps et al., 1999). Fixatives and Embed812 resin were from Electron Microscopy Sciences (Fort Washington, PA); other reagents were from Sigma. Samples were examined with a FEI Tecnai 12 transmission electron microscope operating at 80 kV. Digital images were taken with a TVIPS (Tietz) TemCam-F214 high-resolution digital camera.
Acknowledgments
This work was supported by Muscular Dystrophy Association Research Grant 3682 and NIH R01AR055958 to S.I.B., an American Heart Association (Western States Affiliate) postdoctoral fellowship to G.C.M., and NSF Equipment Grant DBI-030829. Deposited in PMC for release after 12 months.
References
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2195 [DOI] [PubMed] [Google Scholar]
- Amorim M. J., Mata J. (2009). Rng3, a member of the UCS family of myosin co-chaperones, associates with myosin heavy chains cotranslationally. EMBO Rep. 10, 186-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao W., Pilgrim D. (2000) Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J. Cell Biol. 148, 375-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson S. J., Stewart M. (1991). Expression in Escherichia coli of fragments of the coiled-coil rod domain of rabbit myosin: influence of different regions of the molecule on aggregation and paracrystal formation. J. Cell Sci. 99, 823-836 [DOI] [PubMed] [Google Scholar]
- Barral J. M., Bauer C. C., Ortiz I., Epstein H. F. (1998). unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 143, 1215-1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U., Epstein H. F. (2002). Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669-671 [DOI] [PubMed] [Google Scholar]
- Bernstein S. I., O'Donnell P. T., Cripps R. M. (1993). Molecular genetic analysis of muscle development, structure, and function in Drosophila. Int. Rev. Cytol. 143, 63-152 [DOI] [PubMed] [Google Scholar]
- Chow D., Srikakulam R., Chen Y., Winkelmann D. A. (2002). Folding of the striated muscle myosin motor domain. J. Biol. Chem. 277, 36799-36807 [DOI] [PubMed] [Google Scholar]
- Cripps R. M., Becker K. D., Mardahl M., Kronert W. A., Hodges D., Bernstein S. I. (1994). Transformation of Drosophila melanogaster with the wild-type myosin heavy-chain gene: rescue of mutant phenotypes and analysis of defects caused by overexpression. J. Cell Biol. 126, 689-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. M., Suggs J. A., Bernstein S. I. (1999). Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J. 18, 1793-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. F., Thomson J. N. (1974). Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature 250, 579-580 [DOI] [PubMed] [Google Scholar]
- Estrada B., Choe S. E., Gisselbrecht S. S., Michaud S., Raj L., Busser B. W., Halfon M. S., Church G. M., Michelson A. M. (2006). An integrated strategy for analyzing the unique developmental programs of different myoblast subtypes. PLoS Genet. 2, e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard C., Behra M., Fischer N., Hutcheson D., Geisler R., Strahle U. (2007). The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 308, 133-143 [DOI] [PubMed] [Google Scholar]
- Etard C., Roostalu U., Strahle U. (2008). Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol. 180, 1163-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth B. J., Goedecke M. C., Soldati D. (2006). New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA 103, 3681-3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geach T. J., Zimmerman L. B. (2010). Paralysis and delayed Z-disc formation in the Xenopus tropicalis unc45b mutant dicky ticker. BMC Dev. Biol. 10, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman M. J., Kaufman T. C. (1995). Genetic analysis of embryonic cis-acting regulatory elements of the Drosophila homeotic gene sex combs reduced. Genetics 140, 557-572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A. H., Landsverk M. L., Price M. G., Epstein H. F. (2002). The UCS family of myosin chaperones. J. Cell Sci. 115, 3983-3990 [DOI] [PubMed] [Google Scholar]
- Kachur T., Ao W., Berger J., Pilgrim D. (2004). Maternal UNC-45 is involved in cytokinesis and colocalizes with non-muscle myosin in the early Caenorhabditis elegans embryo. J. Cell Sci. 117, 5313-5321 [DOI] [PubMed] [Google Scholar]
- Kachur T. M., Pilgrim D. B. (2008). Myosin assembly, maintenance and degradation in muscle: Role of the chaperone UNC-45 in myosin thick filament dynamics. Int. J. Mol. Sci. 9, 1863-1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur T. M., Audhya A., Pilgrim D. B. (2008). UNC-45 is required for NMY-2 contractile function in early embryonic polarity establishment and germline cellularization in C. elegans. Dev. Biol. 314, 287-299 [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Feghali R. (1986). Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103, 1517-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lowe T., Hoppe T. (2008). Protein quality control gets muscle into shape. Trends Cell Biol. 18, 264-272 [DOI] [PubMed] [Google Scholar]
- Kosman D., Mizutani C. M., Lemons D., Cox W. G., McGinnis W., Bier E. (2004). Multiplex detection of RNA expression in Drosophila embryos. Science 305, 846 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685 [DOI] [PubMed] [Google Scholar]
- Landsverk M. L., Li S., Hutagalung A. H., Najafov A., Hoppe T., Barral J. M., Epstein H. F. (2007). The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J. Cell Biol. 177, 205-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. F., Hauenstein A. V., Fleming J. K., Gasper W. C., Engelke V., Sankaran B., Bernstein S. I., Huxford T. (2011). X-ray crystal structure of the UCS domain-containing UNC-45 myosin chaperone from Drosophila melanogaster. Structure (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Srikakulam R., Winkelmann D. A. (2008). Unc45 activates Hsp90-dependent folding of the myosin motor domain. J. Biol. Chem. 283, 13185-13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D. W., Vigoreaux J. O. (1999). An integrated view of insect flight muscle: genes, motor molecules, and motion. News Physiol. Sci. 14, 87-92 [DOI] [PubMed] [Google Scholar]
- Melkani G. C., Lee C. F., Cammarato A., Bernstein S. I. (2010). Drosophila UNC-45 prevents heat-induced aggregation of skeletal muscle myosin and facilitates refolding of citrate synthase. Biochem. Biophys. Res. Commun. 396, 317-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., D'souza V. M., Chang K. C., Huang Y., Balasubramanian M. K. (2005). Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot. Cell 4, 567-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M. R., Cripps R. M. (2001). Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 109, 51-59 [DOI] [PubMed] [Google Scholar]
- Price M. G., Landsverk M. L., Barral J. M., Epstein H. F. (2002). Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 115, 4013-4023 [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. (1988). A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118, 461-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. (1982). Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353 [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. (2001). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Srikakulam R., Winkelmann D. A. (1999). Myosin II folding is mediated by a molecular chaperonin. J. Biol. Chem. 274, 27265-27273 [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Straceski A. J., Leinwand L. A., Tikunov B. A., Faust L. (1994). Heterologous expression of a cardiomyopathic myosin that is defective in its actin interaction. J. Biol. Chem. 269, 1603-1605 [PubMed] [Google Scholar]
- Thummel C. S., Pirrotta V. (1992). Technical notes: new pCasper P-element vectors. Drosoph. Inf. Serv. 71, 150 [Google Scholar]
- Toi H., Fujimura-Kamada K., Irie K., Takai Y., Todo S., Tanaka K. (2003). She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol. Biol. Cell 14, 2237-2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M. (1994). Regulation of expressed truncated smooth muscle myosins. Role of the essential light chain and tail length. J. Biol. Chem. 269, 20819-20822 [PubMed] [Google Scholar]
- Venolia L., Waterston R. H. (1990). The unc-45 gene of Caenorhabditis elegans is an essential muscle-affecting gene with maternal expression. Genetics 126, 345-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigoreaux J. O. (2006). Nature's Versatile Engine: Insect Flight Muscle Inside and Out (Molecular Biology Intelligence Unit). New York: Springer Science+Business Media; [Google Scholar]
- Wesche S., Arnold M., Jansen R. P. (2003). The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr. Biol. 13, 715-724 [DOI] [PubMed] [Google Scholar]
- Willis M. S., Schisler J. C., Portbury A. L., Patterson C. (2009). Build it up-Tear it down: protein quality control in the cardiac sarcomere. Cardiovasc. Res. 81, 439-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth S. L., Crawford B. D., Pilgrim D. B. (2007). The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev. Biol. 303, 483-492 [DOI] [PubMed] [Google Scholar]
- Wong K. C., Naqvi N. I., Iino Y., Yamamoto M., Balasubramanian M. K. (2000). Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 113, 2421-2432 [DOI] [PubMed] [Google Scholar]
- Young P. E., Pesacreta T. C., Kiehart D. P. (1991). Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development 111, 1-14 [DOI] [PubMed] [Google Scholar]
- Yu Q., Bernstein S. I. (2003). UCS proteins: managing the myosin motor. Curr. Biol. 13, R525-R527 [DOI] [PubMed] [Google Scholar]