Abstract
It was recently shown that adaptive immunity plays a key role in cognitive function. T cells appear to be major players in learning and memory; thus, mice devoid of functional T cells are impaired in performance of cognitive tasks such as Morris Water Maze (MWM), Barnes maze and others. This is a reversible phenomenon; injection of immune deficient mice with T cells from wild type counterparts improves their cognitive function. Recently we described a critical role for T cell-derived IL-4 as having beneficial effects on learning and memory through regulation of meningeal myeloid cell phenotype. In the absence of IL-4, meningeal myeloid cells acquire a pro-inflammatory skew. Thus, the presence of IL-4 in the meningeal spaces maintains a delicate balance of pro- and anti-inflammatory myeloid cell phenotype. Here we show that macrophages alternatively activated in vitro (M2 cells) can circumvent the need for ‘pro-cognitive’ T cells when injected intravenously into immune deficient mice. These results show for the first time that M2 myeloid cells are new and unexpected players in cognitive function, conferring beneficial effects on learning and memory without adaptive immune influence. These results might lead to development of new therapeutic approaches for cognitive pathologies associated with malfunction of adaptive immunity, such as chemo-brain, age-related dementia, HIV-dementia, and others.
Introduction
The negative effects of pro-inflammatory cytokines on cognitive function have been recognized for decades (Barrientos et al., 2003; Cumiskey et al., 2007; Golan et al., 2005; Goshen et al., 2007; Jain et al., 2002; Lee et al., 2009; Maher et al., 2005; McAfoose et al., 2009). Systemic infections were correlated with cognitive impairment, and systemic injection of TNFα or IL-1β resulted in impairment of cognitive function in rodents (Burgess et al., 1998; Dantzer, 2001; Dantzer et al., 2008; Heyser et al., 1997; Kelley et al., 2003; Shen et al., 2004; Sparkman et al., 2006). Aging, which is associated with reduced cognitive function, has been also correlated in mice with increased levels of pro-inflammatory cytokines (Loane et al., 2009; Maher et al., 2005; Salek et al., 2008).
Most of the works linking immunity and cognition were dedicated to the negative aspects of pro-inflammatory cytokines originating from innate immune cells (Cumiskey et al., 2007; Dantzer, 2001; Lin et al., 2009; McAfoose and Baune, 2009; McAfoose et al., 2009; Sparkman et al., 2006; Tanaka et al., 2006). Therefore, the role of adaptive immunity and its derived cytokines were largely neglected in these complex neuroimmune interactions.
Recently, we and others have shown that adaptive immunity plays a major role in brain function (Brynskikh et al., 2008; Cao et al., 2009; Derecki et al., 2010; Kipnis et al., 2004; Lewitus et al., 2008; Wolf et al., 2009; Ziv et al., 2006). Mice deficient in adaptive immunity (i.e. T cell deficient nude mice or T and B cell deficient SCID mice) exhibit cognitive impairment, which could be remedied following passive transfer of T cells from wild type counterparts (Brynskikh et al., 2008; Kipnis et al., 2004). Moreover, acute depletion of T cells from adult wild type mice, via transfer of SCID-derived bone marrow following irradiation, also resulted in cognitive impairment (Brynskikh et al., 2008; Derecki et al., 2010; Ron-Harel et al., 2008). These results suggested for the first time that peripheral adaptive immunity affects learning and memory.
Questions still remained regarding the location and the mechanism underlying T cell effect on cognitive function. No T cells were detectable in the parenchyma of naïve or trained mice, but in the areas immediately adjacent to the parenchyma, such as the sub-arachnoid meningeal spaces and choroid plexus, substantial immunological changes were apparent when the animals were introduced to a cognitive task (Derecki et al., 2010). These areas have been previously shown to be substantially populated by immune cells, but a majority of published works focused primarily on pathological conditions, such as meningitis, or Multiple Sclerosis (MS) (Kivisakk et al., 2009; Kivisakk et al., 2003).
We found that overall T cell numbers in the meninges increased significantly following the performance of a visuo-spatial learning and memory task. These T cells exhibited an activation phenotype and expressed high levels of IL-4, suggesting a possible molecular link (Derecki et al., 2010; Kipnis et al., 2008). Indeed, meningeal myeloid cells were also activated in response to a cognitive task, and interestingly, their phenotype was largely dependent on the influx of meningeal T cells. In mice lacking T cells altogether, meningeal myeloid cells adopted a skewed pro-inflammatory phenotype, expressing high levels of TNFα and IL-12. Similar results were obtained when T cell trafficking into the meningeal spaces was inhibited acutely via pharmacological blockade: When mice were treated with FTY720, a sphingosine-1 phosphate-1 receptor agonist that induces general lymphopenia, or with an antibody to VLA4, an integrin that governs T cell migration into the CNS, meningeal myeloid cells also adopt a pro-inflammatory (M1) skew, and display learning and memory impairment (Derecki et al., 2010). Moreover, mice with a full T cell repertoire—but deficient in IL-4—were profoundly impaired in cognitive function. IL-4 deficiency limited specifically to the immune system, as was shown by chimera studies, was sufficient to result in M1-skewed meningeal myeloid cells and impaired cognitive function, which could be improved by injection of wild type IL-4-competent T cells (Derecki et al., 2010).
These results led us to suggest that T cell-derived IL-4 is the major cytokine that regulates the phenotype of meningeal myeloid cells. However, we have also shown that IL-4 could directly affect astrocytes and induce them to produce high levels of brain derived neurotrophic factor (BDNF) (Derecki et al., 2010) associated with successful cognitive task performance. Therefore, the question is—what is the primary mechanism of IL-4 action? Is the cytokine working through regulation of meningeal myeloid cells, through induction of BDNF in astrocytes, or both? Based on these studies, we proposed that T cell malfunction might significantly contribute to the etiology of some cognitive conditions, like HIV-dementia, age-related dementia, and ‘chemo-brain.’ Important to note, however, is the following caveat: the boost of T cell function under the above-mentioned pathological conditions would not be feasible, and thus therapies that circumvent the need for T cells, instead driving downstream pathways, are needed (Kipnis et al., 2008).
In this study we show that induction of an alternatively activated (M2) phenotype of myeloid cells (Mantovani et al., 2005), achieved by skewing them in vitro with IL-4, and their subsequent injection into T cell-deficient recipients, results in the reverse of pro-inflammatory skew and in improved learning and memory. Our results suggest that M2 cells could contribute directly (in a T cell-independent manner) to cognitive function, and might be developed in the future as an eminently feasible new therapeutic approach for cognitive conditions associated with T cell malfunction.
Materials and Methods
Animals
Inbred male adult (8–10-wk-old) CBySmn.CB17-Prkdcscid/J (recipients) and C57BL/6-Tg(UBC-GFP)30Scha/J (donors) mice were purchased from the Jackson Laboratory. All animals were housed in temperature- and humidity-controlled rooms, maintained on a 12-h light/dark cycle (lights on at 7:00 a.m.), and age matched in each experiment. All strains were kept in identical housing conditions. The lifespan of SCID mice is comparable to that of wild-type mice, and there are no specific dietary or housing requirements for this mutant strain. Animal protocols were approved by the University of Virginia Institutional Animal Care and Use Committee. All procedures complied with regulations of the Institutional Animal Care and Use Committee at the University of Virginia.
Morris Water Maze (MWM)
Mice were given four trials per day, for 4 consecutive days, to find a hidden 10-cm diameter platform located 1 cm below the water surface in a pool 1 m in diameter. The water temperature was kept between 21 and 22°C. Water was made opaque with non-toxic tempera paint. Within the testing room, only distal visual shape and object cues were available to the mice to aid in location of the submerged platform. The escape latency, i.e., the time required by the mouse to find and climb onto the platform, was recorded with a 60s limit. Each mouse was allowed to remain on the platform for 30 s and was then moved from the maze to its home cage. If the mouse did not find the platform within 60 s, it was manually placed on the platform and returned to its home cage after 30 s. The inter-trial interval for each mouse was 5 min. On day 5, the platform was removed from the pool, and each mouse was tested by a probe trial for 60 s, and the amount of time spent in the quadrant where the platform was previously located was recorded. On days 6 and 7, the platform was placed in the quadrant opposite the original training quadrant, and the mouse was retrained for four sessions each day. On day 8 mice were introduced to the pool with a visible platform in a third quadrant, different from the first two training quadrants, and were recorded for four trials. Data from all experiments were recorded using the EthoVision automated tracking system (Noldus Information Technology). Statistical analysis was performed using analysis of variance (ANOVA) and the Bonferroni post-hoc test. Groups were counterbalanced, i.e., run in alternating order on successive training days. All MWM testing was performed between 10 a.m. and 3 p.m. during the lights-on phase. All behavior experiments were performed by an experimenter blinded to the identity of experimental groups.
FACS
Bone marrow-derived macrophages were cultured for 9 days, and then incubated with either 20 ng/ml rIL-4 for 72 hours or IFN-gamma for 48 hrs followed by the addition of lipopolysaccharide for 24 hours (72 hours total for both conditions). Extracellular and intracellular staining was done with cultured macrophages, which were labeled with antibodies to TNFα or CD206 and analyzed by FACS. After 8 days MWM training, viable myeloid cells (CD45+CD11b+) were isolated from meninges and spleens of control and M2-injected animals and were compared for expression of granulocyte and monocyte markers (Gr-1, Ly6C), extracellular markers indicating M2 skew (CD206), and meningeal myeloid expression of intracellular cytokines IL-10 and TNF by FACS.
Bone marrow isolation
Mice were sacrificed using CO2 and saturated with 70% alcohol. Skin was removed from the lower part of the body. Tissue was removed from hind legs with scissors and dissected away from the body. Remaining tissue was cleaned from the tibial and femoral bones and bones were separated at the knee joint. Bone ends were cut off. Cells were forced out of bones with a stream of 0.1 M PBS, pH 7.4, containing 10% fetal calf serum using a 10-cc syringe with a 25-gauge needle. Cells were centrifuged and resuspended at a concentration of 2 × 107 cells/ml in PBS and plated in TC-coated 24-well plates in Hi Glucose DMEM (Invitrogen) + 10% Heat inactivated FBS (Invitrogen) + 10 ng/ml recombinant MCSF (R & D) at 37° 5% CO2, 10^6 BM cells/well in 1.5 ml medium, which was changed every 3 days. On the ninth day, macrophages were skewed to M2 with 20ng/ml rIL4 (R and D) for 72 hours, or M1 with IFN-g (50ng/ml) for 48 hours, then LPS for 24 hours. The cells were released with 10mM EDTA for 15 mins and vigorous pipetting to mix. The cell solution was then centrifuged at 300 X G for 10 minutes and re-suspended in either sterile PBS (for i.v. injection) or artificial cerebrospinal fluid (ACSF) (for i.c.v. injection) at 2×106/ml.
Injections
I.v. injection was performed wherein 250 ul (0.5X106) of either M1- or M2- skewed macrophages suspended in isotonic PBS or plain PBS was injected via the tail vein. The animals were then rested for 5 days before initiating MWM training. For i.c.v. injections, mice were anesthetized with ketamine/xylazine anesthesia; holes were drilled in skulls and stereotactic coordinates (0.5 mm lateral to Bregma and 2 mm deep) were used for injection. All mice received injections of 1 ul each side (total of 2ul) of M1 macrophages, M2 macrophages or ACSV. The animals were then rested for five days; wound clips were removed, and animals were rested for an additional two days (seven days total) before initiating MWM training.
FACS of meningeal isolates
Mice were thoroughly transcardially perfused with pH 7.4 0.1M PBS immediately after the last training trial. Heads were removed and skulls were quickly stripped of all flesh. Mandibles were next removed, as was all skull material rostral to maxillae. Surgical scissors (Fine Science Tools, Foster City, CA) were used to remove tops of skulls, cutting clockwise, beginning and ending inferior to the right post-tympanic hook. Brains and superior skulls were immediately placed in ice-cold FACS buffer (pH 7.4 0.1M PBS, 1mM EDTA, 1% BSA). Meninges (dura mater, arachnoid mater and pia mater) were carefully removed from the interior aspect of skulls and surfaces of brains with Dumont #5 forceps (Fine Science Tools, Foster City, CA). Meninges from each group were pooled. Meningeal tissue was gently pressed through 70 m nylon mesh cell strainers with sterile plastic plungers (BD Biosciences, Franklin Lakes, NJ) to yield a single cell suspension. Cells were then centrifuged at 1100 RPM at 4°C for 10 minutes, the supernatant was removed, and cells were re-suspended in ice-cold FACS buffer. Cells were stained for extracellular markers with antibodies to CD11b conjugated to FITC, PE, APC-Cy7, or PE-Cy7; CD45 conjugated to APC, APC-Cy7, or efluor 450, Ly6C conjugated to PE-Cy5 Ly6G conjugated to PE-Cy7; cells were stained for intracellular markers with antibodies to IL-10 conjugated to FITC, TNFα conjugated to PE. (eBioscience, San Diego, CA). For IL-10 and TNFα staining, meningeal isolates were incubated with 10 g/ml Brefeldin-A at 37°C for 5 hours, then labeled with appropriate antibodies as above (eBioscience). All cells were then fixed in 1% PFA in 0.1M pH 7.4 PBS. Fluorescence data was collected with a CyAn™ ADP High-Performance Flow Cytometer (Dako, Carpenteria, CA) and then analyzed using Flowjo (Tree Star). To obtain equivalent and accurate cell counts cells were gated first using the LIVE/DEAD® Fixable Dead Cell Stain Kit per manufacturer's instructions, (Invitrogen), forward scatter vs. side scatter to eliminate debris, pulse width vs. area to select singlet cells, then by appropriate markers for cell type (e.g. CD11b for myeloid-derived cells).
Results
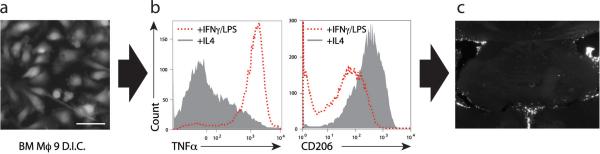
Bone marrow-derived macrophages (BMDM) from UBC-GFP mice [C57BL/6-Tg(UBC-GFP)30Scha/J] were grown in culture for 9 days (Fig. 1a) and then treated with either IL-4 or IFN-γ/LPS to obtain either alternatively (M2) or classically (M1) activated macrophages (Mantovani et al., 2005) prior to their injection into the mice. After the treatment the cells were labeled with CD206 (a marker of M2 cells) and with TNFα (characteristic of M1 cells) and examined by FACS. BMDM treated with IL-4 exhibit low levels of TNFα and high levels of CD206 and those classically activated with IFN-γ/LPS exhibit high levels of TNFα and lower levels of CD206 expression (Fig. 1b). SCID mice were injected intracerebroventricularly (i.c.v.) with either artificial cerebrospinal fluid (ACSF) or M2 cells suspended in ACSF. The injected cells could be visualized in the meningeal spaces of the injected mice for at least 2 weeks post injection (Fig. 1c).
Figure 1. Generation, characterization and intracerebroventricular injection of alternatively activated macrophages.
(a) Representative image of alternatively activated bone marrow-derived macrophages after nine days in culture. (b) FACS analysis of M1 and M2 skewed macrophages labeled for TNFα and CD206 expression. (c) Representative images of CFSE-labeled macrophages in ventricles 3d after i.c.v. injection are shown.
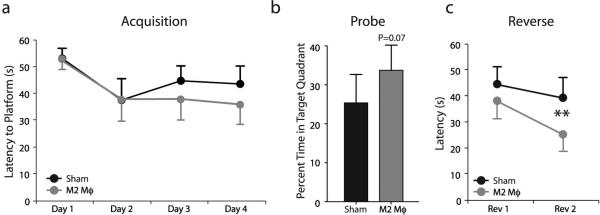
One week after the cells were placed into the ventricular spaces of SCID mice, the animals were examined on the MWM spatial learning and memory cognitive task. No significant effect of M2 cell injection as compared to control treated mice was evident in the acquisition stage of the MWM (Fig. 2a). There was a slight but statistically insignificant increase in the time spent by the M2-treated mice in the training quadrant on the probe trial (Fig. 2b). Finally, a significant improvement of the M2-treated mice was obtained on the last day of the reverse trial (Fig. 2c), suggesting that M2 cells are only having a marginal effect under these experimental conditions. It should be noted that SCID mice are very poor learners, as was previously published by us and others (Brynskikh et al., 2008; Ron-Harel et al., 2008). However, upon passive transfer of T cells their learning significantly improves (Kipnis et al., 2004), suggesting that these mice are amenable to learning improvement. It should be noted that the velocities of both groups of mice did not differ and thus data analysis based on the distance traveled to the platform has revealed identical results (data not shown).
Figure 2. I.c.v. injection of M2 cells into SCID mice marginally benefits MWM performance.
SCID mice (C57BL/6J) were injected i.c.v. with either M2-skewed macrophages (N=8) or ACSV vehicle (N=8) and monitored during MWM task performance. (a) During the acquisition and (b) the probe trial phases, no significant difference between the two groups was obtained. (c) During the reverse phase, M2-injected mice took significantly less time to locate the hidden platform as compared to their controls. Two-way repeated measures ANOVA with post hoc Bonferroni test was used for statistical analysis (**, p < 0.01). All behavior experiments were recorded with the EthoVision video tracking system and performed by an experimenter blinded to the identity of experimental groups. Representative experiments are shown out of two independently performed.
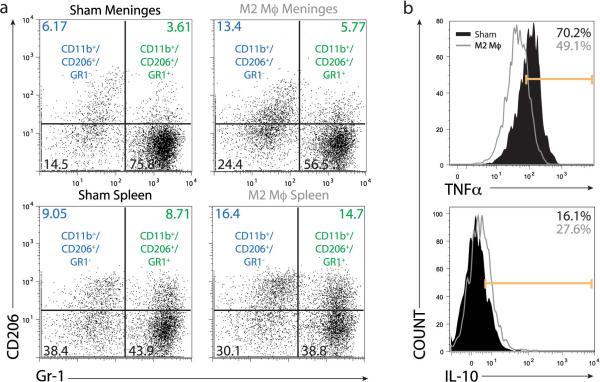
We examined the systemic and the local (meningeal) adaptive immune response in the recipients SCID mice after the training. To examine the changes induced by our implanted M2 cells on the resident myeloid cells, we examined the GFP negative fraction of isolated CD11b+ cells (i.e. excluding the injected GFP cells from the analysis). The numbers of CD11b+ myeloid cells expressing high levels of the mannose receptor (CD206), a marker of anti-inflammatory phenotype, in the meninges of M2-treated mice were increased as compared to controls (Fig. 3a). Interestingly, similar results were obtained in the spleens on the treated mice, suggesting that meningeal immunity has a more broad systemic effect. The cytokine profile of meningeal myeloid cells was also changed and the characteristic pro-inflammatory skew of meningeal myeloid cells in SCID mice was ameliorated in mice treated with M2 as is evident by a reduction in TNFα and increase in IL-10 expression by CD11b+ meningeal cells (Fig. 3b).
Figure 3. I.c.v. injection of M2 cells into SCID mice results in an anti-inflammatory skew of endogenous meningeal myeloid cells.
FACS analyses were performed on viable, CD45+/CD11b+ cells isolated from (a, c) meninges and (b) spleen of MWM-trained mice. Numbers on histograms indicate percentages. CD11b+ cells from both spleens and meninges of M2-injected animals showed up-regulation of CD206 as compared to vehicle-injected control. (c) Endogenous meningeal myeloid cells of M2-injected animals also displayed decreased production of the pro-inflammatory cytokine TNFα and increased production of anti-inflammatory IL-10. Representative experiment out of two independently performed is presented (n = 8 mice in each group).
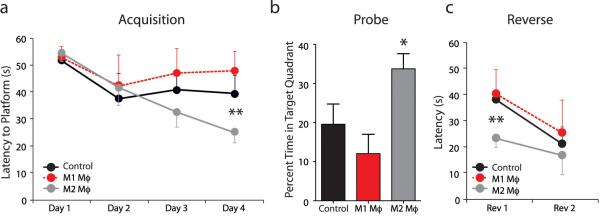
Since the i.c.v. injection of M2 cells did not yield a robust effect on cognitive behavior but did have a substantial effect on meningeal myeloid cell phenotype, we presumed that the injury associated with i.c.v. injection and its accompanying inflammation might have masked the real benefit induced by M2 cells. To address this question we changed the route of M2 administration from i.c.v. to intravenous (i.v.) injection. SCID mice were treated with i.v. injection of PBS, M1 or M2 cells, and 5 days after were examined on the MWM task. A significant improvement of M2 treated SCID mice was evident in the acquisition phase of the task and a slight but insignificant impairment was obtained in M1-treated mice (Fig. 4a). In a probe trial, M2 treated mice performed significantly better then their control or M1-treated mice (Fig. 4b). Finally, in the reverse phase, M2-treated mice again showed a significant superiority in the task performance compared to control or M1-treated mice (Fig. 4c). These results demonstrate for the first time that i.v. treatment of SCID mice with alternatively activated macrophages improves cognitive function as tested in MWM task. As mentioned above, the velocities of all three groups did not differ and thus data analysis based on the distance traveled to reach the platform revealed similar results (data not shown).
Figure 4. Intravenous injection of M2 improves cognitive performance of SCID mice on MWM task.
SCID mice (C57BL/6J) were injected i.v. with either M1-skewed macrophages, M2-skewed macrophages or PBS vehicle (N=8 in each group) and monitored during MWM task performance. During (a) the acquisition and (c) the reversal phases of the task, M2-injected mice took significantly less time than M1- or vehicle-injected controls to locate the hidden platform. Two-way repeated measures ANOVA with post hoc Bonferroni test was used for statistical analysis (**, p < 0.01). (b) During the probe trial, M2-injected mice spent significantly more time in the training quadrant than M1- or vehicle-injected controls. Student's t-test; (*, p < 0.05). Representative experiments are shown out of two independently performed.
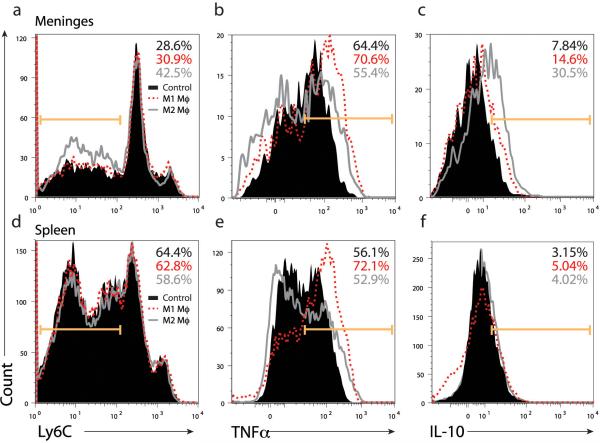
We then analyzed the meningeal and peripheral (splenic) immunity of the trained mice. As before, we concentrated on the GFP negative (presumably induced) fraction of CD11b+ cells, although it should be mentioned that low levels of GFP+CD11b+ cells were found in examined tissues (meninges and spleen), which is expected since mature macrophages do not typically exit circulation as do immature monocytes, and probably mediate their effect through ‘infectious’ regulation of blood monocytes. It was evident that M2-treated mice had higher numbers of Ly6Clo cells specifically in meninges as compared to control and M1-treated mice. These Ly6Clo cells are most likely mature, tissue-building monocytes, rather than classical ‘inflammatory monocytes’, which typically express high levels of Ly6C antigen. The levels of TNFα were significantly reduced in M2-treated mice as compared to M1-treated mice (Fig. 5b), whereas IL-10 expression was higher in meningeal myeloid cells obtained from M2-treated mice that from control mice (Fig. 5c). Interestingly, here as well, differences were evident in peripheral immunity as exhibited by splenic cells. Increased expression of TNFα (Fig. 5e) and reduced levels of IL-10 expression (Fig. 5f) were seen in splenocytes from M1-treated mice, while Ly6C expression did not differ in splenocytes as it did in meningeal myeloid cells (Fig. 5d).
Figure 5. I.v. injection of M2 cells into SCID mice results in an anti-inflammatory skew of endogenous meningeal myeloid cells.
FACS analyses were performed on viable, CD45+/CD11b+ cells isolated from (a-c) meninges and (d-f) spleen of MWM-trained mice. Numbers on histograms indicate percentages. CD11b+ cells from both spleens and meninges of M1- or M2- injected animals and their vehicle-injected controls were examined for (a, d) Ly6C, (b, e) TNFα, and (c, f) IL-10 expression. Representative experiment out of two independently performed is presented (n = 8 mice in each group).
Discussion
Here we demonstrate that the transfer of alternatively activated myeloid cells benefits cognitive function in mice devoid of adaptive immunity. The improved cognitive function in this model is associated with alleviation of a skewed pro-inflammatory (M1) phenotype of meningeal myeloid cells, characteristic of mice deficient in normal meningeal T cell immune response. These results suggest that M2 cells could be utilized in future as a potential therapy for cognitive disorders associated with T cell dysfunction, such as HIV- dementia, age- related dementia, or ‘chemo-brain.’
The precise mechanism (whether cell migration of release of soluble factors to mediate this effect) underlying the changes in peripheral immunity as a result of i.c.v. injection of M2 cells, is beyond the scope of this paper and is being currently studied in the lab. Along these lines, this study aimed to address two major, and likely interconnected questions: First, are T cells working primarily through regulation of meningeal myeloid cell phenotype in their beneficial effect on cognitive function? Second, can immune-mediated benefit on cognitive function be achieved in a T cell-independent manner, or, in other words, can IL-4-activated myeloid cells circumvent the need for T cells and their derived IL-4?
We have shown that T cell derived IL-4 is the major soluble factor identified thus far that regulates meningeal immunity and contributes to cognitive function (Derecki et al., 2010). However, since IL-4 has multiple potential targets—we have previously shown two, i.e. the phenotype of meningeal myeloid cells and BDNF expression by astrocytes—we did not know the extent to which the anti-inflammatory cytokines produced by T cell-induced meningeal myeloid cells might be important for cognitive benefit. Our current results indicate that myeloid cells may play a critical role in the T cell-mediated effect on cognitive function, at least under these experimental conditions. These results do not eliminate the possibility that the T cell-derived IL-4 regulation of astrocyte-produced BDNF is also involved in the T cell-mediated benefit in cognition. To perfectly address this question, a separate depletion of IL-4 receptor on meningeal myeloid cells or on astrocytes is needed. These experiments are currently underway in our laboratory, using IL-4R floxed mice (Marillier et al., 2010).
It should also be noted that all of the experiments presented in this manuscript were performed in immune deficient, SCID, mice. These mice are shown to present a skewed pro-inflammatory myeloid phenotype in both periphery and meninges, due to the lack of T cells. It will be important to examine the effect of M2 cells on cognitive function under conditions of partial T cell malfunction more analogous to human conditions, such as in aged mice and mouse models of chemotherapeutic response. It is possible that in the presence of low levels of T cells, that would not be sufficiently supportive of cognitive function on their own, injection of M2 cells would result in even greater beneficial effect, since beyond their effect on innate immunity, M2 cells could also shift T cell immunity towards an anti-inflammatory TH2 phenotype, leading to a self-propagating beneficial response.
The i.c.v. injection of M2 cells did not yield a very significant behavioral effect, although a molecular effect was indeed observed. While prima facie surprising, this could, in fact, be due to the injury associated with the application of cells. Indeed, it is quite possible that the directly injected M2 cells would be marshaled by the CNS to participate in wound healing—rather than in support of cognition. The likelihood of this scenario is further supported by the fact that the number of cells injected was quite small (a necessity, given the tight constraints of intraventricular fluid pressure that limit injection volume), and that the injury was directly available to the skewed macrophages. Furthermore, microscopic examination post-injury shows significant intercalation of labeled cells within the brain parenchyma (not shown).
The effect of i.c.v. injection on peripheral immunity is intriguing, though not unprecedented. It has been recently shown that the CNS, via the splenic nerve, may be able to directly effect downregulation of macrophage-derived TNFα in spleen (Rosas-Ballina, PNAS, 2008). Whether the observations described in this paper are a result of meningeal or parenchymal immunity affecting peripheral immunity are unclear, and will require further study.
Collectively, we have shown here evidence that anti-inflammatory myeloid cells administered i.v. can significantly ameliorate cognitive impairment in mice lacking adaptive immune cells. While it has been amply demonstrated by our group and others that CD4+ T cells function in support of cognition (Derecki et al., 2010), to our knowledge this is the first time that an immune-based T cell-independent boost of cognitive function has been demonstrated. The obviation of T cells as a necessary component in the support of learning and memory by the immune system is critical in terms of bridging the gap between bench and bedside application of basic neuroimmunology. Therapies that necessitate direct T cell manipulation are inherently risky. A boost of T cell function, if not well-controlled, can result in catastrophic autoimmunity, while suppression of T cell function increases the risk of cancer (Mantovani and Sica, 2010; Sica et al., 2008), and, as demonstrated recently, can also result in unanticipated inflammation and cognitive consequences (Derecki et al., 2010). Myeloid cells, on the other hand, provide an opportunity to harness the therapeutic power of the immune system, and its ability to innately target anatomical sites of dysfunction—such as the meningeal spaces—without substantial risk of autoimmune consequences.
Acknowledgments
We thank Joanne Lannigan and Michael Solga for assistance with FACS acquisition and data analysis. We also thank Gina Wimer, Jeremy Gatesman, and Bonnie Tomlin for animal care assistance. This work was supported by NIA (R01AG034113) award to JK. NCD was supported by NIH grant T32HD007323.
Abbreviations used
- CNS
central nervous system
- MWM
Morris water maze
- M2
alternatively activated macrophages
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Burgess W, Gheusi G, Yao J, Johnson RW, Dantzer R, Kelley KW. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol. 1998;274:R1829–1833. doi: 10.1152/ajpregu.1998.274.6.R1829. [DOI] [PubMed] [Google Scholar]
- Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW. Abeta-specific Th2 cells provide cognitive and pathological benefits to Alzheimer's mice without infiltrating the CNS. Neurobiol Dis. 2009;34:63–70. doi: 10.1016/j.nbd.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumiskey D, Pickering M, O'Connor JJ. Interleukin-18 mediated inhibition of LTP in the rat dentate gyrus is attenuated in the presence of mGluR antagonists. Neurosci Lett. 2007;412:206–210. doi: 10.1016/j.neulet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002;133:369–376. doi: 10.1016/s0166-4328(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Kwak DH, Oh KW, Nam SY, Lee BJ, Yun YW, Kim YB, Han SB, Hong JT. CCR5 deficiency induces astrocyte activation, Abeta deposit and impaired memory function. Neurobiol Learn Mem. 2009;92:356–363. doi: 10.1016/j.nlm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lin HB, Yang XM, Li TJ, Cheng YF, Zhang HT, Xu JP. Memory deficits and neurochemical changes induced by C-reactive protein in rats: implication in Alzheimer's disease. Psychopharmacology (Berl) 2009;204:705–714. doi: 10.1007/s00213-009-1499-2. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, Lynch MA. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2009;30:920–931. doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Marillier RG, Brombacher TM, Dewals B, Leeto M, Barkhuizen M, Govender D, Kellaway L, Horsnell WG, Brombacher F. IL-4R{alpha}-responsive smooth muscle cells increase intestinal hypercontractility and contribute to resistance during acute Schistosomiasis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G943–951. doi: 10.1152/ajpgi.00321.2009. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Koerner H, Baune BT. The effects of TNF deficiency on age-related cognitive performance. Psychoneuroendocrinology. 2009;34:615–619. doi: 10.1016/j.psyneuen.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Ron-Harel N, Segev Y, Lewitus GM, Cardon M, Ziv Y, Netanely D, Jacob-Hirsch J, Amariglio N, Rechavi G, Domany E, Schwartz M. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008;11:903–913. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- Salek RM, Colebrooke RE, Macintosh R, Lynch PJ, Sweatman BC, Emson PC, Griffin JL. A metabolomic study of brain tissues from aged mice with low expression of the vesicular monoamine transporter 2 (VMAT2) gene. Neurochem Res. 2008;33:292–300. doi: 10.1007/s11064-007-9542-3. [DOI] [PubMed] [Google Scholar]
- Shen WH, Jackson ST, Broussard SR, McCusker RH, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW. IL-1beta suppresses prolonged Akt activation and expression of E2F-1 and cyclin A in breast cancer cells. J Immunol. 2004;172:7272–7281. doi: 10.4049/jimmunol.172.12.7272. [DOI] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83:557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]