Abstract
Naive B lymphocytes are generally thought to be poor APCs, and there is limited knowledge of their role in activation of CD8+ T cells. In this article, we demonstrate that class I MHC Ag presentation by human naive B cells is enhanced by TLR9 agonists. Purified naive B cells were cultured with or without a TLR9 agonist (CpG oligodeoxynucleotide [ODN] 2006) for 2 d and then assessed for phenotype, endocytic activity, and their ability to induce CD8+ T cell responses to soluble Ags. CpG ODN enhanced expression of class I MHC and the costimulatory molecule CD86 and increased endocytic activity as determined by uptake of dextran beads. Pretreatment of naive B cells with CpG ODN also enabled presentation of tetanus toxoid to CD8+ T cells, resulting in CD8+ T cell cytokine production and granzyme B secretion and proliferation. Likewise, CpG-activated naive B cells showed enhanced ability to cross-present CMV Ag to autologous CD8+ T cells, resulting in proliferation of CMV-specific CD8+ T cells. Although resting naive B cells are poor APCs, they can be activated by TLR9 agonists to serve as potent APCs for class I MHC-restricted T cell responses. This novel activity of naive B cells could be exploited for vaccine design.
Within the population of APCs that includes dendritic cells (DCs), B cells, and macrophages, DCs are proposed to display a unique competence to cross-present Ags and activate naive CD8+ T cells (1, 2). In contrast, the role of B cells in CD8+ T cell priming has been controversial (3–6). Ag-specific B cells have the capacity to induce primary CD8+ T cell responses in the absence of other competent APCs (7, 8). Memory B cells can be APCs and prime naive CD4+ T cells in a CD40L-CD40–dependent manner (4, 9). In contrast, experiments in mice implied that resting and activated H-Y–presenting B cells induce naive CD8+ T cell tolerance (3, 10). Furthermore, recent studies in mice suggested that Ag presentation by naive B cells may lead to peripheral T cell tolerance by induction of regulatory T cells (11). The failure of naive B cells to induce CD8+ T cell immune responses to Ag may stem, in part, from the relatively low expression of costimulatory molecules, because induction of co-stimulatory molecules on naive B cells tends to increase their APC function (6, 12–18). Another significant limitation for naive B cell APC activity is the low frequency of naive B cells that bear receptors for a particular Ag, making Ag recognition and receptor-mediated uptake by naive B cells a relatively rare event. In contrast, other types of APCs more readily sample environmental Ags by endocytic pathways.
Unmethylated CpG oligodeoxynucleotides (ODNs) enhance innate and adaptive immunity and, consequently, have potential usefulness for immunotherapeutic and vaccine adjuvant applications (19, 20). These molecules mediate their effects by interacting with TLR 9 that is expressed within certain APC populations (20). In humans, the professional APCs that express TLR9 include plasmacytoid DCs (pDCs) and B lymphocytes (21, 22). Activation of TLR9 on pDCs may lead to maturation of these cells, as well as production of type I IFN (23–25). Activation of memory B cells by TLR9 can lead to cellular expansion and Ig production, and this may play an important role in memory B cell homeostasis and sustained Ab production (26). The consequences of TLR9 activation in human naive B cells are less well characterized, although we recently demonstrated that TLR9 agonists are sufficient to induce naive B cell proliferation and to enhance survival of these cells (27).
In this study, we considered the possibility that CpG ODNs may enhance naive B cell APC function. We measured the ability of CpG ODN-treated naive B cells to process and to present soluble Ag to CD8 T cells. We found that human primary naive B cells can take up and present Ags to autologous CD8 T cells after TLR9 ligation. The enhanced cross-presentation of Ags to CD8+ T cells is also accompanied by increased surface expression of class I HLA and increased endocytic activity. Thus, human naive B cells have the potential to become potent APCs once activated by TLR9 agonists.
Materials and Methods
Reagents
Endotoxin-free CpG ODN 2216 (5′-GGGGGACGATCGTCGGGGGG-3′), CpG ODN 2395 (5′-TCGTCGTTTTCGGCGCGCGCCG-3′), CpG ODN 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′), and non-CpG ODN 2137 (GC control for CpG ODN 2395 and CpG ODN 2006) were provided by Coley Pharmaceutical Group Inc. (Wellesley, MA). Titration experiments using a range of concentrations, from 1–18 μg/ml, indicated that 6 μg/ml provided an optimal effect. Recombinant human IL-4 was obtained from R&D Systems (Minneapolis, MN). Human CMV lysate (1:40 dilution) was purchased from BioWhittaker (Walkersville, MD). Tetanus toxoid Ag was purchased from Wyeth-Ayerst Lab Inc. (Marietta, PA).
Study subjects
This study was approved by the institutional review board at the University Hospitals of Cleveland. All blood samples were obtained with informed consent. Blood was drawn into heparin-coated tubes from healthy volunteer donors. All participants reported that they had received a tetanus booster shot as adults
Cell isolation
PBMCs were isolated over a Ficoll-Hypaque cushion. Naive B cells were purified by negative selection immunoaffinity using magnetic beads (naive B cell isolation kit II; Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of naive B cells was determined by flow cytometry and was consistently >97%, whereas viability was >95% based on trypan blue dye exclusion.
T cells were isolated by negative selection (Pan T cell isolation kit II; Miltenyi Biotec); purified total T cells were labeled with 10 μmol CFSE (Molecular Probes). Briefly, cells were washed and resuspended in PBS/0.1% BSA buffer and incubated with 10 μmol CFSE solution at 37°C for 10 min. Then cells were incubated in FBS on ice for 5 min. T cells were mixed with unstimulated or stimulated naive B cells for assays of Ag-induced T cell expansion.
In all assay systems, pDCs were depleted from isolated B and T cells using BDCA-4 microbeads (Miltenyi Biotec). The purified B and T cells contained <0.01% pDCs, as determined by flow cytometry.
Ag processing and presentation assays
Purified naive B cells were cultured with or without CpG ODN 2006 or IL-4 (25 ng/ml) for 2 d and then irradiated (2000 rad). B cells were washed, resuspended, and plated in 96-well U-bottom plates (BD Labware), along with autologous CFSE-labeled T cells at APC/T cell ratios of 1:2, 1:10, and 1:50. Ags (CMVor tetanus toxoid [TT]) were added to 0.2 ×106 total cells in each well. T cell proliferation was quantified by measuring the percentage of cells that diluted tracking dye at day 7 or 8. To assess the frequency of CMV tetramer-reactive CD8+ T cells, 10 μl MHC-I HLA-A*0201 human CMV pp65495–503 (NLV9)-PE tetramer (Beckman Coulter Immunomics) was used to stain cells for 30 min at room temperature. Cells were washed with 4 ml PBS/BSA/azide solution, fixed with PBS/BSA/paraformaldehyde solution, examined using a FACSCalibur flow cytometer (BD Immunocytometry Systems), and analyzed by CellQuest software.
In studies that assessed intracellular granzyme B expression in proliferating T cells, CFSE-labeled purified T cells were cultured with naive B cells or CpG-treated naive B cells at a T cell/B cell ratio of 1:10 or 1:5 for 7 d. As above, some of these cell cultures were pulsed with TTAg. After 7 d, cells were surface labeled with anti-human CD8 Ab, fixed, permeabilized, and stained with anti-granzyme B or isotype control Ab (Abs from BD PharMingen, San Jose, CA). Samples were analyzed by flow cytometry.
Staining for intracellular cytokines
Ag-reactive T cells were expanded for use as responder cells in intracellular cytokine-expression assays by stimulating PBMCs with Ag for 10 d. These T cells were washed at day 3 to remove Ag and were washed again before secondary culture. rIL-2 (72 IU/ml; Chiron) was added at day 4. Assays of surface expression of CD69, as well as Ag-induced IFN-γ and TNF-α production, were performed using 2-d cultured naive B cells (0.2 × 106) as APCs and autologous T cell lines (2 × 106) incubated with Ag for 6 h at 37°C in 5% CO2. Brefeldin A (10 μg/ml) was added, and cells were incubated for an additional 3 h. Surface expression of CD69, as well as intracellular IFN-γ and TNF-α expression in CD8 T cells, was measured by flow cytometry.
Assays of Ag uptake
To examine uptake of FITC-labeled dextran in vitro, naive B cells were cultured with or without CpG ODN 2006 (6 μg/ml) for 2 d. Dextran particles (20 KDA; Sigma, St. Louis, MO) were added to 2 d cultures of naive B cells (0.5 × 106 cells/ml) at a ratio of 10 microspheres per cell. Cultures were maintained at 37°C or, as a negative control, on ice for 2 h prior to harvesting. Cells were washed and analyzed using a FACSCalibur flow cytometer. FITC-dextran uptake was calculated by subtracting the background staining of the sample incubated with particles on ice from the staining of the cells incubated with particles at 37°C.
Flow cytometry
Cells were washed in PBS/BSA staining buffer and stained with the following mAbs: anti–IgD-biotin followed by secondary streptavidin-PerCP, anti–CD19-allophycocyanin, anti–HLA-A,B,C (clone G46-2.6), anti–CD27-allophycocyanin, anti–CD4-allophycocyanin, anti–CD8-PERCP, anti–CD40-FITC, anti–CD80-PE, anti–HLA-DR–allophycocyanin, anti–IFN-γ–FITC, anti–TNF-α–PE, anti-granzyme B-Alex 700, and appropriate isotype control mAbs (BD PharMingen, San Jose, CA).
Statistical methods
Variables were compared by the Wilcoxon rank test, and a p value of 0.05 was considered nominally significant.
Results
CpG ODN induces class I MHC and CD86 expression in primary human naive B lymphocytes
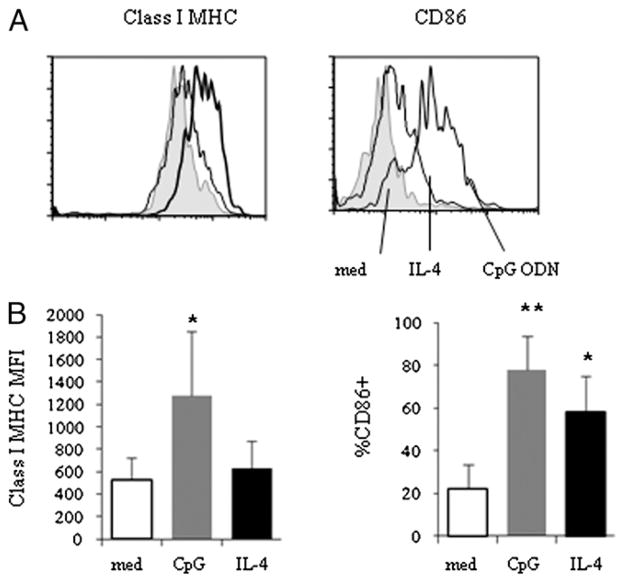
To explore the effects of TLR9 agonists on naive B cell APC function, we first asked whether CpG ODN influences expression of class I MHC and the costimulatory molecule CD86. As anticipated, CpG ODN induced increased surface expression of CD86 on naive B cells. Furthermore, class I MHC expression was also increased after naive B cells were incubated with CpG ODN (Fig. 1). In contrast, cells incubated in IL-4, a cytokine known to enhance B cell survival, did not increase class I MHC expression and produced a smaller increase in CD86 expression than did CpG ODNs on naive B cells (Fig. 1). Our previous study indicated that other costimulatory molecules, including CD40 and CD80, are also induced by CpG ODNs in naive B cells (27).
FIGURE 1.
Increased class I MHC and costimulatory molecule expression in purified naive B cells after CpG ODN 2006 stimulation. Purified naive B cells were isolated and cultured in medium alone (med) or medium supplemented with CpG ODN 2006 (CpG) or with IL-4 (25 ng/ml) for 20 h. Surface expression of class I MHC (A, B, C) and CD86 among naive B cells was analyzed by flow cytometry. A, Representative graphs of class I MHC and CD86 expression on naive B cells. B, Summary data representing median fluorescence intensities (mean ± SD, n = 9) of class I MHC expression (p = 0.008 compared with untreated cells) and percentage of CD86-expressing cells. *p < 0.05, **p < 0.001.
Enhanced endocytosis by naive B cells treated with CpG ODNs
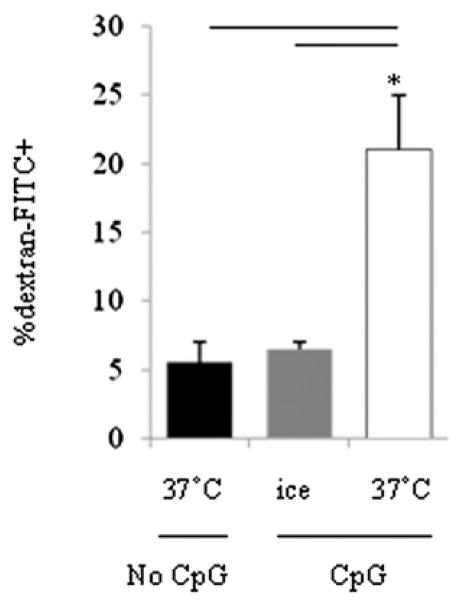
An important limitation of naive B cell APC function may reside in the relatively poor capacity of these cells to take up Ags by endocytic processes. Thus, only rare naive B cells with Ag-specific receptors would be expected to mediate Ag uptake under resting conditions. We tested the endocytic activity of naive B cells by measurement of dextran uptake. Purified naive B cells were stimulated with or without CpG ODN 2006 for 20 h, washed, and incubated with dextran for 2 h on ice or at 37°C. Cells were harvested and analyzed by flow cytometry for dextran uptake. As shown in Fig. 2, naive B cells incubated in medium alone at 37°C or CpG-treated naive B cells incubated on ice demonstrated relatively poor uptake of dextran, whereas naive B cells incubated with CpG ODNs had markedly greater dextran uptake (Fig. 2). CpG incubation had no effect on latex bead uptake (data not shown). These results suggested that CpG ODNs are better at taking up small particles, such as dextran or TT Ag (Fig. 3), but not big particles, such as latex beads. These observations suggested that CpG ODNs induce endocytic mechanisms in naive B cells.
FIGURE 2.
Enhanced endocytosis by purified naive B cells after CpG ODN 2006 stimulation. Purified naive B cells were cultured with or without CpG ODN 2006 (6 μg/ml) for 2 d. Cells were irradiated, washed, and cocultured with dextran on ice or at 37°C for another 2 h and analyzed by flow cytometry. Data shown are mean percentages (± SD) of naive B cells that ingested FITC dextran (n = 5). *p < 0.05.
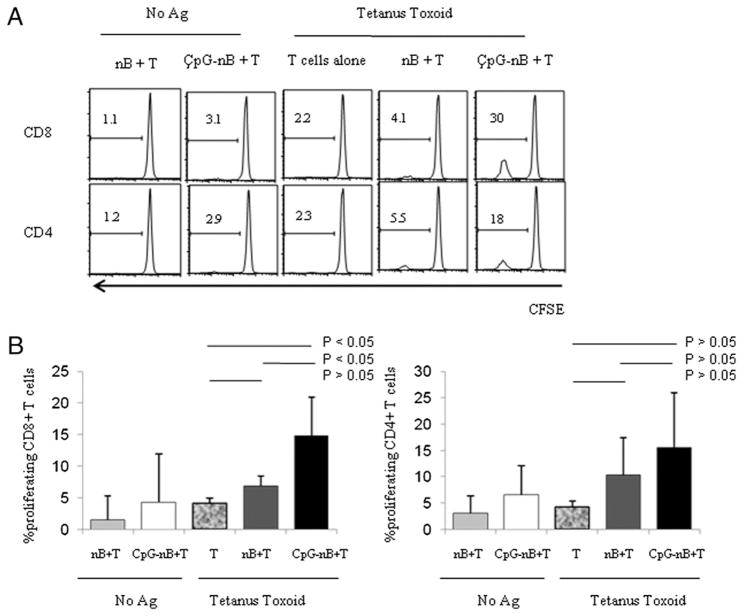
FIGURE 3.
TT-specific CD8+ T cell proliferation is enhanced by CpG ODN-stimulated naive B cells. Purified naive B cells (nB) were cultured with (CpG-nB) or without CpG ODN 2006 (6 μg/ml) for 2 d. Cells were irradiated, washed, and then cocultured with purified autologous CFSE-labeled CD3+ T cells in the presence or absence of TT for 8 d. Proliferation of gated CD8+ and CD4+ T cells was assessed by dilution of CFSE dye. A, Graphs of one representative experiment. B, Percentages of proliferating CD8+ and CD4+ T cells (mean ± SD). The results were analyzed using blood from six donors. The comparisons were analyzed by the non-parametric Wilcoxon rank test.
CpG-activated naive B cells induce expression of CD69, IFN-γ, and TNF-α in TT-stimulated CD8+ T cells
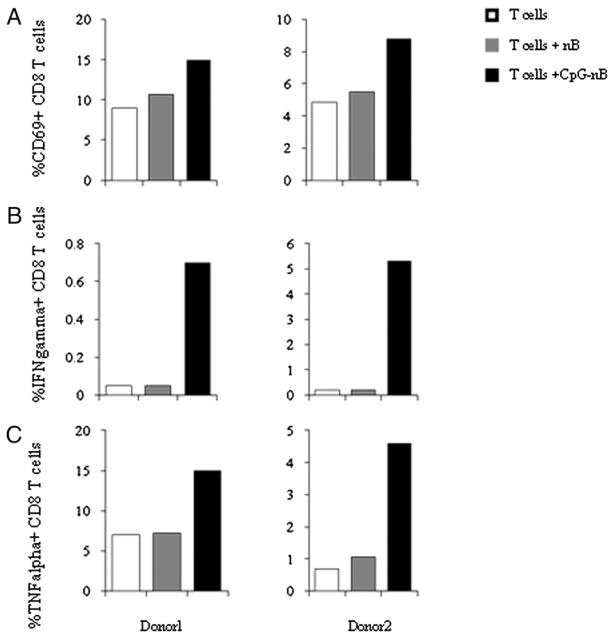
To investigate the potential for naive B cells to present Ag to CD8+ T cells, we incubated purified naive B cells in medium alone or in medium plus CpG ODNs for 2 d. B cells were then mixed with tetanus-reactive autologous T cell lines in the presence of TT for an additional 16 h. T cells were assessed for responses to Ag by measuring CD69 expression and intracellular production of TNF-α and IFN-γ. We found that TT-dependent responses of CD8+ T cells were induced by CpG ODN-stimulated naive B cells but not untreated naive B cells (Fig. 4). Purified T cells incubated with Ag but without B cells failed to respond to TT, attesting to the lack of DC or other APC contamination in the T cell samples.
FIGURE 4.
CpG ODN-activated naive B cells enhance CD8+ T cell surface CD69, as well as intracellular TNF-α and IFN-γ expression in response to TT stimulation. Purified naive B cells (nB) from two individuals were incubated with (CpG-nB) or without CpG ODN 2006 (6 μg/ml) for 20 h. Cells were irradiated, washed, and cocultured with autologous T cell lines in the presence of TT (4 LFU/ml) for 16 h. Brefeldin A was added to inhibit Golgi-mediated export of cytokines. Percentages of CD69+ CD8+ T cells (A), IFN-γ+CD8+ T cells (B), and TNF-α+CD8+ T cells (C) are shown under the following three conditions: purified T cells with Ag, purified T cells with Ag and medium-cultured naive B cells, and purified T cells with Ag and CpG-cultured naive B cells.
Induction of TT-mediated CD8+ T cell proliferation by CpG ODN-activated naive B cells
To evaluate the capacity of CpG-treated naive B cells to induce T cell proliferation responses to recall Ag, we cultured CpG-treated and untreated naive B cells with purified, CFSE-labeled autologous T cells and TT Ag. As shown in Fig. 3, unstimulated naive B cells were able to induce modest CD8+ T cell-proliferation responses in the presence of TT that were not significantly different from CD8+ T cell responses to Ag in the absence of B cells (Fig. 3A); however, pretreatment of naive B cells with CpG ODN dramatically enhanced the ability of these cells to induce CD8+ T cell proliferation in response to TT. In contrast, CpG ODN pretreatment of naive B cells had only modest effects on the ability of these cells to induce CD4+ T cell-proliferation responses to TT in comparison with B cells that were not treated with CpG ODN (Fig. 3). Notably, the mean percentage of proliferating CD8+ T cells among T cells cultured with CpG-treated naive B cells in the absence of Ag was low (4.95%, n = 6) compared with cells incubated in the presence of Ag (14.8%), suggesting that the effects of CpG B cells in the latter circumstance could not simply be explained by enhancement of autologous MLRs.
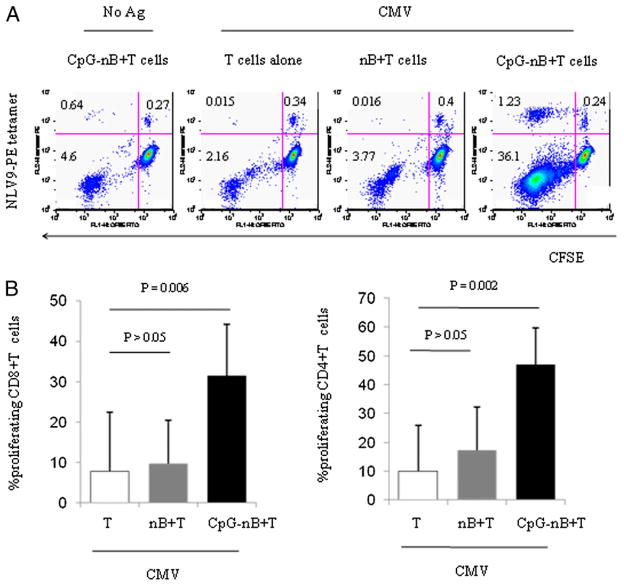
Induction of CMV-pp65–specific CD8 T cell response by CpG-treated naive B cells
To assure that the effects of CpG-ODN–primed naive B cells on class I MHC restricted T cell responses were also demonstrable among CD8+ T cells recognizing viral Ags, we tested CD8+ T cell-proliferation responses to whole CMV Ag and confirmed the specificity of these responses by staining with NLV9 (NLV-PMAVTV) HLA2-specific tetramers. Naive B cells incubated with CpG ODNs or medium alone for 2 d were then mixed with purified autologous CFSE-labeled T cells and CMV Ag for 7–8 d. CD4 and CD8 (Fig. 5) T cells proliferated in response to CMV Ags presented by CpG ODN-primed naive B cells. The proliferating cells included NLV9-tetramer–specific CD8+ T cells, confirming the specificity of the CD8+ T cell response (Fig. 5A). CpG-treated naive B cells caused a significant increase in the magnitude of the CMV-induced CD8+ T cell response and the expansion of CD8+ T cells that bound this NLV9-tetramer compared with the responses of untreated naive B cells. Thus, CpG ODN exposure enhances naive B cell APC function, resulting in an increased capacity for these cells to induce Ag-specific CD8+ T cell responses through the TCR in response to exogenously delivered soluble Ags.
FIGURE 5.
CMV-tetramer–binding CD8+ T cells expand in response to CpG-activated naive B cells in the presence of CMV Ags. Naive B cells (nB) from five CMV seropositive donors were treated with (CpG-nB) or without CpG ODN 2006 (6 μg/ml). Cells were irradiated, washed, and cocultured with autologous CFSE-labeled CD3+ T cells in the absence or presence of CMV Ags for 8 d. Proliferation of CD8+ T cells was assessed by dilution of CFSE dye and by binding of PE-conjugated HLA-A2 NLVPMVATV tetramer complexes. A, Dot plots represent dilution of CFSE dye by proliferating T cells and tetramer staining from one of two representative experiments using blood of HLA-A*0201 donors. B, Percentages of proliferating CD8+ and CD4+ T cells (mean ± SD, n = 5). The comparisons were analyzed using the Wilcoxon rank test.
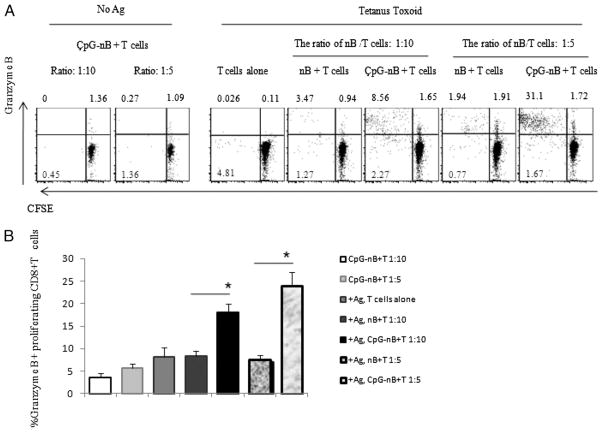
Induction of granzyme B expression in proliferating CD8+ lymphocytes by CpG-treated naive B cells
To determine whether Ag presentation by CpG-treated naive B cells also affected expression of molecules involved in CTL function, we examined granzyme B expression in CD8+ T cells that proliferated in response to TT stimulation. Granzyme B is an important component of lytic granules that mediates apoptosis of target cells (28). The percentages of CD8+ T cells that were proliferating and expressing intracellular granzyme B were markedly greater in CD8+ T cells that were stimulated with Ag presented by CpG-treated naive B cells compared with untreated naive B cells (Fig. 6). Roughly 80–90% of CFSElow cells expressed granzyme B among CD8 cells that proliferated in response to TT presented by CpG-treated naive B cells (Fig. 6A, data not shown).
FIGURE 6.
CpG-treated naive B cells induce granzyme B expression in proliferating CD8+ lymphocytes. Purified naive B cells were cultured with (CpG-nB) or without (nB) CpG ODN 2006 (6 μg/ml) for 2 d. Cells were washed and cocultured with purified autologous CFSE-labeled CD3+ T cells at a ratio of 1:10 and 1:5 in the presence or absence of TT for 7 d. A, Intracellular expression of granzyme B and proliferation of CD8+ T cells from one representative donor is shown in dot plots. B, Percentages of CD8+ T cells that are proliferating and expressing intracellular granzyme B from seven donors (mean ± SEM, n = 7). The comparisons were analyzed using the Wilcoxon rank test. *p < 0.05.
Discussion
CpG ODN provides a sufficient signal, which we showed in earlier studies, to induce naive B cell expansion and protect activated naive B cells from apoptosis (27). Despite induction to proliferate, naive B cells exposed to CpG ODN do not undergo phenotypic maturation into a memory phenotype, yet they differentiate to express increased surface levels of costimulatory molecules (27).
Because costimulation is an important component of APC function, we hypothesized that naive B cells exposed to CpG ODN may have more potent APC function. Thus, in contrast to resting naive B cells that express low levels of costimulatory molecules and that generally induce a tolerant T cell response (5, 13), naive B cells activated to express costimulatory molecules may provide more effective APC function for induction of T cell immunity (4, 14). A variety of stimuli, including cytokines, CD40L (4, 14), and TLR agonists (e.g., CpG ODNs) (6, 29), were shown to enhance costimulatory function of resting B cells in mice; our work points to a similar effect of CpG ODNs on human naive B cells.
The role of human naive B cells in priming T cell responses in vivo is not well recognized; however, in general, it is thought that other cell types, especially DCs, play a more central role in this process. A significant role for naive B cells in Ag presentation is thought to be limited by the relatively rarity of cells that express specificity for a given Ag that would be capable of receptor-mediated Ag uptake. Our results demonstrated that CpG ODN enhances endocytic uptake by naive B cells, providing these cells with an enhanced ability to ingest Ags prevalent in the microenvironment without a need for Ag specificity. However, a potential limitation of naive B cell APC function in vivo is the rarity (or failure) of their migration to tissue sites and the resultant diminished opportunity to encounter Ag. Nevertheless, naive B cells circulate through the blood and lymph and home to secondary lymphoid organs where they may encounter extrafollicular Ag-bearing DCs and T cells; these cells may have opportunities to contribute to initial phases of immune responses (30, 31).
A striking observation that we made in this study is the unique capacity of CpG ODN-primed naive B cells to cross-present soluble Ag to CD8+ T cells. Generally, cross-presentation is thought to be mediated largely by DCs, with limited contributions from other cell types. Nonetheless, previous studies indicated that resting mouse B cells exposed to CpG ODN expressed increased levels of CD40 and CD86 and can cross-present exogenous Ag to CD8 T cells (6, 32). The present studies demonstrated that human naive B cells also respond to CpG ODN with enhanced cross-presentation capabilities that coincide with increased surface expression of class I MHC. The increase in class I MHC expression on naive B cells likely potentiates the enhanced presentation of Ag to CD8+ T cells, but it is also likely that other changes in these cells contribute to B cell cross-presentation. These may include changes in Ag uptake or changes in the expression of proteolytic enzymes, TAP, or other molecules implicated in cross-processing and cross-presentation. Further studies into the mechanisms that facilitate human naive B cell cross-presentation may provide important insights into the regulation of these functions and may help to clarify the potential role of naive B cells in the generation of the adaptive T cell response. These observations provide insight on a novel function of naive human B cells and have the potential to inform the design of new vaccine strategies.
Recent studies in humans demonstrated that administration of CpG ODN adjuvant can enhance humoral and cell-mediated immunity to hepatitis B vaccine (33–35). Importantly, no major adverse events have been reported with CpG ODN adjuvant administration in humans (35). The involvement of CD8+ T cell responses to Ag in these trials has not been carefully evaluated; however, experiments in mice suggest that CpG ODN can enhance CD8+ T cell responses to recombinant protein Ag in a B cell-dependent manner (36). These data and our in vitro observations with human cells suggest that strategies that target Ags and CpG ODN to B cells may be an effective approach for induction of CD8+ T cell immunity.
Acknowledgments
This work was supported in part by National Institutes of Health Grants AI55793, AI68636, and AI034343 and the Center for AIDS Research at Case Western Reserve University, University Hospitals of Cleveland (AI36219).
We thank Arthur M. Krieg for helpful advice and the Coley Pharmaceutical Group for providing the CpG ODN. We also appreciate the assistance of Robert Asaad in the collection of clinical samples.
Abbreviations used in this article
- CMV-NLV9
human CMV HLA-A*0201 pp65495-503
- DC
dendritic cell
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid dendritic cell
- TT
tetanus toxoid
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pozzi LA, Maciaszek JW, Rock KL. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol. 2005;175:2071–2081. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- 3.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 5.Raimondi G, Zanoni I, Citterio S, Ricciardi-Castagnoli P, Granucci F. Induction of peripheral T cell tolerance by antigen-presenting B cells. II. Chronic antigen presentation overrules antigen-presenting B cell activation. J Immunol. 2006;176:4021–4028. doi: 10.4049/jimmunol.176.7.4021. [DOI] [PubMed] [Google Scholar]
- 6.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–1507. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 7.Castiglioni P, Gerloni M, Zanetti M. Genetically programmed B lymphocytes are highly efficient in inducing anti-virus protective immunity mediated by central memory CD8 T cells. Vaccine. 2004;23:699–708. doi: 10.1016/j.vaccine.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Filaci G, Gerloni M, Rizzi M, Castiglioni P, Chang HD, Wheeler MC, Fiocca R, Zanetti M. Spontaneous transgenesis of human B lymphocytes. Gene Ther. 2004;11:42–51. doi: 10.1038/sj.gt.3302132. [DOI] [PubMed] [Google Scholar]
- 9.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 11.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, Gunzer M. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 12.Wu HY, Monsonego A, Weiner HL. The mechanism of nasal tolerance in lupus prone mice is T-cell anergy induced by immature B cells that lack B7 expression. J Autoimmun. 2006;26:116–126. doi: 10.1016/j.jaut.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Buhlmann JE, Foy TM, Aruffo A, Crassi KM, Ledbetter JA, Green WR, Xu JC, Shultz LD, Roopesian D, Flavell RA, et al. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995;2:645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 14.Clatza A, Bonifaz LC, Vignali DA, Moreno J. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J Immunol. 2003;171:6478–6487. doi: 10.4049/jimmunol.171.12.6478. [DOI] [PubMed] [Google Scholar]
- 15.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000;164:688–697. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov R, Aarts T, Hagenbeek A, Hol S, Ebeling S. B-cell expansion in the presence of the novel 293-CD40L-sCD40L cell line allows the generation of large numbers of efficient xenoantigen-free APC. Cytotherapy. 2005;7:62–73. doi: 10.1080/14653240510018055. [DOI] [PubMed] [Google Scholar]
- 17.Tobian AA, Harding CV, Canaday DH. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J Immunol. 2005;174:5209–5214. doi: 10.4049/jimmunol.174.9.5209. [DOI] [PubMed] [Google Scholar]
- 18.Yuschenkoff VN, Sethna MP, Freeman GJ, Parker DC. Coexpression of B7-1 and antigen blocks tolerance induction to antigen presented by resting B cells. J Immunol. 1996;157:1987–1995. [PubMed] [Google Scholar]
- 19.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 22.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 23.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray RC, Kuchtey J, Harding CV. CpG-B ODNs potently induce low levels of IFN-alphabeta and induce IFN-alphabeta-dependent MHC-I cross-presentation in DCs as effectively as CpG-A and CpG-C ODNs. J Leukoc Biol. 2007;81:1075–1085. doi: 10.1189/jlb.1006606. [DOI] [PubMed] [Google Scholar]
- 25.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naïve B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 28.Kam CM, Hudig D, Powers JC. Granzymes (lymphocyte serine proteases): characterization with natural and synthetic substrates and inhibitors. Biochim Biophys Acta. 2000;1477:307–323. doi: 10.1016/s0167-4838(99)00282-4. [DOI] [PubMed] [Google Scholar]
- 29.Heit A, Maurer T, Hochrein H, Bauer S, Huster KM, Busch DH, Wagner H. Cutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8 T cells. J Immunol. 2003;170:2802–2805. doi: 10.4049/jimmunol.170.6.2802. [DOI] [PubMed] [Google Scholar]
- 30.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Kenji A, Norihiro T, Eisaku K, Takashi O, Kazuhiko H, Tadashi Y, Tadaatsu A. Morphological interactions of interdigitating dendritic cells with B and T cells in human mesenteric lymph nodes. Am J Pathol. 2001;159:131–138. doi: 10.1016/S0002-9440(10)61680-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobian AA, Potter NS, Ramachandra L, Pai RK, Convery M, Boom WH, Harding CV. Alternate class I MHC antigen processing is inhibited by Toll-like receptor signaling pathogen-associated molecular patterns: Mycobacterium tuberculosis 19-kDa lipoprotein, CpG DNA, and lipopolysaccharide. J Immunol. 2003;171:1413–1422. doi: 10.4049/jimmunol.171.3.1413. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, Rasmussen WL, Waldschmidt M, Sajuthi D, Purcell RH, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617–1624. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 34.Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–1479. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 35.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 36.Hoft DF, Eickhoff CS, Giddings OK, Vasconcelos JR, Rodrigues MM. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J Immunol. 2007;179:6889–6900. doi: 10.4049/jimmunol.179.10.6889. [DOI] [PubMed] [Google Scholar]