Abstract
Golgi-to-plasma-membrane trafficking of synaptic-like microvesicle (SLMV) proteins, vesicular acetylcholine transporter (VAChT) and synaptophysin (SYN), and a large dense-core vesicle (LDCV) protein, chromogranin A (CgA), was investigated in undifferentiated neuroendocrine PC12 cells. Live cell imaging and 20°C block–release experiments showed that VAChT–GFP, SYN–GFP and CgA–RFP specifically and transiently cohabitated in a distinct sorting compartment during cold block and then separated into synaptic protein transport vesicles (SPTVs) and LDCVs, after release from temperature block. We found that in this trans-Golgi subcompartment there was colocalization of SPTV and LDCV proteins, most significantly with VAMP4 and Golgin97, and to some degree with TGN46, but not at all with TGN38. Moreover, some SNAP25 and VAMP2, two subunits of the exocytic machinery, were also recruited onto this compartment. Thus, in neuroendocrine cells, synaptic vesicle and LDCV proteins converge briefly in a distinct trans-Golgi network subcompartment before sorting into SPTVs and LDCVs, ultimately for delivery to the plasma membrane. This specialized sorting compartment from which SPTVs and LDCVs bud might facilitate the acquisition of common exocytic machinery needed on the membranes of these vesicles.
Keywords: Synaptic vesicles, Large dense-core vesicles, PC12 cells, Protein trafficking
Introduction
Classical cholinergic neurons and neuroendocrine cells contain both synaptic and peptidergic vesicles. Synaptic vesicle proteins such as vesicular acetylcholine transporter (VAChT) and synaptophysin are sorted at the Golgi into specific transport (constitutive) vesicles for delivery to the active zone at the presynaptic or plasma membrane (PM), where they are recycled for the production of synaptic vesicles (De Camilli and Jahn, 1990; Kelly, 1991). Neuropeptides, however, are sorted to large dense-core vesicles (LDCVs), which are also delivered to the release site, but are not fused to the presynaptic membrane or PM under resting conditions (Gondré-Lewis et al., 2006; Park and Loh, 2008). Thus far, no clear distinction has been made between sorting compartments and transport routes from the Golgi complex to the presynaptic membrane or PM for synaptic vesicle and LDCV proteins. One study has reported that VAChT is sorted separately from LDCV proteins within the Golgi to constitutive vesicles (Liu and Edwards, 1997) in the PC12 neuroendocrine cell line, but the study could not distinguish clearly where and when synaptic vesicle versus LDCV proteins are segregated from each other before being sorted to their target vesicles. Four-dimensional (space and time) dissection of their segregation and sorting is necessary to understand how neurons and neuroendocrine cells orchestrate spatial and temporal resolution of sorting of synaptic vesicle and LDCV proteins to the cell periphery for regulated secretion at synapses or into the bloodstream.
VAChT is a presynaptically localized transmembrane protein that imports acetylcholine synthesized by choline acetyltransferase (ChAT) into synaptic vesicles for release at the synaptic cleft (Eiden, 1998; Ferguson et al., 2003). ChAT and VAChT are expressed in cholinergic neurons in the basal forebrain, hippocampus, hypothalamus, at neuromuscular junctions, and in other sympathetic and parasympathetic nerve terminals (Roghani et al., 1996; Weihe et al., 1996). Proper in situ expression of ChAT and VAChT, which is altered in Alzheimer's disease (Blusztajn and Berse, 2000), is necessary for efficient sympathetic and parasympathetic neurotransmission. Some cholinergic neurons also have LDCVs containing neuropeptides and the vesicular monoamine transporters (VMATs) that import monoamine into LDCVs (Schäfer et al., 1997) for later secretion at neurite terminals during neurophysiological functions.
Undifferentiated PC12 cells have synaptic-like microvesicles (SLMVs), which contain acetylcholine (Weihe et al., 1996), as well as LDCVs that contain monoamines and neuropeptides (Liu and Edwards, 1997). Because the content and mechanisms of biogenesis are similar in neuronal synaptic vesicles and SLMVs (Clift-O'Grady et al., 1990; Mundigl et al., 1993), the PC12 cell is a useful model system for studying how vesicular proteins are sorted to different types of vesicle. VMATs are used as tracking markers for LDCVs in comparison with VAChT in PC12 cells, to distinguish sorting routes between LDCV and SLMV proteins (Krantz et al., 2000; Liu and Edwards, 1997; Yao et al., 2004). Chromogranin A (CgA), a neuropeptide precursor, is also a good marker for LDCVs in PC12 cells (Kim et al., 2001), as well as for peptidergic vesicles in various neurons of the central and peripheral nervous systems (Schafer et al., 1994). Results on the sorting of SLMVs and LDCVs in PC12 cells have been extrapolated to sorting mechanism(s) in neurons.
It has become increasingly evident that there is co-sharing of the sorting routes taken by SLMV and LDCV proteins from the Golgi complex. A small amount of VAChT was found in LDCVs of PC12 cells (Liu and Edwards, 1997; Tao-Cheng and Eiden, 1998) and in neurons (Agoston and Whittaker, 1989). Although many groups have shown that VMATs and CgA are the primary proteins associated with LDCVs and not with SLMVs (Liu and Edwards, 1997; Tao-Cheng and Eiden, 1998; Weihe et al., 1996), the possibility exists that LDCV and SLMV proteins co-traffic through similar sorting compartments before segregation.
In this study, we have examined the sorting and trafficking of the SLMV proteins VAChT and synaptophysin, and the LDCV protein CgA in undifferentiated PC12 cells to determine where these proteins are sorted and when they are segregated into synaptic protein transport vesicles (SPTVs) and LDCVs for delivery to the PM. We adopted a ‘20°C temperature block’ methodology that is used to stop Golgi-to-PM trafficking of lumenal proteins (Griffiths et al., 1985; Kuliawat and Arvan, 1992; Simon et al., 1996). Cold temperature block at 20°C allows coat assembly and budding of post-Golgi vesicles whereas it inhibits membrane lipid mobilization required for vesicle fission, resulting in accumulation of secretory and membrane proteins at the trans-Golgi network (TGN) (Simon et al., 1996). Quantitative live cell imaging and 3D reconstructions show that VAChT, synaptophysin and CgA co-traffic for 15 minutes through a previously uncharacterized trans-Golgi subcompartment containing Golgin97 and TGN46, but not TGN38, before parting ways to SPTVs and LDCVs. Moreover, the trans-Golgi subcompartment recruits VAMP4, and some VAMP2 and SNAP25, the subunits of SNARE complex required for regulated secretion, suggesting that this compartment is the place where the vesicular membranes of SPTVs and LDCVs are equipped with similar exocytosis machinery. A similar compartment which synaptophysin and CgA cohabit transiently before segregating into SPTVs and LDCVs was also found in rat cortical neurons.
Results
The SLMV marker VAChT–GFP and the LDCV marker CgA–RFP partially colocalize in a Golgi subcompartment at steady state
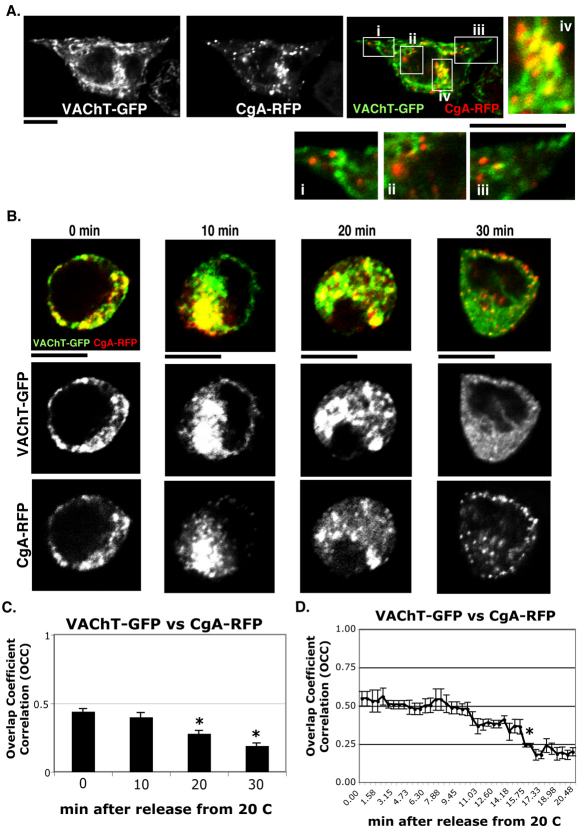
We investigated how SLMVs and LDCVs are distributed with respect to each other in steady state PC12 cells. GFP was tagged to the C-terminus of VAChT (VAChT–GFP) and monomeric RFP was C-terminally tagged to CgA (CgA–RFP) and they were transfected into undifferentiated PC12 cells, which are known to have both SLMVs and LDCVs (Greene and Rein, 1977; Greene and Tischler, 1976; Melega and Howard, 1981). We examined the distribution of VAChT–GFP and CgA–RFP 18 hours after transfection. VAChT–GFP did not show any colocalization with CgA–RFP at the cell periphery (Fig. 1A, inset i–iii). This suggests that VAChT and CgA do not co-exist in the same vesicles at the cell periphery, supporting previous findings that SLMV and LDCV proteins are not targeted to the same vesicles (Krantz et al., 2000; Liu and Edwards, 1997; Yao et al., 2004). However, we found that VAChT–GFP and CgA–RFP colocalized (Fig. 1A, inset iv) in some perinuclear areas of PC12 cells. The extent of colocalization between VAChT–GFP and CgA–RFP was measured by calculating the overlap coefficient correlation (OCC), which is determined by similarity of shapes or areas between two different color images, but not by intensity merge between them. The formula for calculating the OCC is described in the Materials and Methods. OCC yields lower numbers than the colocalization coefficient (CC), which depends on the intensities of overlapped area, whole region of interest and background. In our measurement of colocalization, an OCC of 0.4 is equivalent to a CC of 0.65, which is regarded as significant colocalization. Therefore, only OCC values higher than 0.25 (CC of 0.5) are considered evidence of significant colocalization. The average VAChT and CgA OCC values of all PC12 cells at steady state (i.e. at 37°C) was 0.14±0.02 (mean OCC ± s.e.m.), which indicates little colocalization throughout the cell, whereas yellow fluorescence at the peri-Golgi area indicates extensive colocalization in this area. This result suggests that VAChT–GFP and CgA–RFP co-exist in a specific compartment in the Golgi at steady state.
Fig. 1.
VAChT–GFP and CgA–RFP pass through a common intermediate compartment. (A) PC12 cells transfected with VAChT–GFP (green) and CgA–RFP (red) under steady state conditions at 37°C were imaged. The magnified insets i–iii and iv show VAChT–GFP and CgA–RFP at the cell periphery and at the cell center, respectively. (B) PC12 cells transfected with VAChT–GFP (green) and CgA–RFP (red) were incubated at 20°C to arrest Golgi-to-PM trafficking and then moved to 37°C to induce it. (C) Overlap coefficient correlations (OCCs) between VAChT–GFP and CgA–RFP were measured at 0, 10, 20, 30 minutes after 20°C block and release. The average OCC ± s.e.m. was calculated from three different experiments (n=30). (D) The real-time OCCs between VAChT–GFP and CgA–RFP for 20 minutes after 20°C block and release were measured (n=6 cells). Average OCC ± s.e.m. at every fifth shot from the beginning to the end of the time-lapse movie (supplementary material Movie 1) was picked and shown on the line graph (*P<0.05). Scale bars: 5 μm.
VAChT–GFP and CgA–RFP transiently colocalize in an intermediate compartment during 20°C block and release
To monitor the morphodynamics of trafficking of the secretory membrane proteins, VAChT–GFP and CgA–RFP, as they exit the Golgi complex, we synchronized the Golgi-to-PM vesicular traffic by incubating PC12 cells at 20°C for 30 hours following a 12 hour transfection with different vesicular markers. After the 30 hours, the cells were transferred to a 37°C incubator to induce Golgi-to-PM trafficking. First, we examined whether GFP-labeled GPI-anchored protein (GPI–GFP), a protein trafficked between the Golgi and the PM (Lippincott-Schwartz, 2004), was effectively accumulated in the TGN after cold temperature block. In the cells fixed immediately after incubation at 20°C, there was more GFP–GPI protein found in the p115-positive Golgi compartment compared with that in cells maintained at 37°C where the protein was mainly at the PM (supplementary material Fig. S1), indicating that our cold temperature block was effective in stopping exit of PM proteins from the Golgi.
After cells transfected with VAChT–GFP and CgA–RFP were subjected to cold temperature block, some cells were placed in fixative immediately to indicate time zero after 20°C block, whereas others were fixed after 10, 20 or 30 minutes at 37°C. We examined only cells with a moderate level of expression of both VAChT–GFP and CgA–RFP. The extent of colocalization between VAChT–GFP and CgA–RFP during early Golgi-to-PM trafficking after release from 20°C block was much higher than that at steady state (Fig. 1B). This suggests that the trafficking of VAChT–GFP and CgA–RFP was successfully synchronized to maximize the amount of VAChT–GFP and CgA–RFP passing through a common compartment during early Golgi-to-PM trafficking. However, the maximum colocalization was transient and maintained only until ~10 minutes after release from 20°C block. Then, the OCC values decreased and were significantly reduced by 30 minutes. The whole-cell OCC value between VAChT–GFP and CgA–RFP was 0.44±0.02 at 0 minutes and gradually decreased to 0.40±0.03, 0.28±0.02 and 0.19±0.02 at 10, 20 and 30 minutes, respectively, post release (Fig. 1C). Next, we imaged live cells to monitor this transient colocalization between VAChT–GFP and CgA–RFP and their subsequent trafficking. Our time-lapse movies showed that the merging between VAChT–GFP and CgA–RFP was maintained until ~15 minutes and gradually decreased thereafter (supplementary material Movie 1). OCC values were also calculated in real time. The OCC value (Fig. 1D) began at 0.47±0.04 at 0 time and ended at 0.22±0.04 after 30 minutes at 37°C. These values were similar to the OCC values of fixed cells (Fig. 1C). Based on the analysis of six live-cell movies, it is clear that the converging and diverging events between VAChT–GFP and CgA–RFP during the Golgi-to-PM trafficking are very dynamic processes. These findings suggest that VAChT–GFP and CgA–RFP pass through the common compartment during early Golgi-to-PM traffic before separating.
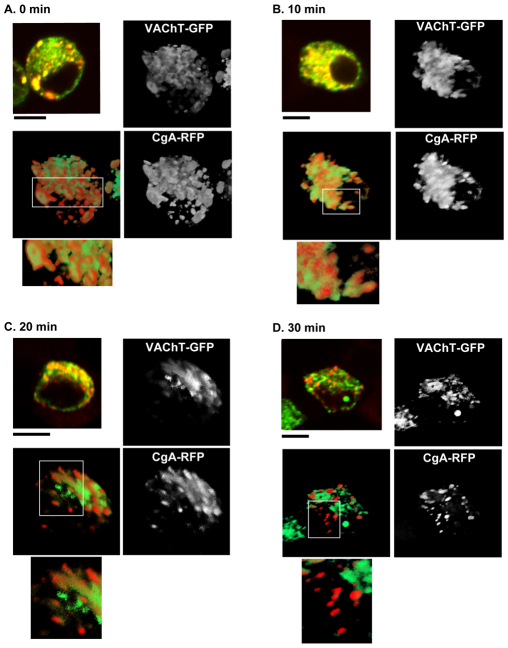
VAChT–GFP and CgA–RFP show transient colocalization in three dimensions
Positional overlap of two different markers through the z-axis can sometimes generate apparent colocalization in two-dimensional images even though two markers might not actually reside in the same compartment. Therefore, to obtain more accurate information about the colocalization between VAChT–GFP and CgA–RFP, we took a series of images through the z-axis of fixed cells (~4 μm thick) that expressed both VAChT–GFP and CgA–RFP, at an interval of 0.2 μm from the top to the bottom surface. The stacks of images were reconstructed to create 3D images. A typical 3D image of a cell incubated at 20°C showed that most of the CgA–RFP was accumulated in the compartment containing VAChT–GFP described above (Fig. 2A). However, some VAChT was distributed outside the compartment, suggesting that not all VAChT–GFP accumulated in the compartment upon 20°C block. At 10 minutes after release, the high levels of colocalization were maintained (Fig. 2B) whereas some CgA–RFP appeared to start budding from the compartment (Fig. 2B, inset). There were more elongated tubulovesicular buds of CgA–RFP at 20 minutes after release (Fig. 2C). At 30 minutes after release, CgA–RFP was accumulated in LDCVs that were free of VAChT–GFP (Fig. 2D) whereas a minimal level of colocalization between VAChT–GFP and CgA–RFP was still detected, similarly to that seen at steady state of a cell that has never been exposed to 20°C block. Our analysis of the colocalization profiles in three dimensions confirms that CgA–RFP merges with the VAChT–GFP after 20°C block and dynamically separate into distinct vesicular and tubulovesicular profiles 10 minutes after release.
Fig. 2.
Three-dimensional analysis of the colocalization between VAChT–GFP and CgA–RFP during Golgi-to-PM transport. The colocalization between VAChT–GFP and CgA–RFP was analyzed through the z-axis in cells expressing both proteins. The images taken through the z-axis were combined to generate a 3D image. The images show 3D colocalization between VAChT–GFP and CgA–RFP at 0 minutes (A), 10 minutes (B), 20 minutes (C) and 30 minutes (D). Inset: magnified merge. Confocal images were used to generate adjacent 3D images. Scale bars: 5 μm.
SYN–GFP traffics with VAChT–GFP and CgA–RFP
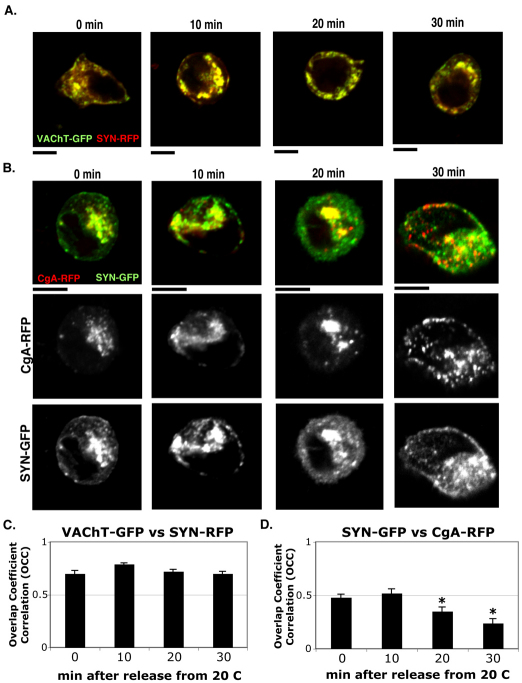
We tested another SLMV marker, synaptophysin, using the same experimental paradigm to determine whether its trafficking pattern is similar to VAChT–GFP relative to CgA–RFP. We used synaptophysin tagged C-terminally with either GFP or monomeric RFP (SYN–GFP or SYN–RFP). Both SYN–GFP and SYN–RFP transfected into PC12 cells cofractionated with endogenous SLMVs in velocity sucrose gradient (data not shown). First, we compared the distribution pattern of SYN–RFP with that of VAChT–GFP during 20°C block and release, and found that SYN–RFP and VAChT–GFP were constantly and significantly colocalized (Fig. 3A), yielding OCC values close to 0.8 (Fig. 3C). At 10 minutes, both SYN–RFP and VAChT–GFP were accumulated in an intermediate perinuclear compartment. Later, both were gradually sorted into smaller SPTVs and high OCC values (~0.8) were always maintained. These data indicate that SYN–RFP and VAChT–GFP pass through the same compartments and vesicles during Golgi-to-PM trafficking.
Fig. 3.
Syn–RFP co-migrates with VAChT–GFP and is passed through the CgA–RFP-containing compartment. (A) VAChT–GFP (green) and Syn–RFP (red) co-transfected into PC12 cells were tracked together through 20°C block and release. (B) Syn–GFP (green) and CgA–RFP (red) in PC12 cells were tracked together during 20°C block and release. (C) The OCCs between VAChT–GFP and SYN–RFP were measured at 0, 10, 20 and 30 minutes after release from 20°C block. (D) The OCCs between Syn–GFP and CgA–RFP throughout the temperature block and release were measured. The average OCC ± s.e.m. was calculated from three different experiments (n=30; *P<0.05, compared with the OCC at 10 minutes). Scale bars: 5 μm.
Next, we compared the trafficking of SYN–GFP with that of CgA–RFP. SYN–GFP partially merged with CgA–RFP after 20°C block and the extent of the merge was maintained until ~10 minutes after release from the block (Fig. 3B,D), yielding ~0.5 for the OCC value (Fig. 3D). At 20 minutes, the departure of CgA–RFP from SYN–GFP was more prominent and at 30 minutes, CgA–RFP was clearly observed to be separate from SYN–GFP. The timing of merging and diverging between SYN–GFP and CgA–RFP was very similar to that between VAChT–GFP and CgA–RFP (Fig. 1C,D). These data suggest that the trafficking pattern of SLMV proteins, SYN–GFP and VAChT–GFP, relative to CgA–RFP is representative of the endogenous SLMV trafficking pathway.
The time-dependent changes of colocalization between SYN–GFP and CgA–RFP were reconstructed in 3D (supplementary material Fig. S2). At 0 minutes, most CgA–RFP was accumulated in intermediate compartments containing SYN–GFP (supplementary material Fig. S2A). The convergence between SYN–GFP and CgA–RFP increased in 3D volume after 10 minutes of release (supplementary material Fig. S2B). At 20 minutes, CgA–RFP appeared to bud off from the common compartment (supplementary material Fig. S2C) and more separation between SYN–GFP and CgA–RFP was observed at 30 minutes (supplementary material Fig. S2D). Thus, the 3D analysis of colocalization between SYN–GFP and CgA–RFP confirmed that SYN–GFP transiently merges with and then diverges from CgA–RFP in a similar manner to VAChT–GFP.
SLMVs and LDCVs converge in a Golgin97-positive trans-Golgi subcompartment
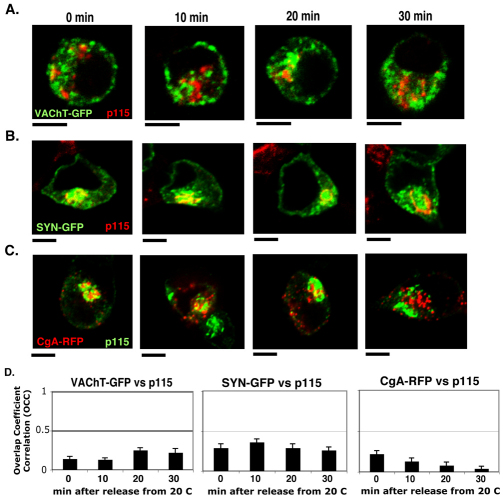
First, we examined the possibility that the incubation at 20°C disrupts the Golgi complex, thereby causing accumulation of vesicular proteins in a non-Golgi structure. Thus, we quantitatively compared Golgi morphology at 37°C and 20°C. The comparison showed that there was no disruptive effect on Golgi structure when cells were incubated at 20°C (supplementary material Fig. S3). Given that 20°C block should prevent the exit of secretory and PM proteins from the Golgi compartments (Simon et al., 1996), we expected that VAChT–GFP, SYN–GFP and CgA–RFP would accumulate in one of the Golgi compartments, such as the TGN. We fixed cells expressing those constructs at different time points after release from the 20°C block before immunolabeling them with the antibodies against p115, a marker for cis- and medial-Golgi cisternae. VAChT–GFP, SYN–GFP and CgA–RFP did not accumulate in the p115-containing compartment, but rather in the area around the cis- and medial-Golgi cisternae (Fig. 4A–C). After 20 minutes, most CgA had diffused away from the peri-p115 area. VAChT–GFP and CgA–RFP did not show significant levels of colocalization with p115 during cold block and release (Fig. 4D, OCCVAChT–GFP=0.19±0.06, OCCCgA–RFP=0.12±0.04, OCCSYN–GFP= 0.3±0.02), whereas a small pool of SYN–GFP appeared to associate with the cis and medial Golgi. Therefore, the SPTV and LDCV proteins seem to have accumulated in a p115-negative compartment with similarity to that described by Lippincott-Schwartz (Lippincott-Schwartz, 2004).
Fig. 4.
VAChT–GFP, SYN–GFP and CgA–RFP are not accumulated in p115-containing cis–medial Golgi cisternae after 20°C block and release. The cis and medial Golgi (red) was visualized with a monoclonal antibody against p115 and compared with VAChT–GFP (A), SYN–GFP (B) and CgA–RFP (C). (D) The average OCC ± s.e.m. of VAChT–GFP, SYN–GFP and CgA–RFP with respect to p115 was calculated from three different experiments (n=30). Scale bars: 5 μm.
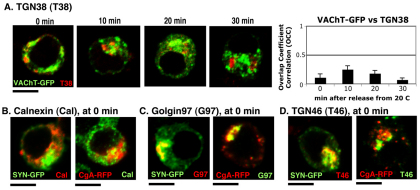
To further characterize this compartment, we examined whether VAChT–GFP was accumulated in a TGN38-containing trans- Golgi compartment upon 20°C block, but found that VAChT–GFP was neither accumulated in, nor even passed through the TGN38 compartment during 20°C block and release (Fig. 5A; OCCVAChT–GFP for 30 minutes=0.15±0.04). Consistent with other studies using the 20°C block strategy, we confirmed that none of the proteins accumulated in the endoplasmic reticulum (ER) by measuring colocalization with the ER marker, calnexin (Fig. 5B; OCCSYN–GFP=0.06±0.01 and OCCCgA–RFP=0.04±0.02). Given that the TGN is heterogeneous with respect to its lipid and protein composition, we hypothesized that there might be a non-TGN38 trans-Golgi subcompartment where VAChT–GFP, SYN–GFP and CgA–RFP were accumulated upon 20°C block. We labeled cells expressing SYN–GFP and CgA–RFP with antibodies against Golgin97 and TGN46, which are other well-characterized TGN markers. Both SYN–GFP and CgA–RFP colocalized with Golgin97 (OCCSYN–GFP=0.36±0.04 and OCCCgA–RFP=0.34±0.05), whereas SYN–GFP, but not CgA showed significant colocalization with TGN46 (OCCSYN–GFP=0.32±0.04 and OCCCgA–RFP=0.19±0.04) (Fig. 5C,D). Thus, the Golgin97-positive trans-Golgi subcompartment is the place where most of SYN–GFP and CgA–RFP is accumulated upon 20°C block, whereas the TGN46-positive trans-Golgi subcompartment appears to retain SYN–GFP and VAChT–GFP, but not CgA–RFP.
Fig. 5.
SPTV and LDCV proteins are accumulated in a TGN subcompartment that contains Golgin97 and some TGN46, but no TGN38 or calnexin (ER) after 20°C block. (A) The location of the trans-Golgi marker TGN38 (red) tagged with HA visualized by anti-HA polyclonal antibody was compared with that of VAChT–GFP during temperature block and release. The average OCC ± s.e.m. of VAChT–GFP with respect to TfnR was calculated from three different experiments (n=30). SYN–GFP- or CgA–RFP-expressing cells were incubated at 20°C and labeled with primary antibodies against calnexin (ER) (B), Golgin97 (TGN) (C) and TGN46 (D). Scale bars: 5 μm.
A subpopulation of synaptic vesicle proteins remains associated with recycling endosomes during 20°C block and release
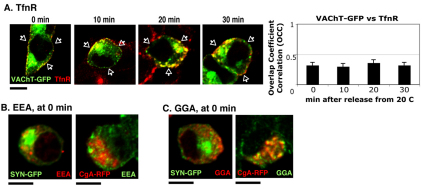
Recycling endosomes located close to the PM are known to house some of the SLMV proteins recycled from the PM even at temperatures as low as 18°C (Desnos et al., 1995; Schmidt et al., 1997). Generation of SLMVs by endocytosis from the PM has been demonstrated by tracking VAMP (Desnos et al., 1995) and synaptophysin (Schmidt et al., 1997) trafficking, and by assaying for recycling endosomes containing transferrin receptor (Desnos et al., 1995; Grote and Kelly, 1996; Lichtenstein et al., 1998; Wiedenmann and Franke, 1985). These studies showed that mature SLMVs come from recycling endosomes, and are not necessarily directly derived from the Golgi. Therefore, we hypothesized that some of the VAChT–GFP arriving at the PM before 20°C block might be present in the recycling endosomes. The recycling endosomes were visualized with antibodies against transferrin receptor (TfnR). The majority of the TfnR-labeled recycling endosomes highly overlapped with VAChT–GFP from the beginning (time 0) to the end of the 30 minute incubation at 37°C (Fig. 6A) yielding OCC values that were consistently above 0.4. In addition to recycling endosomes just beneath the PM, a trace amount of TfnR was found inside the cell that did not significantly overlap with VAChT–GFP (Fig. 6A). These results suggest that the pool of VAChT–GFP that is TfnR positive was neither mobilized by 20°C block and release, nor re-routed to the newly described sorting Golgin97-positive TGN, but rather remained in the recycling endosome pool apposed to the PM. The time-dependent changes of distribution of SYN–GFP and CgA–RFP with respect to the TfnR-positive recycling endosomes were also examined (supplementary material Fig. S4). As with VAChT, a subpopulation of SYN–GFP remained within the recycling endosomes during 20°C block and release (supplementary material Fig. S4A), yielding an OCC range of 0.38–0.50 with TfnR (supplementary material Fig. S4C), whereas the other SYN–GFP pool remained centrally in the cell. By contrast, CgA–RFP never contacted the TfnR-positive recycling endosomes (supplementary material Fig. S4B) as evidenced by OCC values of CgA–RFP with TfnR lower than 0.1 at any time point (supplementary material Fig. S4D). This indicates that CgA–RFP is never routed to the recycling endosomes.
Fig. 6.
A pool of SPTV proteins, but not LDCV proteins, remains unchanged in recycling endosomes during 20°C block and release. (A) The recycling endosomes were marked with the monoclonal antibody against transferrin receptor (TfnR, red) in cells expressing VAChT–GFP during 20°C block and release. The average OCC ± s.e.m. of VAChT–GFP with respect to TfnR was calculated from three different experiments (n=30). Note that cells of interest are indicated by arrowheads. SYN–GFP- or CgA–RFP-expressing cells were incubated at 20°C and labeled with primary antibodies against EEA1 (EEA, early endosomes) (B) and GGA (C). Scale bars: 5 μm.
In addition to TfnR, possible colocalization of SYN–GFP and CgA–RFP with EEA1-containing early endosomes after 20°C block was examined. This analysis yielded an OCCSYN–GFP=0.20±0.02 and OCCCgA–RFP=0.04±0.01 for SYN–GFP and CgA–RFP relative to EEA1, respectively, suggesting that some SYN–GFP, but no CgA reached EEA1-positive early endosomes during 20°C block (Fig. 6B). We then examined whether adaptor proteins involved in clathrin-dependent endocytosis are recruited to the recycling endosomes using antibodies against adaptor protein-2 (AP-2) or Golgi-associated γ-adaptin ADP-ribosylation factor binding protein (GGA). Anti-AP2 antibodies yielded poor immunostaining. However, using anti-GGA antibody, we found that GGA did not colocalize with SYN–GFP, whereas it appeared to surround CgA–RFP-positive compartments (Fig. 6B; OCCSYN–GFP=0.09±0.04 and OCCCgA–RFP=0.20±0.04).
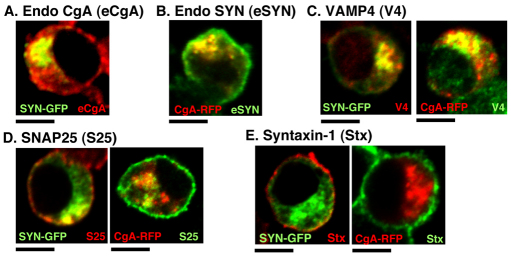
The trans-Golgi subcompartment containing SYN–GFP and CgA–RFP recruits VAMP4 and some SNAP25 and VAMP2
Given that both SLMVs and LDCVs share similar exocytosis machinery (De Camilli and Jahn, 1990; Eaton et al., 2000; Kelly, 1991; Kim et al., 2006), it is possible that this newly identified TGN subcompartment where both synaptic and LDCV proteins are accumulated upon 20°C block might recruit SNARE proteins such as VAMP2, VAMP4, SNAP25 and syntaxin-1, which are involved in exocytosis. We examined this possibility after confirming that the TGN subcompartment when transfected with SYN–GFP, can recruit endogenous CgA and conversely that CgA–RFP-containing TGN can recruit endogenous synaptophysin (Fig. 7A,B; OCCSYN–GFP and OCCCgA–RFP reached 0.57 and 0.64, respectively). Among the SNARE subunits, VAMP4, a vesicular SNARE protein (v-SNARE), showed the highest levels of colocalization with both SYN–GFP and CgA–RFP (Fig. 7C; OCCSYN–GFP=0.56±0.04 and OCCCgA–RFP=0.41±0.05). VAMP2 was also found in the new TGN subcompartment after 20°C block but showed large variations in the degree of its colocalization within the subcompartment in different cells (data not shown). SNAP25 partly colocalized with SYN–GFP (OCC=0.20±0.05) but not with CgA–RFP (OCC=0.06±0.02) (Fig. 7D). However, the t-SNARE syntaxin-1, which aligned along the PM, colocalized with neither SYN–GFP nor CgA–RFP (Fig. 7E). Based on these results, we deduce that the TGN subcompartment in which synaptic and LDCV proteins cohabit occurs before post-Golgi trafficking and recruits SNARE proteins to equip both SPTVs and LDCVs with similar exocytosis machinery for regulated secretion.
Fig. 7.
The TGN subcompartment containing SYN–GFP and CgA–RFP recruits the subunits of SNARE complex after 20°C block. (A) SYN–GFP-expressing cells were incubated at 20°C and labeled with antibody against endogenous CgA (eCgA). (B) CgA–RFP-expressing cells incubated at 20°C are labeled with antibody against endogenous synaptophysin (eSYN). Cells expressing either SYN–GFP or CgA–RFP are incubated at 20°C and labeled with primary antibodies against VAMP4 (C), SNAP25 (D) or syntaxin-1 (E). Scale bars: 5 μm.
Both VAChT–GFP and SYN–GFP exhibit partial colocalization with CgA–RFP at steady state
Thus far, we have demonstrated that there is a newly described trans-Golgi subcompartment where the colocalization of CgA–RFP with VAChT–GFP and SYN–GFP is prominent after 20°C block and can be maintained up to 15 minutes after release. The larger VAChT–GFP and CgA–RFP colocalization subcompartment observed after 20°C block is probably an expanded form of the small subcompartment seen at steady state where VAChT–GFP and CgA–RFP colocalized (Fig. 1A). The steady state colocalization between VAChT–GFP and CgA–RFP was confirmed by 3D reconstitution (supplementary material Fig. S5A). We also examined the steady state colocalization between VAChT–GFP and SYN–RFP; and between SYN–GFP and CgA–RFP in parallel. The 3D image showed an overall colocalization between VAChT–GFP and SYN–RFP throughout the cell (supplementary material Fig. S5B). Conversely, the cells expressing SYN–GFP and CgA–RFP only showed partial overlap in the cytoplasm (supplementary material Fig. S5C). In colocalization analysis, VAChT–GFP and SYN–RFP extensively overlapped in the same compartment whereas VAChT–GFP versus CgA–RFP and SYN–GFP versus CgA–RFP showed only partial colocalization (supplementary material Fig. S5D). This suggests that in this special sorting subcompartment VAChT–GFP, SYN–GFP and CgA–RFP are co-sorted transiently, even at steady state.
We compared the steady state distribution of VAChT–GFP, SYN–GFP and CgA–RFP with that of the cis–medial Golgi (p115). Both VAChT–GFP and SYN–GFP partially overlapped with the Golgi (supplementary material Fig. S6A,B), yielding OCC values of 0.27±0.05 and 0.32±0.06, respectively (supplementary material Fig. S6G), whereas CgA–RFP never showed any overlap with the Golgi marker (OCC=0.02±0.02; supplementary material Fig. S6C,G). We also compared their steady state distribution with the recycling endosomes using TfnR as a marker. Both VAChT–GFP and SYN–GFP were highly enriched in the recycling endosomes (supplementary material Fig. S6D,E) as they were after 20°C block and release, suggesting that the population of VAChT–GFP and SYN–GFP in the recycling endosomes is constantly refilled at 37°C and 20°C and distinct from the population in the sorting subcompartment. The OCC values of VAChT–GFP or SYN–GFP with TfnR were 0.37±0.06 and 0.46±0.06, respectively (supplementary material Fig. S6H). By contrast, no CgA–RFP at steady state was found in the TfnR-containing compartment (supplementary material Fig. S6F), yielding OCC values close to 0.03 (supplementary material Fig. S6H). This indicates that CgA–RFP is never recycled or trafficked to the TfnR-positive recycling endosomes at either 37°C or 20°C.
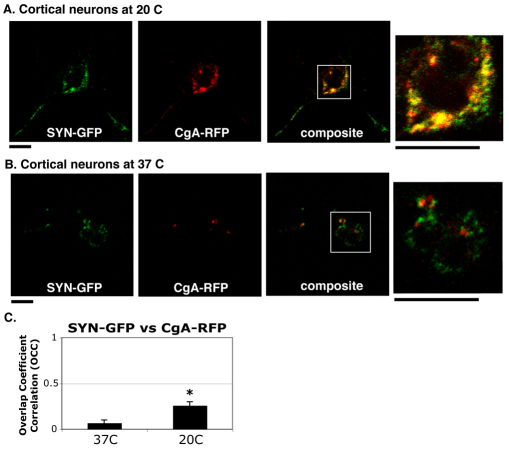
SYN–GFP partially colocalizes with CgA–RFP in rat cortical neurons after cold temperature block
Given that CgA also exists in cortical neurons (Adams et al., 1993), the distinct sorting compartment where CgA and synaptic vesicle protein cohabit in PC12 cells is expected to also exist in these neurons. To test this possibility, E18 primary cortical neurons were transfected with SYN–GFP and CgA–RFP and then incubated at 20°C or 37°C before being fixed for colocalization analysis. SYN–GFP was found in small compartments where CgA–RFP accumulated in cortical neurons after cold block (Fig. 8A). Conversely, little to no colocalization between SYN–GFP and CgA–RFP was observed at 37°C (Fig. 8B). The average OCC value of ~0.26 (CC=~0.5) at 20°C was much higher than ~0.07 at 37°C (Fig. 8C). Thus, there appears to be a neuronal compartment in primary cortical neurons where synaptic vesicle and LDCV proteins cohabit transiently, which is similar to those in PC12 cells.
Fig. 8.
Cortical neurons show colocalization of SYN–GFP and CGA–RFP after cold temperature block at 20°C. (A) After cold temperature block, SYN–GFP and CGA–RFP colocalize in the membranous compartment of cortical neurons. (B) The colocalization of SYN–GFP and CGA–RFP is not detectable at steady state at 37°C. (C) The average OCC between SYN–GFP and CGA–RFP in cortical neurons is higher at 20°C than at 37°C (*P<0.05). Scale bars: 5 μm.
Discussion
Peptidergic neurons and neuroendocrine cells simultaneously operate two regulated secretory systems comprising synaptic vesicles and LDCVs to respond to different physiological requirements. Synaptic vesicles (and SLMVs in PC12 cells) package and release neurotransmitters such as acetylcholine, γ-aminobutyric acid and glutamate, to mediate crosstalk between neurons (Blusztajn and Berse, 2000; Parsons et al., 1993; Vizi et al., 1989; Weihe et al., 1996) and between neuroendocrine cells (Bauerfeind et al., 1993), whereas LDCVs secrete monoamines and various neuropeptides for neurotransmission and to modulate neurophysiological homeostasis. Nonetheless, it is thought that synaptic vesicles and SLMVs share many components (e.g. synaptotagmin and VAMP2) of the exocytosis machinery with LDCVs (De Camilli and Jahn, 1990; Eaton et al., 2000; Kelly, 1991; Kim et al., 2006). The commonality in the usage of exocytosis machinery components suggests a shared sorting compartment where SLMVs and LDCVs converge and acquire similar proteins necessary for exocytosis. In this study, we report the existence of such a compartment. We discovered a previously uncharacterized TGN subcompartment where SLMV and LDCV proteins cohabit transiently during transport from the Golgi to the PM. The TGN subcompartment contains Golgin97, some TGN46, but little TGN38, and recruits VAMP4, and some VAMP2 and SNAP25, subsequently. We propose that this compartment is the final sorting compartment to generate SPTVs and LDCVs, to allow more efficient acquisition of their common exocytosis machinery.
Cold temperature block has been used to stop the exit of secretory and transmembrane proteins from the ER at 15°C (Milgram and Mains, 1994) and the Golgi at 20°C in different cell lines (Griffiths et al., 1985; Kuliawat and Arvan, 1992; Milgram and Mains, 1994; Simon et al., 1996). Incubation of cells at 20°C has been reported to have minimal effect on the rate of protein synthesis (Griffiths et al., 1985) and clathrin coat formation for vesicle budding (Simon et al., 1996), but has significant effects on retarding the mobility and metabolism of membrane lipids required for generation of membrane curvature and fission, resulting in co-accumulation of secretory and Golgi resident proteins at the TGN (Simon et al., 1996).
In our studies, cold temperature block of PC12 cells resulted in the accumulation at the Golgi of a GFP-tagged GPI-anchored protein, which traffics between the Golgi and the PM (Lippincott-Schwartz, 2004). By contrast, SYN–GFP, a SPTV marker that co-migrates with VAChT–GFP during post-Golgi transport, showed high levels of colocalization with CgA–RFP at 20°C in a TGN subcompartment containing Golgin97 and some TGN46, but little TGN38 and p115 (cis–medial Golgi). From these results, we conclude that this Golgin97-positive TGN subcompartment is where SPTV and LDCV proteins transiently cohabit for more refined sorting of these proteins into separate vesicles. In cortical neurons, which are known to have synaptic and peptidergic vesicles, as well as a regulated secretory pathway, we found a TGN subcompartment that was similar to that in PC12 chromaffin cells.
SLMV-targeted proteins, such as VAChT and synaptophysin, are constantly transported to the PM in SPTVs and then recycled directly to SLMVs, or to the recycling endosomes where SLMVs are generated (Desnos et al., 1995; Grote and Kelly, 1996; Lichtenstein et al., 1998; Wiedenmann and Franke, 1985). Consistent with the latter, we observed a pool of SYN–GFP and VAChT–GFP, which stayed in the TfnR-positive recycling endosomes, and was not significantly affected by 20°C block. By contrast, LDCV proteins such as the neuropeptide precursor, chromogranin A (CgA), are sorted and packaged at the inner face of the TGN at cholesterol- and sphingolipid-rich membrane microdomains that bud off to form LDCVs (Dhanvantari and Loh, 2000; Gondré-Lewis et al., 2006; Kim et al., 2006). These LDCVs are transported to secretion sites and stored until stimulated release, without recycling at the PM to recycling endosomes. Since SLMVs (or SVs) are separated from LDCVs in density gradient fractionation (Liu and Edwards, 1997; Yao et al., 2004), it is thought that SLMVs (or SVs) and LDCVs do not cross each other's traffic route. However, under normal conditions, some LDCV proteins are found in SLMVs (or SVs) and vice versa, in both neuroendocrine cells (De Camilli and Jahn, 1990; Kelly, 1991; Liu and Edwards, 1997; Yao et al., 2004) and neurons (Agoston and Whittaker, 1989; Nirenberg et al., 1997; Nirenberg et al., 1995), suggesting that the idea of complete separation of their sorting and trafficking routes might not be entirely accurate.
Indeed, our studies show that even at steady state, there exists a compartment where VAChT–GFP and CgA–RFP colocalize adjacent to the Golgi (Fig. 1A). This compartment might be similar to structures described in previous studies as large immature granules or immobile vesicular compartments in the cell soma (Arvan and Castle, 1998; Rudolf et al., 2001; Santos et al., 2001; Thoidis and Kandror, 2001). However, an additional function of those structures as a sorting compartment where synaptic vesicle and LDCV proteins converge was never attributed to them. Using a temperature block at 20°C and release at 37°C, we were able to clearly visualize induced accumulation and flow of VAChT–GFP and CgA–RFP through this compartment. The OCC values and three-dimensional reconstitution of VAChT–GFP or SYN–GFP and CgA–RFP after temperature block and release demonstrate that a significant amount of VAChT–GFP or SYN–GFP and CgA–RFP pass through this sub-TGN sorting compartment for about 15 minutes after release from 20°C block. Later, VAChT–GFP or SYN–GFP and CgA–RFP are split into SPTVs and LDCVs, respectively. We tried to separate this intermediate compartment from other floating membranous compartments, such as PM and cis- and medial-Golgi cisternae, by subcellular fractionation, but could not (data not shown), suggesting that it has similar buoyancy to those cell components.
Why would the cell sort synaptic and LDCV proteins into a common compartment? Synaptic vesicles, SLMVs and LDCVs appear to use a similar set of exocytic machinery proteins (e.g. SNAP25, VAMP proteins, etc.) for regulated exocytosis. For example, VAMP2/synaptobrevin2, an exocytic machinery protein in SLMVs or synaptic vesicles (Pennuto et al., 2003) and LDCVs (Eaton et al., 2000; Kim et al., 2006; Nevins and Thurmond, 2005) mediates exocytosis for both. Indeed, we found that the Golgin97-positive TGN subcompartment where both SPTV and LDCV proteins transiently cohabit contained a significant amount of VAMP4. VAMP2 also appears to surround the TGN subcompartment, although the extent of its colocalization with the compartment varied considerably in different cells. SNAP25 was recruited to SPTVs later, but not so much to LDCVs. This observation substantiates our idea that the newly found Golgin97 TGN subcompartment is the additional sorting station where a similar set of exocytosis machinery proteins are conferred on the surface of SLMVs and LDCVs. Mechanisms have been described in endocrine cells for budding off of ‘immature’ LDCVs from the TGN at cholesterol- and sphingolipid-rich membrane microdomains (‘lipid rafts’), which might also be enriched with components of the exocytosis machinery (Dhanvantari and Loh, 2000; Zhang et al., 2003). To overcome the great heterogeneity of proteins associated with the Golgi that renders it more difficult for SPTVs to specifically obtain a similar set of vesicular membrane proteins as LDCVs, we propose that both SPTV and LDCV proteins are co-accumulated in this TGN subcompartment, which recruits SNARE machinery subunits simultaneously at its cytoplasmic surface.
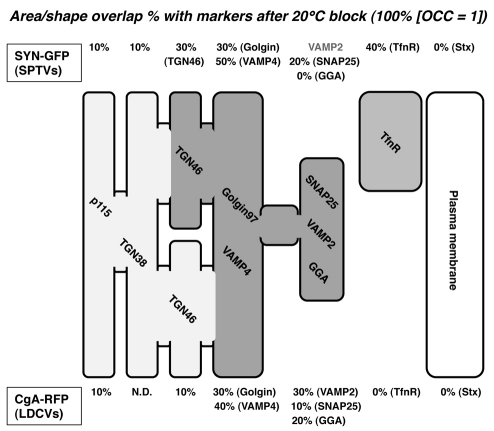
We provide a model of the intracellular distribution of SPTV and LDCV proteins in undifferentiated PC12 cells after 20°C block (Fig. 9). We estimate the percentage of intracellular partition of SPTV and LDCV proteins after 20°C block based on the principle of OCC values that are determined by the extent of similarity of shapes and areas overlapped by two different markers. At 20°C, SPTV proteins appear to reside in two major compartments: the VAMP4-positive TGN subcompartment (~50%) and TfnR-containing recycling endosomes (~40%). The VAMP4-positive TGN subcompartment shares SPTV proteins with the compartments containing TGN46 (~30%), Golgin97 (~30%), SNAP25 (~20%) and some VAMP2. The TfnR-positive recycling endosomes appear to contain a pool of SPTV proteins in early endosomes (~20%). However, the majority of LDCV proteins appear to accumulate in the VAMP4 (~40%)-positive TGN subcompartments, which partly overlap with those containing Golgin97 (~30%), SNAP25 (~10%), GGA (~20%) and VAMP2. No LDCV proteins associated with the TfnR recycling endosomes. The residual population of LDCV proteins might be scattered throughout other Golgi compartments (p115, TGN38, TGN46). Based on the highest OCC values shown by both SYN–GFP and CgA–RFP, the Golgin97-positive TGN subcompartment is where SPTV and LDCV proteins are co-sorted for acquisition of SNARE subunits for the regulated exocytosis.
Fig. 9.
Model of intracellular partitioning of SPTV and LDCV proteins after 20°C block. Percentage overlap of SPTV or LDCV markers within each compartment was estimated from the OCC values that were determined by the area and shape superimposed by two different markers. For example, the OCC=0.3 is converted to 30% overlap. SYN–GFP, a SPTV marker, appears to be divided into central and peripheral populations. The central SYN–GFP population is distributed within the Golgi compartment with overlap with various markers as follows: p115 (10%), TGN38 (VAChT–GFP-based, 10%), TGN46 (30%), Golgin97 (Golgin, 30%), VAMP4 (50%) SNAP25 (20%) and an undefined amount of VAMP2. 40% of the peripheral SYN–GFP overlaps with endosomes containing TfnR (REs: 40%). Conversely, most CgA–RFP, a LDCV marker, is located centrally in the Golgi compartment with overlap with various markers as follows: p115 (10%), TGN38 (not determined: N.D.), TGN46 (10%), Golgin97 (30%), VAMP4 (40%), VAMP2 (30%), GGA (20%) and SNAP25 (10%). Neither SYN–GFP nor CgA–RFP is associated with syntaxin-1 (Stx)-labeled plasma membrane. None of the CgA–RFP has contact with recycling endosomes. The VAMP4- and Golgin97-positive TGN subcompartment (dark shaded area) appears to harbor the majority of SPTV and LDCV proteins after 20°C block.
In conclusion, we show that in undifferentiated PC12 cells, SLMV and LDCV proteins are initially sorted and move together into a previously uncharacterized Golgin97 trans-Golgi subcompartment where they are later packaged separately into SPTVs and LDCVs. Unknown factors might contribute to the separation, which is worthy of future study. Our study does not support co-packaging of SLMV or synaptic vesicle proteins into LDCVs for delivery to the cell surface. Sorting of SLMV (or SV) and LDCV proteins into this newly identified trans-Golgi subcompartment before parting ways allows for an economic and effective means for SPTVs and LDCVs to acquire some exocytosis machinery, such as VAMP4, VAMP2 and SNAP25. A similar route exists in cortical neurons. This implies a role for this compartment in CNS neuronal function.
Materials and Methods
DNA constructs and antibodies
VAChT–GFP, synaptophysin–GFP (Syn–GFP), and synaptophysin–RFP (Syn–RFP) were given by Zu-Hwang Sheng (NINDS, NIH). CgA–RFP was made in our laboratory. HA-tagged TGN38 (HA-TGN38) GPI-anchored protein–GFP and GalT–GFP were from Jennifer Lippincott-Schwartz (NICHD, NIH). The mouse antibodies against p115, SNAP25 and transferrin receptor were obtained from BD Bioscience (San Diego, CA). The rabbit antibodies against HA tag, calnexin, GGA and EEA1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibody against CgA (HL6013) was made in our laboratory. The rabbit antibodies against VAMP4, Golgin97, GGA, mouse antibodies against syntaxin-1, synaptophysin, TGN46, and chicken antibodies against VAMP2 were purchased from AbCam (Cambridge, MA).
Cell culture, transfection and immunocytochemistry
PC12 cells were grown in DMEM medium (GIBCO-BRL, Life Technologies, Grand Island, NY) supplemented with 10% FBS (GIBCO-BRL) and 5% horse serum (GIBCO-BRL). 2×104 cells were seeded on 25×25 mm coverslips in 35 mm dishes or six-well plates and grown for 18 hours in growth medium before subjecting them to transfection and immunocytochemistry procedures. Transfection of DNA constructs was performed using Lipofectamine™ 2000 according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). DNA (4 μg) plus lipofectamine (10 μl) in OPTI-MEM I (GIBCO-BRL) and 1.5 ml DMEM medium were added to the cells, followed by incubation for 18 hours.
For 20°C block and release, PC12 cells grown on coverslips in six-well plates were transfected with DNA constructs and incubated at 37°C and 5% CO2, 95% air for 12 hours to allow active protein synthesis. Cells were incubated at 20°C, 5% CO2, 95% air for 20°C block of protein trafficking. After 30 hours, cells on one six-well plate were fixed immediately (0 minutes after release from 20°C block). The other plates were incubated at 37°C for 10, 20, 30 minutes before fixation, equivalent to 10, 20, 30 minutes after release from cold (20°C) block. We minimized the time for transfer of cells from 20°C to 37°C by placing both 20°C and 37°C incubators close together.
E18 rat cortical neurons were purchased from GenLantis (San Diego, CA) and transfected with 8 μg of SYN–GFP and CgA–RFP (4 μg each) using Amaxa Nucleofector (program: O-003). The neurons were incubated in 10% FBS plus DMEM for 2 days for recovery and in B27 neurobasal medium (Genlantis, San Diego, CA) for two more days for differentiation. The transfected cortical neurons were incubated for 30 hours either at 20°C or 37°C with 5% CO2, 95% air and then fixed.
For immunocytochemistry, all steps were performed at room temperature unless otherwise noted. Cells were rinsed with PBS, fixed in 3.5% formaldehyde in PBS for 30 minutes, and permeabilized in 0.1% Triton X-100 in PBS for 30 minutes. Cells were then blocked in TTBS (TBS, 0.1% Tween-20 and 2% BSA), incubated for 30 minutes in primary antibodies, washed in TTBS (5 minutes, three times), and incubated in Alexa Green (488 nm) or Alexa Red (568 nm) secondary antibody (Molecular Probes, Eugene, OR) for 15 minutes. Samples were washed again and mounted on slides in GEL/MOUNT (Biomeda, Foster City, CA) for analysis.
Microscopy
The expression of VAChT–GFP, Synaptophysin–GFP/RFP (Syn–GFP/RFP), and CgA–RFP in PC12 cells was determined by visual inspection under a Nikon upright LABPHOT microscope (Nikon, Kanagawa, Japan). Immunofluorescence microscopy was performed at room temperature using a Zeiss Axiovert 200 M inverted microscope (Carl Zeiss, Thornwood, NY) equipped with 100× Zeiss alpha plan fluor oil, 1.45 NA, DIC objectives. Images were acquired by a Meta detector for spectral imaging (Carl Zeiss) and digitized using ‘LSM 510 Meta’ software version 3.5 (Carl Zeiss). The LSM510 Meta software was also used to calculate the Overlap Coefficient Correlation (OCC) using an absolute frequency of moderate (50 arbitrary units) intensity pixels in the red and green channels. Region of Interest was limited to one cell per image.
According to the developer of the software, OCC=Σi[Sc1i × Sc2 i]/SQRT(Σi[S1i]2 × Σi[S2i]2), where i is ‘i’th pixel in the region of interest, Sc1 is signal intensity of pixel involved in colocalization in the first channel, Sc2 the signal intensity of pixel involved in colocalization in the second channel, S1 the signal intensity of pixel, regardless of colocalization, in the first channel, S1 is the signal intensity of pixel, regardless of colocalization, in the second channel and SQRT represents square root.
For time-lapse imaging, PC12 cells expressing VAChT–GFP or Syn–RFP along with CgA–RFP were maintained in Phenol-Red-free DMEM plus 10% FBS with 5% horse serum in a temperature-controlled (37°C) Bioptechs Delta T live-cell environmental chamber (Bioptechs, Butler, PA) and imaged using the Zeiss Axiovert 200M inverted microscope and ‘LSM 510 Meta’ software (Carl Zeiss) at 1.57 second exposure per image acquisition for 1500 acquisitions for 30 minutes. Images were converted to 8-bit movies using ‘LSM 510 Meta’ software.
Supplementary Material
Acknowledgments
We thank the lab members in SCN, NICHD for technical assistance and helpful discussions. We thank Zu-Hang Sheng (NINDS, NIH) for the synaptophysin constructs and Jennifer Lippincott-Schwartz (NICHD, NIH) for the GPI–GFP construct and her helpful discussions. We thank Vincent Schram and Chip Dye in the NICHD Microscopy Imaging Core for technical support. This research was supported by the Intramural Research Program of the NICHD, NIH. J.J.P. is supported by NICHD K22 and ARRA grants. M.G.L. is supported by the Howard University Medical Alumni Association (HUMAA) Endowed Chair in the Basic Sciences, an intramural SEED grant, and NINDS/NIH Grant # NS065385. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/5/735/DC1
References
- Adams L. A., Ang L. C., Munoz D. G. (1993). Chromogranin A, a soluble synaptic vesicle protein, is found in cortical neurons other than previously defined peptidergic neurons in the human neocortex. Brain Res. 602, 336-341 [DOI] [PubMed] [Google Scholar]
- Agoston D. V., Whittaker V. P. (1989). Characterization, by size, density, osmotic fragility, and immunoaffinity, of acetylcholine- and vasoactive intestinal polypeptide-containing storage particles from myenteric neurones of the guinea-pig. J. Neurochem. 52, 1474-1480 [DOI] [PubMed] [Google Scholar]
- Arvan P., Castle D. (1998). Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem. J. 332, 593-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R., Regnier-Vigouroux A., Flatmark T., Huttner W. B. (1993). Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron 11, 105-121 [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Berse B. (2000). The cholinergic neuronal phenotype in Alzheimer's disease. Metab. Brain Dis. 15, 45-64 [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Linstedt A. D., Lowe A. W., Grote E., Kelly R. B. (1990). Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J. Cell Biol. 110, 1693-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Jahn R. (1990). Pathways to regulated exocytosis in neurons. Annu. Rev. Physiol. 52, 625-645 [DOI] [PubMed] [Google Scholar]
- Desnos C., Clift-O'Grady L., Kelly R. B. (1995). Biogenesis of synaptic vesicles in vitro. J. Cell Biol. 130, 1041-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanvantari S., Loh Y. P. (2000). Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J. Biol. Chem. 275, 29887-29893 [DOI] [PubMed] [Google Scholar]
- Eaton B. A., Haugwitz M., Lau D., Moore H. P. (2000). Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J. Neurosci. 20, 7334-7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden L. E. (1998). The cholinergic gene locus. J. Neurochem. 70, 2227-2240 [DOI] [PubMed] [Google Scholar]
- Ferguson S. M., Savchenko V., Apparsundaram S., Zwick M., Wright J., Heilman C. J., Yi H., Levey A. I., Blakely R. D. (2003). Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J. Neurosci. 23, 9697-9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondré-Lewis M. C., Petrache H. I., Wassif C. A., Harries D., Parsegian A., Porter F. D., Loh Y. P. (2006). Abnormal sterols in cholesterol-deficiency diseases cause secretory granule malformation and decreased membrane curvature. J. Cell Sci. 119, 1876-1885 [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73, 2424-2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Rein G. (1977). Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature 268, 349-351 [DOI] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. (1985). Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J. Cell Biol. 101, 949-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E., Kelly R. B. (1996). Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J. Cell Biol. 132, 537-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. (1991). Secretory granule and synaptic vesicle formation. Curr. Opin. Cell Biol. 3, 654-660 [DOI] [PubMed] [Google Scholar]
- Kim T., Tao-Cheng J. H., Eiden L. E., Loh Y. P. (2001). Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106, 499-509 [DOI] [PubMed] [Google Scholar]
- Kim T., Gondre-Lewis M. C., Arnaoutova I., Loh Y. P. (2006). Dense-core secretory granule biogenesis. Physiology (Bethesda) 21, 124-133 [DOI] [PubMed] [Google Scholar]
- Krantz D. E., Waites C., Oorschot V., Liu Y., Wilson R. I., Tan P. K., Klumperman J., Edwards R. H. (2000). A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles. J. Cell Biol. 149, 379-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R., Arvan P. (1992). Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J. Cell Biol. 118, 521-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein Y., Desnos C., Faundez V., Kelly R. B., Clift-O'Grady L. (1998). Vesiculation and sorting from PC12-derived endosomes in vitro. Proc. Natl. Acad. Sci. USA 95, 11223-11228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. (2004). Dynamics of secretory membrane trafficking. Ann. N. Y. Acad. Sci. 1038, 115-124 [DOI] [PubMed] [Google Scholar]
- Liu Y., Edwards R. H. (1997). Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J. Cell Biol. 139, 907-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega W. P., Howard B. D. (1981). Choline and acetylcholine metabolism in PC12 secretory cells. Biochemistry 20, 4477-4483 [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Mains R. E. (1994). Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J. Cell Sci. 107, 737-745 [DOI] [PubMed] [Google Scholar]
- Mundigl O., Matteoli M., Daniell L., Thomas-Reetz A., Metcalf A., Jahn R., De Camilli P. (1993). Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites. J. Cell Biol. 122, 1207-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins A. K., Thurmond D. C. (2005). A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J. Biol. Chem. 280, 1944-1952 [DOI] [PubMed] [Google Scholar]
- Nirenberg M. J., Liu Y., Peter D., Edwards R. H., Pickel V. M. (1995). The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc. Natl. Acad. Sci. USA 92, 8773-8777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M. J., Chan J., Liu Y., Edwards R. H., Pickel V. M. (1997). Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse 26, 194-198 [DOI] [PubMed] [Google Scholar]
- Park J. J., Loh Y. P. (2008). How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol. Endocrinol. 22, 2583-2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. M., Prior C., Marshall I. G. (1993). Acetylcholine transport, storage, and release. Int. Rev. Neurobiol. 35, 279-390 [DOI] [PubMed] [Google Scholar]
- Pennuto M., Bonanomi D., Benfenati F., Valtorta F. (2003). Synaptophysin I controls the targeting of VAMP2/synaptobrevin II to synaptic vesicles. Mol. Biol. Cell 14, 4909-4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghani A., Shirzadi A., Kohan S. A., Edwards R. H., Butcher L. L. (1996). Differential distribution of the putative vesicular transporter for acetylcholine in the rat central nervous system. Brain Res. Mol. Brain Res. 43, 65-76 [DOI] [PubMed] [Google Scholar]
- Rudolf R., Salm T., Rustom A., Gerdes H. H. (2001). Dynamics of immature secretory granules: role of cytoskeletal elements during transport, cortical restriction, and F-actin-dependent tethering. Mol. Biol. Cell 12, 1353-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. S., Barbosa J., Jr, Veloso G. S., Ribeiro F., Kushmerick C., Gomez M. V., Ferguson S. S., Prado V. F., Prado M. A. (2001). Trafficking of green fluorescent protein tagged-vesicular acetylcholine transporter to varicosities in a cholinergic cell line. J. Neurochem. 78, 1104-1113 [DOI] [PubMed] [Google Scholar]
- Schäfer M. K., Nohr D., Romeo H., Eiden L. E., Weihe E. (1994). Pan-neuronal expression of chromogranin A in rat nervous system. Peptides 15, 263-279 [DOI] [PubMed] [Google Scholar]
- Schäfer M. K., Schutz B., Weihe E., Eiden L. E. (1997). Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc. Natl. Acad. Sci. USA 94, 4149-4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hannah M. J., Huttner W. B. (1997). Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J. Cell Biol. 137, 445-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. P., Ivanov I. E., Adesnik M., Sabatini D. D. (1996). The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J. Cell Biol. 135, 355-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng J. H., Eiden L. E. (1998). The vesicular monoamine transporter VMAT2 and vesicular acetylcholine transporter VAChT are sorted to separate vesicle populations in PC12 cells. Adv. Pharmacol. 42, 250-253 [DOI] [PubMed] [Google Scholar]
- Thoidis G., Kandror K. V. (2001). A Glut4-vesicle marker protein, insulin-responsive aminopeptidase, is localized in a novel vesicular compartment in PC12 cells. Traffic 2, 577-587 [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Kobayashi O., Torocsik A., Kinjo M., Nagashima H., Manabe N., Goldiner P. L., Potter P. E., Foldes F. F. (1989). Heterogeneity of presynaptic muscarinic receptors involved in modulation of transmitter release. Neuroscience 31, 259-267 [DOI] [PubMed] [Google Scholar]
- Weihe E., Tao-Cheng J. H., Schafer M. K., Erickson J. D., Eiden L. E. (1996). Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc. Natl. Acad. Sci. USA 93, 3547-3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. (1985). Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41, 1017-1028 [DOI] [PubMed] [Google Scholar]
- Yao J., Erickson J. D., Hersh L. B. (2004). Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic 5, 1006-1016 [DOI] [PubMed] [Google Scholar]
- Zhang C. F., Dhanvantari S., Lou H., Loh Y. P. (2003). Sorting of carboxypeptidase E to the regulated secretory pathway requires interaction of its transmembrane domain with lipid rafts. Biochem. J. 369, 453-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.