Abstract
Glutamatergic inputs to the nucleus accumbens (NAc) modulate both appetitive and fearful motivation. Pathological disturbances of glutamate signaling in NAc have been suggested to contribute to motivation disorders, ranging from excessive desire in drug addiction to paranoia in schizophrenia. Metabotropic glutamate receptors are of special interest, as metabotropic Group II receptor (mglu2/3) agonists have been proposed as potential treatments for both addiction and schizophrenia. Here we tested whether local mglu2/3 receptor blockade in medial shell of the rat NAc can generate intense distortions of motivation or affect, which might model clinical dysfunction. We found that microinjection of the mglu2/3 antagonist LY341495 suppressed appetitive motivation to eat and drink at sites throughout medial shell. Simultaneously, LY341495 microinjections generated fearful motivation in the form of defensive treading or burying. To assess whether the valence shift extended into a parallel hedonic shift from affective ‘liking’ to ‘disliking,’ we employed the taste reactivity test, which measures affective orofacial reactions to the sensory pleasure or displeasure of tastes. We found that LY341495 microinjections reduced positive ‘liking’ reactions to sucrose and enhanced ‘disliking’ reactions. Overall, mglu2/3 antagonism at most shell sites produced a similar valence shift from positive to negative. This pattern comprised a) generation of fearful behaviors, and b) induction of aversive affective reactions, together with c) loss of appetitive ingestion and d) loss of ‘liking’ for rewards. These results are discussed in terms of implications for clinical disorders and the influence of corticolimbic glutamate inputs to NAc in the generation of motivation and affect.
Keywords: motivation, eating, defensive treading, taste reactivity, rat
Introduction
The nucleus accumbens (NAc) is best known for its role in appetitive motivated behaviors like reward seeking (Ikemoto & Panksepp, 1999; Cardinal & Everitt, 2004; Kalivas & Volkow, 2005; Kelley et al., 2005; Nicola, 2007; Sesack & Grace, 2010), but also plays a role in negatively valenced motivation and affect, such as fear, pain, stress and disgust (Blackburn et al., 1992; Salamone, 1994; Horvitz, 2000; Levita et al., 2002; Reynolds & Berridge, 2002; Scott et al., 2006; Kerfoot et al., 2007; Delgado et al., 2008; Levita et al., 2008; Carlezon & Thomas, 2009). Glutamate inputs to NAc medial shell participate in the bivalent generation of motivations (Maldonado-Irizarry et al., 1995; Kelley & Swanson, 1997; Reynolds & Berridge, 2003), and anatomically arise predominantly from prefrontal cortex, and cortical related-structures such as basolateral amygdala, hippocampus and thalamus (Beckstead, 1979; Christie et al., 1987; Fuller et al., 1987; Sesack et al., 1989; McDonald, 1991; Swanson, 2005; Wolf et al., 2005; Zahm, 2006; Belujon & Grace, 2008). Disruptions of glutamate signaling are implicated in a variety of motivation disorders including addiction, depression and schizophrenia (Moghaddam, 2004; Lapish et al., 2006; Uys & LaLumiere, 2008; Floresco et al., 2009; Guo et al., 2009; Kalivas, 2009).
Metabotropic glutamate receptors may be especially implicated in psychopathology: specifically, the Group II (mglu2/3) subtypes have been suggested as targets for the pharmacological treatment of depression, anxiety, schizophrenia and addiction (Kenny & Markou, 2004; Moghaddam, 2004; Swanson et al., 2005; Patil et al., 2007). Group II mglu receptors are prevalent in NAc as well as other limbic structures (Kenny & Markou, 2004; Gu et al., 2008) and there is evidence that agonists for NAc mglu2/3 receptors may suppress reinstatement of drug seeking and conditioned place preference (Markou et al., 2004; Bossert et al., 2006; Gerdjikov & Beninger, 2006; Liechti et al., 2007). Given the increasing interest in agonists for mglu2/3 receptors as potential medications for disorders of motivation, we wanted to understand whether blocking mglu2/3 receptors could conversely induce any pathological motivational states.
Here, we investigated whether localized blockade of mglu2/3 receptors in NAc medial shell modulates the generation of motivated behavior or affective reactions to tastes. Following microinjections of the mglu2/3 antagonist LY341495 into NAc shell, rats were tested for spontaneous appetitive eating and drinking, and for fearful behavior in the form of defensive treading, an anti-predator behavior which rodents emit in the wild to rattlesnakes, and in the laboratory to shock prods and other noxious objects (Coss & Owings, 1978; Treit et al., 1981). We also tested for modulation of affective reactions elicited by sweet or bitter tastes infused into the rat’s mouth. Disruptions of mglu2/3 transmission were found to suppress feeding and drinking behavior, while promoting defensive treading behavior, suggesting a fearful or anxious state. Independently, in the taste reactivity paradigm we found that mglu2/3 disruptions suppressed ‘liking’ reactions to sweetness and conversely increased ‘disliking’ reactions of disgust that are normally reserved for bitter tastes.
Materials and Methods
Synopsis
In order to independently test the effect of mglu2/3 blockade on motivated behaviors and affective reactions to taste, rats were randomly separated into food intake and taste reactivity groups. Independent groups of rats were tested for the effects of mglu2/3 blockade on generation of spontaneous appetitive and fearful motivated behavior or modulation of ‘liking’ reactions to the taste of sweet sucrose solution and for ‘disliking’ reactions to bitter quinine solution in the taste reactivity test. Additionally, we analyzed local Fos expression in order to assess the functional impact and spread of drug microinjections on surrounding tissue.
Subjects
Sprague-Dawley rats [male, n= 44 (food intake testing, n=16; taste reactivity testing, n=14; Fos plume analysis, n =14), 300 – 500 grams at the time of surgery] were housed on a 12:12 reverse light/dark cycle at ~21°C with ad lib access to food (Purina Rat Chow) and water (tap water). All experimental procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Surgery
All rats were implanted with permanent bilateral cranial cannulae aimed at points throughout the anatomical extent of medial shell of NAc or at anatomical control sites. The coordinates used for each rat were bilaterally symmetrical, but placements were staggered across rats in anteroposterior and dorsoventral planes so that the group’s placements filled the medial shell. Rats were placed under surgical anesthesia using ketamine (80 mg/kg) and xylazine (5 mg/kg) and pretreated with atropine (0.04 mg/kg) to prevent respiratory distress, after which they were placed in a stereotaxic apparatus (David Kopf Instruments). All rats received bilateral implantation of 14mm, 23 gauge stainless-steel guide cannulae aimed 2mm above target sites. The incisor bar was set at 5.0 mm above intra-aural zero so that cannulae were angled to avoid the lateral ventricles. For medial shell rats (n=41) placements were adjusted in order to achieve microinjection sites spaced throughout the shell, and were aimed at coordinates anteroposterior (AP) +2.4 to +3.4, mediolateral (ML) +/− 0.8 to 1.0mm, and dorsoventral (DV) −5.5 to −5.7 mm in comparison to bregma. Anatomical control rats (n=3) received cannulae aimed at sites dorsal and medial to NAc shell, including the intermediate and ventral areas of the lateral septum. Cannulae were anchored to the skull using four surgical screws and dental acrylic, and stainless steel obturators were inserted to avoid cannulae occlusion. An independent group (n=14), designated for taste reactivity testing, received implantation of permanent oral cannulae for the infusion of taste solutions in addition to the microinjection cannulae in medial shell. Oral cannula were made of ethyl vinyl acetate microbore tubing (0.04-in. inner diameter, Cole-Parmer, Vernon Hills, IL), and each was inserted into the mouth just lateral to the first maxillary molar and run subcutaneously along the zygomatic arch to the top of the skull, where the cannula exited through an incision. There, the tubing was secured to a 15mm, 19 gauge stainless-steel cannula with wiring and dental acrylic. Post-surgery, each rat received chloramphenicol sodium succinate (60 mg/kg) to prevent infection, and buprenorphine hydrochloride (0.3 mg/kg) for pain relief. Rats recovered for at least 7 days before testing began.
Drugs and Intracerebral Microinjections
We dissolved the selective and highly potent antagonist for mglu2/3 receptors, (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xan th-9-yl) propanoic acid (LY341495, Tocris) in ~10 μl of .1 M NaOH and brought to dose volume using 0.9% sterile saline solution. The pH was normalized to 7.4 using HCl for both drug and vehicle microinjections. Rats received the following microinjections, counterbalanced for order and spaced 48 hours apart: either 5 μg LY341495 in 0.5μl vehicle per side, or vehicle alone 0.5 μl per side as within-subject control. At a speed of 0.3 μl/m, drugs were bilaterally infused through stainless-steel injectors (29 gauge) which extended 2mm beyond the guide cannula into the NAc shell and were attached to the syringe pump via PE-20 tubing. Following infusion, injectors were left in place for 1 min to allow drug to diffuse away from the microinjector tip, after which obturators were replaced and rats were placed in the testing chamber.
Behavioral Tests of Fear and Feeding
To assess spontaneous motivated behaviors, following 3 days of preexposure in the home cage to palatable chocolates (M&Ms) in order to counter any neophobia, and 3 days of handling, rats implanted with intracranial cannulae (n = 16) were preliminarily habituated to the food intake procedure and apparatus on 4 days. On the 4th day of habituation rats also received mock microinjection of vehicle prior to entering the testing chamber, in order to habituate them to the microinjection procedure. On the test day, rats either received LY341495 or vehicle and were placed immediately in the transparent testing chamber, which contained ~3cm deep of bedding, ad lib water, and ~20 g of preweighed M&Ms. These stimuli allowed the expression of eating and drinking behavior as well as of defensive treading behavior (using the granular bedding). Behavior was taped for 60 min post-microinjection, and scored later offline for analysis.
Behavioral Taste Reactivity Test
Rats (n=14) implanted with oral cannulae and intracranial cannulae were habituated to the taste reactivity chambers on 4 days prior to testing. On the 4th day of habituation rats received mock microinjection of vehicle, and mock infusions of distilled water. On the test days, rats either received LY341495 or vehicle and PE-50 tubing was attached to an oral cannula. Rats were then placed immediately in the taste reactivity chamber, which had a transparent floor, under-which was angled a mirror from which reactions were taped. After a 10 min delay a 1ml volume of either sucrose (0.1 M) or quinine (3×10−5M) was infused into the rat’s mouth over a 1m period. Each rat was re-tested again at 35 min after microinjection with a second 1-ml infusion of the same taste solution to examine the time course of modulation. Sucrose solution and quinine solution were presented on separate days, for a total of 4 test days (once after LY341495 microinjection; once after vehicle microinjection) in an alternating order that was counterbalanced across rats. Orofacial reactions to taste were videorecorded and analyzed later offline by an observer who was blind to the drug condition.
Behavioral Video Scoring
Spontaneous fear and feeding behaviors
An experimenter blind to treatment scored each tape for the total time (seconds) rats spent eating (mouth in contact with food pellet or continual chewing movements), drinking (tongue in contact with spout), defensive treading (rapid, alternating thrusts of the forepaws, which spray or push bedding material to the front of the rat), and grooming. In addition to total time spent in each behavior, the experimenter recorded the number of bouts, or episodes, of appetitive behavior as well. For appetitive motivated behaviors (eating and drinking) the end of a bout was marked by a pause in behavior of at least 5 seconds (which typically extended into a much longer break in behavior). The total number of each behavior was scored for more discrete events including food carrying (grasping and transportation of food pellets in the mouth), food sniffs, rears and cage crosses.
Hedonic taste reactivity scoring
A trained observer, blind to experimental and taste solution condition, scored the hedonic, aversive and neutral reactions to tapes in slow motion (1/25 to 1/10 speed) on Observer software (Noldus, Netherlands). Totals for each type of reaction were tallied using a bin system which was designed to allow comparison of hedonic and aversively valenced reactions, and is described elsewhere (Berridge & Grill, 1984; Berridge, 2000). Hedonic reactions include rhythmic tongue protrusions along the midline, lateral tongue protrusions, and paw-licks, whereas aversive reactions consist of face washing, gapes, chin rubs, forelimb flails, and headshakes. Neutral reactions to tastes are less consistently linked to hedonic valence, and consist of neutral mouth movements, passive drip, and grooming behavior. Behaviors which typically occur as discrete events were counted each time they occurred, including lateral tongue protrusions, gapes, chin rubs, forelimb flails and headshakes. Behaviors which occur generally in succession were binned based on the typical length and frequency of these reactions; tongue protrusions were scored in 2-sec bins and paw-licks, face washing, mouth movements, and grooming consisted of 5-sec bins.
Histology
Following all testing, rats used for behavioral tests were deeply anesthetized with an overdose of sodium pentobarbital and decapitated. Brains were removed and fixed in 10% paraformaldehyde overnight, and then cryoprotected in 25% sucrose solution for at least 2d. Brains were then sliced at 60 microns on a freezing microtome, and stained with cresyl violet for verification of microinjection sites. Bilateral microinjection sites were placed on coronal slices from a rat brain atlas (Paxinos & Watson, 2007). Later these placements were used to position bilateral injection sites for each rat onto sagittal and horizontal slices, which were then color-coded for behavioral changes in the figures.
In order to quantify localization of function for mglu2/3 receptors within medial shell, we also measured functional spread of drug via changes in local Fos expression in a separate group of rats (n = 14 [vehicle = 6, LY341495 = 8]). Local plumes of modulated Fos expression are produced surrounding the microinjection site of drugs such as DNQX, which blocks AMPA-kainate receptors, as well for drugs that modulate neurotransmission at opioid, cannabinoid, dopamine, or GABAergic synapses (Peciña & Berridge, 2000; Peciña & Berridge, 2005; Smith & Berridge, 2005; Pecina et al., 2006; Mahler et al., 2007; Faure et al., 2008; Faure et al., 2010). Here, following a single microinjection of either LY341495 or vehicle, brain slices were processed for Fos expression and analyzed for local Fos plumes as described previously (Peciña & Berridge, 2005; Smith & Berridge, 2005; Mahler et al., 2007; Smith & Berridge, 2007; Faure et al., 2008; Reynolds & Berridge, 2008).
Statistical Analysis
The effect of drug microinjection on behaviors of interest was tested using both one-factor (drug) repeated measures ANOVA and/or a two-factor mixed within- and between-subjects ANOVA (drug x anatomical level, rostral, middle, and caudal, or dorsal and ventral). Taste reactivity data was also analyzed using a two-factor within-subject ANOVA (drug x time). For statistical analysis rostral or caudal classification was determined by placing rats with AP coordinates >+1.8mm ahead of bregma in the rostral group, and rats with AP coordinates <+1.6 mm ahead of bregma in the caudal group. Dorsal versus ventral classification was determined by placing rats with DV coordinates >7.6 mm below the skull in the ventral group, and rats with DV coordinates <7.4 mm below the skull in the dorsal group. Rats with placements between these cut-offs, or with asymmetrical placements in both halves were designated as middle. For the purposes of visualizing changes in behavior produced at particular sites, the anatomical placements of rats were divided further into 6 rostrocaudal and 6 dorsoventral bins and data from each bin was represented in a bar graph superimposed next to maps of behavioral effects produced at individual sites in each figure.
Results
LY341495 suppresses appetitively motivated behaviors throughout medial shell
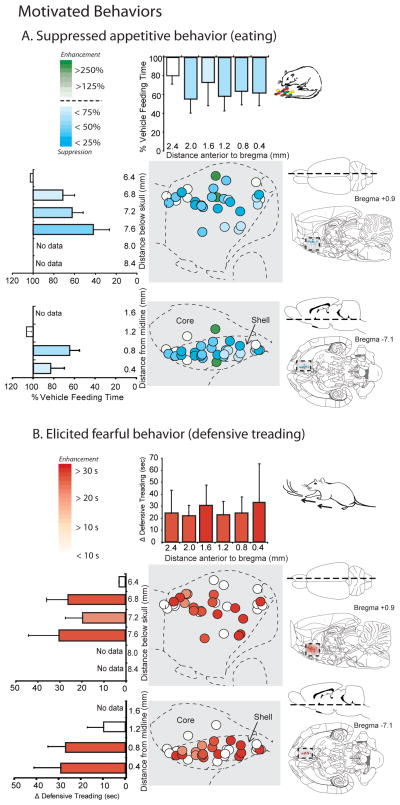
Microinjections of the mglu2/3 antagonist, LY341495, in medial shell suppressed eating behavior and intake of palatable chocolate to below half of vehicle control levels (main effect of drug: M&M intake grams, F(1,15) = 14.837, p = .002; eating duration, F(1,15) = 10.522, p = .006) (Fig. 1a, 1c and 2). The reduction in eating behavior reflected primarily a decrease in the number of ingestive bouts initiated (F(1,15) = 8.770, p = .011) more than a decrease in the duration spent eating after initiation (main effect of drug, F(1,15) = 1.672, p = .111), suggesting that LY341495 disrupted the initiation of eating more than its maintenance. Similarly, LY341495 microinjections also suppressed drinking behavior and water intake to nearly zero (main effect of drug, F(1,15) = 7.835, p = .015) (Fig. 1b). Nearly all sites within the medial shell were equally effective for LY341495 suppression of eating and drinking behaviors, suggesting the entire medial shell is relatively homogeneous for mglu2/3 appetitive suppression. Specifically, there were no differences between the intensity of suppression induced at rostral sites versus caudal sites (drug x rostrocaudal level interaction: M&M intake grams, F(2,14) = .900, p = .449; eating duration, F(2,14) = 2.153, p = .187; drinking time, F(2,14) = 1.597, p = .268) or dorsal versus ventral sites (drug x dorsoventral level interaction: M&M intake grams, F(2,14) = .812, p = .482; eating duration, F(2,14) = 1.700, p = .250; drinking time, F(2,14) = 2.366, p = .164) (Fig. 2a). The suppression of behavior by mglu2/3 blockade was relatively specific to consumption of food and water, as LY341495 did not significantly affect other food-related behaviors such as food carrying or food sniffs (main effect of drug, food carrying, F(1,15) = .147, p = .482; average of 7.94 +/− 1.42 SEM versus 8.75 +/− 1.70 SEM on vehicle; food sniffs, F(1,15) = .066, p = .909; average of 17.1 +/− 2.40 SEM versus 17.5 +/− 1.56 SEM on vehicle).
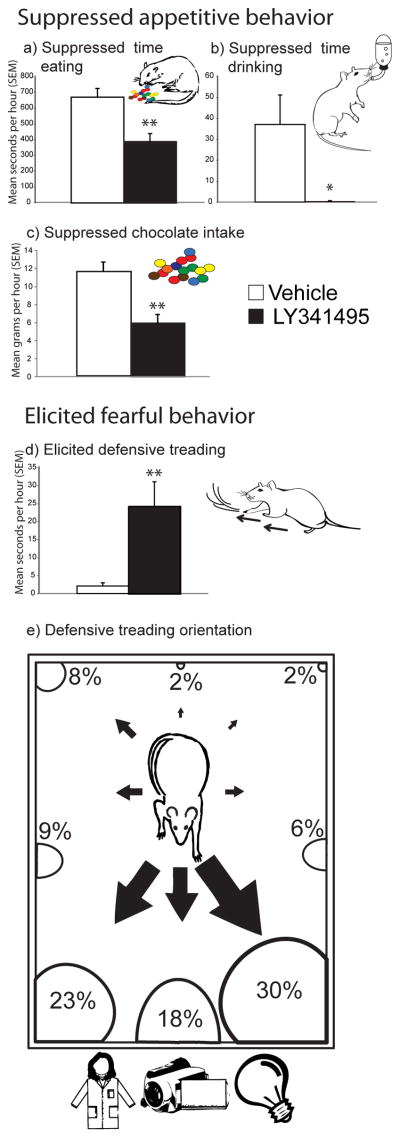
Figure 1. Motivated behavior summary.
Changes in eating duration (A), drinking duration (B), food intake (C) and defensive treading behavior (D) elicited by vehicle or LY341495 microinjections into NAc shell. Directional orientation of defensive treading (E) elicited by LY341495 is shown by size of arrows pointing from rat and size of semi-circles in each direction (numbers are mean percentage of treading in each direction). Defensive treading was typically pointed at the transparent front of the chamber (which revealed sights of the experimenter and objects in the room beyond), or at corners of the plastic tub chambers (which may have reflected back bright patterns of light). We interpret the directedness of treading as indicating that the behavior was fearfully motivated and targeted toward specific stimuli that the rat perceived as most threatening, rather than merely a motor stereotypy. Data shown are combined for rats with rostral and caudal microinjection sites. Error bars indicate SEM, *p < 0.05, **p < 0.01.
Figure 2. Motivation shifts: LY341495 in NAc shell suppresses appetitive eating and drinking but stimulates fearful motivated behavior.
Sagittal and horizontal maps of appetitive eating (A) and defensive treading (B) behavior generated by LY341495 mglu2/3 antagonism. Changes in the cumulative duration of eating behavior is shown as percentage of vehicle, while defensive treading behavior is shown as unit change from vehicle (given near zero levels of treading baseline). LY341495 microinjections in medial shell suppressed appetitive eating and enhanced defensive behavior throughout medial NAc shell. Histograms bars below and to the sides of the maps show mean values (error bars = SEM) for each behavior at rostrocaudal, dorsoventral and mediolateral levels.
LY341495 generates fearful antipredator behavior throughout medial shell
Microinjections of the mglu2/3 antagonist, LY341495, also generated defensive treading behavior at an intensity almost 10 times above vehicle levels (which are generally near zero) (main effect of drug, F(1,15) = 6.310, p = .026; average of 24.1 +/− 6.90 sec SEM versus 2.59 +/− 1.21 sec SEM on vehicle) (Fig. 1d). Supporting the idea that defensive treading was a motivated fearful behavior emitted towards particular stimuli perceived as threatening by the rat, the treading behavior after LY341495 was not randomly directed throughout the cage (i.e., as a motor stereotypy), but was typically 3 to 10 times more likely to be focused and directed toward a small set of specific stimulus targets: either the transparent front of the cage which revealed objects in the room and lights (18% +/− 3% SEM to the revealed front versus 2% +/− 1.53% SEM to the back) or its brightly lit, reflective corners (23% and 31% +/− 7% SEM to the front concerns versus 6 and 9% to the sides of the chamber) (main effect of direction, F(1,13)=4.590, p<.001) (Fig. 1e). The strong directional focus of the defensive burying and treading movements stimulated by LY341495 supports the interpretation of this behavior as a coordinated defensive reaction, reflecting a fearful motivated state. Like the feeding and drinking effects, defensive treading was elicited by LY341495 at nearly every site throughout the medial shell, and not limited to a particular rostral or caudal zone (drug x rostrocaudal level, F(2,14) = .243, p = .790) or dorsal or ventral zone (drug x dorsoventral level, F(2,14) = 1.076, p = .436) (Fig. 2b).
Other behaviors
General changes in activity level or capacity do not appear to account for the changes in motivated behavior described above, as LY341495 did not alter normal levels of locomotor behavior (main effect of drug: rears, F(1,15) = .299, p = .723, average of 97.1 +/− 13.1 SEM versus 101.4 +/− 8.62 on vehicle; cage crosses, F(1,15) = 1.302, p = .307, average of 91.5 +/−13.5 SEM versus 77.4 +/− 8.24 on vehicle). Burrowing and burrow-treading were generally not observed and were not elevated by LY341495 microinjections. The drug did moderately suppress the time spent in grooming behavior (main effect of drug, F(1,15) = 5.320, p = .038) to about two-thirds of vehicle levels (average of 196.7 +/− 49.2 sec SEM versus 291.1 +/− 34.4 sec SEM on vehicle), but that may have been due partly to the reduction in number of eating bouts, which would in turn reduce the incidence of post-prandial grooming. In general, LY341495 microinjections in medial shell appeared to most specifically suppress appetitive behavior and to stimulate defensive behavior.
LY341495 suppresses hedonic reactions and increases aversive reactions to sucrose
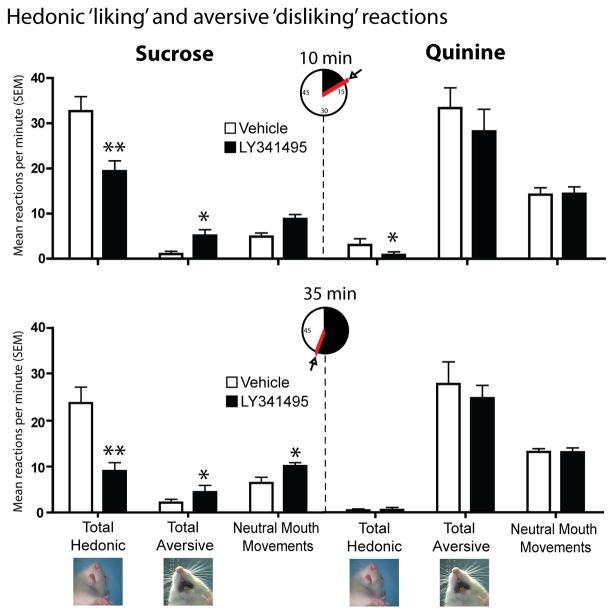
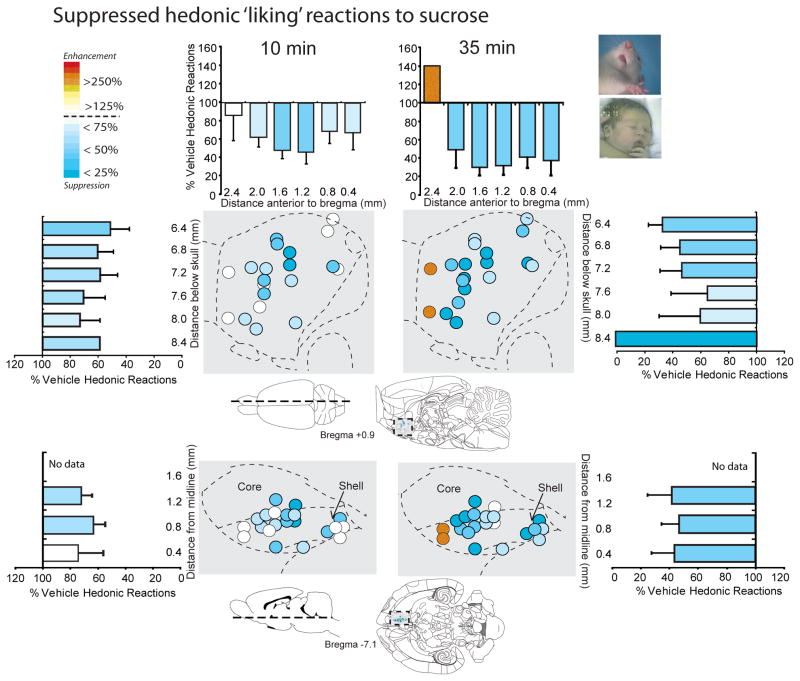
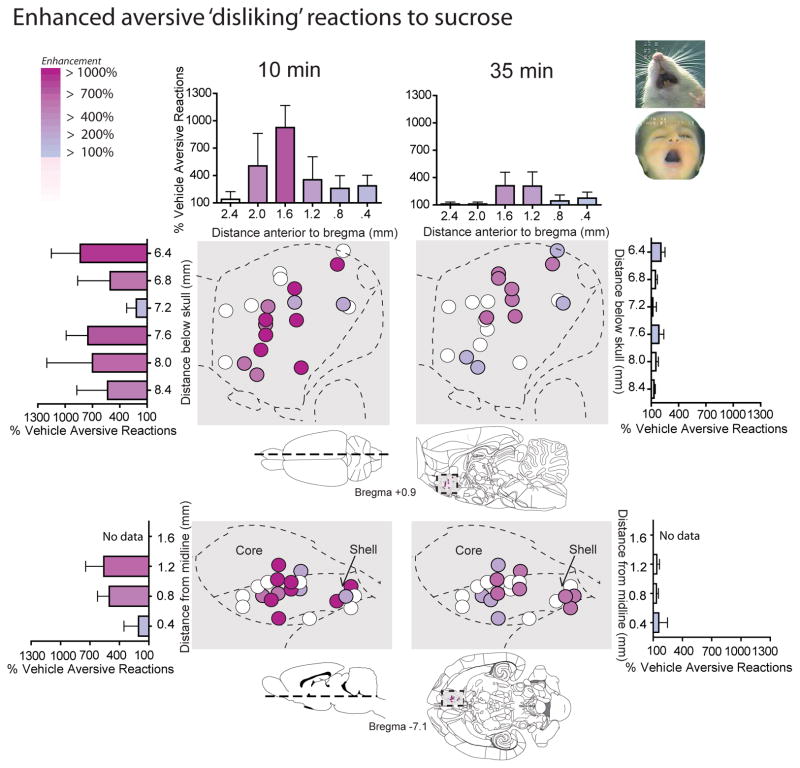
Because LY341495 strongly suppressed motivation ‘wanting’ to eat, at least as reflected in intake of palatable chocolates, we further tested for any corresponding change in ‘liking’ reactions to the hedonic impact of food reward. In the taste reactivity test, liquid sucrose infusions into the mouth ordinarily elicit primarily positive hedonic reactions (rhythmic midline tongue protrusions, lateral tongue protrusions or lip licking, and paw licking behaviors) after control vehicle microinjections, and all these hedonic components were suppressed by LY341495 (Fig. 3 and 4). The number of positive hedonic reactions to sucrose taste was reduced by blockade of mglu2/3 receptors with LY341495 in medial shell to less than half of vehicle control levels (main effect of drug, F(1,10) = 20.171, p = .001; average of 9.07 +/− 1.79 SEM versus 23.9 +/− 3.46 SEM on vehicle) (Fig. 3). Blockade of mglu2/3 receptors suppressed hedonic reactions homogeneously at all sites throughout medial shell (drug x rostrocaudal level interaction, F(1,9) = 1.018, p = .426; drug x dorsoventral level interaction, F(1,9) = 1.091, p = .344) (Fig. 4). Suppression of positive hedonic reactions by LY341495 was consistent at both 10 and 35 minutes after microinjection (time x drug interaction, F(1,10) = .413, p = .535), though there was an overall reduction in hedonic reactions between the 10 and 35 minute infusions, regardless of drug condition (main effect of time F(1,10) = 11.419, p =.007) (Fig. 3). Conversely, mglu2/3 receptor blockade with LY341495 increased overall aversive reactions to sucrose (e.g., gapes,), which normally are elicited only by bitter quinine or other avoided tastes (main effect of drug, F(1,10) = 12.653, p = .005; drug x rostrocaudal level interaction, F(1,9) = .916, p = .458; drug x dorsoventral interaction, F(1,9) = .163, p = .703) (Fig. 3 and 5). Aversive reactions were almost quadrupled over near-zero control levels when sucrose was tested at 10 min after LY341495 microinjection (average of 3.93 +/− 1.29 SEM versus 1.07 +/− .578 SEM on vehicle), and still more than doubled later at 35 min (average of 4.43 +/− 1.49 SEM versus 2.14 +/− .725 SEM) (Fig. 3 and 5). Finally, the relatively non-valenced neutral mouth movements were moderately enhanced by about 50% to 75%, which may also be consistent with a shift in the perception of sweet taste from highly positive to more mixed in valence (main effect of drug, F(1,10) = 6.223, p = .032; average of 6.82 +/− 0.98 at 10 min and 8.18 +/− 1.36 at 35 min versus 5.00 +/− 1.01 at 10 min and 4.63 +/− 1.03 at 35 min on vehicle) (Fig. 3). The combination of strong suppression of highly hedonic reactions, moderate increase of neutral reactions, and strong increase of negative aversive reactions to sucrose indicates a shift in palatability from positive to negative, rather than an overall motor effect on orofacial reactions as a whole (Fig. 3). Hedonic suppression and aversive magnification was produced at similar intensities by most sites throughout the 90% of medial shell that was posterior to the far-rostral strip (i.e. at or caudal to the genu of corpus callosum), without evidence of localization or of a rostrocaudal gradient (Fig. 4 and 5).
Figure 3. Affective reactions summary.
Changes in hedonic, aversive and neutral mouth movements elicited by sucrose (left) at 10 minutes (top) and 35 minutes (bottom), or by quinine (right) at 10 minutes (top) and 35 minutes (bottom) after microinjection of vehicle or LY341495 microinjections into accumbens shell. Data shown are combined for rats with rostral and caudal microinjection sites. Error bars indicate SEM, *p < 0.05, **p < 0.01.
Figure 4. Hedonic ‘liking’ suppression: LY341495 in NAc shell suppresses sucrose hedonic reactions.
Sagittal and horizontal maps of sites where LY341495 suppressed positive hedonic ‘liking’ reactions to sucrose taste at 10 minutes after the microinjection (left) and at 35 minutes after the microinjection (right; both 1-min tests). The entire medial shell supported LY341495 suppression of positive hedonic impact, with the possible anatomical exception of moderately far rostral strip (0.1 mm AP width directly beneath the genu of the corpus callosum). Histograms bars show mean change from vehicle (error bars = SEM) for both each reaction pattern at rostrocaudal, dorsoventral and mediolateral levels throughout medial shell.
Figure 5. Aversive ‘disliking’ enhancement: LY341495 in NAc shell enhances sucrose aversive reactions.
Sagittal and horizontal maps of sites where LY341495 enhanced aversive ‘disliking’ reactions to sucrose taste at 10 minutes after the microinjection (left) and at 35 minutes after the microinjection (right; both 1-min tests). Histograms bars show mean change from vehicle (error bars = SEM) for both each reaction pattern at rostrocaudal, dorsoventral and mediolateral levels throughout medial shell.
However, a possible anatomical exception to the pattern of hedonic suppression and aversive magnification may have occurred in an anterior 10% strip of medial shell located just ahead of the genu of the corpus callosum and immediately adjacent to the rostral pole (AP +2.4mm). This was suggested by data from the rat with the furthest rostral placements, where LY341495 actually elevated hedonic reactions to sucrose to 140% of vehicle control levels (Fig. 4). While more data would be needed to draw firm conclusions, we note that this anterior strip in medial shell has been found to generate anomalous patterns of taste reactivity in previous studies (e.g., unique positive hedonic enhancement by GABAergic agonist muscimol microinjections that contrasts to a gradient of hedonic suppression and aversive enhancement in the rest of medial shell) (Reynolds & Berridge, 2002; Faure et al., 2010).
LY341495 does not affect aversive reactivity to quinine
Aversive reactions were ordinarily elicited robustly by quinine, and remained high and unchanged after LY341495 injection at either 10 min or 35 min (main effect of drug, F(1,10) = 4.141, p = .072; time x drug interaction, F(1,10) = 2.280, p = .165) (Fig. 3). Only modest numbers of positive hedonic reactions were ever elicited by quinine in control condition, but hedonic reactions were suppressed even further by the mglu2/3 blockade to <40% of vehicle control levels (main effect of drug, F(1,10) = 5.918, p = .038; time x drug interaction, F(1, 10) = 2.562, p = .111) (Fig. 3). LY341495 blockade did not alter neutral mouth movements elicited by quinine, which remained at relatively low levels (main effect of drug, F(1,10) = 5.053, p = .051) (Fig. 3).
Anatomical control sites
LY341495 microinjection at control placements dorsal to medial shell, largely contained in lateral septum, had no significant effect on taste reactivity behaviors: mglu2/3 receptor blockade had no significant effect on hedonic reactions (sucrose, main effect of drug, F(1,2) = 16.333, p = .056; quinine, main effect of drug, (F(1,2) = .730, p = .483), aversive reactions (sucrose, main effect of drug, F(1,2) = 3.000, p = .225; quinine, main effect of drug, F(1,2) = 3.510, p = .202), or neutral mouth movements (sucrose, main effect of drug, F(1,2) = 7.737, p = .109; quinine, main effect of drug, F(1,2) = .964, p = .430).
Although our microinjection placements nucleus accumbens specifically targeted the medial shell, it is worth addressing whether there was any contribution from diffusion into the core. In our study, two rats in the food intake test had at least a unilateral placement that impinged into the core (with the contralateral placement in medial shell). However, these unilateral-core rats did not show the same suppression of intake seen in those rats with bilateral medial shell hits, and one even showed an increase in eating time (Fig. 2a). Additionally, neither rat met the lowest criteria for defensive treading behavior (10 seconds, Fig. 2b). Those observations suggest that medial shell makes much more of a contribution than core to mglu2/3 influences in NAc over fear induction and appetitive suppression, just as for ionotropic AMPA glutamate modulation of the same behaviors (Reynolds & Berridge, 2003). Even so, while microinjection of LY341495 that are centered in medial shell of NAc produce the behavioral effects described here, future studies would be needed to unequivocally state whether the core contributes anything to mglu2/3 effects.
Local Fos plumes not induced by LY341495
Microinjections of LY341495 produced no discernable change in Fos expression immediately surrounding the microinjection site when compared to vehicle microinjections (main effect of drug, F(1,11) = .464, p=.504). Blockade of mglu2/3 receptors failed to cause either excitatory local Fos plumes of elevated protein translation or inhibitory anti-plumes of suppressed translation compared to vehicle, as drug and vehicle microinjections induced identical Fos expression levels surrounding the center of injection (both slightly elevated over normal tissue baselines). The lack of any local change in Fos expression after a LY341495 microinjection is unusual and contrasts to the excitatory plumes produced by the ionotropic glutamate AMPA antagonist (DNQX), and by agonists for mu opioid (DAMGO), CRF (CRF) or endocannabinoid (anandamide) receptors, as well as amphetamine and GABA blockade (bicuculline) in NAc medial shell; lack of any local Fos change at the mglu2/3 blockade site also contrasts to the inhibitory antiplumes produced in medial shell at sites of a GABA agonist (muscimol) or an opioid antagonist (naloxone) (Peciña & Berridge, 2000; Peciña & Berridge, 2005; Smith & Berridge, 2005; Pecina et al., 2006; Mahler et al., 2007; Faure et al., 2008; Faure et al., 2010). One possible explanation for absence of any Fos change for LY341495 might be that mglu2/3 receptors are located predominantly on presynaptic terminals, rather than on the postsynaptic medium spiny neurons, which are more directly impacted by the other drugs mentioned above. However, assessment of the spread of microinjection impact was relatively unimportant for interpretation of this experiment given that the behavioral effects of LY341495 microinjections described earlier were homogeneous throughout the entire medial shell. Therefore all maps of behavioral effects constructed for figures were based only on the pinpoint locations of the center for each microinjection placement. For any future experiment that finds LY341495 to involve functional localization, other markers of neuronal activation that are more sensitive to terminal mglu2/3 blockade would be required.
Discussion
Here, we found that local blockade of metabotropic glutamate (mglu2/3) receptors by LY341495 microinjections in NAc medial shell suppressed appetitive eating of palatable chocolates and suppressed positive hedonic (‘liking’) reactions normally induced by the sensory pleasure of sweetness. The effects on motivation and hedonic impact could be related, as a reduction in ‘liking’ for sweet taste could contribute to reduced ‘wanting,’ though it is also possible that appetitive motivation is directly suppressed by NAc mglu2/3 blockade. Concomitantly the same LY341495 microinjections generated increases in spontaneous fearful motivation as reflected by 10-fold increases in a species-specific antipredator behavior, defensive treading, and in parallel increased the number of aversive ‘disliking’ reactions such as bitter-typical gapes to the normally pleasant taste of sucrose. These shifts of motivation and affect from positive valence to negative valence occurred after LY341495 microinjections homogenously throughout the entire medial shell, and were not anatomically segregated or localized to any subregion of shell. Overall, NAc mglu2/3 blockade appears to push the animals from appetitive motivation and positive hedonic reactions into a more affectively negative, but still highly motivated state of fear and disgust.
Metabotropic versus ionotropic glutamate disruptions
Our finding of a negative shift in motivation and affective valence produced throughout the entire medial shell by metabotropic glutamate antagonism contrasts strongly with the anatomically-segregated ‘keyboard pattern’ of desire and dread generation produced by disruptions of ionotropic AMPA glutamate signals in the same structure. Local microinjections of the AMPA antagonist DNQX in the rostral half of medial shell are known to produce intense appetitive behaviors such as hyperphagia (Maldonado-Irizarry et al., 1995; Stratford et al., 1998; Reynolds & Berridge, 2003; Faure et al., 2008; Reynolds & Berridge, 2008), whereas the equivalent DNQX microinjections in the caudal half of medial shell instead produce fearful anti-predator responses similar to observed here such as defensive treading and burying in a standard laboratory setting, distress vocalizations, escape attempts, etc. (Reynolds & Berridge, 2003; Faure et al., 2008; Reynolds & Berridge, 2008). A similar rostrocaudal gradient of bivalent motivation is also produced by local GABAA stimulation caused by muscimol microinjections in medial shell: appetitive stimulation at rostral sites, and fearful stimulation (with appetitive suppression) at caudal sites (Stratford & Kelley, 1997; Reynolds & Berridge, 2001; Reynolds & Berridge, 2002). Yet, while both local GABA receptor activation and local ionotropic glutamate disruption generate similar keyboard gradients of appetitive versus fearful motivated behaviors, there are also differences between GABA and glutamate gradients in medial shell. Only GABA muscimol microinjections additionally cause a corresponding affective gradient of positive ‘liking’ versus negative ‘disliking’ in hedonic reactions similar to the hedonic shift from ‘liking’ to disgust seen here after LY31495, whereas local blockade of ionotropic glutamate AMPA receptors by DNQX anywhere in medial NAc shell fails to alter ‘liking’ or ‘disliking’ (Reynolds & Berridge, 2002; Faure et al., 2010)
Thus, two features of our current results of metabotropic glutamate signal blockade stand in stark contrast to that gradient pattern of effects produced by AMPA blockade of ionotropic glutamate receptors in NAc medial shell. First, mglu2/3 receptor blockade by LY341495 penetrated as successfully into sensory pleasure reactions as into motivated behavior, suppressing positive ‘liking’ and amplifying negative ‘disliking’ reactions to sweetness, while simultaneously suppressing appetitive eating and generating motivated fearful behaviors. Second, mglu2/3 blockade homogeneously generated fear and shifted affective valence toward negative at all locations in medial shell, rostral as well as caudal, without the bivalent keyboard pattern (rostral=appetitive and caudal=fearful) of ionotropic AMPA blockade (or of GABAergic inhibition).
Limits to top-down penetration of sensory pleasure: not the case for mglu2/3?
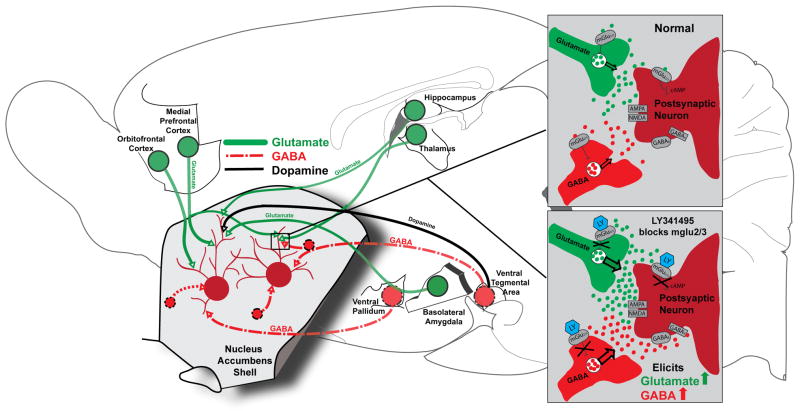
We previously proposed that fast corticolimbic glutamate signals mediated by ionotropic receptors on NAc neurons were unable to control hedonic emotional processes generated in NAc, based on observations that DNQX local AMPA blockade in NAc shell sites failed to alter ‘liking’ reactions to tastes (Faure et al., 2010). The ability of metabotropic mglu2/3 blockade here to suppress hedonic ‘liking’ and stimulate ‘disliking’ may possibly be reconciled with our previous conclusion, that ionotropic AMPA manipulations of motivation in shell do not penetrate into hedonic ‘liking’ or ‘disliking,’ by distinguishing between fast ionotropic (AMPA) and slower metabotropic (mglu2/3) functions of glutamate in NAc. For example, metabotropic receptors more widely regulate the release of other neurotransmitters besides glutamate, including GABA, which more effectively influence hedonic impact in NAc (Fig. 6). Therefore, a hypothetical explanation for LY341495 effects on hedonic ‘disliking’ shifts might be that mglu2/3 microinjections recruit local GABA release by acting on pre-synaptic axons of GABA-releasing neurons. This hypothesis is based on reports that stimulation of mglu2/3 receptors can suppress GABA release (Cartmell & Schoepp, 2000; Schoepp, 2001) and our findings that GABA agonist and LY341495 microinjections can both suppress ‘liking’ and enhance ‘disliking’ in much of NAc shell.
Figure 6. Corticolimbic circuits impacted by metabotropic glutamate receptor blockade induced by LY341495 microinjectionin NAc shell.
Close-up of glutamatergic (green) and GABAergic (red) neurotransmission under normal conditions and in the presence of LY341495 (LY, blue), which blocks presynaptic mglu2/3 receptors and consequently enhances release of both glutamate and GABA from their respective neurons (insets). Glutamatergic inputs (green) are shown from medial prefrontal cortex, orbitofrontal cortex, hippocampus, thalamus, and basolateral amygdale. GABAergic medium spiny neurons (deep red) and GABAergic inputs (red) to NAc shell are shown from ventral pallidum and ventral tegmental area. Dopaminergic inputs are shown from the ventral tegmental area (black). Circuit diagram is modified from Faure et al., 2010, with inset based on Swanson et al., 2005.
Alternatively, mglu2/3 blockade may access hedonic impact via direct glutamatergic actions, but via metabotropic receptor functions that recruit intracellular cascades absent from ionotropic receptor function (Spooren et al., 2003). Generally, the responses of metabotropic glutamate receptors are slower in onset and longer-lasting than the fast excitatory synaptic transmission of ionotropic receptors (Conn & Pin, 1997; Simeone et al., 2004). Additionally, Group II metabotropic glutamate receptors are located primarily presynaptically, as well as on glial cells and on the periphery of synaptic zones (Schoepp, 2001; Swanson et al., 2005; Imre, 2007), while AMPA receptors are found almost entirely on the spines of postsynaptic membranes of medium spiny neurons in NAc (Meredith et al., 1990; Sesack & Pickel, 1992; Johnson et al., 1994; Chen et al., 1998; Tarazi et al., 1998b, a; ). Given that blockade of mglu2/3 receptors triggers intracellular processes not impacted by AMPA receptors, conceivably metabotropic receptors might alter hedonic processing through differential modulation of NAc neurons.
Expansion of fear-generating zone to rostral shell
We note two points relevant to understanding why LY341495 induced negative valence shifts homogeneously throughout the entire medial shell. First, there is at least a similarity to ionotropic effects in caudal shell, where both LY341495 and DNQX microinjections generate fearful behaviors. Second, an anatomical expansion of fear generation into rostral shell also has one known parallel in the gradients observed after ionotropic AMPA disruptions: DNQX microinjections at many rostral sites in medial shell can generate fearful defensive treading (nearly as well as at caudal sites) when rats are tested in a stressful, highly-noisy and brightly-lit environment (Reynolds & Berridge, 2008). We hypothesize that mglu2/3 blockade may act similarly to just such a stressful environmental ambience, at least in terms of modulating the function of local sites in medial shell, and consequently expand the negative fear generation from caudal into rostral shell. The mechanism of such expansion, by either mglu2/3 blockade or stressful environment combined with AMPA blockade, will of course require further explanation.
Role of mglu2/3 receptors in fearful salience: relevance to schizophrenia
Aberrant and intense fearful salience, generated in part by NAc circuits, has been suggested to be involved in the paranoia of human schizophrenia (Kapur, 2003; Barch, 2005; Taylor et al., 2005; Kienast & Heinz, 2006; Schmidt & Beninger, 2006; Jensen et al., 2008; Walter et al., 2010). Potential future treatment options for schizophrenic paranoia have been suggested to include mglu2/3 agonists, which would have opposite receptor effects from those of the antagonist LY341495 tested here, which generated fear. Group II agonists have anxiolytic and antipsychotic-like effects in animal models of schizophrenia (Swanson et al., 2005; Schlumberger et al., 2009). For example, systemic administration of mglu2/3 agonists blocks the expression of fear-potentiated startle (Helton et al., 1998; Tizzano et al., 2002; Grillon et al., 2003; Rorick-Kehn et al., 2007), increases the time spent on the open-arms of an elevated plus maze (Monn et al., 1997; Helton et al., 1998; Ferris et al., 2001) and inhibits conditioned-avoidance responding (Rorick-Kehn et al., 2007). Similarly, in phase II clinical trials LY2140023, an oral prodrug of LY404039, reduced both positive and negative symptoms of human schizophrenia (Patil et al., 2007). The effectiveness of mglu2/3 agonists as potential antipsychotics might have more to do with effects on affective dysfunction involving motivation and emotion, than on cognitive dysfunction, as the drugs do not appear effective in animal models of the cognitive deficits found in schizophrenia (Schlumberger et al., 2009). Given these considerations and our results, we hypothesize that the effectiveness of mglu2/3 agonists may be linked to the dampening of fearful motivational salience, extending beyond the cognitive dysfunction these drugs were first considered to treat (Moghaddam, 2004).
Similar dampening of fearful salience has been proposed by Kapur and others to be the mechanism of action of typical and atypical antipsychotic medications, which block D2 dopamine receptors to prevent the enhancement of salience of negative stimuli (Kapur & Mamo, 2003; Kapur, 2004; Howes & Kapur, 2009; Heinz & Schlagenhauf, 2010). Presynaptic alterations in dopamine release may contribute to the fearful motivational state produced by mglu2/3 antagonism. Agonism at mglu2/3 receptors inhibits KCl-stimulated dopamine release (Chaki et al., 2006) and blockade of these receptors increases extracellular dopamine levels in NAc shell (Karasawa et al., 2006). Additionally, we have previously reported that endogenous dopamine is necessary for the generation of fearful motivation by local disruptions of ionotropic glutamate in the caudal shell, as well as for the generation of appetitive motivation in rostral shell (Faure et al., 2008). All this suggests that interactions between glutamate and dopamine signals in NAc modulate negative fearful salience as well as positive incentive salience. In contrast, we suggest that dopaminergic transmission in NAc shell is unlikely to contribute to LY341495’s suppression of hedonic ‘liking’ or amplification of ‘disliking’ reactions to sucrose. Neither suppression of dopamine function (using either systemic dopamine blockade or 6-OHDA lesions of dopamine projections) nor dopamine stimulation in NAc (by systemic administration or local microinjection of amphetamine, or by genetic mutation) have been found to alter hedonic reactions to tastes, and the bulk of available evidence indicates that dopamine does not actually mediate ‘liking’ or the hedonic impact of sensory pleasure (Berridge et al., 1989; Peciña et al., 1997; Berridge & Robinson, 1998; Wyvell & Berridge, 2000; Wyvell & Berridge, 2001; Peciña et al., 2003; Tindell et al., 2005; Berridge, 2007).
Conclusions
Local blockade of mglu2/3 receptors by LY341495 microinjections in NAc shell shifts both motivation and hedonic affective reactions from positive valence to negative valence. The ability of LY341495 to negatively shift motivation from appetitive to fearful, and hedonic impact of a sensory reward from pleasant to disgusting, demonstrates the potential importance of normal mglu2/3 neurotransmission to appetitive motivation and positive affective reactions. This role of metabotropic Group II glutamate signals also may be related to why drugs which act as agonists at these receptors might help to restore normality in some cases of aberrant motivation, such as that found in schizophrenia and drug addiction.
Acknowledgments
This research was supported by National Institutes of Health Grants DA015188 and MH63649. JMR was supported by NIMH NRSA MH090602. We thank Ryan Selleck for assistance with Fos histology.
Reference List
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Beckstead RM. Autoradiographic examination of cortico-cortical and sub-cortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. Journal of Comparative Neurology. 1979;184:43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical Role of the Prefrontal Cortex in the Regulation of Hippocampus-Accumbens Information Flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience & Biobehavioral Reviews. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ. Isohedonic tastes support a two-dimensional hypothesis of palatability. Appetite. 1984;5:221–231. doi: 10.1016/s0195-6663(84)80017-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: Implications for arousal and anhedonia hypotheses of dopamine function. Behavioral Neuroscience. 1989;103:36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. Journal of Neurochemistry. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Okuyama S. Group II metabotropic glutamate receptor-mediated regulation of dopamine release from slices of rat nucleus accumbens. Neuroscience Letters. 2006;404:182–186. doi: 10.1016/j.neulet.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Chen Q, Veenman L, Knopp K, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience. 1998;83:749–761. doi: 10.1016/s0306-4522(97)00452-1. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino-acid projections to the nucleus accumbens-septi in the rat: a retrograde transport study utilizing D[H-3] aspartate and [H-3] GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Coss RG, Owings DH. Snake-Directed Behavior By Snake Naive and Experienced California Ground Squirrels in a Simulated Burrow. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1978;48:421–435. [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: Cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PloS one. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris P, Seward E, Dawson GR. Interactions between LY354740, a group II metabotropic agonist and the GABA(A)-benzodiazepine receptor complex in the rat elevated plus-maze. J Psychopharmacol. 2001;15:76–82. doi: 10.1177/026988110101500203. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamatergic aspartergic afferents to the rat ventral striatopallidal region. Journal of Comparative Neurology. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by local blockade of Group II metabotropic glutamate receptors in rats. BMC Neuroscience. 2006:7. doi: 10.1186/1471-2202-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine LR, Morgan CA., 3rd Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist ( LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl) 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- Gu GB, Lorrain DS, Wei HB, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Research. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang HL, Xiang XH, Zhao Y. The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neuroscience and Biobehavioral Reviews. 2009;33:864–873. doi: 10.1016/j.neubiorev.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward- seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RLM, Totterdell S. Synaptic organization of the amygdalar input to the nucleus-accumbens in the rat. In: Percheron G, McKenzie JS, Feger J, editors. Basal Ganglia Iv - New Ideas and Data on Structure and Function. 1994. pp. 109–114. [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kapur S. How antipsychotics become anti-‘psychotic’ - from dopamine to salience to psychosis. Trends In Pharmacological Sciences. 2004;25:402–406. doi: 10.1016/j.tips.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D-2 receptors. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Karasawa JI, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neuroscience Letters. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behavioural Brain Research. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: A FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. 2006;5:109–131. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcoholism-Clinical and Experimental Research. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behavioural Brain Research. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare T, Voss H, Glover G, Ballon D, Casey B. The bivalent side of the nucleus accumbens. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. Journal of Neuroscience. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. Journal of Neuroscience. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement - Potential pharmacotherapies for smoking cessation. In: Ali SF, Nabeshima T, Yanagita T, editors. Current Status of Drug Dependence/Abuse Studies: Cellular and Molecular Mechanisms of Drugs of Abuse and Neurotoxicity. New York Acad Sciences; New York: 2004. pp. 491–503. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG, Pattiselanno A. Hippocampal fibers make synaptic contacts with glutamate decarboxylase-immunoreactive neurons in the rat nucleus accumbens. Brain Research. 1990;513:329–334. doi: 10.1016/0006-8993(90)90476-r. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology. 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid ( LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Medicine. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid eating site in accumbens shell mediates food intake and hedonic ‘liking’: map based on microinjection Fos plumes. Brain Research. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav. 1997;58:801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. Journal of Neuroscience. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behavioral Brain Research. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behavioural Pharmacology. 2009;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Beninger RJ. Behavioural sensitization in addiction, schizophrenia, Parkinson’s disease and dyskinesia. Neurotox Res. 2006;10:161–166. doi: 10.1007/BF03033244. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2001;299:12–20. [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus-vulgaris leucoagglutinin. Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. Journal of Comparative Neurology. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19:343–360. doi: 10.1177/088307380401900507. discussion 361. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behavioural Pharmacology. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. Journal of Neuroscience. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behavioural Brain Research. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Reviews Drug Discovery. 2005;4:131–U134. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Anatomical localization of striatolimbic ionotropic glutamate (NMDA, AMPA and kainate) receptors. Naunyn-Schmiedebergs Archives of Pharmacology. 1998a;358:119. [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of ionotropic glutamate receptors in caudate-putamen and nucleus accumbens septi of rat brain: Comparison of NMDA, AMPA, and kainate receptors. Synapse. 1998b;30:227–235. doi: 10.1002/(SICI)1098-2396(199810)30:2<227::AID-SYN13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tizzano JP, Griffey KI, Schoepp DD. The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacol Biochem Behav. 2002;73:367–374. doi: 10.1016/s0091-3057(02)00850-x. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology, Biochemistry & Behavior. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. Glutamate: The New Frontier in Pharmacotherapy for Cocaine Addiction. Cns & Neurological Disorders-Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- Walter H, Heckers S, Kassubek J, Erk S, Frasch K, Abler B. Further evidence for aberrant prefrontal salience coding in schizophrenia. Front Behav Neurosci. 2010;3:62. doi: 10.3389/neuro.08.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA Ratio Impacts State Transitions and Entrainment to Oscillations in a Computational Model of the Nucleus Accumbens Medium Spiny Projection Neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. Journal of Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive-sensitization by previous amphetamine exposure: Increased cue-triggered ‘wanting’ for sucrose reward. Journal of Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. The evolving theory of basal forebrain functional--anatomical ‘macrosystems’. Neuroscience & Biobehavioral Reviews. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]