Abstract
Vertebrate cranial placodes contribute vitally to development of sensory structures of the head. Amongst posterior placodes, the otic placode forms the inner ear whereas nearby epibranchial placodes produce sensory ganglia within branchial clefts. Though diverse in fate, these placodes show striking similarities in their early regulation. In zebrafish, both are initiated by localized Fgf signaling plus the ubiquitous competence factor Foxi1, and both express pax8 and sox3 in response. It has been suggested that Fgf initially induces a common otic/epibranchial field, which later subdivides in response to other signals. However, we find that otic and epibranchial placodes form at different times and by distinct mechanisms. Initially, Fgf from surrounding tissues induces otic expression of pax8 and sox3, which cooperate synergistically to establish otic fate. Subsequently, pax8 works with related genes pax2a/pax2b to downregulate otic expression of foxi1, a necessary step for further otic development. Additionally, pax2/8 activate otic expression of fgf24, which induces epibranchial expression of sox3. Knockdown of fgf24 or sox3 causes severe epibranchial deficiencies but has little effect on otic development. These findings clarify the roles of pax8 and sox3 and support a model whereby the otic placode forms first and induces epibranchial placodes through an Fgf-relay.
Keywords: zebrafish, heat shock, Fgf, Pax2/5/8, SoxB1
INTRODUCTION
In vertebrate embryos, cranial placodes form as a series of epithelial thickenings around the anterior neural plate and contribute to sensory structures of the head (Baker and Bronner-Fraser, 2001; Brugmann and Moody, 2005; Schlosser, 2006). All placodes are derived from a contiguous zone of preplacodal ectoderm, which forms in the head along the neural-nonneural interface during gastrulation (Streit, 2007). The preplacodal ectoderm then generates the diverse array of placodal fates in response to different regional signals. The otic placode, which gives rise to the inner ear, has been the most extensively characterized of all cranial placodes. Otic development is initiated by Fgf ligands secreted by the hindbrain and subjacent mesendoderm (Alvarez et al., 2003; Ladher et al., 2000; Ladher et al., 2005; Léger and Brand, 2002; Liu et al., 2003; Maroon et al., 2002; Park and Saint-Jeannet, 2008; Phillips et al., 2001; Riley and Phillips, 2003; Wright and Mansour, 2003). Some of the earliest markers of otic development are members of the Pax2/8 family of transcription factors (Pfeffer et al., 1998). The functions of Pax2 and Pax8 in regulating early otic development have been most extensively studied in zebrafish (Hans et al., 2004; Mackereth et al., 2005). Otic expression of pax8 begins during late gastrulation and requires both Fgf signaling and the otic-competence factor Foxi1 (Hans et al., 2004; Hans et al., 2007; Phillips et al., 2001; Solomon et al., 2003, 2004;). By early somitogenesis stages, expression of related genes pax2a and pax2b is also detected in the preotic placode (Pfeffer et al., 1998). Otic expression of pax2a/b requires Fgf, but not foxi1 (Hans et al., 2004; Léger and Brand, 2002; Solomon et al., 2003, 2004). Despite these slight differences in regulation, pax8 and pax2a/b function together and provide substantial redundancy during otic development. Impairment of both pax2a and pax2b has little effect on otic induction, whereas impairment of pax8 leads to production of a reduced otic placode (Hans et al., 2004; Mackereth et al., 2005). In pax2a/pax2b/pax8-depleted embryos, a small otic placode initially forms but eventually disperses as cells lose otic identity (Mackereth et al., 2005). Thus, pax2/8 genes are together necessary for normal induction and maintenance of the otic placode. How pax2/8 genes mediate these functions is still unknown. Moreover, because some otic tissue initially forms in the absence of pax2/8 function, there must be additional genes that help mediate the effects of Fgf during otic induction.
Another gene coexpressed with pax2/8 in the otic primordium is sox3 (Nikaido et al., 2007; Sun et al., 2007). Like pax8, otic expression of sox3 also requires Fgf and foxi1 (Lee et al., 2003; Nechiporuk et al., 2007; Sun et al., 2007). In mouse and zebrafish, disruption of Sox3 causes mild-to-moderate reduction in the size of the otic vesicle (Dee et al., 2008; Okuda et al., 2010; Rizzoti and Lovell-Badge, 2007). However, otic patterning has not been examined in detail in these backgrounds. Additionally, genetic interactions between sox3 and pax8 have not been investigated, leaving open the question of whether these genes cooperate to mediate otic induction.
Epibranchial placodes constitute a distinct set of placodes with fates quite different from the otic placode, yet there are striking parallels between early development of these placode-types (Ladher et al., 2010). Epibranchial placodes give rise to a series of sensory ganglia associated with the mouth and throat, including the facial, glossopharyngeal and vagal ganglia. Like the otic placode, epibranchial placodes require the same upstream regulators, Fgf and Foxi1, and both express pax8 and sox3 as early response factors. Moreover, fate-mapping studies show that otic and epibranchial precursors lie close together during early development, with epibranchial placodes emerging from an arc of ectoderm wrapping around the lateral edge of the otic territory (Streit, 2002; Sun et al, 2007). These similarities have led to the hypothesis that Fgf initially specifies a common otic/epibranchial field, which later splits into adjacent compartments with distinct fates (Freter et al., 2008; Ladher et al., 2010; Ohyama et al., 2006; Schlosser and Ahrens, 2004; Sun et al., 2007). However, close examination of early markers suggests that otic and epibranchial placodes are induced at different times, possibly by distinct mechanisms. Initially, pax8 and sox3 are coexpressed within a relatively small domain adjacent to rhombomere 4 of the hindbrain. This appears to correspond to the otic domain in zebrafish because at least two otic-specific markers, atoh1b and fgf24, are soon induced within the same domain (Draper et al., 2003; Millimaki et al., 2007). A dramatic transition occurs Between 3 and 6 somites stage (11 hpf and 12 hpf) as sox3 downregulates within the otic domain and spreads outward into the prospective epibranchial domain (Nikaido et al., 2007; Sun et al., 2007). Similarly, expression of foxi1 is abruptly lost from otic cells but is maintained at high levels in epibranchial cells. In contrast, pax8 and pax2a remain highly expressed in the otic domain but shows only weak expression in the epibranchial domain (Pfeffer et al., 1998; Phillips et al., 2001). The regulation and functional significance of these dynamic changes have not been established.
Here we have reexamined early regulation of otic and epibranchial development. Our data confirm that the otic placode forms first and that pax8 and sox3 interact synergistically to promote otic induction. Subsequently, pax8 works redundantly with pax2a and pax2b to promote two distinct functions in the otic placode. First, pax2/8 repress otic expression of foxi1. This is necessary to maintain otic fate, as artificially maintaining foxi1 expression blocks further otic development. Second, pax2/8 activate otic expression of fgf24. Fgf24 in turn downregulates sox3 in the otic domain and induces sox3 in the epibranchial domain. Knockdown of fgf24 has little effect on otic development but causes a severe deficiency of epibranchial ganglia, similar to knocking down sox3 directly. These data support a new model wherein the otic placode forms first and subsequently induces formation of epibranchial placodes through pax2/8-dependent Fgf24 signaling. The data also support a key role for pax8 in orchestrating the dynamic changes in early gene expression that distinguish otic from epibranchial fates.
MATERIALS AND METHODS
Strains and developmental conditions
The wild type strain was derived from AB line (Eugene, OR). The noitu29a mutation is a null allele (Lun and Brand, 1998) and was used to assess function of pax2a. Transgenic lines used in this study include Tg(hsp70:fgf8a)x17 (Millimaki et al., 2010), Tg(hsp70:foxi1)x19 (Kwon et al., 2010) and Tg(brn3c:gap43-GFP)s356t (Xiao et al., 2005). For convenience, these transgenes are referred to in the remainder of the text as hs:fgf8, hs:foxi1 and brn3c:GFP, respectively. Embryos were developed at standard conditions of 28.5°C in fish water containing methylene blue and were staged based on standard protocols (Kimmel et al., 1995).
In situ hybridization
In situ hybridization was carried out at 67°C as described previously (Jowett and Yan, 1996; Phillips et al., 2001).
Morpholinos
Translation–blocking morpholino oligomers (MOs) obtained from Gene Tools Inc. were used to block gene function. MOs were injected into embryos at one-cell. All MO sequences used in this study have been previous described and tested for efficacy and specificity. To knockdown pax8, wild-type embryos were injected with 2.5 ng each of variant 1 MO (5′-GTTCACAAACATGCCTCCTAGTTGA-3′) and variant 2/3 MO (5′-GACCTCGCCCAGTGCTGTTGGACAT-3′) as previously described (Mackereth et al., 2005). To knock down fgf24, embryos were coninjected with 5 ng fgf24-MO, 5′-GACGGCAGAACAGACATCTTGGTCA-3′ (Fischer et al., 2003) and, to inhibit non-specific cell death, 7.5 ng of p53-MO (Robu et al., 2007). Other morpholinos used in this study include pax2b-MO, 5′-GGTCTGCCTTACAGTGAATATCCAT-3′ (5 ng/embryo, Mackereth et al., 2005); and sox3-MO1 5′-TACATTCTTAAAAGTGGTGTGCCAAGC-3′ (5 ng/embryo, Okuda et al., 2010).
Gene misexpression
To misexpress foxi1 or fgf8 from heat shock-inducible transgenes, heterozygous transgenic embryos were heat shocked at 39 °C for 30 min at the indicated times. After heat shock, embryos were incubated at 33°C until fixation.
Cell transplantation
Donor embryos were injected with lineage tracer (lysine-fixable biotinylated dextran, 10000 MW, in 0.2 M KCl) at the one-cell stage. Labeled cells were transplanted from blastula stage donors into non-labeled hosts of the same stage. Transplanted cells were identified in the hosts by streptavidin-FITC antibody staining.
RESULTS
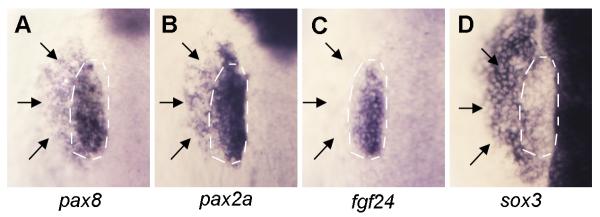
Previous studies have shown the importance of Fgf signaling in otic and epibranchial induction, but there is still much to learn about the factors that mediate Fgf signaling. Fgf initially induces expression of pax8 and sox3 in the otic primordium by 9.5 hpf (late gastrulation). Another otic marker, pax2a, is coexpressed in the otic domain by 11 hpf (1-3 somites stage). By 12 hpf, expression of sox3 begins to downregulate in the otic placode as it expands outward into prospective epibranchial ectoderm (Nikaido et al., 2007; Sun et al., 2007, Fig. 1D). Weaker expression of pax8 and pax2a is also detected in the epibranchial anlagen by 12 hpf, whereas higher levels persist in otic domain (Fig. 1A, B). By comparison, expression of fgf24 remains restricted to the otic domain throughout placodal development (Draper et al., 2003; Fig. 1C).
Figure 1. Spatial domains of otic and epibranchial markers at 12 hpf.
Dorsal views showing expression of pax8 (A), pax2a (B), fgf24 (C) and sox3 (D) in wild-type embryos at 12 hpf. Otic domains (white dashed lines) and epibranchial domains (black arrows) are indicated. Unlike the other genes, fgf24 expression is limited to the otic domain.
The roles of pax8 and pax2a in otic induction have been partially characterized, but their roles in epibranchial development have not been determined, nor have the roles of sox3 and fgf24 been determined. To address these questions, we injected morpholino oligomers (MOs) to knock down these genes and assessed the effects on otic and epibranchial development.
sox3 and pax8 cooperate to regulate otic and epibranchial induction
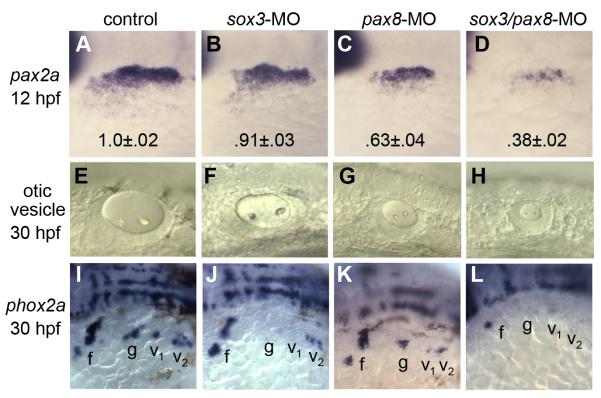
We first examined the effects of knocking down the earliest otic markers, pax8 and/or sox3, on otic development. Knockdown of sox3 alone caused a 9±2% reduction in the area of the otic/epibranchial domain of pax2a at 12 hpf, but subsequent formation of the otic vesicle was nearly normal (Fig. 2B, F). Consistent with previous findings (Mackereth et al., 2005), knockdown of pax8 reduced the area of pax2a expression to 63±4% of normal, with a similar reduction in the size of the otic vesicle (Fig. 2C, G). The expression domains of pax8 and sox3 were similarly reduced at 10 hpf (not shown). Knockdown of both genes caused further reduction in otic development, such that the area of the pax2a domain was only 38±2% of normal and the otic vesicle was similarly reduced (Fig. 2D, H). Patterning in the otic vesicle was relatively normal in embryos knocked down for pax8 and/or sox3, though expression domains of all markers were reduced in proportion to the overall size of the otic vesicle (Fig. S1 A-L). Thus sox3 and pax8 both regulate otic placode induction. pax8 function appears more critical than sox3, but the strong enhancement of otic deficiency in pax8-sox3 double morphants shows that each gene provides unique functions required for early otic development.
Figure 2. pax8 and sox3 interact in otic and epibranchial induction.
(A-D) pax2a expression in the otic/epibranchial domain at 12 hpf in a control embryo (A), sox3 morphant (B), pax8 morphant (C) and sox3-pax8 double morphant (D). Numbers indicate normalized values for the mean ± standard deviation of the area of the pax2a expression domain (n=10 specimens for each background). Area was calculated by outlining otic-epibranchial domains in Photoshop and measuring the number of pixels within. Differences between the morphants and the control were highly significant (p<.0005) as determined by t-tests. (E-H) otic vesicles at 30 hpf in a live control embryo (E), sox3 morphant (F), pax8 morphant (G) and sox3-pax8 double morphant (H). (I-L) expression of phox2a at 30 hpf in a control embryo (I), sox3 morphant (J), pax8 morphant (K) and sox3-pax8 double morphant (L). Positions of the facial ganglion (f) glossopharyngeal ganglion (g), and vagal ganglia (v1 and v2) are indicated. All images show lateral views with anterior to the left.
Because Sox3 has been implicated in regulation of sensory-neural regions of the otic vesicle in chick (Abelló et al., 2010), we also examined formation of sensory epithelia and neurons of the stato-acoustic ganglion (SAG) in embryos knocked down for sox3 and/or pax8. sox3 morphants produced sensory epithelia with roughly normal numbers of hair cells, as marked by brn3c:GFP expression. SAG development was also normal based on expression of proneural gene neuroD, as well as accumulation of mature Islet1-positive SAG neurons (Fig. S1 M-T, Table 1). In pax8 morphants and pax8-sox3 double morphants, sensory epithelia and SAG neurons formed but were reduced in size as expected from the diminished size of the otic vesicle. These data suggest that pax8 and sox3 are not directly required for development of sensory epithelia or SAG neurons in zebrafish. Instead these genes interact to control the amount of otic tissue induced, which indirectly affects the amount of sensory-neural tissue produced.
Table 1.
Number of SAG neurons and hair cells in 30 hpf embryos.
| Control | sox3-MO | pax8-MO | sox3/pax8MO | fgf24-MO | |
|---|---|---|---|---|---|
| No. of SAG neurons a |
28.9 ± 2.1 n = 20 |
24 ± 2.2 n = 20 |
12.5 ± 1.8 n = 20 |
8.1 ± 1.4 n = 20 |
27.2 ± 1.9 n = 20 |
| No. of hair cells in the utricular macula a |
6.3 ± 0.6 n = 14 |
6.2 ± 0.4 n = 14 |
4.8 ± 0.4 n = 14 |
3.1 ± 0.5 n = 14 |
6.3 ± 0.7 n = 20 |
| No. of hair cells in the saccular macula a |
3.9 ± 0.8 n = 14 |
3.8 ± 0.7 n = 14 |
2.2 ± 0.4 n = 14 |
2.1 ± 0.3 n = 14 |
3.8 ± 0.4 n = 20 |
values expressed as mean ± SD. n, sample size. MO, morphants, hpf, hours post fertilization.
To monitor epibranchial development following gene knockdown, we examined expression of phox2a, which marks all epibranchial ganglia by 30 hpf (Begbie et al., 1999; Lee et al., 2003; Nechiporuk et al., 2005). Previous studies have shown that sox3 is required for normal development of epibranchial ganglia (Dee et al., 2008; Rizzoti and Lovell-Badge, 2007). We confirmed that sox3 morphants develop with a substantial deficiency of phox2a-expressing epibranchial ganglia, with almost total loss of the glossopharyngeal and anterior vagal ganglia (Fig. 2J). Pax8 has not previously been shown to regulate epibranchial placode development, but we tested this possibility because pax8 is expressed at a low level in at least part of the epibranchial primordium by 12 hpf (Hans et al., 2004; Phillips et al., 2001; Fig. 1A). Although pax8 morphants developed with only a slight reduction in epibranchial ganglia (Fig. 2K), pax8-sox3 double morphants showed complete loss of all epibranchial ganglia (Fig. 2L). Similar results were obtained by visualizing expression of the general neurogenic marker, ngn1, though a few small disorganized clusters of ngn1-expressing cells were still produced in pax8-sox3 double morphants (not shown). However, these clusters appear to be derived from neural crest as simultaneous ablation of neural crest eliminated all residual neurogenesis in the epibranchial region (our unpublished observations). Thus, pax8 and sox3 are together indispensable for development of epibranchial ganglia.
Downregulation of foxi1 and sox3 in the otic placode
Although pax8 and sox3 are initially coinduced in the otic anlagen by Fgf, sox3 soon downregulates in otic cells as they develop. Because pax8 expression persists in the otic domain, we speculated that pax8 might directly or indirectly repress otic expression of sox3. Indeed, downregulation of sox3 in the otic placode was delayed by at least 3 hours in pax8 morphants (Fig. 3E, and data not shown). Surprisingly, induction of sox3 in the epibranchial domain was also delayed by 3 hours, consistent with a non-autonomous role for pax8 (see below).
Figure 3. Requirement for pax2/8 in otic and epibranchial development.
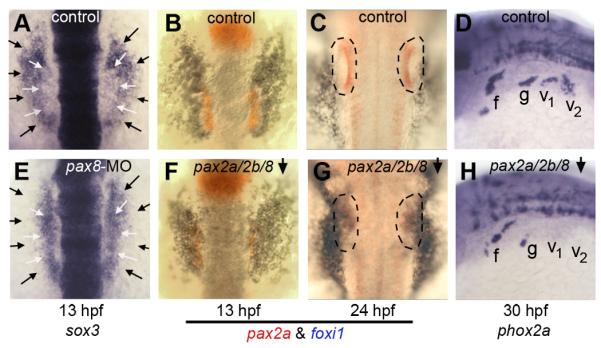
(A, E) expression of sox3 at 13 hpf in a control embryo (A) and pax8 morphant (E). White arrows indicate the lateral edges of the otic domain and black arrows indicate the edges of the epibranchial domain. (B, C, F, G) two color in situ hybridization of embryos at 13 hpf (B, F) and 24hpf (C, G) showing expression of pax2a (red) and foxi1(blue). Outlines indicate the otic vesicle (C) or vestigial otic region (G). Expression patterns are shown in control embryos (B, C) and pax2a/pax2b/pax8-deficient embryos (F, G). (D, H) expression of phox2a at 30 hpf in a control embryo (D) and pax2a/2b/8-deficient embryo (H). Images show dorsal views with anterior to the top (A-C, E-G); dorsolateral views with anterior to the left and dorsal to the top (D, H). Positions of the facial ganglion (f), glossopharyngeal ganglion (g) and vagal ganglia (v1 and v2) are indicated.
We also tested whether pax8 modulates foxi1 expression during otic/epibranchial development. Foxi1 initially serves as a competence factor for establishing the entire preplacodal ectoderm (Kwon et al., 2010), and its expression later becomes restricted to the otic and epibranchial primordia where its function is especially critical (Hans et al., 2007; Lee et al., 2003; Nissen et al., 2003; Solomon et al., 2003). As development proceeds, foxi1 expression normally begins to downregulate in the otic domain by 11 hpf whereas it is maintained in epibranchial ganglia through at least 36 hpf (Lee et al., 2003). In pax8 morphants, however, exclusion of foxi1 from the otic placode was delayed by about 2 hours (data not shown). Because pax2a and pax2b are later coexpressed in the otic placode and are partially redundant with pax8, we tested the effects of disrupting all known pax2/8 function. In pax2a/pax2b/pax8-deficient embryos, strong foxi1 expression was maintained in the otic region through at least 24 hpf (Fig. 3F, G), by which time otic identity is lost (Mackereth et al., 2005). These data show that Pax2/8 proteins directly or indirectly repress foxi1 transcription in the otic placode.
Although foxi1 is required to initiate otic development, we hypothesized that failure to downregulate foxi1 at later stages impedes further otic development. To test this idea, we made use of a stable transgenic line to misexpress foxi1 under the control of a heat shock promoter (Kwon et al., 2010). Global activation of hs:foxi1 expression at 11 hpf caused a dramatic reduction in the size of the otic placode by 14 hpf (Fig. 4B). Additionally, otic expression of pax2a was irregular and spotty. Because global misexpression of foxi1 possibly interferes with essential signals from other tissues, we generated mosaic embryos by transplanting cells from hs:foxi1 transgenic embryos into non-transgenic host embryos. Activation of hs:foxi1 in mosaic embryos caused loss of expression of pax2a in transgenic cells within the otic region (Fig. 4C, D). These data indicate that maintaining foxi1 expression after 11 hpf impairs completion of otic development in a cell-autonomous manner. This could explain why otic cells eventually lose otic identity in pax2a/pax2b/pax8-deficient embryos (Mackereth et al., 2005).
Figure 4. Misexpression of foxi1 inhibits otic development.
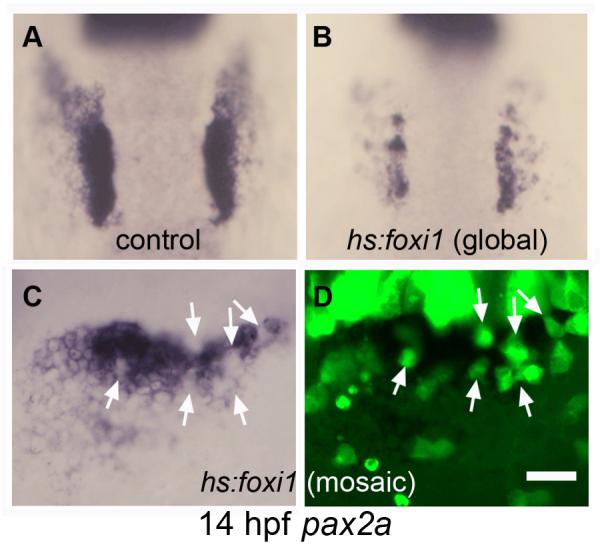
(A, B) expression of pax2a at 14 hpf in a control embryo (A) and a hs:foxi1 transgenic embryo (B) heat shocked at 11 hpf. (C, D) expression of pax2a at 14 hpf in a mosaic embryo as seen under bright field (C) and fluorescence imaging (D). The mosaic was produced by transplanting lineage-labeled hs:foxi1 transgenic cells (green fluorescence) into a non-transgenic host. The embryo was heat shocked at 11 hpf to activate the transgene. Note the absence of pax2a expression in transgenic cells (white arrows). Images show dorsal views with anterior to the top (A-B); lateral views with anterior to the left (C-D). Scale bar, 50 μm (A, B), 25 μm (C, D).
Loss of otic fate in pax2a/pax2b/pax8-deficient embryos does not involve death of the otic placode, as these cells persist in the otic-epibranchial area through at least 24 hpf (Mackereth et al., 2005). We hypothesized that some of these cells might switch fate and contribute to epibranchial tissue instead. However, development of epibranchial ganglia was severely impaired in pax2a/pax2b/pax8-deficient embryos (Fig, 3H). These data are consistent with loss of epibranchial expression of sox3 (Fig. 3E, and data not shown), further indicating that pax2/8 genes are required directly or indirectly for development of epibranchial placodes.
Pax2/8 regulate expression of fgf24 in the otic placode
Expression of fgf24 is limited to the otic placode and is first expressed there by 10.5 hpf, shortly after the onset of pax8 expression (Draper et al., 2003; Fig. 1C, and our unpublished observation). We therefore tested whether pax8, which is critical for controlling the size of the otic placode, is required to activate this domain of fgf24 expression. Indeed, otic expression of fgf24 is delayed until 13 hpf in pax8 morphants (Fig. 5B, D). We hypothesized that belated expression of fgf24 reflects the activation of pax2a and pax2b. In support, pax2a/pax2b/pax8-deficient embryos fail to express fgf24 in otic tissue through at least 18 hpf, although fgf24 expression occurs normally in pharyngeal arches (Fig. 5F-H). Thus, one of the functions of Pax2/8 during otic induction is to activate expression of fgf24. In contrast, knockdown of sox3 had no effect on the onset of fgf24 expression (not shown).
Figure 5. pax2/8 regulates otic expression of fgf24.
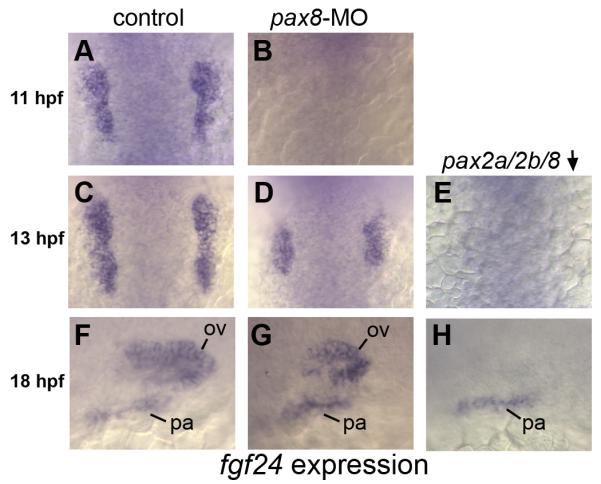
(A, C, F) fgf24 expression in the otic placode in control embryos at 11 hpf (A), 13 hpf (C) and 18 hpf (F). (B, D, G) fgf24 expression in pax8 morphants at 11hpf (B), 13 hpf (D) and 18 hpf (G). Expression of fgf24 is lost from preotic placodes in pax8 morphants at 11 hpf (B) and is reduced in pax8 morphants at 13 hpf (D) and 18 hpf (G). (E, H) noi (pax2a) mutants co-injected with pax8-MO and pax2b-MO showing loss of otic expression of fgf24 at all time points. Expression in pharyngeal (pa) arches and the otic vesicle (ov) is indicated. Images show dorsal views with anterior to the top (A-E); lateral views with anterior to the left and dorsal to the top (F-H).
Fgf24 is not required for otic development
The function of fgf24 in otic development has not been investigated. To test this we injected wild-type embryos with morpholino to knockdown fgf24. fgf24 morphants develop with a normally sized otic placode, judging by expression of pax8 at 11 hpf (Fig. 6E). Like pax8 morphants, fgf24 morphants fail to downregulate expression of sox3 in the otic placode (Fig. 6F). In contrast, expression of foxi1 showed a normal pattern of exclusion from otic cells in fgf24 morphants (Fig. 6C, G). Furthermore, we could detect no changes in expression of regional markers within the otic vesicle, nor in development of sensory epithelia and SAG neurons (Fig. S2 and Table 1). Thus, fgf24 is not required for otic placode induction or subsequent development and patterning of the otic vesicle. Additionally, the data show that failure to downregulate sox3 in the otic placode in fgf24 morphants does not adversely affect patterning and differentiation within the otic placode and vesicle. The latter conclusion was further supported by the finding that elevating sox3 expression by activating a heat shock-inducible transgene at 11.5 hpf does not detectably alter patterning or neurogenesis within the otic vesicle (Fig. S3).
Figure 6. fgf24 is required for epibranchial development.
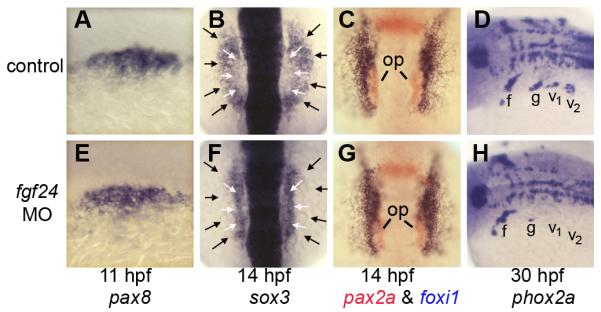
(A, E) expression of pax8 at 11 hpf in a control embryo (A) and fgf24 morphant (E). (B, F) expression of sox3 at 14 hpf in a control embryo (B) and fgf24 morphant (F). White arrows indicate the lateral edges of the otic domain and black arrows indicate the lateral edges of the epibranchial domain. (C, G) two color in situ hybridization showing pax2a (red) and foxi1 (blue) in a control embryo (C) and fgf24 morphant (G) at 14 hpf. Positions of otic placodes (op) are indicated. (D, H) expression of phox2a at 30 hpf in a control embryo (D) and fgf24 morphant (H). Positions of the facial ganglion (f), glossopharyngeal ganglion (g) and vagal ganglia (v1 and v2) are indicated. Images show dorsolateral views with anterior to the left (A, D, E, H); dorsal views with anterior to the left and dorsal to the top (B, C, F, G).
Fgf24 regulates epibranchial development
We next examined whether the otic domain of fgf24 acts non-autonomously to regulate epibranchial development. As in pax8 morphants, fgf24 morphants fail to show expansion of sox3 into the epibranchial domain (Fig. 6F). Moreover, development of glossopharyngeal and vagal ganglia was almost completely blocked (Fig. 6H). These are the same ganglia adversely affected in sox3 morphants (Fig. 2J), suggesting that the role of Fgf24 is to induce expression of sox3 in these primordia. In contrast, development of the facial ganglion was relatively normal in fgf24 and sox3 morphants, indicating that other genes are able to compensate in these cells. The facial ganglion arises from the anterior-most region of the epibranchial domain, relatively far from the otic domain of fgf24. It is possible that some other source of Fgf regulates development of the facial ganglion, and that subsequent expression of pax8 can partially compensate for loss of sox3.
Modulation of sox3 by a threshold response to Fgf
Although Fgf signaling is required to activate sox3 expression, the observation that fgf24 is required to downregulate sox3 in the otic domain suggested that sox3 is subject to repression by high levels of Fgf signaling. To test this, we used a heat shock line to misexpress fgf8 beginning at 11 hpf. This caused sox3 to be expressed throughout the otic and epibranchial domains, but at a significantly reduced level compared to the control embryo (Fig. 7). The low level of sox3 expression in hs:fgf8 embryos was comparable to the level normally seen in the otic domain of control embryos (compare Figs. 7A and C). In another control experiment, heat shock did not alter the effects of fgf24 knockdown; sox3 expression remained elevated in the otic domain and failed to expand into the epibranchial domain (Fig. 7B). These data support the hypothesis that sox3 shows two distinct responses to Fgf signaling, explaining how otic expression of fgf24 differentially regulates sox3 in the otic and epibranchial domains.
Figure 7. Response of sox3 to elevated Fgf signaling.
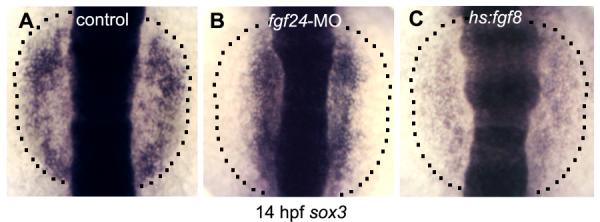
Expression of sox3 at 14 hpf in a control embryo (A), fgf24 morphant (B) and hs:fgf8 transgenic embryo (C). The lateral edges of the prospective epibranchial domain are indicated (dashed lines). All embryos were heat shocked at 11 hpf.
DISCUSSION
The data provided here clarify early steps in otic placode development and support a new model for sequential induction of otic and epibranchial placodes (Fig. 8). The otic placode forms first by a previously established mechanism involving Fgf3 and Fgf8 acting locally within a broader domain of foxi1 expression (Hans et al., 2004; Hans et al., 2007; Léger and Brand, 2002; Liu et al., 2003; Maroon et al., 2002; Nissen et al., 2003; Phillips et al., 2001; Solomon et al., 2003). As an initial response, pax8 and sox3 are coinduced in the otic domain (Nikaido et al., 2007; Sun et al., 2007). Otic expression of pax8 stabilizes otic fate through downregulation of foxi1, and non-autonomously induces the majority of epibranchial placodes through activation of fgf24. This model is compatible with previous studies showing that Fgf3 and Fgf8 regulate otic and epibranchial development but adds important mechanistic details, as described below. Only the facial ganglion appears to develop independently of Fgf24, and its regulation will be considered separately.
Figure 8. Summary and model of otic and epibranchial induction.
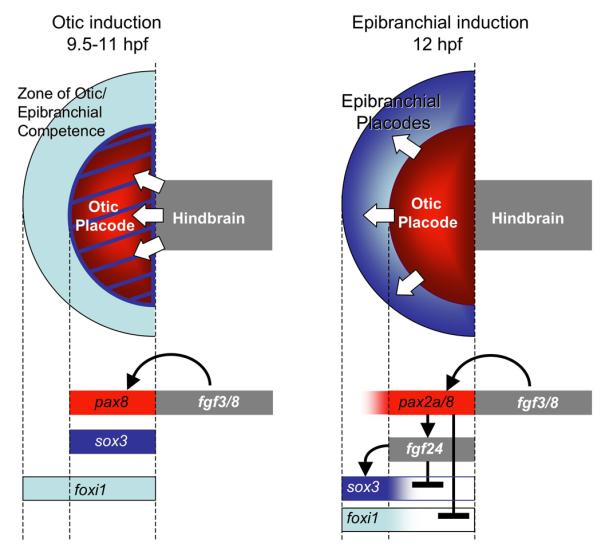
During otic induction (9.5-11 hpf), Fgf3/8 from the mesendoderm (not shown) and hindbrain (gray) induce expression of pax8 (red) and sox3 (blue) in preotic cells. Specific responsiveness to Fgf requires the competence factor Foxi1, which becomes restricted to the otic and epibranchial regions during this period. By 12 hpf, Pax8 has induced expression of fgf24 and repressed otic expression of foxi1. Pax2a can mediate the same functions in the absence of Pax8, albeit belatedly. Fgf24 in turn downregulates otic expression of sox3 and induces strong expression of sox3 in adjacent epibranchial cells. Arrows represent positive regulation and cross-bars indicate negative regulation.
The roles of pax8 and sox3 in early otic development
Our data provide important new insights into the mechanisms by which Fgf-target genes control early otic development. Expression of pax8 is especially critical for establishing the size of the otic placode, as shown by the production of roughly half-sized otic placodes in pax8 morphants (Mackereth et al., 2005, and Fig. 2G). Because pax8 is required to activate otic expression of fgf24, we initially hypothesized that this additional source of Fgf would serve to recruit more distant cells into the otic placode. Surprisingly, however, fgf24 appears to provide no essential function for otic development: fgf24 morphants and mutants show no deficit in the size of the otic placode, and there appear to be no defects in subsequent patterning in the otic vesicle (Fig. S2). Instead, the primary function of otic fgf24 is to initiate epibranchial development through induction of sox3 in the surrounding ectoderm (described in more detail below). How then, does pax8 control the size of the otic domain? Because Pax2/8 genes are auto-regulatory in other developmental settings (Lun and Brand, 1998; Pfeffer et al., 1998), we speculate that Pax8 forms a feedback amplification loop in pre-otic cells, allowing cells further from the Fgf signaling source to achieve detectable expression of pax8 and sox3. In pax8 morphants, therefore, otic induction is limited to a smaller field of cells closer to the Fgf source where signal amplification is less critical. Another Fgf target gene, sox3, cooperates with pax8 during otic induction. Knockdown of sox3 alone causes only a 9% reduction in the size of the otic placode. However, knocking down both pax8 and sox3 causes a synergistic loss of nearly two-thirds of otic tissue. In this case, we presume that only cells immediately adjacent to the sources of Fgf are able to initiate otic development through the activation of additional as yet unknown target genes.
After helping to establish the otic placode, pax8 later represses foxi1 in the otic domain. This function is shared with pax2a and pax2b and appears to be essential for maintaining otic fate. In pax2a/pax2b/pax8-deficient embryos, foxi1 expression persists in the otic domain (Fig. 3F, G) and expression of all otic markers is lost by 24 hpf (Mackereth et al., 2005). Furthermore, experimentally maintaining expression of foxi1 from an inducible transgene also causes loss of otic markers. It is not clear why foxi1 must be repressed in the otic domain since it is absolutely required for Fgf’s ability to induce otic development in the first place. However, our analysis of the early role of foxi1 in establishing preplacodal ectoderm indicates that it functions in part by repressing other regulatory genes (Kwon et al., 2010 and our unpublished observations). Thus, pax8-dependent downregulation of foxi1 may alleviate repression of other genes necessary for otic differentiation.
After the onset of otic development, the later role(s) of sox3 are still unclear. Although the otic vesicle is slightly smaller than normal in sox3 morphants, all regional markers are expressed normally. Based on studies in chick it has been suggested that Sox3 regulates formation of the sensory-neural domain of the otic vesicle (Abelló et al.). However, we find that knockdown of sox3 causes no appreciable deficiency in development of sensory epithelia or SAG neurons (Fig. S1-N, R and Table 1). Otic development in Sox3 null mice has not been studied in detail, but otic vesicles appear grossly normal and produce at least some SAG neurons (Rizzoti and Lovell-Badge, 2007). It is possible that other SoxB1 genes compensate for loss of Sox3 in mouse, but no other appropriately expressed genes have been identified in zebrafish. It is interesting that fgf24 morphants fail to downregulate sox3 in the otic placode (Fig. 6F), yet all other aspects of otic development appear normal (Fig. S2). Likewise, misexpressing sox3 from a heat shock-inducible transgene does not detectably alter otic development. However it must be acknowledged that failure to downregulate sox3 could cause defects too subtle to detect using the markers at hand, even though such changes could be quite deleterious in the long-run.
The role of Fgf24
A novel finding central to our model is that Fgf24 emitted by the nascent otic placode is essential for development of all epibranchial placodes posterior to the facial placode (Fig. 6H). A prominent target of Fgf24 appears to be sox3. Within 1-2 hours of activation of fgf24 in the otic placode, sox3 begins to downregulate in the otic domain while it is induced in the abutting epibranchial domain. Differential spatial regulation of sox3 could reflect a threshold response to changing levels of Fgf24 within a diffusion gradient. Indeed, overexpression of Fgf8 causes sox3 to be expressed at a low level throughout the domain of foxi1 expression (Fig. 7). In the absence of Fgf24, sox3 remains highly expressed in the otic domain and is not detected in the epibranchial domain. Disruption of sox3 has little effect on otic development but blocks all epibranchial development posterior to the facial ganglion. This phenotype strongly resembles that of fgf24 morphants, again supporting the notion that sox3 is the primary mediator of Fgf24 signaling. Otic expression of fgf24 is in turn regulated redundantly by pax2 and pax8 genes. Accordingly, loss of pax8 alone causes a 2-3 hour delay in fgf24 expression, with negligible effects on epibranchial development. In contrast, disruption of all pax2/8 function eliminates otic expression of fgf24 entirely and causes a deficiency in epibranchial development comparable to fgf24-MO. Together these data support the existence of a pathway in which otic expression of pax8 activates expression of fgf24, which induces formation of epibranchial placodes in adjacent ectoderm through sox3.
In contrast to sox3, pax2/8 genes are normally maintained at a high level in the otic placode but show only weak expression in the epibranchial domain. This pattern remains unchanged in fgf24 morphants. Epibranchial expression of pax2/8 appears after otic expression, possibly reflecting a delayed response to low levels of Fgf3 and Fgf8 from the hindbrain and subotic mesendoderm (Alvarez et al., 2003; Freter et al., 2008; Ladher et al., 2000; Ladher et al., 2005; Léger and Brand, 2002; Liu et al., 2003; Maroon et al., 2002; Nechiporuk et al. 2007; Park and Saint-Jeannet, 2008; Phillips et al., 2001; Riley and Phillips, 2003; Wright and Mansour, 2003). It is possible that pax2/8 provides a cell-autonomous requirement for epibranchial development, but such function(s) are evidently not sufficient to support epibranchial development in the absence of Fgf24.
Our model is distinct from an earlier model proposing that epibranchial placodes are induced by Fgf3 and Fgf8 from paraxial cephalic mesoderm (Nechiporuk et al., 2007). It is formally possible that mesodermal Fgf3 and Fgf8 work in parallel with otic Fgf24 to regulate certain aspects of epibranchial development. Indeed we have confirmed that ablation of mesoderm blocks differentiation of epibranchial neurons, as shown by loss of phox2a and ngn1 expression (Nechiporuk et al., 2007; and our unpublished observations). However, we find that genetic ablation of mesoderm does not block otic or epibranchial induction. For example, pax8, sox3 and fgf24 are all expressed in the otic domain by 12 hpf, after which sox3 shows downregulation in the otic domain and upregulation in the epibranchial domain (Kwon and Riley, 2009; Mendonsa and Riley, 1999; and our unpublished observations). These data are consistent with our model and indicate that mesodermal signals are not required for epibranchial specification but are instead required for maintenance or differentiation of epibranchial ganglia.
Regulation of the facial ganglion
Epibranchial placodes and ganglia appear to follow similar regulation in general, but our data show that the facial (geniculate) placode shows key differences from more posterior epibranchial placodes. First, development of the facial ganglion does not require fgf24 (Fig. 6H). Similarly, there are only minor deficiencies in the facial ganglion following knockdown of sox3 alone, pax8 alone, or all pax2/8 functions, whereas the other epibranchial ganglia are severely impaired or ablated under these conditions (Figs. 2J, 2K and 3H). However, combined knockdown of sox3 and pax8 ablates formation of facial ganglion (Fig. 2L). This indicates that sox3 and pax8 serve redundant functions in the facial placode, unlike more posterior epibranchial placodes. Such early differences in regulation could confer unique functional attributes to the facial ganglion that distinguish it from other epibranchial ganglia.
Other essential signals
In chick, frog and zebrafish, Fgfs and various Bmps secreted from pharyngeal endoderm are also required for development of epibranchial ganglia (Begbie et al., 1999; Holzschuh et al., 2005; Nechiporuk et al., 2005; Nikaido et al., 2007). However, these signals operate at a later stage, well after Fgf-dependent induction of sox3, and are required to initiate neurogenic differentiation. It is still unknown whether these endodermal signals act sequentially or are required as parallel inputs.
In mouse and chick, Wnt8a from the hindbrain is thought to distinguish otic from epibranchial fates. Accordingly, disruption of Wnt signaling blocks completion of otic development whereas elevating Wnt signaling expands otic tissue as it blocks epibranchial development (Freter et al., 2008; Ohyama et al., 2006). At first glance this appears to be an entirely different mechanism than what we describe for zebrafish, but this is not necessarily the case. Chick and mouse embryos show prominent otic expression of multiple Fgf genes around the time of otic induction, the functions of which have not been examined (Adamska et al., 2001; Alsina et al., 2004; Chapman et al., 2006; Pirvola et al., 2004; Wright et al., 2003). Conceivably, Wnt signaling could help modulate expression of these otic Fgf genes, or work in parallel with them, to affect epibranchial development. In zebrafish, Wnt signaling influences otic development indirectly through modulation of hindbrain expression of fgf3 and fgf8 (Phillips et al., 2004). Additional studies are needed to assess the degree to which underlying mechanisms have been conserved between zebrafish and amniotes.
Supplementary Material
Figure S1. Patterning of the otic vesicle in sox3-pax8 morphants. Expression of dlx3b, otx1, pax5, and neurod in the otic vesicle of control embryos (A, E, I, Q), sox3 morphants (B, F, J, R), pax8 morphants (C, G, K, S) and sox3-pax8 double morphants (D, H, L, T) at 24 hpf. (M-P) brn3c:GFP expression at 30 hpf in a control embryo (M), sox3 morphant (N), pax8 morphant (O) and sox3-pax8 double morphant (P). Positions of utricular (u) and saccular (s) maculae are indicated. Images show dorsolateral views with anterior to the left (A - P); dorsal views with anterior to the top (Q - T).
Figure S2. Patterning of the otic vesicle in fgf24 morphants. Expression of dlx3b, otx1, pax5, and neurod in the otic vesicle of control embryos (A, C, E, I) and fgf24 morphant (B, D, F, J) at 24 hpf. brn3c:GFP expression at 30 hpf in control embryo (G) and fgf24 morphant (H). Positions of utricular (u) and saccular (s) maculae are indicated. Images show dorsolateral views with anterior to the left (A-H); dorsal views with anterior to the top (I, J).
Figure S3. Patterning of the otic veiscle following misexpression of sox3. Expression of dlx3b, otx1, pax5, and neurod in control embryos (A, C, E, G) and in embryos transiently trasnfected with hs:sox3 (B, D, F, H). Embryos were heat shocked for 30 minutes at 39°C beginning at 11.5 hpf, after which embryos were maintained at 33°C. These conditions yield maximal expression of the heat shock vector for 90 minutes, followed by sustained lower level misexpression until fixation at 26 hpf.
ACKNOWLEDGMENTS
This work was supported by NIH-NIDCD grant R01-DC03806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abelló G, Khatri S, Radosevic M, Scotting PJ, Giráldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 2010;339:166–178. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Adamska M, Herbrand H, Adamski M, Krüger M, Braun T, Bober E. FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech. Dev. 2001;109:303–313. doi: 10.1016/s0925-4773(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Alsina B, Abelló G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev. Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–38. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. Vertebrate Cranial Placodes I. Embryonic Induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Moody SA. Induction and specification of the vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biol. Cell. 2005;97:303–319. doi: 10.1042/BC20040515. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Cai Q, Bleyl SB, Schoenwolf GC. Restricted expression of Fgf16 within the developing chick inner ear. Dev. Dyn. 2006;235:2276–2281. doi: 10.1002/dvdy.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee CT, Hirst CS, Shih Y-H, V.B., Patient RK, Scotting PJ. Sox3 regulates both neural fate and differentiation in the zebrafish ectoderm. Dev. Biol. 2008;320:289–301. doi: 10.1016/j.ydbio.2008.05.542. [DOI] [PubMed] [Google Scholar]
- Draper BW, Stock DW, Kimmel CB. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–4654. doi: 10.1242/dev.00671. [DOI] [PubMed] [Google Scholar]
- Fischer S, Draper BW, Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak S-S, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev. Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuβ A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Jowett T, Yan YL. Double fluorescent in situ hybridization to zebrafish embryos. Trends Genet. 1996;12:387–389. doi: 10.1016/s0168-9525(96)90091-8. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kwon H, Riley BB. Mesendodermal signals required for otic induction: Bmpantagonists cooperate with Fgf and can facilitate formation of ectopic otic tissue. Dev. Dyn. 2009;238:1582–1594. doi: 10.1002/dvdy.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development. PLoS Genet. 2010;6(9):e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1968. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, O’Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Lee SA, Shen EL, Fiser A, Sali A, Guo S. The zebrafish forkhead transcription factor Foxi1 specifies epibranchial placode-derived sensory neurons. Development. 2003;130:2669–2679. doi: 10.1242/dev.00502. [DOI] [PubMed] [Google Scholar]
- Léger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech. Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan Y-L, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–3062. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Mackereth MD, Kwak S-J, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Mendonsa ES, Riley BB. Genetic analysis of tissue interactions required for otic otic placode induction in zebrafish. Dev. Biol. 1999;206:100–112. doi: 10.1006/dbio.1998.9134. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sxo2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–3730. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev. Dyn. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM. Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development. 2003;130:2543–2554. doi: 10.1242/dev.00455. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Ogura E, Kondoh H, Kamachi Y. B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo. PLoS Genet. 2010;6:e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-Y, Saint-Jeannet J-P. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev. Biol. 2008;324:108–121. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Büsslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 Encode Redundant Functions Required for Otic Placode Induction. Dev. Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Storch EM, Lekven AC, Riley BB. A direct role for Fgf but not Wnt in otic placode induction. Development. 2004;131:923–931. doi: 10.1242/dev.00978. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev. Biol. 2004;273:350–360. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Riley BB, Phillips BT. Ringing in the new ear: resolution of cell interactions in otic development. Dev. Biol. 2003;261:289–312. doi: 10.1016/s0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. SOX3 activity during pharyngeal segmentation is required for craniofacial morphogenesis. Development. 2007;134:3437–3448. doi: 10.1242/dev.007906. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 Activation by Knockdown Technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev. Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kwak S-J, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev. Dyn. 2004;230:419–433. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive Cell Movements Accompany Formation of the Otic Placode. Dev. Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contributes to the sense organs and cranial ganglia. Int. J. Dev. Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Sun S-K, Dee CT, V.B., Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev. Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Hatch EP, Karabagli H, Karabagli P, Schoenwolf GC, Mansour SL. Expression of mouse fibroblast growth factor and fibroblast growth factor receptor genes during early inner ear development. Dev. Dyn. 2003;228:267–272. doi: 10.1002/dvdy.10362. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser R, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2967–2995. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patterning of the otic vesicle in sox3-pax8 morphants. Expression of dlx3b, otx1, pax5, and neurod in the otic vesicle of control embryos (A, E, I, Q), sox3 morphants (B, F, J, R), pax8 morphants (C, G, K, S) and sox3-pax8 double morphants (D, H, L, T) at 24 hpf. (M-P) brn3c:GFP expression at 30 hpf in a control embryo (M), sox3 morphant (N), pax8 morphant (O) and sox3-pax8 double morphant (P). Positions of utricular (u) and saccular (s) maculae are indicated. Images show dorsolateral views with anterior to the left (A - P); dorsal views with anterior to the top (Q - T).
Figure S2. Patterning of the otic vesicle in fgf24 morphants. Expression of dlx3b, otx1, pax5, and neurod in the otic vesicle of control embryos (A, C, E, I) and fgf24 morphant (B, D, F, J) at 24 hpf. brn3c:GFP expression at 30 hpf in control embryo (G) and fgf24 morphant (H). Positions of utricular (u) and saccular (s) maculae are indicated. Images show dorsolateral views with anterior to the left (A-H); dorsal views with anterior to the top (I, J).
Figure S3. Patterning of the otic veiscle following misexpression of sox3. Expression of dlx3b, otx1, pax5, and neurod in control embryos (A, C, E, G) and in embryos transiently trasnfected with hs:sox3 (B, D, F, H). Embryos were heat shocked for 30 minutes at 39°C beginning at 11.5 hpf, after which embryos were maintained at 33°C. These conditions yield maximal expression of the heat shock vector for 90 minutes, followed by sustained lower level misexpression until fixation at 26 hpf.