Abstract
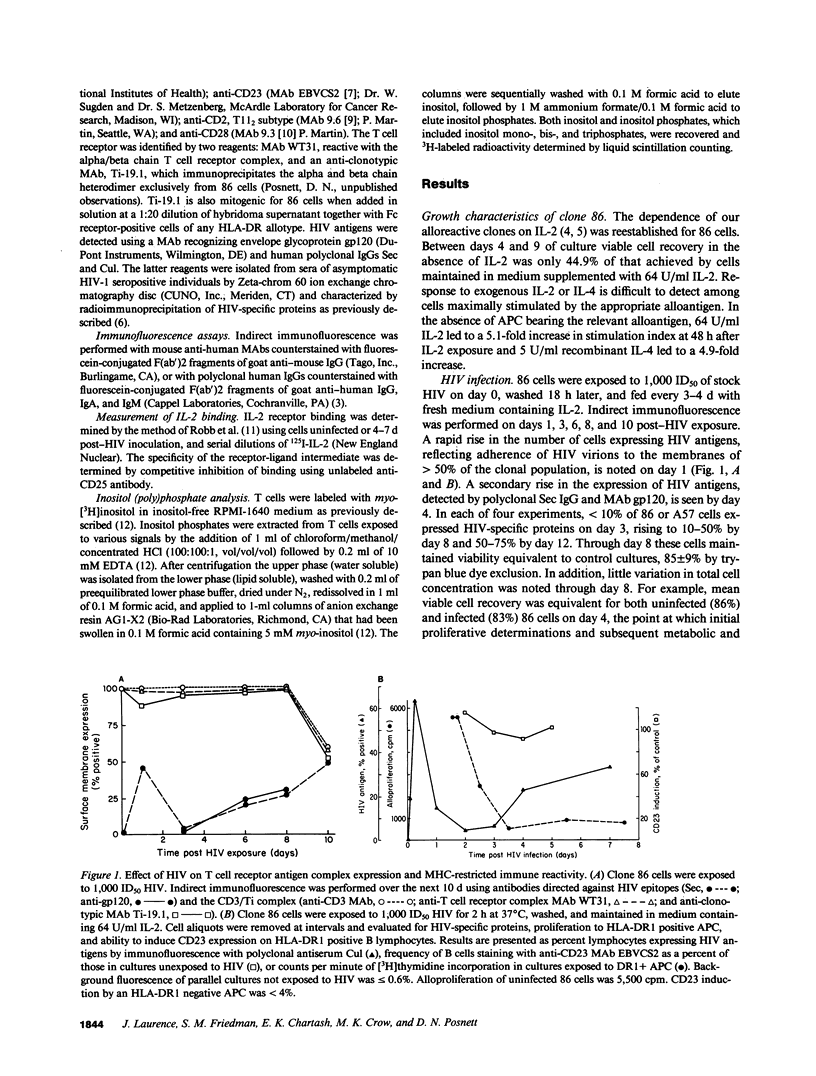
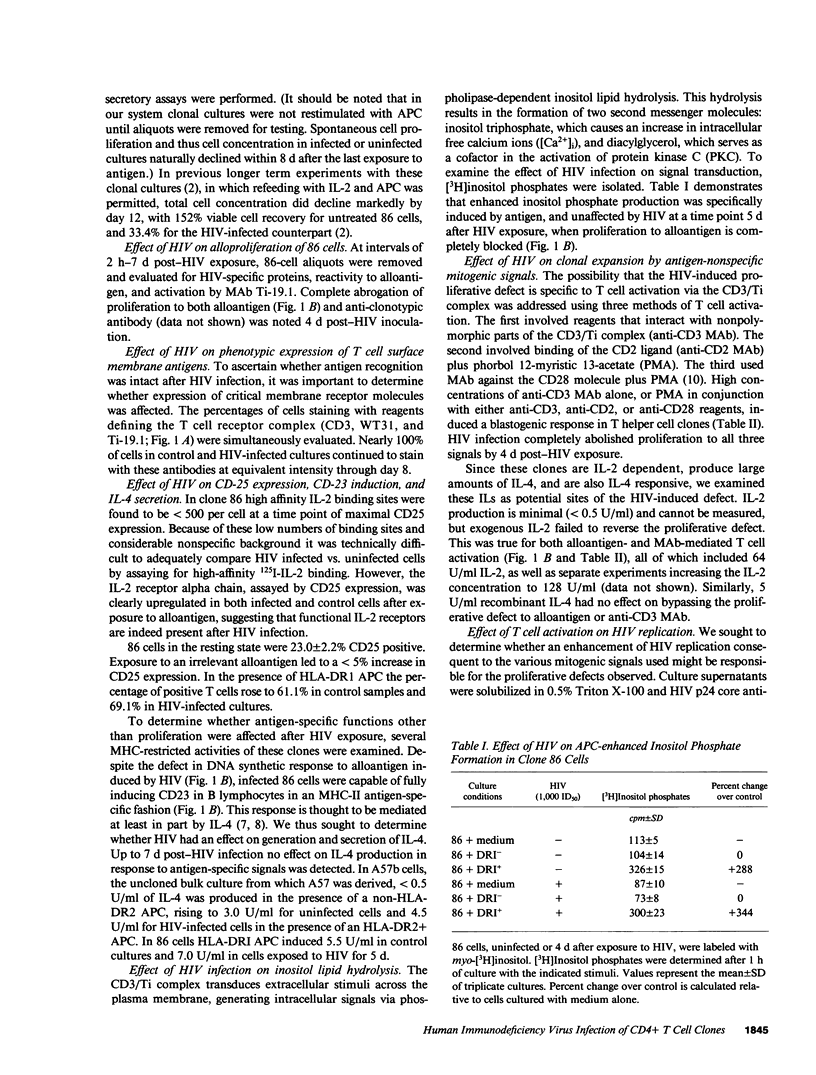
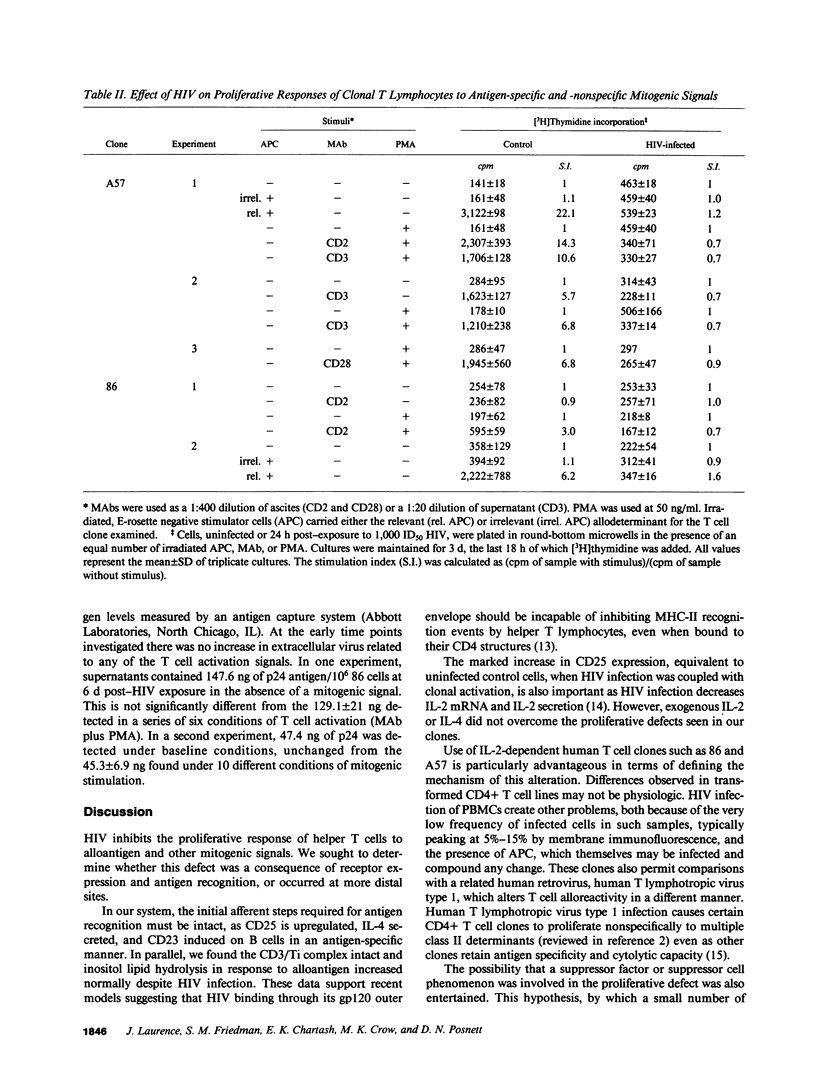
HIV selectively inhibited the proliferative response of clonal CD4+ T lymphocytes to alloantigen while other alloantigen-dependent responses were unperturbed. Specifically, impaired blastogenesis could be dissociated from alloantigen-specific induction of the B cell activation molecule CD23, IL-4 release, and inositol lipid hydrolysis. In addition, membrane expression of pertinent T cell receptor molecules, including CD2, CD3, and T cell antigen receptor (Ti), remained intact. Using two MHC class II-specific human CD4+ helper T cell clones, the proliferative defect was shown to be an early consequence of HIV infection, occurring within 4 d of viral inoculation and preceding increases in mature virion production. It was generalizable to three distinct methods of T cell activation, all independent of antigen-presenting cells: anti-CD3 mediated cross-linking of the CD3/Ti complex; anti-CD2 and phorbol 12-myristic 13-acetate (PMA); and anti-CD28 plus PMA. These abnormalities were not mitigated by addition of exogenous IL-2, even though expression of the IL-2 receptor (CD25) was unaltered. These studies define a selective blockade in T cell function early after HIV exposure that could serve as a model for certain in vivo manifestations of AIDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chartash E. K., Imai A., Gershengorn M. C., Crow M. K., Friedman S. M. Direct human T helper cell-induced B cell activation is not mediated by inositol lipid hydrolysis. J Immunol. 1988 Mar 15;140(6):1974–1981. [PubMed] [Google Scholar]
- Crow M. K., Jover J. A., Friedman S. M. Direct T helper-B cell interactions induce an early B cell activation antigen. J Exp Med. 1986 Nov 1;164(5):1760–1772. doi: 10.1084/jem.164.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Crimmins M. A., Mentzer S. J. Human T-cell leukemia virus type I infection of CD4+ or CD8+ cytotoxic T-cell clones results in immortalization with retention of antigen specificity. J Virol. 1988 Aug;62(8):2942–2950. doi: 10.1128/jvi.62.8.2942-2950.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Thompson G. S. Functionally restricted, allospecific, human helper T cell lines that amplify either B cell or cytolytic T cell responses. J Exp Med. 1983 May 1;157(5):1675–1680. doi: 10.1084/jem.157.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D., Green A., Gottlieb A. B., Crow M. K., Lewison A., Friedman S. M. Cloned allospecific human helper T cell lines induce an MHC-restricted proliferative response by resting B cells. J Immunol. 1985 Aug;135(2):1012–1019. [PubMed] [Google Scholar]
- Gupta S., Vayuvegula B. Human immunodeficiency virus-associated changes in signal transduction. J Clin Immunol. 1987 Nov;7(6):486–489. doi: 10.1007/BF00915060. [DOI] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Nakashima H., Kobayashi N., Yamamoto N. Tumor promoter, TPA, enhances replication of HTLV-III/LAV. Virology. 1986 Oct 30;154(2):249–258. doi: 10.1016/0042-6822(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Bouvier M., Malim M. H., Cullen B. R. Phosphorylation of the rev gene product of human immunodeficiency virus type 1. J Virol. 1988 Dec;62(12):4801–4804. doi: 10.1128/jvi.62.12.4801-4804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld H., Cruikshank W. W., Pyle S. W., Berman J. S., Center D. M. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988 Sep 29;335(6189):445–448. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- Laurence J., Gottlieb A. B., Kunkel H. G. Soluble suppressor factors in patients with acquired immune deficiency syndrome and its prodrome. Elaboration in vitro by T lymphocyte-adherent cell interactions. J Clin Invest. 1983 Dec;72(6):2072–2081. doi: 10.1172/JCI111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Kulkosky J., Friedman S. M., Posnett D. N., Ts'o P. O. PolyI.polyC12U-mediated inhibition of loss of alloantigen responsiveness viral replication in human CD4+ T cell clones exposed to human immunodeficiency virus in vitro. J Clin Invest. 1987 Dec;80(6):1631–1639. doi: 10.1172/JCI113251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Saunders A., Kulkosky J. Characterization and clinical association of antibody inhibitory to HIV reverse transcriptase activity. Science. 1987 Mar 20;235(4795):1501–1504. doi: 10.1126/science.2435004. [DOI] [PubMed] [Google Scholar]
- Linette G. P., Hartzman R. J., Ledbetter J. A., June C. H. HIV-1-infected T cells show a selective signaling defect after perturbation of CD3/antigen receptor. Science. 1988 Jul 29;241(4865):573–576. doi: 10.1126/science.2899908. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Cheng-Mayer C., Levy J. A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn W. S., Tweedale A., Cloyd M. W. Human immunodeficiency virus (HIV-1) cytotoxicity: perturbation of the cell membrane and depression of phospholipid synthesis. Virology. 1988 Mar;163(1):43–51. doi: 10.1016/0042-6822(88)90232-2. [DOI] [PubMed] [Google Scholar]
- Maggi E., Macchia D., Parronchi P., Mazzetti M., Ravina A., Milo D., Romagnani S. Reduced production of interleukin 2 and interferon-gamma and enhanced helper activity for IgG synthesis by cloned CD4+ T cells from patients with AIDS. Eur J Immunol. 1987 Dec;17(12):1685–1690. doi: 10.1002/eji.1830171202. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Richardson N. E., Brown N. R., Hussey R. E., Vaid A., Matthews T. J., Bolognesi D. P., Reinherz E. L. Binding site for human immunodeficiency virus coat protein gp120 is located in the NH2-terminal region of T4 (CD4) and requires the intact variable-region-like domain. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6102–6106. doi: 10.1073/pnas.85.16.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S., Des Jarlais D. C., Friedman S. R., Spira T. J., Marmor M., Holzman R., Mildvan D., Yancovitz S., Mathur-Wagh U., Garber J. Nonrandom development of immunologic abnormalities after infection with human immunodeficiency virus: implications for immunologic classification of the disease. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5404–5408. doi: 10.1073/pnas.84.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]


