Abstract
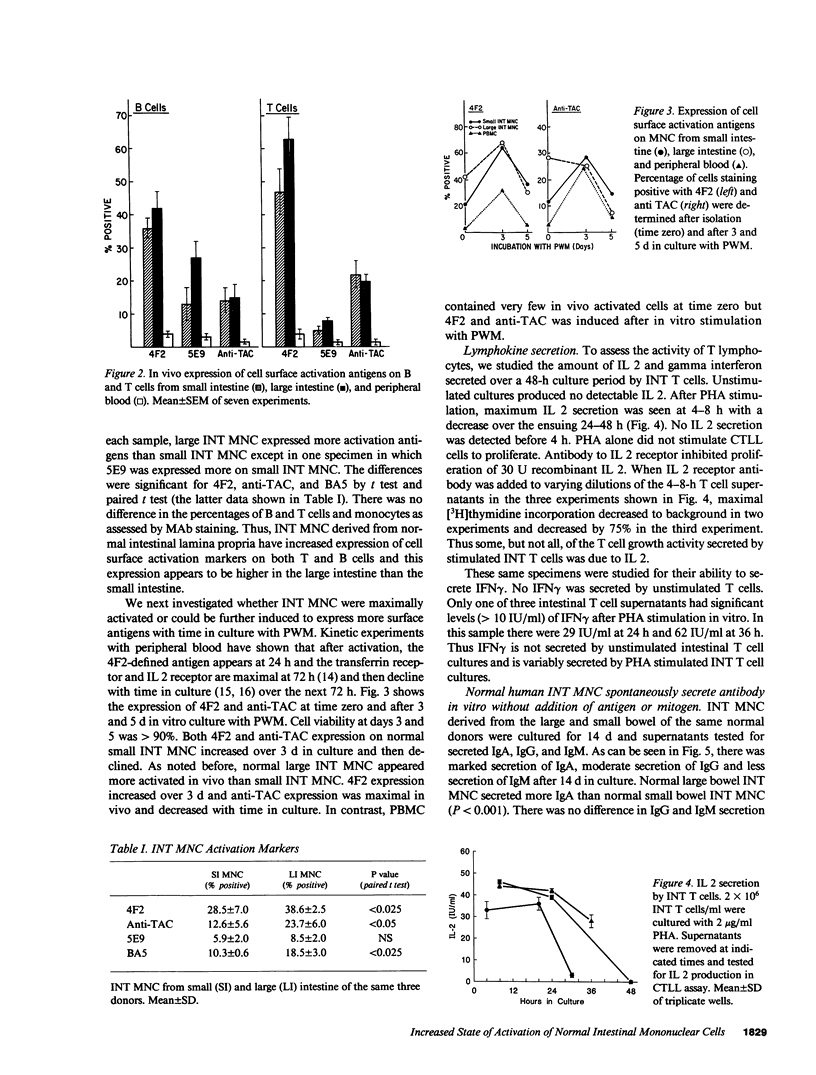
The state of activation of normal human intestinal mononuclear cells obtained from transplant donors was studied. Compared with PBMC, freshly isolated intestinal mononuclear cells expressed significantly more cell surface activation antigens on both B and T lymphocytes. Intestinal mononuclear cells contained significant numbers of immunoglobulin secreting cells immediately after cell separation. This population included CD5-positive B cells that secreted predominantly IgA. Cells from the large bowel consistently revealed higher numbers of IgA secreting cells than cells from the small bowel. Thus, intestinal B cells are markedly activated in vivo compared with PBMC and this increased activation correlates with increased spontaneous antibody secretion. B cells from the large intestine are more highly activated and secrete more antibody than do cells from the small intestine. The intestinal lamina propria lymphoid compartment exhibits a heightened state of activation that may be important for its distinct role in mucosal defense.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Jr, Jurgensen C. H., Brown E. J., McFarland P., Fauci A. S. Identification of a receptor for high molecular weight human B cell growth factor. J Immunol. 1988 Aug 1;141(3):861–869. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Efrat S., Pilo S., Kaempfer R. Kinetics of induction and molecular size of mRNAs encoding human interleukin-2 and gamma-interferon. Nature. 1982 May 20;297(5863):236–239. doi: 10.1038/297236a0. [DOI] [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
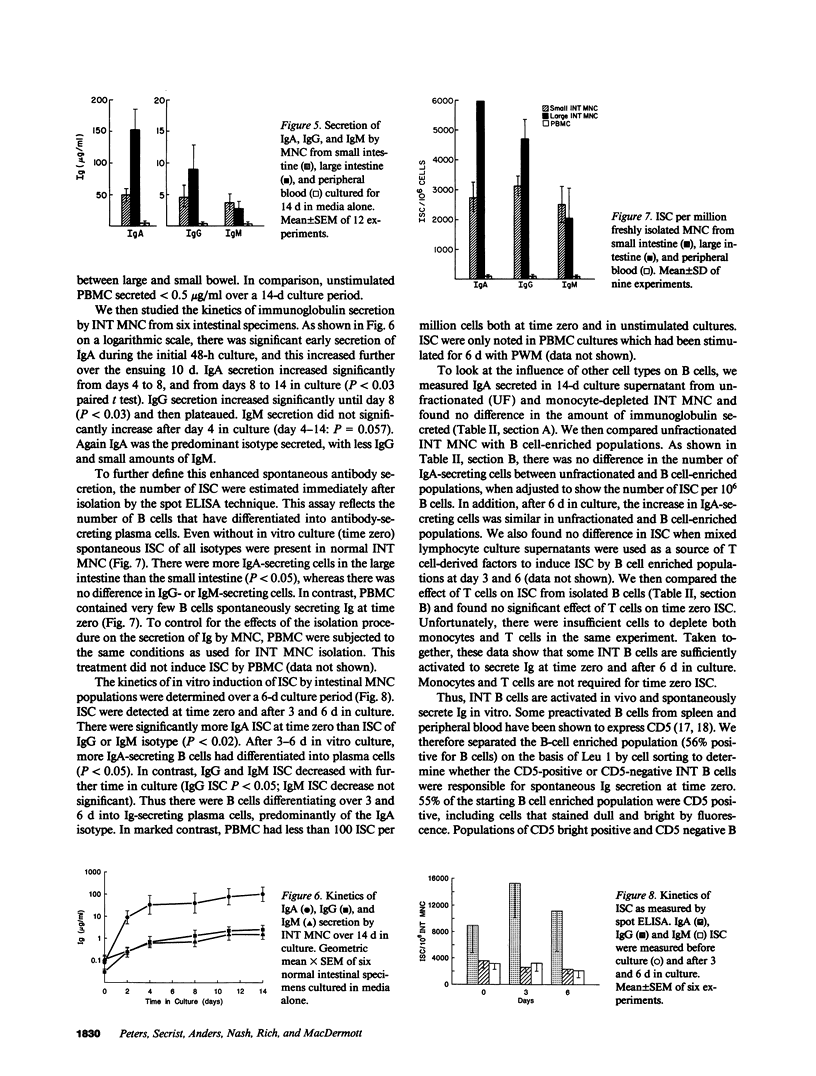
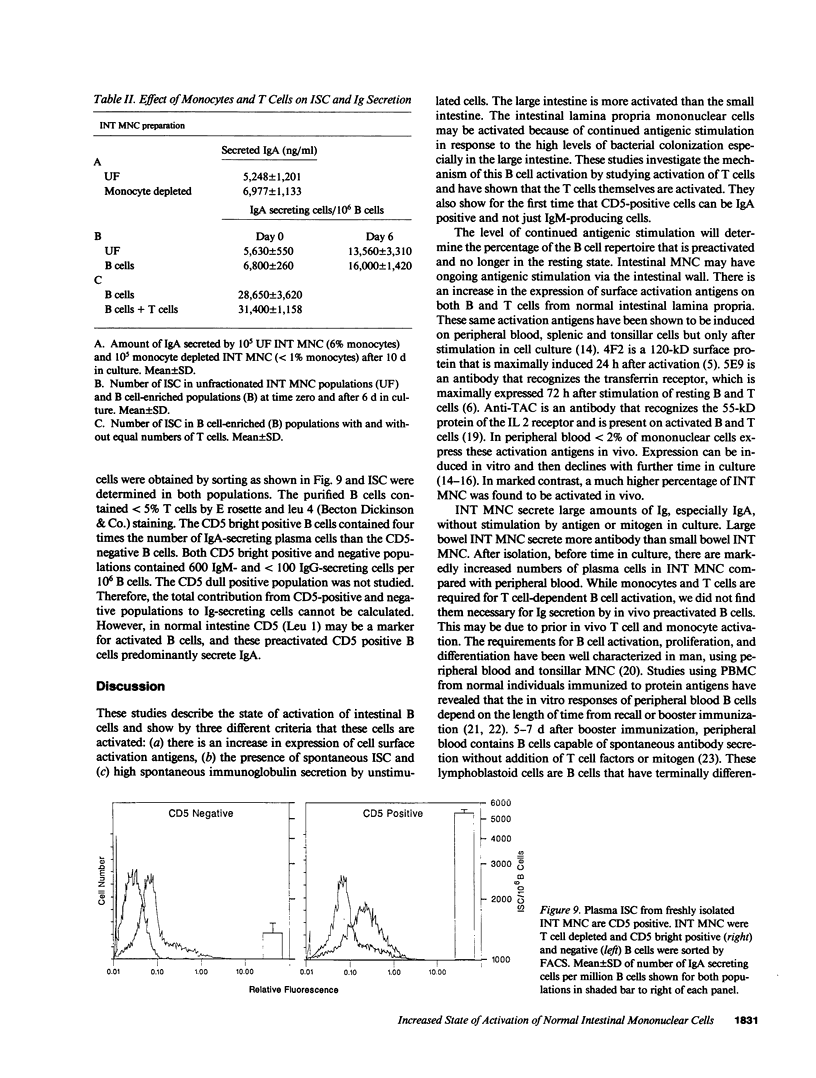
- Kehrl J. H., Muraguchi A., Butler J. L., Falkoff R. J., Fauci A. S. Human B cell activation, proliferation and differentiation. Immunol Rev. 1984 Apr;78:75–96. doi: 10.1111/j.1600-065x.1984.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinikki P. O., Calderon J., Luquette M. H., Schreiber R. D. Reduced receptor binding by a human interferon-gamma fragment lacking 11 carboxyl-terminal amino acids. J Immunol. 1987 Nov 15;139(10):3360–3366. [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Mohrman R. F., Kodner I. J., Delacroix D. L., Vaerman J. P. Altered patterns of secretion of monomeric IgA and IgA subclass 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology. 1986 Aug;91(2):379–385. doi: 10.1016/0016-5085(86)90572-x. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985 Jan 1;161(1):181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Yenokida G., James S. P. The role of the transferrin receptor in human B lymphocyte activation. J Immunol. 1984 Nov;133(5):2437–2441. [PubMed] [Google Scholar]
- Peters M., Ambrus J. L., Zheleznyak A., Walling D., Hoofnagle J. H. Effect of interferon-alpha on immunoglobulin synthesis by human B cells. J Immunol. 1986 Nov 15;137(10):3153–3157. [PubMed] [Google Scholar]
- Peters M., Butler J. L., Margolick J. B., Gerrard T. L., Dinarello C. A., Fauci A. S. Synergy of helper factors in the differentiation of in vivo-preactivated antigen-specific human B cells. Cell Immunol. 1985 Mar;91(1):33–42. doi: 10.1016/0008-8749(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Peters M., Fauci A. S. Selective activation of antigen-specific human B cells in recently immunized individuals by nonspecific factors in the absence of antigen. J Immunol. 1983 Feb;130(2):678–680. [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Taniguchi O., Miyajima H., Hirano T., Noguchi M., Ueda A., Hashimoto H., Hirose S., Okumura K. The Leu-1 B-cell subpopulation in patients with rheumatoid arthritis. J Clin Immunol. 1987 Nov;7(6):441–448. doi: 10.1007/BF00915053. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Zeitz M., Greene W. C., Peffer N. J., James S. P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988 Mar;94(3):647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]