Abstract
Bone morphogenic protein 2 (BMP-2) plays a key role in skeletal development, repair and regeneration. To gain a better understanding of the role of BMP-2 in periosteum-mediated bone repair, we deleted BMP-2 postnatally at the initiation stage of healing utilizing a Tamoxifen-inducible CreER mouse model. To mark the mutant cells, we further generated a BMP-2f/f; CreER; RosaR mouse model that enabled the activation of a LacZ reporter gene upon treatment of Tamoxifen. We demonstrated that deletion of BMP-2 at the onset of healing abolished periosteum-mediated bone/cartilage callus formation. In a chimeric periosteal callus with cells derived from both wild type and the mutant, over 90% of the mutant mesenchymal progenitors remained undifferentiated. Within differentiated bone and cartilage tissues, only a few cells could be identified as mutants. Using a bone graft transplantation approach, we further showed that transplantation of a mutant bone graft into a wild type host failed to rescue the deficient differentiation of the mutant cells at day 10 post-grafting. These data strongly suggest that the endogenous expression of BMP-2 plays a critical role in osteogenic and chondrogenic differentiation of periosteal progenitors during repair. To determine whether BMP-2 deficient cells remained responsive to exogenous BMP-2, we isolated periosteal mesenchymal progenitors from BMP-2 deficient bone autografts. The isolated cells demonstrated a 90% reduction of endogenous BMP-2 expression, accompanied by significant decrease in cellular proliferation and a near blockade of osteogenic differentiation. The addition of exogenous BMP-2 partially rescued impaired proliferation and further enhanced osteogenic differentiation in a dose dependent manner. Taken together, our data show that the initiation of the cortical bone repair in vivo is controlled by endogenous BMP-2. Future studies are necessary to determine the mechanisms by which the BMP-2 pathway is activated in periosteal progenitor cells at the onset of cortical bone repair.
INTRODUCTION
Adult cortical bone repair is a well orchestrated process precisely controlled by the presentation of molecular factors that play key roles in bone and cartilage developments. Analogous to embryonic skeletal development, adult bone repair involves both endochondral and intramembranous bone formation processes that proceed in a sequential and organized manner in order to reconstruct the damaged bone [1–2]. While adult bone repair recapitulates some essential regulatory mechanisms in skeletal development, it remains as a unique bone morphogenic process that involves the ensemble of genes distinctive from embryonic skeletogenesis [3–4]. Understanding the temporal and spatial control of adult bone repair is vital for devising novel therapeutics aimed at improving skeletal healing and repair.
Bone morphogenetic proteins (BMPs) are a group of multifunctional growth factors that play key roles in the development of bone and cartilage across all species. BMPs were first identified and purified from demineralized bone matrix capable of inducing ectopic bone formation in adult animals [5–9]. The unique bone regeneration capacity of the BMPs allows them to be successfully used in animal models as well as in humans for the treatment of bone repair [10]. Among all identified BMPs, BMP-2 has recently emerged as a key regulator for the maintenance of postnatal skeleton. Targeted deletion of BMP-2 in limb bud via Prx-1Cre does not affect the development or growth of the limbs in mice. However, the absence of BMP-2 in limb completely disrupted bone and cartilage formation at the initiation stage of fracture healing [11]. Furthermore, the limb BMP-2 knockout mouse develops micro-fractures as early as two weeks after birth. By 10-week these mice have a 70–80% incidence of spontaneous fracture which become worsen with aging [12].
To the end of further understanding the role of BMP-2 in the initiation and completion of adult bone repair and regeneration, in this current study, we utilized a bone graft transplantation approach to examine the role of BMP-2 in periosteum mediated cortical bone repair [13–16]. To achieve the temporal deletion of genes during adult repair, we utilized a Tamoxifen inducible CreER mouse model which permits efficient recombination of floxed gene prior to or during the initiation stage of cortical bone repair [17]. The incorporation of a ROSA26 reporter (R26R) that enabled the activation of a LacZ gene in mutant cells upon treatment of Tamoxifen further allowed us to track the fate of the BMP-2 deficient progenitor cells during bone repair. Our data showed that temporal deletion of BMP-2 blocked the osteogenic and chondrogenic differentiation of periosteal progenitor cells, disrupting the initiation of endochondral and intramembraneous bone repair. Interestingly, the defective differentiation could not be rescued in vivo by placing the cells in a wild type bone healing milieu. Furthermore, in a chimeric form of cartilage callus, only wild type cells were capable of differentiate into mature chondrocytes. These data strongly suggest that endogenous BMP-2 in periosteal mesenchymal progenitors plays a critical role in the initiation of endochondral and intramembranous bone healing.
MATERIALS AND METHODS
Animal models
All mice used were about 8–10 wks-old and all surgical procedures were approved by the University Committee on Animal Resources. Conditional BMP-2 f/f mice were kindly provided by Dr. James Martin at Texas A&M Health Science Center [18]. Tamoxifen inducible CreER transgenic mice were purchased from the Jackson Laboratory [19]. By crossing CreER with a LacZ reporter mouse RosaR, we generated CreER; RosaR which allowed us to examine the CreER-mediated recombinant efficiency during fracture healing following TM treatment [17]. Triple transgenic mice, BMP-2f/f; CreER; RosaR mice were generated by crossing BMP-2f/f mice with double transgenic CreER; RosaR mice. The BMP-2f/f; RosaR littermates (Cre-negative) were used as controls for some experimental group. To induce temporal gene deletion, Tamoxifen (TM) (Sigma-Aldrich, St. Louis, MO) at a dose of 1mg/10g of body weight was administered via peritoneal injection every other day prior to and after surgery for a total of 4 times. Upon treatment of Tamoxifen, the LacZ reporter gene was activated together with the deletion of BMP-2 gene in mutant cells.
Segmental bone graft transplantation
The segmental femoral bone graft transplantation was performed as previously described [13–14, 20]. Prior to transplantations, either donor or recipient mice were treated with TM twice to induce BMP-2 gene deletion in all tissues. Briefly, ten-week-old recipient mice were anesthetized by peritoneal injection of a mix of 10 mg/ml Ketamine and 1 mg/ml Xylazine. A 4 mm mid-diaphyseal segment was removed from the femur of the donor mice using a sharp diamond-cutting wheel attached to a cordless Dremel (Dremel Minimite model 750). Grafts were carefully dissected to remove the muscles without compromising the periosteum and immediately used to reconstruct a 4 mm segmental defect in the same mouse (autograft), or a mouse of the same strain (isograft). Bone graft transplantations were performed between CreER negative wild type mice (BMP-2f/f) and CreER positive (BMP-2f/f; CreER) mutant littermates. The grafted femurs were harvested at day 10 for histology and X-Gal staining, and day 14 for MicroCT analyses for bone formation.
Isolation of mesenchymal progenitor cells from early periosteal callus
To isolate mesenchymal progenitor cells from autograft periosteum, live bone autograft transplantation was performed on either wild type control or TM-treated BMP-2-deficient mice. Grafts were collected at day 5 post-transplantation. Bone marrow was removed by repeatedly flushing of the marrow cavities with serum free a-MEM medium. At least three grafts of the same genotypes were pooled and tissues attached to the periosteal surface of the donor grafts were scraped off and placed in a petri dish. Following digestion with Collagenase D (Roche Applied Science, Indianapolis, IN) at a concentration of 1mg/ml for 1 hour, cells released from the tissue were suspended in α-MEM medium containing 1% penicillin and streptomycin, 1% glutamine and 20% fetal bovine serum. Cells were allowed to adhere to the culture dish and grow to confluence. Cells collected from the second or third passage were used for differentiation assay [21]. For osteogenesis assay, cells were cultured with fresh osteogenic differentiation media containing 10 mM β-glycerolphosphate and 50 µM ascorbic acid. Cells were harvested at the indicated times for alkaline phosphatase (ALP) staining and gene analyses. For chondrogenesis assay, a total of 2 × 105 cells in 10 µL of media were placed as micromass in the center of a 24-well plate and incubated in 5% CO2 at 37°C for 1 h. Basic chondrogenic differentiation medium containing high glucose DMEM supplemented with 1% ITS-Premix, L-ascorbic acid-2-phosphate (0.1 mM), dexamethasone (1×107M), proline (400 µg/mL) was used. To examine the proliferation of the cells, Brdu labeling assay was performed using a Cell Proliferation ELISA kit from Roche Molecular Biochemicals. Cells at passage 1 were used in the assay. Wild type and BMP-2-deficient cells were seeded at a density of 4000 cells/well in a 96-well-plate in complete culture medium containing 20% fetal bovine serum. After 24 hours, cultures were replaced with the medium containing 2% fetal bovine serum with or without 100ng/ml of human recombinant BMP-2 for additional 24 hours. Brdu was added to a final concentration of 1 mM in the culture medium following BMP-2 treatment. After incubation for 3 hours, Brdu incorporation was measured according to the instruction from the manufacture. Brdu activity was read using a multi-mode microplate reader (BioTek, Highland park, VT).
Real-time PCR analyses
Total RNA was prepared using Qiagen RNA extraction kit. Exactly 0.5 µg of mRNA per callus was pooled and used in reverse transcription to make single-strand cDNA. Single-strand cDNA was synthesized using a commercial first strand cDNA synthesis kit (Invitrogen). RT-PCR reaction was performed using SyberGreen (ABgene, Rochester, NY) in a RotorGene real time PCR machine (Corbett Research, Carlsbad, CA). All genes were compared to a standard β-actin control. Data were assessed quantitatively using ANOVA comparing relative levels of transcript expression as a function of time. All primers used for the assessment are listed below.
| Genes | Primer forward | Primer reverse |
| murine Osx | 5'-ACTGGCTAGGTGGTGGTCAG-3' | 5'-GGTAGGGAGCTGGGTTAAGG-3' |
| murine BMP-2 | 5'-TGGAAGTGGCCCATTTAGAG-3' | 5'-GCTTTTCTCGTTTGTGGAGC-3' |
| murine ALP | 5'-TTGTGCGAGAGAAAGAGAGAGA-3' | 5'-GTTTCAGGGCATTTTTCAAGGT-3' |
| Murine Osteocalcin | 5'-AGGGAGGATCAAGTCCCG-3' | 5'-GAACAGACTCCGGCGCTA-3' |
MicroCT Imaging analyses
Femurs were harvested at day 14 post-grafting and scanned using a Viva MicroCT system (Scanco Medical, Bassersdorf, Switzerland) at a voxel size of 10.5 mm to image bone. The threshold was chosen using 2D evaluation of several slices in the transverse anatomic plane so that mineralized callus was identified while surrounding soft tissue was excluded. An average threshold of 220 was optimal and used uniformly for all samples. Each sample was contoured around the external callus and along the edge of the cortical bone, excluding the marrow cavity. New bone volume was measured on the surface of the host and donor side in grafted femurs as previously described [14, 22]. Briefly, to measure the new bone volume, contour lines were drawn manually by a specialized MircoCT technician in the 2D slice images to exclude the host cortical bone and marrow space. The volumes of interest (VOIs) on the host side and donor side were subsequently determined by manually tracing of the new bone formed on the entire length of the donor bone graft surface (New Bone Volume on donor site) or the host cortical bone surface at both bone graft junctions (New Bone Volume on host side). The new bone formation associated with either donor or host bone can be easily differentiated on the 2D images of the grafted femurs harvested on day 14.
Histology and analyses
X-Gal staining was performed on cryosections obtained from bone callus of BMP-2f/f; CreER; RosaR mice. Staining was carried out as we have previously described [14, 22]. Briefly, fresh femur samples were fixed in 0.2% glutaraldehyde at 4°C for 4 days and decalcifed in EDTA at 4°C for 14 days. Following complete decalcification, samples were immersed in 30% sucrose at 4°C overnight, and then embedded in OCT medium for cryosectioning. Sections were cut at 5 µm thick and stained in X-gal solution (0.02% NP40, 10mM EDTA, 0.02% glutaraldehyde, 0.05% X-gal and 2mM MgCL2 in phosphate buffer, PH 7.5) for 12 hours. X-gal positive cells were visualized and photographed under light microscopy. For analysis of cell fate, at least three samples were examined in each group and representative images were presented in the final figures. For specimens harvested for histomorphometric analyses, all samples were prepared via paraffin sections. Alcian blue and Orange G staining was used to examine the bone and cartilage formation as previously described [4]. Histomorphometric analyses were performed using Osteometrics™ software to determine the area of bone, cartilage and mesenchyme (a subtraction of total callus from bone and cartilage tissue) by manual tracing of the callus in computer program. The percent areas of bone, cartilage and mesenchyme were used for analyses. At least three non-consecutive longitudinal sections spanning 300 µm of the central region of the callus were used for histomorphometric analyses and a mean of three represents one sample. At least 6 samples were included in each group for analyses. The mean from 6 samples was used in statistical analyses to determine the composition of the bone graft callus. Cortical bone was excluded from the histomorphometric analysis [14, 22].
Statistical analysis
Each of the in vitro culture condition described consists of n=3 samples and each experiment were repeated 3 times. The statistical significance among each group was determined by analysis of variance (ANOVA). Data are expressed as a mean value plus or minus the standard error of the mean. Statistical significance between experimental groups was determined using one-way ANOVA and a Tukey's posthoc test. A P value <0.05 was considered statistically significant. Data analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Temporal deletion of BMP-2 blocks chondrogenesis and osteogenesis during cortical bone repair
Based on our previously published treatment schematic, TM was administered twice prior to surgery and another 2 doses at day 1 and day 3 following bone autografting to delete BMP-2 in all cells. Due to the propagation of the early stem/progenitors in periosteum, the treatment schematic shown in Figure 1A led to efficient gene recombination in early stem/progenitor cells and subsequently efficient removal of the targeted gene in periosteal callus [17].
Figure 1.
Temporal deletion of BMP-2 at the onset of bone autograft healing blocked periosteal callus formation. A) Schematic of Tamoxifen treatment during bone autograft healing. Representative MicroCT images show periosteal callus on day 14 post-surgery in BMP-2 f/f (B) and BMP-2 f/f; CreER mice (C). Volumetric analyses demonstrate new bone volume on host side (D) and Graft side (E), respectively. n= 6, * p<0.05.
To determine the outcome of bone graft healing, MicroCT analyses were performed at day 14 following bone autograft surgery in BMP-2f/f (wild type, WT) and BMP-2f/f; CreER (BMP-2 deficient, KD) mice. Treatment of Tamoxifen completely abolished bone callus formation at cortical bone junctions in BMP-2f/f; CreER mice (Fig. 1B and C). Volumetric analyses by MicroCT demonstrated a 3.7-fold reduction of total bone volume in BMP-2 deficient mice compared to the control mice treated with the same amount of Tamoxifen (Fig. 1D and E, n=6, p<0.01). The deletion of BMP-2 resulted in severe disruption of the healing such that minimal callus formation was observed at 4 weeks following osteotomy.
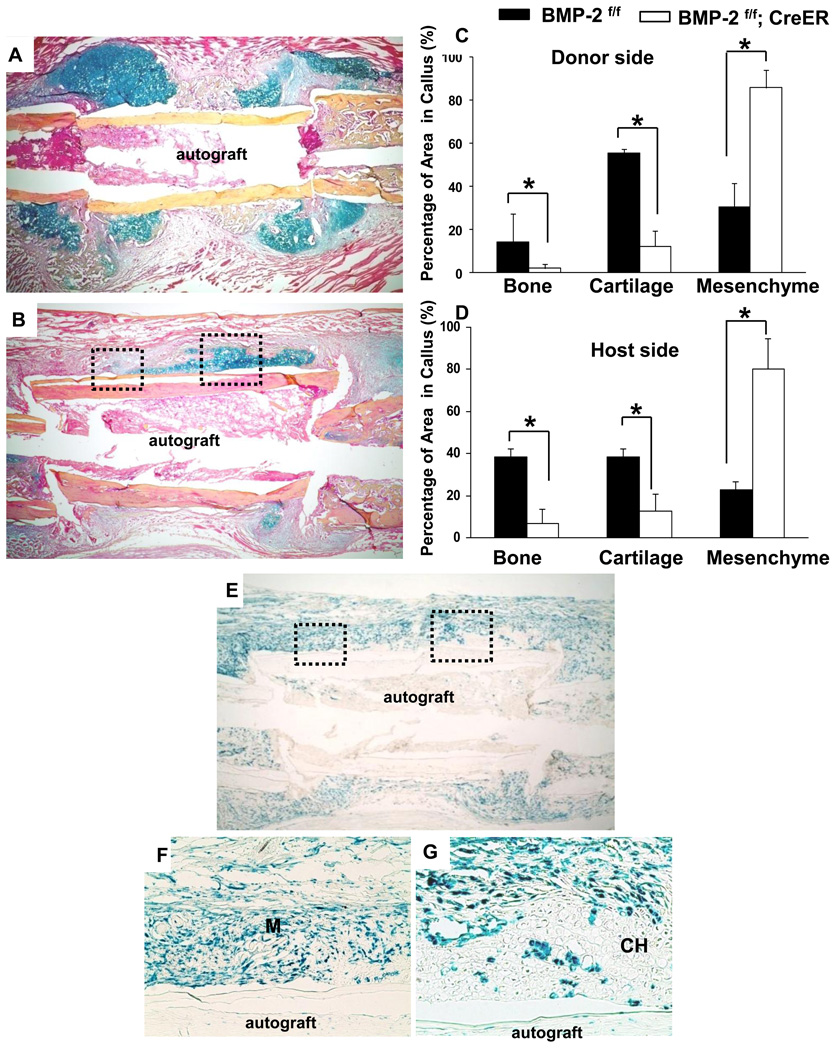
Histological analyses further demonstrated limited callus formation in BMP-2f/f; CreER mice treated with TM. Compared to the Cre negative control mice, the mutant mice showed persistent presence of undifferentiated mesenchymal cells at cortical bone junctions. This result indicated that mesenchymal cells were recruited to the site of injury in the mutant mice. However, these mutant cells were incapable of differentiating into chondrocytes or osteoblasts (Fig. 2A and B). Histomorphometric analyses demonstrated a 90% and an 80% reduction of bone formation at the donor side and host side, respectively. Cartilage formation was also reduced by 85% and 75% at the donor and host side. As a result of the lack of chondrogenesis and osteogenesis, percentages of undifferentiated mesenchymal cells were markedly increased at both host and donor sites in the mutant mice (Fig. 2C and D, n=6, p<0.05).
Figure 2.
Histologic analyses demonstrate impaired graft healing in BMP-2f/f; CreER; RosaR mice following gene deletion. Representative H&E Alcian Blue stained histologic sections show robust chondrogenesis and bone formation at the cortical bone junction in BMP-2f/f mice (A). In contrast, periosteal bone callus was nearly abolished in BMP-2f/f; CreER; RosaR mice following BMP-2 gene deletion (B). Quantitative histomorphometric analyses further demonstrates the marked reduction of bone and cartilage formation in BMP-2 deficient autografts at the donor site (C) and host site (D). n=6, * indicates p<0.05. X-Gal staining demonstrates efficient and chimeric gene recombination in BMP-2f/f; CreER; Rosa R mice following treatment of Tamoxifen (E). Boxed regions in E are shown in F and G at higher magnification. Of note is the presence of LacZ positive mesenchymal cells at the graft healing site (F). M: indicates mesenchyme. The majority of differentiated chondrocytes are LacZ negative indicating that these cells were derived from the wild type cells with endogenous BMP-2 expression (G). CH: indicates chondrocytes.
X-Gal staining was performed to track the fate of the mutant cells in triple transgenic BMP-2f/f; CreER; RosaR mice during healing. As previously described, TM treatment not only induced an efficient gene recombination within periosteal callus, but also resulted in formation of a chimeric callus with the presence of both mutant and wild type cells (Fig. 2E–G). Careful examination of the callus at higher magnification demonstrated that over 90% of the LacZ positive, mutant BMP-2-null mesenchymal cells remained undifferentiated at the periosteal site and cortical bone junctions (Fig. 2F). Remarkably, in the residue cartilaginous tissues, the majority of the differentiated chondrocytes remained as LacZ negative (wild type cells with endogenous BMP-2 expression). Only a few LacZ positive chondrocytes were found embedded in a large number of LacZ negative, fully differentiated wild type chondrocytes (Fig. 2G). This data strongly indicates that endogenous BMP-2 expression is critical and sufficient to induce mesenchymal progenitors to differentiate into bone forming cells at the healing site.
Endogenous BMP-2 is essential for periosteal progenitor cell differentiation in vivo
To further determine whether mutant cells can be induced to differentiate into chondrocytes and osteoblasts in a wild type healing environment, we transplanted the mutant BMP-2-deficient (KD) bone graft into a wild type mouse (WT) and vice versa. In these experiments, TM treatment was initiated prior to grafting for two times as described previously and followed by another two times at day 1 and day 3 post-grafting in the host mice. All wild type host mice received the same treatment as the mutant mice. MicroCT analyses demonstrated that the BMP-2-deficient donor bone graft generated significant less bone when transplanted into a host with normal expression of BMP-2 (Cre negative control floxed mice, WT) (Fig. 3A and B). Although abundant bone formation was found at the host side, osseointegration between host and donor bone graft was impaired due to deficient bone formation on the donor grafts. When compared to WT to WT transplantation, quantitative analyses further showed a 2.3 fold reduction of bone formation at the donor side with a similar amount of bone formation on the host side in KD-to-WT transplantations (Fig. 3E and F, n=6). Conversely, when a wild type donor graft was transplanted into a BMP-2 deficient BMP2f/f; CreER mice, MicroCT imaging showed that bone formation on the host side was significantly reduced, indicating the efficient gene deletion in the host mice (Fig. 3C). However, if compared with KD-KD transplantation (Fig. 3D), a nearly 2-fold increase of bone formation was found at the donor graft site (Fig. 3E and F, n=6, p<0.05). Although bone formation was significantly increased on the grafts, total bone formation as well as bone formation at the donor site was reduced compared with WT-WT transplantation, suggesting that host environment is critical for healing. Taken together, our results demonstrated that the aberrant BMP-2 expression in both transplantations (WT-KD or KD-WT) led to impaired osseointegration.
Figure 3.
Transplantation of BMP-2 deficient grafts (KD) into BMP-2 wild type (WT) mice failed to rescue the impaired differentiation of periosteal mesenchymal cells on BMP-2 deficient bone grafts. Representative MicroCT images show graft healing on post-surgery day 14 in WT to WT (A), KD to WT (B), WT to KD (C) and KD to KD (D) live bone graft transplantations. Volumetric analyses demonstrate new bone volume on the host side (E) and graft side (F). n= 6, * p<0.05. Of note is that transplantation of a BMP-2 deficient bone graft into a host with BMP-2 expression does not rescue the impaired periosteal healing. Tracking the fate of BMP-2 deficient cells in a wild type host (KD-WT transplantation) shows impaired periosteal callus formation along donor bone graft (G). X-Gal staining demonstrates impaired differentiation of LacZ positive BMP-2 deficient cells into chondrocytes (H). High magnification image of the boxed region shows that few LacZ positive BMP-2 deficient cells adopt chondrocyte morphology (I). Conversely, transplantation of a WT grafts derived from CreER; RosaR mice following treatment of TM into a BMP-2 deficient host (BMP-2f/f; CreER treated with TM) demonstrates abundant differentiation of donor cells into chondrocytes (LacZ positive indicates WT cells) (J). X-Gal stained histologic section at low (K) and high magnification (L) shows chimeric chondrocytes at the grafted femurs. CH: indicates chondrocytes.
Throughout our experiment, we found that changes in callus size corresponded well to the changes in bone volume. WT transplantation gave rise to a contiguous callus encompassing both cortical bone junctions. In contrast, in the BMP-2 mutant transplantations, minimal bone callus was observed along both host and donor periosteal surface. In the mixed transplantations, we found bone formation either favored host or donor depending on which side of BMP-2 was deleted.
To further determine the fate of the BMP-2 mutant cells in a wild type host injury environment, we transplanted donor bone graft from triple transgenic mice (BMP-2f/f; CreER;RosaR), which permit the tracking of the mutant cell fate via X-Gal staining in wild type host (BMP-2f/f; RosaR). As shown in Figure 3G–I, X-Gal staining showed a typical chimeric expression of LacZ in early repair callus at day 10 post-surgery. Remarkably, in all tissue sections examined, we found that only a few mutant cells (LacZ positive) were capable of differentiating into chondrocytes. And even fewer of the LacZ positive cells could differentiate into hypertrophic chondrocytes. The majority of LacZ positive cells remained as elongated mesenchymal cells in a wild type host (Fig. 3 H and I). And nearly all mature chondrocytes and osteoblasts were negative for X-Gal staining. In contrast to KD-WT transplantation, when we transplanted a wild type CreER; RosaR graft into a BMP-2 deficient BMP2f/f; CreER mouse that was treated with TM for 4 times, we found a large amount of LacZ positive chondrocyte and osteoblasts (WT) at the donor site (Fig. 3J–L). At high magnification, we observed chimeric differentiation of the donor cells into chondrocytes (Fig. 3L).
Deletion of endogenous BMP-2 blocked periosteal progenitor cell differentiation in vitro
To further determine the role of endogenous BMP-2 in the differentiation of periosteal progenitor cells, mesenchymal progenitor cells were isolated from wild type (BMP-2f/f) and BMP-2 deficient (BMP-2f/f; CreER) periosteal callus 5 days following bone autograft surgery. The isolated cells express several mesenchymal stem cell markers, namely Sca-I, SSEA-4 and CD105, and can be differentiated into multiple lineages such as osteoblasts, chondrocytes and adipocytes [21]. We found that using the described TM treatment scheme we were able to knock down 90% of the BMP-2 expression in the cells isolated from BMP-2 deficient periosteal callus (Fig. 4B). And remarkably, we found that osteogenic differentiation of the isolated progenitor cells was nearly blocked in BMP-2 deficient cells, as indicated by markedly reduction of ALP staining and osteoblastic marker gene expression (Fig. 4 A and B). Chondrogenesis was also examined in a micromass culture using cells isolated from wild type and BMP-2 deficient bone grafts. BMP-2 deficient cells formed a very loose micromass culture that was quickly dissociated from the culture dish (data not shown), indicating impaired chondrogenesis in the mutant cells.
Figure 4.
Impaired proliferation and differentiation in BMP-2-deficient periosteal mesenchymal progenitors. Mesenchymal cells derived from periosteal callus were isolated from BMP-2f/f and BMP-2f/f; CreER callus following Tamoxifen treatment as described in the Method section. Cells were cultured in the differentiation medium for 6 days. ALP staining demonstrates significant inhibition of osteoblastic differentiation following deletion of BMP-2. The addition of exogenous BMP-2 further enhances the differentiation of BMP-2 deficient cells (A). Quantitative analyses of the same culture by real time PCR demonstrate markedly reduction of the expression of BMP-2 and osteogenic gene, Osteocalcin, OSX and ALP in BMP-2 deficient cells (B). Cellular proliferation assay via Brdu labeling demonstrates significant reduction of the proliferation of BMP-2 deficient cells. Exogenous BMP-2 can partially rescue the impaired proliferation in BMP-2 deficient cells (C). n=3, * p<0.05.
To determine whether BMP-2 deficient cells remained responsive to exogenous BMP-2, we treated wild type and BMP-2 deficient cells with increasing doses of exogenous BMP-2. As indicated in Fig. 4 A and B, BMP-2 deficient cells responded to exogenous BMP-2 in a dose-dependent manner. However, the enhanced differentiation failed to reach the level of the wild type cells (Fig. 4B), as indicated by both ALP staining and RT-PCR analyses. In addition to impaired differentiation, Brdu labeling of the culture further showed a 23% reduction of cellular proliferation in BMP-2 deficient cells. The impaired proliferation could also be rescued partially by addition of exogenous BMP-2 in vitro (Fig. 4C).
DISCUSSION
To gain better understanding of the molecular pathways that control the mesenchymal progenitor cell fate during the early stage of cortical bone repair, we devised a CreER mediated gene deletion approach combined with a bone graft transplantation model to examine the role of BMP-2 at the initiation stage of cortical bone healing. Here, we provided several lines of evidence to demonstrate that the expression of endogenous BMP-2 was critical for osteogenic and chondrogenic differentiation of the periosteal progenitor cells during bone repair. Deletion of BMP-2 at the early stage of healing led to severe disruption of bone callus formation at the cortical bone junction.
Using BMP-2f/f; Prx-1Cre mouse model, Tsuji et al show that deletion of BMP-2 in limb bud at the embryonic stage results in deletion of BMP-2 in the adult limbs. As a result, bone healing is completely disrupted [23]. Since BMP-2 is found to be absent in bone matrix as well as in all cells derived from the limb mesenchymal progenitors including muscles, fibroblasts and ligaments, the Prx-1Cre mediated BMP-2 deletion does not allow tracking cell fate and further examination of the autocrine and paracrine effects of BMP-2 on bone healing. Using a temporal gene deletion approach via CreER-mediated gene recombination in adult animals, in this study, we deleted BMP-2 in adult animals during the early stage of cortical bone healing. By incorporation of a ROSA26 reporter (R26R) that enables the activation of a LacZ gene in the mutant cells upon treatment of Tamoxifen, we further created a chimeric bone callus that allowed us to track mutant cell fate and further examine the interaction of the wild type and the mutant cells during healing. Our data showed that only mesenchymal progenitors expressing endogenous BMP-2 were capable of differentiating into bone forming cells, namely chondrocytes and osteoblasts. The majority of the BMP-2 null cells remained undifferentiated at the site of healing. These data strongly indicates that the expression of the endogenous BMP-2 is essential for the differentiation of the bone progenitor cells in vivo.
To take the advantage of the graft transplantation approach that permits the miss-and-match of the different donor and host, we further examined the differentiation of BMP-2 null cells in a wild type and a BMP-2 deficient injury milieu. Our data showed that when a BMP-2 deficient bone graft was transplanted into a wild type host, the donor site bone formation was markedly reduced. By tracking the fate of the mutant cells, we demonstrate that transplantation of BMP-2 null cells into a wild type host did not rescue the fate of the mutant cells. Most of the LacZ positive, BMP-2 null cells remained as poorly differentiated mesenchymal progenitors (Fig. 3I). The lack of the differentiation of the mutant cells in a wild type environment further supports the notion that endogenous BMP-2 induces differentiation via a largely autocrine rather than a paracrine mechanism.
Another line of evidence for the critical role of endogenous BMP-2 in repair comes from in vitro experiments using isolated progenitors from the wild type and BMP-2-deficient periosteal callus. Culturing of the wild type cells for 48-hour did not produce detectable amount of BMP-2 protein in the supernatant as determined by Enzyme-linked immunosorbent assay (ELISA) for BMP-2 (data not shown). However, deletion of BMP-2 gene endogenously blocked the osteogenic and chondrogenic differentiation of the isolated mutant periosteal progenitors. The impaired differentiation could be partially reversed by additional of exogenous BMP-2 in vitro. These data suggest that BMP-2-deficient cells remain responsive to exogenous BMP-2. The impaired differentiation of bone progenitor cells in vivo is due to the absence of endogenous BMP-2 and the insufficient presence of BMP-2 in their surroundings. These data again underscore the essential role of endogenous BMP-2 signaling in control of the bone progenitor cell differentiation in vivo.
In addition to reduced differentiation, we also found a significant reduction of cellular proliferation in BMP-2 mutant cells, strongly indicating that endogenous BMP-2 was involved in the regulation of proliferation, and potentially survival of the periosteal progenitors. The addition of exogenous BMP-2 could further increase the proliferation of the mutant cells in vitro (Fig. 4C). Although the proliferative effect of BMP-2 is not well characterized in bone progenitors, BMP-2 has been shown to exhibit a paradoxical role in modulating cell proliferation and cell fate decisions in neural precursor cells [24]. BMP-2 has also been shown to prevent apoptosis in a chondrocytic cell line as well as in human vascular smooth muscle cells and mouse embryonic fibroblasts [25–26]. It is not surprising that endogenous BMP-2 is involved in the regulation of the proliferation and survival of the bone progenitors. The regenerative activity of BMP-2 is often coupled to an increase in mesenchymal progenitor numbers and a decrease in apoptosis [27]. These actions show that BMP-2 as a strong osteoinductive factor coordinates both differentiation and proliferation in order to precisely control the pace of bone and cartilage regeneration.
It has been speculated that endogenous BMP-2 is crucial for bone formation and bone repair [28–30]. However, so far no direct evidence has been provided. Expression of BMP-2 is induced in the periosteal cells as well as chondrocyte and osteoblasts during the initiation of the healing [31–35]. The timing and localization of BMP-2 are consistent with our current conclusions, which suggest that the activation of BMP-2 signaling in early progenitor cells is critical for the initiation of healing. While BMP-2 has been identified as a secreted factor that primarily functions as an autocrine and paracrine factor for cell proliferation and differentiation, a recent study demonstrates the existence of a nuclear variant of BMP-2 in the 10T1/2 mesenchymal cells and a rat chondrosarcoma (RCS) cell line, associated with cell cycle progression[36]. Although the study remained descriptive, it raises an interesting question as to a potential intracrine function of the nuclear variant of BMP-2 in modulating cellular proliferation, survival, and differentiation.
In addition to the critical role of endogenous BMP-2 in progenitor cells, interestingly, we also found that host plays a key role in cortical bone graft repair. When a wild type donor bone was transplanted into a BMP-2 deficient host, bone formation at the donor site was increased significantly compared to the KD-to-KD transplantation (Fig. 3F). Tracking of the donor cells showed efficient differentiation of WT donor progenitor cells to chondrocytes (Fig 3J–L). However, the induction of the bone formation on the graft did not restore to the level of WT-to-WT transplantation as indicated by MicroCT analyses. The reduced bone formation from the WT donor graft in a BMP-2 deficient host is likely due to the systemic effects resultant from BMP-2 deletion in the host. These data reinforce the notion that efficient osseointegration of the cortical bone requires coordinated bone formation from both host and donor. The absence of BMP-2 in either host or donor could lead to impaired bone graft repair.
Our current study embodies a series of novel approaches to examine the role of endogenous BMP-2 in repair. These approaches are useful in dissecting the molecular controls of periosteal progenitor cell fate during bone repair and regeneration. Although our current study focuses on the early stage of healing, CreER-mediated recombination can also be induced at a late stage of healing (remodeling) by controlling the timing and dosing of Tamoxifen. These experiments are under way to further characterize the role of BMP-2 in the late stage of healing. The drawback of our current approach is the non-selective deletion of BMP-2 in nearly all cells due to the insertion of CreER in a ubiquitous Gt(ROSA)26Sor locus. The development of a drug-inducible mouse model specifically targeting adult periosteum will further advance our understanding of the molecular signaling that controls periosteum-dependent bone repair and regeneration.
Bone morphogenic proteins have been widely used as strong osteoinductive factors for bone tissue repair and regeneration. Although extremely successful in various animal models, the effectiveness of BMPs usually requires large doses and sustained delivery of active proteins, particularly in humans [37–39]. Our current study indicates that stimulation of endogenous BMP-2 expression may represent a more effective approach for enhancing bone repair and regeneration. Future studies will need to focus on elucidating the mechanism by which BMP-2 pathway is activated in bone progenitors at the early stage of healing. A deeper understanding of the regulation of endogenous BMP-2 expression at the early stage of healing will provide novel insight for devising new therapeutic strategies aimed at enhancing skeletal repair and regeneration.
ACKNOWLEDGEMENTS
Funding support: The Musculoskeletal Transplant Foundation
NYSTEM: N08G-495
NIH: RC1AR058435, AR051469, AR048681, P50AR054041
A grant from Finger Lake Eye and Tissue Bank
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampath TK, Muthukumaran N, Reddi AH. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987;84:7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampath TK, Reddi AH. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983;80:6591–6595. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–744. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 8.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 9.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 10.Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009;20:481–488. doi: 10.1016/j.cytogfr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 12.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20:2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie C, Ming X, Wang Q, Schwarz EM, Guldberg RE, O'Keefe RJ, Zhang X. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43:1075–1083. doi: 10.1016/j.bone.2008.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, Guldberg RE, Schwarz EM, O'Keefe RJ, Zhang X. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435–445. doi: 10.1089/ten.2006.0182. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Awad HA, O'Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466:1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie C, Xue M, Wang Q, Schwarz EM, O'Keefe RJ, Zhang X. Tamoxifen-inducible CreER-mediated gene targeting in periosteum via bone-graft transplantation. J Bone Joint Surg Am. 2008;90 Suppl 1:9–13. doi: 10.2106/JBJS.G.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L, Martin JF. Generation of a Bmp2 conditional null allele. Genesis. 2005;42:203–206. doi: 10.1002/gene.20132. [DOI] [PubMed] [Google Scholar]
- 19.Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Huang C, Zeng F, Xue M, Zhang X. Activation of Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: implication for postnatal bone repair. The American Journal of Pathology. 2010 doi: 10.2353/ajpath.2010.100060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Naik A, Xie C, Reynolds D, Palmer J, Lin A, Awad H, Guldberg R, Schwarz E, O'Keefe R. Periosteal stem cells are essential for bone revitalization and repair. J Musculoskelet Neuronal Interact. 2005;5:360–362. [PubMed] [Google Scholar]
- 23.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90 Suppl 1:14–18. doi: 10.2106/JBJS.G.01109. [DOI] [PubMed] [Google Scholar]
- 24.Chen HL, Panchision DM. Concise review: bone morphogenetic protein pleiotropism in neural stem cells and their derivatives--alternative pathways, convergent signals. Stem Cells. 2007;25:63–68. doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- 25.Sugimori K, Matsui K, Motomura H, Tokoro T, Wang J, Higa S, Kimura T, Kitajima I. BMP-2 prevents apoptosis of the N1511 chondrocytic cell line through PI3K/Akt-mediated NF-kappaB activation. J Bone Miner Metab. 2005;23:411–419. doi: 10.1007/s00774-005-0622-7. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Shen J, Pu K, Katus HA, Ploger F, Tiefenbacher CP, Chen X, Braun T. GDF5 and BMP2 inhibit apoptosis via activation of BMPR2 and subsequent stabilization of XIAP. Biochim Biophys Acta. 2009;1793:1819–1827. doi: 10.1016/j.bbamcr.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Turgeman G, Zilberman Y, Zhou S, Kelly P, Moutsatsos IK, Kharode YP, Borella LE, Bex FJ, Komm BS, Bodine PV, Gazit D. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J Cell Biochem. 2002;86:461–474. doi: 10.1002/jcb.10231. [DOI] [PubMed] [Google Scholar]
- 28.Alam N, St-Arnaud R, Lauzier D, Rosen V, Hamdy RC. Are endogenous BMPs necessary for bone healing during distraction osteogenesis? Clin Orthop Relat Res. 2009;467:3190–3198. doi: 10.1007/s11999-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsell R, Einhorn TA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury. 2009;40 Suppl 3:S4–S7. doi: 10.1016/S0020-1383(09)70003-8. [DOI] [PubMed] [Google Scholar]
- 30.Kugimiya F, Kawaguchi H, Kamekura S, Chikuda H, Ohba S, Yano F, Ogata N, Katagiri T, Harada Y, Azuma Y, Nakamura K, Chung UI. Involvement of endogenous bone morphogenetic protein (BMP) 2 and BMP6 in bone formation. J Biol Chem. 2005;280:35704–35712. doi: 10.1074/jbc.M505166200. [DOI] [PubMed] [Google Scholar]
- 31.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 32.Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81:284–294. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:124–134. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46:841–851. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Farhadieh RD, Gianoutsos MP, Yu Y, Walsh WR. The role of bone morphogenetic proteins BMP-2 and BMP-4 and their related postreceptor signaling system (Smads) in distraction osteogenesis of the mandible. J Craniofac Surg. 2004;15:714–718. doi: 10.1097/00001665-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Felin JE, Mayo JL, Loos TJ, Jensen JD, Sperry DK, Gaufin SL, Meinhart CA, Moss JB, Bridgewater LC. Nuclear variants of bone morphogenetic proteins. BMC Cell Biol. 2010;11:20. doi: 10.1186/1471-2121-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, Song F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11:1–150. iii–iv. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Williams K, Umscheid CA, Welch WC. Osteoinductive bone graft substitutes for lumbar fusion: a systematic review. J Neurosurg Spine. 2009;11:729–740. doi: 10.3171/2009.6.SPINE08669. [DOI] [PubMed] [Google Scholar]
- 39.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]