Abstract
Macroautophagy is a dynamic process whereby portions of the cytosol are encapsulated in double-membrane vesicles and delivered to the lysosome for degradation. Phosphatidylinositol-3-phosphate (PtdIns3P) is concentrated on autophagic vesicles and recruits effector proteins that are crucial for this process. The production of PtdIns3P by the class III phosphatidylinositol 3-kinase Vps34, has been well established; however, protein phosphatases that antagonize this early step in autophagy remain to be identified. To identify such enzymes, we screened human phosphatase genes by RNA interference and found that loss of PTPσ, a dual-domain protein tyrosine phosphatase (PTP), increases levels of cellular PtdIns3P. The abundant PtdIns3P-positive vesicles conferred by loss of PTPσ strikingly phenocopied those observed in cells starved of amino acids. Accordingly, we discovered that loss of PTPσ hyperactivates both constitutive and induced autophagy. Finally, we found that PTPσ localizes to PtdIns3P-positive membranes in cells, and this vesicular localization is enhanced during autophagy. We therefore describe a novel role for PTPσ and provide insight into the regulation of autophagy. Mechanistic knowledge of this process is crucial for understanding and targeting therapies for several human diseases, including cancer and Alzheimer's disease, in which abnormal autophagy might be pathological.
Keywords: FYVE, PTPσ, PtdIns3P, RNAi, Autophagy, Phosphatase
Introduction
In addition to the well-characterized role of phosphatidylinositol-3-phosphate (PtdIns3P) in endocytosis, recent evidence has uncovered a crucial requirement for this lipid in macroautophagy (autophagy) (Axe et al., 2008; Obara et al., 2008a; Petiot et al., 2000). Autophagy occurs constitutively in nearly all cells to maintain homeostasis, but is further activated in response to stress as a survival or adaptive mechanism. During autophagy, a double-membrane phagophore forms and elongates around portions of cytosol, matures into an enclosed autophagosome, and delivers its contents to the lysosome for degradation (Klionsky, 2007). Basic biochemical components (i.e. amino acids and fatty acids) are exported from the lysosome and recycled by the cell, representing an energetically favorable alternative to de novo synthesis. In mammalian systems, the lipid kinase Vps34 forms a complex with several proteins, including Vps15, Beclin1, Atg14L, UVRAG and Bif1 to generate PtdIns3P on autophagic vesicles (Itakura et al., 2008; Zhong et al., 2009). PtdIns3P then recruits and tethers effector proteins, such as WIPI-1 (Atg18), which are required for proper membrane formation (Obara et al., 2008b; Proikas-Cezanne et al., 2004). The crucial requirement for PtdIns3P in this process is evidenced by the fact that autophagy is ablated in mutant Vps34 yeast strains and genetic Vps34 knockouts in Drosophila (Juhasz et al., 2008; Kihara et al., 2001). Despite knowledge of PtdIns3P production, the antagonistic phosphatases that regulate PtdIns3P during autophagy have remained elusive. Two myotubularin-related phosphatases, MTMR3 and MTMR14 (hJumpy), have recently been shown to dephosphorylate autophagic PtdIns3P in various contexts (Taguchi-Atarashi et al., 2010; Vergne et al., 2009). However, given the complexity of autophagy execution and the number of proteins in the autophagy network, we predict that additional protein phosphatases exist to regulate this process. Accordingly, we performed a high-content cell-based RNAi screen using a fluorescent PtdIns3P sensor to identify protein phosphatases that function upstream of PtdIns3P during autophagy.
Results
RNAi screening identifies PTPσ as a modulator of PtdIns3P signaling
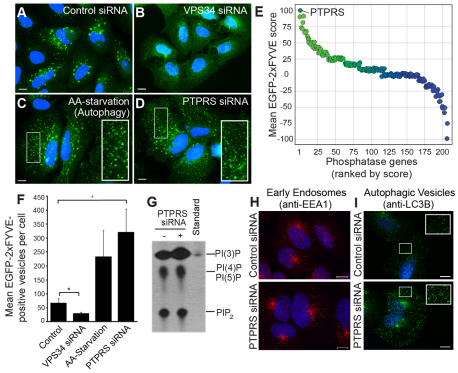
FYVE (Fab1, YOTB, Vac1 and EEA1) domains are cysteine-rich zinc-finger binding motifs that specifically recognize and bind PtdIns3P (Gaullier et al., 1998). An EGFP molecule fused to two tandem FYVE domains, termed EGFP–2xFYVE, serves as an effective cellular sensor of PtdIns3P (Gillooly et al., 2000). We analyzed U2OS cells stably expressing this construct by fluorescent microscopy and found that PtdIns3P predominantly localized to punctate, often perinuclear, vesicles when cultured in complete growth medium with full nutrients (Fig. 1A, supplementary material Movie 1). RNAi-mediated knockdown of Vps34 reduced cellular PtdIns3P content and resulted in a diffuse cytosolic distribution of EGFP–2xFYVE (Fig. 1B,F, supplementary material Fig. S1A). By contrast, a redistribution of EGFP–2xFYVE to small abundant autophagic vesicles occurred when cells were deprived of amino acids to potently induce autophagy (Fig. 1C, supplementary material Movie 2).
Fig. 1.
A cell-based siRNA screen identifies PTPσ as a modulator of PtdIns3P. (A–D) U2OS EGFP–2xFYVE cells transfected with control siRNA (A), VSP34 siRNA (B), starved of amino acids for 3 hours (C), or transfected with PTPRS siRNA (D), were fixed and imaged at 60× magnification by fluorescent microscopy (green, PtdIns3P, EGFP–2xFYVE; blue, nuclei). Insets show 2× magnifications of small EGFP–2xFYVE vesicles. Scale bars: 10 μm. (E) Following knockdown of phosphatases, EGFP–2xFYVE-positive punctae were scored from −100 (decreased from control cells) to +100 (increased). Phosphatases are ranked and plotted by decreasing score (left to right) with genes whose loss increased EGFP–2xFYVE fluorescence highlighted in green and whose loss caused decreases highlighted in blue. PTPRS is identified. (F) Mean EGFP–2xFYVE-positive punctate were quantified from cells under the conditions indicated using image analysis software. Bars represent s.e.m.; *P<0.05. (G) Phospholipids were radiolabeled in vivo, extracted, resolved by TLC, and visualized by autoradiography following transfection with control or PTPRS siRNA. A radiolabeled PtdIns3P standard was resolved adjacent to extracted lipids. (H,I) Endosomes were labeled by immunostaining with anti-EEA1 antibodies (H) and autophagic vesicles were labeled with anti-LC3B antibodies (I) following transfection with control or PTPRS siRNA (red, EEA1; green, LC3B; blue, nuclei). Insets show 2× magnifications of LC3-positive vesicles. Scale bars: 10 μm.
To identify genes that downregulate PtdIns3P signaling, we used several siRNAs targeting over 200 known and putative human phosphatases. The siRNAs were introduced into U2OS EGFP–2xFYVE cells, and the cells were subsequently monitored for PtdIns3P signaling. We identified several genes whose knockdown significantly increased the abundance of cellular EGFP–2xFYVE punctae (Fig. 1E, supplementary material Table S1). Most notably, PtdIns3P was substantially increased following knockdown of the myotubularin family member MTMR6 (supplementary material Fig. S1B,C), as well as the dual-domain protein tyrosine phosphatase PTPRS (PTPσ) (Fig. 1D,E). Although reduced expression of MTMR6 was characterized by the appearance of enlarged perinuclear vesicles, the siRNAs targeting PTPσ caused a dramatic accumulation of abundant smaller vesicles throughout the cytosol, which phenocopied results observed during amino acid starvation (Fig. 1C,D, supplementary material Movie 3). Quantification of PtdIns3P-positive vesicles revealed a 3.5-fold increase in abundance during starvation-induced autophagy and a nearly 5-fold increase caused by knockdown of PTPσ alone (Fig. 1F). This phenotype was confirmed using four unique siRNA sequences targeting PTPσ (supplementary material Fig. S1D-K).
To validate a physiological increase in PtdIns3P following knockdown of PTPσ, phospholipids were radiolabeled with [32P]orthophosphate in vivo, extracted, and resolved by thin-layer chromatography. Indeed, PtdIns3P levels were specifically elevated in the absence of PTPσ, whereas other lipid species remained unchanged (Fig. 1G). To determine the identity of the PtdIns3P-positive vesicles formed by knockdown of PTPσ, we immunostained cells with well-established markers of early endosomes [anti-EEA1 (early endosomal antigen 1)] and autophagic vesicles (AVs) [anti-LC3 (light chain 3)]. We found that knockdown of PTPσ had no substantial effect on the presence of EEA1-positive endosomes (Fig. 1H, supplementary material Fig. S2A), but significantly increased the abundance of LC3-positive vesicles (Fig. 1I). From this, we hypothesized that PTPσ functions during autophagy and focused our attention on this enzyme as a candidate autophagic phosphatase.
Loss of PTPσ hyperactivates constitutive and induced autophagy
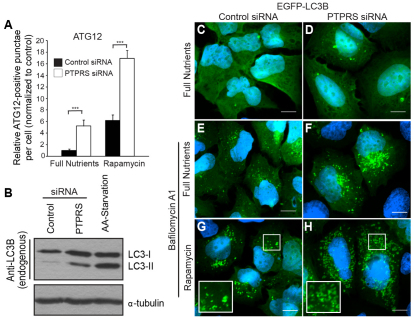
The striking resemblance of PtdIns3P-positive vesicles induced by PTPσ knockdown to AVs formed during amino acid starvation led us to propose that autophagy is hyperactivated in the absence of PTPσ, despite the presence of nutrients. To test this, autophagy was analyzed in U2OS cells by evaluating two ubiquitin-like proteins, Atg12 and LC3 (Atg8), which become conjugated to AVs during autophagy. Following phagophore nucleation, Atg12 covalently binds Atg5 and oligomerizes with Atg16L at the autophagic membrane (Klionsky, 2007). To measure vesicle abundance at this step, we immunostained cells for endogenous Atg12 and quantified Atg12-positive punctae. We found that knockdown of PTPσ increased AV abundance three- or fivefold over the control when cells were cultured with rapamycin, a potent mTOR inhibitor and autophagy inducer, or full nutrients, respectively (Fig. 2A, supplementary material Fig. S2B).
Fig. 2.
Loss of PTPσ hyperactivates autophagy. (A) ATG12-positive punctae were quantified from 60× magnification images of cells transfected with control (black) or PTPRS siRNAs (white) and treated for 1 hour with normal growth medium (full nutrients) or 50 nM rapamycin. Values represent relative ATG12-positive punctae per cell following normalization to control cells cultured with full nutrients. Bars represent s.e.m.; ***P<0.001. (B) LC3-I and LC3-II were analyzed by western blot using whole cell lysates from cells transfected with control or PTPRS siRNA or starved of amino acids. α-tubulin was probed as a loading control. (C–H) EGFP–LC3-positive punctae were visualized in U2OS cells transfected with control (C,E,G) or PTPRS (D,F,H) siRNA following treatment for 2 hours with normal growth medium (full nutrients; C,D), 100 nM bafilomycin A1 (Baf-A1; E,F), or 50 nM rapamycin and 100 nM Baf-A1 (G,H) (green, EGFP-LC3; blue, nuclei). Insets are 2× magnifications of EGFP–LC3-positive AVs. Scale bars: 10 μm.
The membrane-bound Atg5–Atg12–Atg16L complex permits lipidation of LC3, which is a classic marker for AVs (Hanada et al., 2007). LC3 is unique among the autophagy proteins in that a portion of it remains membrane bound and is degraded in the lysosome along with vesicle cargo. Therefore, lysosomal function can be inhibited [i.e. with bafilomycin A1 (Baf-A1) or chloroquine treatment] and LC3 accumulation used as a measure of autophagic flux (Tanida et al., 2005). We found that both knockdown of PTPσ and amino acid starvation increased the abundance of LC3-II, the AV-lipidated form of LC3, when lysates were probed with endogenous antibodies (Fig. 2B). Similarly, we observed an increased number of EGFP–LC3-positive punctae when PTPσ expression was reduced under normal growth conditions, and these structures accumulated substantially when cells were cultured with Baf-A1, indicating their functionality (Fig. 2C–F). Knockdown of PTPσ caused an even greater increase in EGFP–LC3 punctae above the control level when cells were treated with both Baf-A1 and rapamycin (Fig. 2G,H). Similar results were obtained when AVs were quantified from cells immunostained for endogenous LC3 (supplementary material Fig. S2C).
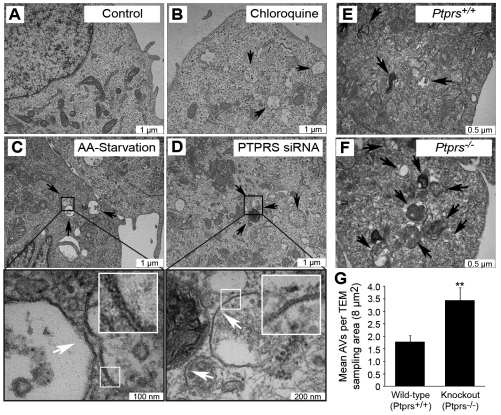
To confirm hyperactive autophagy in the absence of PTPσ independently of fluorescent markers, we detected AVs by transmission electron microscopy (TEM). AVs are hallmarked by unique double membranes and by the presence of engulfed cytosolic content: features that allow them to be easily detected by TEM. Although control cells contained very few AVs, chloroquine treatment increased their abundance, most of which appeared to be autolysosomal, as expected (Fig. 3A,B). Similarly, cells deprived of amino acids for 1 hour harbored elevated numbers of double-membrane AVs, as did cells transfected with siRNAs against PTPσ, but cultured in full nutrients (Fig. 3C,D). To establish this phenotype independently of RNAi, we examined autophagy during PTPσ loss using wild-type (Ptprs+/+) and PTPσ knockout (Ptprs−/−) murine embryonic fibroblasts (MEFs). We have previously generated Ptprs−/− mice by inserting a selectable neomycin resistance gene into the D1 phosphatase (catalytic) domain. From these mice, we generated MEFs that lack both Ptprs transcript and protein, as measured by northern blot and western blot, respectively (Elchebly et al., 1999). TEM analysis showed that both Ptprs+/+ and Ptprs−/− primary MEFs contained a basal level of AVs; however, they were twice as abundant in Ptprs−/− MEFs (Fig. 3E-G). Collectively, these results demonstrate that loss of PTPσ, by RNAi and genetic deletion, increases both constitutive and induced autophagy.
Fig. 3.
Loss of PTPσ increases autophagic vesicle abundance as measured by electron microscopy. (A–D) Few autophagic vesicles (AVs) were found by transmission electron microscopy (TEM) within control cells cultured in full nutrients (A), but were abundant in chloroquine-treated cells (B), amino acid (AA)-starved cells (C) and cells transfected with PTPRS siRNA (D). Black arrows indicate autophagic vesicles. White box insets are 3× magnifications. White arrows indicate double membranes. (E–G) Primary wild-type (Ptprs+/+, E) and knockout (Ptprs−/−, F) MEFs were analyzed by TEM and AVs quantified (G). AVs, defined as double-membrane structures containing cytosolic components, were counted from 8 μm2 sampling regions per cell. Values represent mean AVs per sampling area. Bars represent s.e.m.; **P<0.01.
PTPσ localizes to PtdIns3P-positive vesicles and rescues the siRNA phenotype
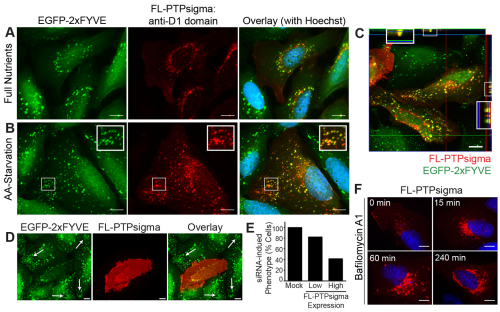
Given the robust PtdIns3P response elicited by knockdown of PTPσ, we hypothesized that PTPσ regulates autophagy by functioning at the level of PtdIns3P-positive vesicles. PTPσ is a bulky receptor-like PTP with an extracellular segment and two tandem cytosolic phosphatase domains (termed D1 and D2). Complex processing events have been reported for PTPσ and related enzymes, including ectodomain shedding and internalization from the cell surface (Aicher et al., 1997; Ruhe et al., 2006). To determine the localization of PTPσ phosphatase domains, untagged full-length protein (FL-PTPσ) was transiently expressed in U2OS EGFP–2xFYVE cells and detected by fluorescent microscopy using D1-targeted monoclonal antibodies. We found that in addition to its presence at the plasma membrane, PTPσ localized to the perinuclear region and to numerous intracellular-vesicle-like structures, many of which were positive for PtdIns3P (Fig. 4A). Strikingly, autophagy induction by amino acid starvation induced a redistribution of PTPσ to smaller vesicles, which were abundant throughout the cytosol and were almost entirely PtdIns3P positive (Fig. 4B,C). In support of the notion that this localization was autophagic, we discovered that PTPσ was also capable of localizing to mRFP–LC3-positive punctae in the context of both basal and induced autophagy (supplementary material Fig. S3B).
Fig. 4.
Exogenous PTPσ localizes to PtdIns3P vesicles and rescues the siRNA phenotype. (A,B) FL-PTPσ was transiently expressed in U2OS EGFP–2xFYVE cells and PtdIns3P and PTPσ imaged by fluorescent microscopy following incubation for 2 hours with full nutrient medium (A) or amino acid starvation medium (B) [green, PtdIns3P; red, anti-PTPσ (D1-targeted antibodies); blue, nuclei]. Insets are 2× magnifications of boxed regions. Scale bars: 10 μm. (C) U2OS EGFP–2xFYVE cells transfected with FL-PTPσ and amino acid starved for 2 hours were imaged using D1-targeted PTPσ antibodies. A Z-stack of 0.25 μm increments was captured using sequential channel acquisition and confocal microscopy, with the third slice displayed and Z-stacks through the X and Y planes shown at the border. Insets are 2× magnifications of boxed regions. Scale bar: 10 μm (green, PtdIns3P, EGFP–2xFYVE; red, anti-PTPσ; yellow, colocalization). (D,E) U2OS EGFP–2xFYVE cells were transfected with PTPRS siRNA-1 for 48 hours, after which FL-PTPσ (which lacks the sequence targeted by siRNA-1) was introduced for an additional 24 hours. PtdIns3P and PTPσ were imaged as previously described. The presence of siRNA-induced phenotype (abundant, non-perinuclear EGFP–2xFYVE-positive vesicles; indicated by white arrows in D) was determined for cells expressing no, low or high levels of FL-PTPσ (E). Scale bars: 10 μm. (F) FL-PTPσ-positive vesicular structures accumulate when lysosomal fusion is inhibited. U2OS cells expressing FL-PTPσ for 24 hours were treated with 100 nM Baf-A1 in full nutrient medium for 0, 15, 60 or 240 minutes and FL-PTPσ imaged using D1-targeted PTPσ antibodies (red). Nuclei were stained with Hoechst 33342 (blue). Scale bars: 10 μm.
We further used exogenous PTPσ expression in an RNAi rescue experiment to demonstrate the specificity of phenotype induced by PTPRS siRNA. The naturally occurring isoform of PTPσ used in our studies lacks the fourth through seventh fibronectin domains (present in the longest isoform): a region encompassing the sequence targeted by a potent siRNA (siRNA-1; see supplementary material Fig. S1E,J,K) (Pulido et al., 1995). The accumulation of small, abundant, non-perinuclear PtdIns3P-positive vesicles induced by siRNA transfection was rescued by exogenous expression of PTPσ, an effect that was dose dependent (Fig. 4D,E). This result suggests a target-specific effect of siRNA-mediated knockdown of PTPσ and a role for this enzyme in PtdIns3P signaling.
To verify that the PTPσ-positive punctate structures were in fact vesicles functioning in a lysosomal pathway, we monitored the localization of PTPσ in Baf-A1-treated cells. Baf-A1 prevents maturing vesicles (e.g. endocytic and autophagic) from fusing with lysosomes and in turn, they accumulate in a perinuclear region. We found that PTPσ-positive vesicular structures began to accumulate quickly (within 15 minutes) and densely populated the perinuclear region within several hours (Fig. 4F). This suggests that PTPσ normally localizes to vesicles destined for the lysosome.
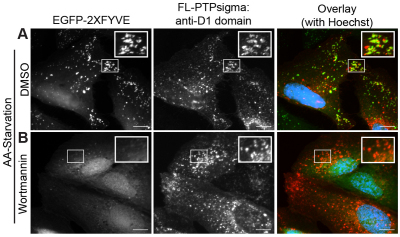
Finally, to determine whether PTPσ functions upstream or downstream of PtdIns3P at the starvation-induced punctae, we analyzed its localization in cells depleted of the phospholipid. Autophagy was induced by amino acid starvation in cells treated with wortmannin, a potent and irreversible inhibitor of Vps34 and other phosphoinositide 3-kinase isoforms, or vehicle (DMSO). In vehicle-treated cells, starvation induced the formation of abundant PtdIns3P-positive vesicles, which also contained PTPσ (Fig. 5A). Conversely, wortmannin treatment essentially ablated the formation of PtdIns3P during starvation; however, PTPσ was still recruited to the abundant punctate structures (Fig. 5B). This finding suggests that the localization of PTPσ to intracellular structures formed during autophagy occurs upstream, or independently, of PtdIns3P.
Fig. 5.
Localization of PTPσ to vesicular structures does not require PtdIns3P. (A,B) U2OS cells expressing FL-PTPσ were treated with vehicle (DMSO; A) or 100 nM wortmannin (PI3K inhibitor; B) for 2 hours while cultured in amino acid starvation medium and PtdIns3P and PTPσ was imaged by fluorescent microscopy [green, PtdIns3P, EGFP–2xFYVE; red, anti-PTPσ (D1-targeted antibodies); blue, nuclei]. Insets are 2× magnifications of boxed regions. Scale bars: 10 μm.
Discussion
Using a high-content cell-based RNAi screen, we have identified phosphatases whose knockdown elevates cellular PtdIns3P. Notably, RNAi-mediated knockdown of MTMR6 and several other phosphatases resulted in swollen and often perinuclear PtdIns3P-positive vesicles. Previous studies have shown similar phenotypes when endocytic PtdIns3P is elevated, for example, by constitutive activation of early endosomal Rab5, or knockdown of the phosphatidylinositol 5-kinase PIKfyve (Murray et al., 2002; Rutherford et al., 2006). Accordingly, the vesicles observed following knockdown of these phosphatases are probably endosomal, and these enzymes, including MTMR6, might function in endocytic signaling. Of note, knockdown of both PTPN11 (SHP2) and PTPN13 (PTPL1) resulted in increased numbers of EGFP–2xFYVE punctae (supplementary material Table S1). PTPN13, a phosphatase that is proposed to have both tumor suppressive and oncogenic functions, has been implicated in several signal transduction pathways. Specifically, PTPN13 was shown to inhibit PI3K/Akt signaling and thus, the PtdIns3P phenotype elicited by knockdown could potentially be explained by altered 3′-phosphoinositide metabolism (Abaan and Toretsky, 2008; Dromard et al., 2007). Mutations in SHP2 are associated with several human diseases, most notably Noonan syndrome, LEOPARD syndrome and juvenile myelomonocytic leukemia (Araki et al., 2004; Kontaridis et al., 2006; Mohi and Neel, 2007; Mohi et al., 2005). Its activity has been linked to numerous signaling pathways, often downstream of receptor tyrosine kinases, and the observed phenotype could be a consequence of disruption of any number of substrates (Chan et al., 2008).
Surprisingly, we did not identify MTMR3 or MTMR14 (hJumpy), the PtdIns3P phosphatases with reported roles in autophagy. The myotubularin phosphatases comprise a large, highly conserved family of enzymes whose members have been shown to function as heteromeric partners (Lorenzo et al., 2006). As one example, MTMR3 and MTMR4, both FYVE-domain containing phosphatases, have been demonstrated to interact with one another and inhibit PtdIns3P (Lorenzo et al., 2006). Accordingly, gene-by-gene loss-of-function analysis of this family might not reveal phenotypes if compensation within the family occurs. Furthermore, these enzymes may serve cell- or context-specific functions that are not revealed in this study.
The most striking result from this study was the presence of abundant PtdIns3P-positive vesicles following knockdown of PTPσ, which phenocopied that of an autophagic cell. We confirmed hyperactive autophagy in the absence of PTPσ through the use of several autophagy markers, as well as electron microscopy. Atg12- and LC3-positive autophagic vesicles were substantially more abundant in the absence of PTPσ when cells were cultured in full nutrients (constitutive AVs) or treated with rapamycin (induced AVs). These autophagic vesicles accumulated upon treatment with the lysosomal inhibitors, Baf-A1 and chloroquine, demonstrating that they were functional and destined for lysosomal degradation. This phenotype suggests that PTPσ regulates an early step in autophagy induction, and its loss results in increased autophagic vesicle generation. This is consistent with the fact that PtdIns3P is generated on early phagophores and is required for proper autophagic vesicle formation. A role for PTPσ in autophagy induction and more specifically in PtdIns3P regulation, is supported by our findings that PTPσ localizes to PtdIns3P-positive vesicles that increase in number during autophagy.
It remains to be addressed how PTPσ is targeted to autophagic vesicles. PTPσ is expressed at the cell surface in a two-subunit complex comprised of a large ectodomain and a membrane-spanning intracellular domain. Accordingly, it is implicated in cell–cell and cell–ECM interactions, and it is a crucial regulator of axon homeostasis and neuronal development (Aicher et al., 1997; Elchebly et al., 1999; Uetani et al., 2006; Wallace et al., 1999). Relevant to our own work, ectodomain shedding and internalization of a membrane-bound C-terminal fragment has been demonstrated previously (Aicher et al., 1997). Through immunofluorescence analysis of PTPσ using D1-domain-specific antibodies, we place intracellular PTPσ on PtdIns3P-positive autophagic vesicles. Autophagosomes frequently fuse with endosomes during their maturation, forming hybrid organelles called amphisomes, establishing the possibility that PTPσ is internalized by endocytosis to arrive at autophagic vesicles (Klionsky, 2007). Furthermore, the close relative of PTPσ LAR (PTPRF), undergoes an additional proteolytic event whereby a soluble intracellular domain is formed and targeted inside the cell, similarly to the Notch receptor (Ruhe et al., 2006). PTPσ contains similar cleavage residues to LAR, making it therefore plausible that PTPσ is targeted from the plasma membrane to autophagic vesicles through a series of proteolytic events in response to autophagic stimuli. Thus, this phosphatase might serve several unique functions during various cellular conditions that are governed by its subcellular localization.
An important finding presented here is the recruitment of PTPσ to vesicular structures during amino acid starvation, which occurs even in the absence of PtdIns3P generation. This finding, together with the hyperactivation of autophagy elicited by knockdown of PTPσ (as measured by PtdIns3P, Atg12 and LC3), suggests that PTPσ regulates autophagy at an early step upstream of this lipid. In further support of this, we found that although almost all PTPσ-positive vesicles are also positive for PtdIns3P (EGFP–2xFYVE presence), fewer harbored LC3, a marker that is incorporated into AVs at later stages of maturation.
There are several potential mechanisms by which PTPσ might function to regulate autophagy. First, it is possible that PTPσ directly dephosphorylates PtdIns3P following recruitment to AVs. We tested the activity of recombinant PTPσ in vitro, and although we could not detect PtdIns3P phosphatase activity, it cannot be entirely excluded that PtdIns3P does not serve as a direct substrate in vivo (supplementary material Fig. S4). It is also possible that PTPσ uses its robust protein phosphatase activity to regulate the function of a PtdIns3P-modifying enzyme, such as a PtdIns3P phosphatase or a phosphoinositide 4- or 5-kinase. Alternatively, PTPσ could control the activity of Vps34, which contains at least one phosphotyrosine site, or another component within the larger Vps34 complex (Imami et al., 2008). Finally, PTPσ might contribute to the regulation of autophagy at the earliest initiation step, which is executed by a complex of autophagy proteins, namely ULK1 (Atg1) and Atg13. The functional formation of this complex, which permits the generation of the PtdIns3P-positive phagophore, was recently found to be tightly regulated by phosphorylation events (Chang and Neufeld, 2009; Ganley et al., 2009; Hosokawa et al., 2009; Jung et al., 2009). The aim of future work will be to determine the precise mechanism by which PTPσ functions to regulate autophagy.
Materials and Methods
siRNA screen and validation
U2OS EGFP–2xFYVE cells were seeded on 96-well plates (2000 cells per well) in McCoy's 5A medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) at 37°C for 24 hours. Four siRNA molecules per phosphatase gene (phosphatase siRNA library version 2.0, Qiagen, Valencia, CA) were transfected per well at a final concentration of 25 nM using 0.2 μl HiPerfect transfection reagent (Qiagen) per well. After 48 hours, cells were fixed with 3.7% formaldehyde and nuclei were stained with Hoechst 33342 (Invitrogen). Cells were visualized at 40× magnification on a Zeiss LSM 510 Meta confocal microscope (Oberkochen, Germany) and EGFP–2xFYVE fluorescence was compared with that of control siRNA transfected cells within each plate. Triplicate wells from each gene were qualitatively scored by two independent scorers on a scale from −100 (decreased EGFP–2xFYVE signal and distribution) to +100 (increased) and mean scores were determined. Twenty-seven phosphatase genes whose knockdown increased EGFP–2xFYVE fluorescence in the primary screen were used in a secondary screen, where four siRNAs were individually transfected to eliminate off-target hits. The primary score was multiplied by a binned secondary screen score (score of 1.0 for 3/4 or 4/4 siRNAs yielding a phenotype; 0.75 for 2/4 siRNAs; and 0 for 0/4 or 1/4 siRNAs). Quantitative real-time PCR (qRT-PCR) assays with SYBR green dye (Roche, Basel, Switzerland) and gene-specific primers confirmed that siRNAs effectively reduced mRNA expression of target genes. For imaging, cells were cultured on number 1.5 coverglass, transfections repeated as above, cells fixed, nuclei stained, and coverglass inverted into microslides with mounting gel. A control siRNA transfected well was cultured for 3 hours in amino acid starvation medium [Dulbecco's phosphate-buffered saline (DPBS) with 10% FBS and 1 g/l D-glucose]. Cells were imaged using a 60× oil objective on a Nikon TE300 fluorescent microscope (Tokyo, Japan). EGFP–2xFYVE-positive vesicles were quantified using image analysis software.
Phospholipid labeling, extraction and thin-layer chromatography
U2OS cells were seeded in McCoy's 5A with 10% FBS at 200,000 cells per well of six-well tissue culture plates. After 24 hours, control or PTPRS siRNAs were transfected at a final concentration of 25 nM using 2 μl HiPerfect transfection reagent per ml medium. Control siRNA was All-star Negative Control (Qiagen) and PTPRS siRNAs were two unique sequences (SI02759288, SI03056284, Qiagen). After 48 hours of transfection, the medium was replaced with phosphate-free DMEM (Invitrogen) supplemented with 10% phosphate-free FBS for 30 minutes. [32P]O4 (0.25 mCi) was added per ml of medium for an additional 2 hours (Perkin Elmer, Waltham, MA). Radiolabeling was quenched with ice-cold TCA (10% final concentration) and cells incubated on ice for 1 hour. Cells were scraped, pelleted and lipids extracted via an acidified Bligh and Dyer method (Bird, 1994). Lipids were lyophilized, resuspended in chloroform:methanol (1:1), spotted on 20 cm × 20 cm silica gel TLC plates (Whatman, Maidstone, UK), and resolved in a chamber using boric acid buffer (Walsh et al., 1991). A PtdIns3P standard was generated by incubating synthetic phosphatidylinositol (diC16 PtdIns; Echelon, Salt Lake City, UT) with immunoprecipitated PI3K (using anti-p85, Cell Signaling, Danvers, MA) and [32P]ATP (Perkin Elmer). The TLC plate was exposed to film for 20 hours at −80°C and developed.
Fluorescent microscopy and western blot analyses of autophagy markers
U2OS cells were seeded at a density of 35,000 cells per well in McCoy's 5A medium with 10% FBS on number 1.5 coverglasses in 24-well tissue culture plates (for fluorescent imaging) or 150,000 cells per well on six-well dishes (for western blot). After 24 hours, cells were transfected with control or PTPRS siRNAs for 48 hours, as described above. Following, cells were treated for 1–2 hours in amino acid starvation medium or with 50 nM rapamycin (Calbiochem, San Diego, CA), 25 μM chloroquine (Sigma, St Louis, MO), 100 nM Baf-A1 (A.G. Scientific, San Diego, CA) or normal growth medium (full nutrients; McCoy's 5A with 10% FBS), as indicated. For western blots, cells were lysed [in 10 mM KPO4, 1 mM EDTA, 10 mM MgCl2, 5 mM EGTA, 50 mM bis-glycerophosphate, 0.5% NP40, 0.1% Brij35, 0.1% sodium deoxycholate, 1 mM NaVO4, 5 mM NaF, 2 mM DTT, and complete protease inhibitors (Sigma)] and 20 μg of total protein was resolved by SDS-PAGE. Proteins were transferred to PVDF membranes and probed with primary antibodies (LC3B, Cell Signaling Technologies; anti-α-tubulin, Sigma) for 16 hours at 4°C followed by secondary antibodies (HRP-linked rabbit or mouse IgG, GE, Piscataway, NJ) for 1 hour at room temperature. Proteins were detected by enhanced chemiluminescence. For EGFP–LC3 imaging, U2OS cells stably expressing ptfLC3 (Addgene plasmid 21074) (Kimura et al., 2007) were fixed with 3.7% formaldehyde and nuclei stained with Hoechst 33342 (2 μg/ml). Coverglasses were inverted onto microslides using mounting gel and cells imaged using a 100× oil-immersion objective on a Nikon Eclipse Ti fluorescence microscope. For immunofluorescence, cells were fixed with 3.7% formaldehyde, permeabilized with 0.2% Triton-X 100, and blocked with 3% bovine serum albumin (BSA) in PBS. Antibodies (LC3B, ATG12, EEA1; Cell Signaling Technologies) were added for 16 hours at 4°C followed by Alexa-Fluor-488-conjugated anti-rabbit IgG (Invitrogen) for 1 hour at room temperature. Nuclei were counterstained with Hoechst 33342, coverglasses were inverted onto microslides using mounting gel and cells were imaged using 60× or 100× oil-immersion objectives on a Nikon TE300 fluorescence microscope (LC3, ATG12) or a 63× water-immersion objective on a Zeiss LSM510 Meta confocal microscope (EEA1). For quantification, punctae were counted using image analysis software after establishing an intensity threshold.
Transmission electron microscopy
U2OS cells in 10 cm dishes were transfected with control or PTPRS siRNAs for 48 hours as described above. Cells were briefly trypsinized, pelleted, rinsed and resuspended in 2% glutaraldehyde fixative (Sigma). Cell pellets were embedded in 2% agarose, postfixed in osmium tetroxide, and dehydrated with an acetone series. Samples were infiltrated and embedded in Poly/Bed 812 resin and polymerized at 60°C for 24 hours. Ultrathin sections (70 nm) were generated with a Power Tome XL (Boeckeler Instruments, Tucson, Arizona) and placed on copper grids. Cells were examined using a JEOL 100C× Transmission Electron Microscope at 100 kV (Tokyo, Japan). Autophagic structures were quantified from images encompassing approximately 8.5 μm2 of cell area each. Electron microscopy services were performed by the Michigan State University Center for Advanced Microscopy (East Lansing, MI).
Exogenous PTPσ expression and immunofluorescence
U2OS EGFP–2xFYVE cells were seeded at a density of 20,000 cells per well in McCoy's 5A medium with 10% FBS on number 1.5 coverglasses in 24-well tissue culture dishes. Full-length PTPRS cDNA (BC104812) was inserted into pRK7 by EcoRI digestion and ligation to yield FL-PTPσ-pRK7 (FL-PTPσ). DNA was transfected at 0.15 μg per well using 0.45 μl FuGeneHD transfection reagent (Roche, Mannheim, Germany) in 50 μl Optimem and 450 μl McCoy's 5A with 10% FBS for 24 hours. For 2 hours, cells were cultured with full nutrient medium or starved of amino acids (Fig. 4A-C), or amino acid starved while treated with DMSO or 100 nM wortmannin (Sigma) (Fig. 5, supplementary material Fig. S3). Alternatively, cells were treated with Baf-A1 (100 nM in full nutrient medium) for 0, 15, 60 or 240 minutes (Fig. 4E). Cells were then fixed with 3.7% formaldehyde, permeabilized with 0.2% Triton X-100, blocked in 3% BSA, and stained with antibodies targeting the D1 domain of PTPσ for 2 hours at room temperature. AF-546-conjugated anti-mouse-IgG (Invitrogen) were incubated for an additional hour at room temperature and nuclei were stained with Hoechst 33342. Cells were imaged using oil-immersion objectives at 60× on a Nikon TE3000 or 100× on an Eclipse Ti fluorescent microscope. For Fig. 4C, cells were treated as above and imaged using a 63× water-immersion objective on a Zeiss LSM510 Meta microscope. Red (AF-546, FL-PTPσ) and green (EGFP–2xFYVE, PtdIns3P) channels were captured with confocality through the Z-plane using 16 increments of 0.25 μm. Stacks through the indicated X and Y planes are shown at the border of an image of the third Z-plane. For supplementary material Fig. S3B, U2OS cells stably expressing mRFP–LC3 (Addgene plasmid 21075) (Kimura et al., 2007) were seeded, transfected, treated (full nutrient or amino acid starvation media for 2 hours), and stained as above. Images were captured at 100× using an oil-immersion objective on an Eclipse Ti fluorescent microscope.
Rescue of siRNA phenotype
U2OS EGFP–2xFYVE cells were seeded on number 1.5 coverglasses in 24-well dishes at 20,000 cells per well in McCoy's 5A medium with 10% FBS. 24 hours later, PTPRS siRNA-1 (CACGGCATCAGGCGTGCACAA; Qiagen) was transfected at a concentration of 25 nM using 1 μl oligofectamine per well per 500 μl McCoy's with 10% FBS (Invitrogen). FL-PTPσ-pRK7 plasmid DNA was transfected 24 hours later at a concentration of 0.15 μg DNA per well using 0.45 μl FuGeneHD transfection reagent in 50 μl Optimem and 450 μl McCoy's 5A with 10% FBS for an additional 24 hours. Cells were fixed and immunostained as described above. Cells were imaged using a 60× oil objective on a Nikon TE300 fluorescent microscope for EGFP–2xFYVE phenotype (green) and FL-PTPσ-pRK7 expression (red). FL-PTPσ-pRK7 expression levels were categorized as high or low. The presence or absence of a robust PTPRS siRNA-induced EGFP–2xFYVE phenotype was determined (phenotype defined as the presence of small, abundant, non-perinuclear EGFP–2xFYVE-positive vesicles; indicated with white arrows in Fig. 4D) for 30–40 cells each of low and high FL-PTPσ-expressing cells, as well as cells transfected with PTPRS siRNA-1 but not FL-PTPσ-pRK7.
In vitro phosphatase assays
GST-tagged recombinant PTPσ containing all residues C-terminal to the transmembrane domain (BC104812 cDNA; aa 883–1501) was generated in pGEXKG (Guan and Dixon, 1991). GST-tagged full-length MTMR6 (NM_004685.2) was generated in pGEXKG and GST-tagged recombinant PTP1B was purchased (Upstate, Billerica, MA). Proteins were purified from BL21 Escherichia coli after isopropyl β-D-1-thiogalactopyranoside (IPTG) induction and used in phosphatase assays. For PtdIns3P phosphatase reactions, 1 μg protein was suspended in 50 μl assay buffer (50 mM sodium acetate, 25 mM Tris-HCl, 10 mM DTT, pH 6.5) with 0, 25, 50 or 200 μM diC8-PtdIns3P and reactions carried out at 37°C for 25 minutes. For Tyr-P phosphatase assays, reactions were carried out as above using 0, 10, 25 or 100 μM Tyr-P peptide (TSTEPQpYQPGENL; Upstate) at 37°C for 15 minutes. Released phosphates were detected with malachite green (Upstate) and absorbance measured at 650 nm. Background levels from enzyme-only and substrate-only (Tyr-P or PtdIns3P) reactions were subtracted and absorbance converted to picomoles free phosphate released per minute using a standard curve.
Supplementary Material
Acknowledgments
We thank the Van Andel Institute Systems Biology lab for advice, analysis, and reagents. This work was supported by the Department of Defense Prostate Cancer Research Program of the Office of Congressionally Directed Medical Research Programs PC081089 to J.P.M. J.P.M. is also supported by Award Number R01CA138651 from the National Cancer Institute. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/5/812/DC1
References
- Abaan O. D., Toretsky J. A. (2008). PTPL1: a large phosphatase with a split personality. Cancer Metastasis Rev. 27, 205-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher B., Lerch M. M., Muller T., Schilling J., Ullrich A. (1997). Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J. Cell Biol. 138, 681-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T., Mohi M. G., Ismat F. A., Bronson R. T., Williams I. R., Kutok J. L., Yang W., Pao L. I., Gilliland D. G., Epstein J. A. (2004). Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat. Med. 10, 849-857 [DOI] [PubMed] [Google Scholar]
- Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. (2008). Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird I. M. (1994). Analysis of cellular phosphoinositides and phosphoinositols by extraction and simple analytical procedures. Methods Mol. Biol. 27, 227-248 [DOI] [PubMed] [Google Scholar]
- Chan G., Kalaitzidis D., Neel B. G. (2008). The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 27, 179-192 [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Neufeld T. P. (2009). An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol. Biol. Cell 20, 2004-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromard M., Bompard G., Glondu-Lassis M., Puech C., Chalbos D., Freiss G. (2007). The putative tumor suppressor gene PTPN13/PTPL1 induces apoptosis through insulin receptor substrate-1 dephosphorylation. Cancer Res. 67, 6806-6813 [DOI] [PubMed] [Google Scholar]
- Elchebly M., Wagner J., Kennedy T. E., Lanctot C., Michaliszyn E., Itie A., Drouin J., Tremblay M. L. (1999). Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat. Genet. 21, 330-333 [DOI] [PubMed] [Google Scholar]
- Ganley I. G., Lam du H., Wang J., Ding X., Chen S., Jiang X. (2009). ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 284, 12297-12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier J. M., Simonsen A., D'Arrigo A., Bremnes B., Stenmark H., Aasland R. (1998). FYVE fingers bind PtdIns(3)P. Nature 394, 432-433 [DOI] [PubMed] [Google Scholar]
- Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaullier J. M., Parton R. G., Stenmark H. (2000). Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577-4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. (1991). Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192, 262-267 [DOI] [PubMed] [Google Scholar]
- Hanada T., Noda N. N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. (2007). The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 282, 37298-37302 [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N. (2009). Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imami K., Sugiyama N., Kyono Y., Tomita M., Ishihama Y. (2008). Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined titania/C18 biphasic column. Anal. Sci. 24, 161-166 [DOI] [PubMed] [Google Scholar]
- Itakura E., Kishi C., Inoue K., Mizushima N. (2008). Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360-5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G., Hill J. H., Yan Y., Sass M., Baehrecke E. H., Backer J. M., Neufeld T. P. (2008). The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J. Cell Biol. 181, 655-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. H., Jun C. B., Ro S. H., Kim Y. M., Otto N. M., Cao J., Kundu M., Kim D. H. (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20, 1992-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A., Noda T., Ishihara N., Ohsumi Y. (2001). Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 152, 519-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Noda T., Yoshimori T. (2007). Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452-460 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J. (2007). Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931-937 [DOI] [PubMed] [Google Scholar]
- Kontaridis M. I., Swanson K. D., David F. S., Barford D., Neel B. G. (2006). PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J. Biol. Chem. 281, 6785-6792 [DOI] [PubMed] [Google Scholar]
- Lorenzo O., Urbe S., Clague M. J. (2006). Systematic analysis of myotubularins: heteromeric interactions, subcellular localisation and endosome related functions. J. Cell Sci. 119, 2953-2959 [DOI] [PubMed] [Google Scholar]
- Mohi M. G., Neel B. G. (2007). The role of Shp2 (PTPN11) in cancer. Curr. Opin. Genet. Dev. 17, 23-30 [DOI] [PubMed] [Google Scholar]
- Mohi M. G., Williams I. R., Dearolf C. R., Chan G., Kutok J. L., Cohen S., Morgan K., Boulton C., Shigematsu H., Keilhack H. (2005). Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell 7, 179-191 [DOI] [PubMed] [Google Scholar]
- Murray J. T., Panaretou C., Stenmark H., Miaczynska M., Backer J. M. (2002). Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic 3, 416-427 [DOI] [PubMed] [Google Scholar]
- Obara K., Noda T., Niimi K., Ohsumi Y. (2008a). Transport of phosphatidylinositol 3-phosphate into the vacuole via autophagic membranes in Saccharomyces cerevisiae. Genes Cells 13, 537-547 [DOI] [PubMed] [Google Scholar]
- Obara K., Sekito T., Niimi K., Ohsumi Y. (2008b). The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 283, 23972-23980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A., Ogier-Denis E., Blommaart E. F., Meijer A. J., Codogno P. (2000). Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992-998 [DOI] [PubMed] [Google Scholar]
- Proikas-Cezanne T., Waddell S., Gaugel A., Frickey T., Lupas A., Nordheim A. (2004). WIPI-1alpha (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene 23, 9314-9325 [DOI] [PubMed] [Google Scholar]
- Pulido R., Serra-Pages C., Tang M., Streuli M. (1995). The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc. Natl. Acad. Sci. USA 92, 11686-11690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe J. E., Streit S., Hart S., Ullrich A. (2006). EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell. Signal. 18, 1515-1527 [DOI] [PubMed] [Google Scholar]
- Rutherford A. C., Traer C., Wassmer T., Pattni K., Bujny M. V., Carlton J. G., Stenmark H., Cullen P. J. (2006). The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J. Cell Sci. 119, 3944-3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi-Atarashi N., Hamasaki M., Matsunaga K., Omori H., Ktistakis N. T., Yoshimori T., Noda T. (2010). Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy. Traffic 11, 468-478 [DOI] [PubMed] [Google Scholar]
- Tanida I., Minematsu-Ikeguchi N., Ueno T., Kominami E. (2005). Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1, 84-91 [DOI] [PubMed] [Google Scholar]
- Uetani N., Chagnon M. J., Kennedy T. E., Iwakura Y., Tremblay M. L. (2006). Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J. Neurosci. 26, 5872-5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Roberts E., Elmaoued R. A., Tosch V., Delgado M. A., Proikas-Cezanne T., Laporte J., Deretic V. (2009). Control of autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO J. 28, 2244-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. J., Batt J., Fladd C. A., Henderson J. T., Skarnes W., Rotin D. (1999). Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat. Genet. 21, 334-338 [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Caldwell K. K., Majerus P. W. (1991). Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc. Natl. Acad. Sci. USA 88, 9184-9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009). Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.