Abstract
Background
Innovative approaches to the widespread delivery of evidence-based dementia care are needed. The aims of this study were to determine whether a telephone screening method could efficiently identify individuals in the community in need of care for dementia and to develop a multidimensional needs assessment tool for identifying the type and frequency of unmet needs related to memory disorders in the home setting.
Methods
This was a cross-sectional evaluation of 292 community-residing individuals aged 70 and older in Maryland. Participants were given a brief cognitive telephone screen. A subsample (n=43) received a comprehensive in-home assessment for dementia and dementia-related needs. Cognitive, functional, behavioral, and clinical factors were assessed. The Johns Hopkins Dementia Care Needs Assessment was used to identify unmet needs related to dementia.
Results
Telephone screening for the sample took 350 hours, and 27% screened positive for dementia. Virtually all participants with dementia who received an in-home assessment had at least one unmet need, with the most frequent unmet needs being for a dementia workup, general medical care, environmental safety, assistance with ADL impairments, and access to meaningful activities. Caregivers, when present, also had a number of unmet needs, with the most common being caregiver education about dementia, knowledge of community resources, and caregiver mental health care.
Conclusions
Effective and efficient means for identifying community-residing individuals with dementia are needed so that Dementia Care interventions can be provided to address unmet care needs of patients and their caregivers.
Keywords: Dementia, Community, Outreach, Caregivers, Needs Assessment
Introduction
Dementia is recognised in primary care settings at a rate of only 30% to 50% (Boustani et al, 2003). Undiagnosed dementia is associated with increased risk for acute hospitalization, exhaustion of the informal caregiver (Baillardy et al, 2005), and increased use of acute care services by caregivers (Shelton et al, 2001, Schubert et al, 2008). The American Association for Geriatric Psychiatry (AAGP) defines best practices in dementia care as including the multidisciplinary, patient-centered management of medical comorbidities, safety, maximization of function, the treatment of behavioral and cognitive symptoms, and assistance with long term planning, caregiver education and support, including access to respite care and other resources (Lyketsos et al, 2006). The US Preventive Task Force (USPTF) has advocated randomized controlled trials of community based dementia screening that prospectively evaluate multiple health outcomes, including the effects of screening and treatment on cognition, function, health care utilization, health-related quality of life, and caregiver burden. (USPTF 2003) While collaborative home-based care initiatives for the elderly have been proposed (Beck et al, 2009), interventions that provide dementia-specific, home-based care at the community level remain unexamined.
No practical, cost-effective method, has yet been devised by which to identify in specific communities persons with dementia who could participate in studies examining what, beyond pharmacological interventions, might help them continue living at home. Case-finding options include targeted direct contact (e.g., mailings and advertising in areas with high concentrations of older residents), screening via primary care practices, or community-participation strategies such as “gatekeeper” training.
Obstacles to dementia detection in primary care practice include the interaction of case-complexity and the negative effects of reimbursement systems (Iliffe et al, 2009, Callahan et al, 2006,), patient reluctance to have the diagnosis confirmed, physician attitudes (e.g., fear of misdiagnosing dementia), patient-provider communication, educational deficits, and system resource (Bradford et al, 2009), and the time and workload limitations of primary care physicians. Among patients with positive screening results for dementia, approximately half (47.7%) refuse further assessment to confirm the screening results (Boustani et al, 2006). Unfortunately, due to these obstacles, dementia is usually already well established before it is recognized by medical providers.
Culture and ethnicity-specific factors can affect access to general as well as to mental health care (United States Department of Health and Human Services, 2005; Trujillo, 2008)). Tailoring casefinding strategies to specific communities might be more effective than a “generic” approach. Maximizing Independence at Home (MIND at Home) Phase 1 was a pilot study of individuals from a targeted population identified using an existing demographic database for the Jewish community living in Northwest Baltimore. The purpose of the study was to evaluate a telephone screening approach to identify persons with memory problems who could be assessed for dementia in their homes and to develop a multidimensional needs assessment tool for determining the type and frequency of unmet needs related to memory disorders in a community-based sample of elders aged 70 and older.
Methods
Participant sample
The sampling frame for the study was derived from a list of individuals who participated in a demographic study in 1999 entitled “Aging in Place in the Baltimore Jewish Community” conducted by the THE ASSOCIATED: Jewish Community Federation of Baltimore. The earlier study systematically identified approximately 12,200 members of the Baltimore Jewish community 65 years of age and older who were living at home in a specific geographic area in Northwest Baltimore (Ukeles, 2001). Based on known community-based age-adjusted prevalence estimates (Plassman et al, 2007), we might have expected approximately 1,664 (13.8%) of these participants to have dementia. From the ASSOCIATED s full participant listing, we limited the sampling frame of the current study to those who were age 70 or older in order to maximize the yield of the telephone screening for dementia case detection, and because the risk of dementia increases with age. The current study enrolled participants between 2006 and 2007. Notices were also placed in local newspapers for several publishing cycles to provide background promotion of the study.
The study was reviewed and approved by the Johns Hopkins Medicine Institutional Review Board. Oral consent was obtained from individuals who participated in the telephone screening stage, and written consent was obtained from those selected and who agreed to participate in an in-home dementia assessment.
Procedure and Measures
To identify the sample, letters were mailed to 800 households that participated in the ASSOCIATED s 1999 survey and in which at least one individual aged 70 or older was reported to reside. The letters explained the nature of the study and that they would be contacted and invited to participate in a brief telephone research interview. A telephone number was provided on the letter for recipients to call if they wished to “opt out” of being contacted for the telephone interview. For those who did not “opt out,” trained telephone evaluators called each household using a standardized script and oral consent procedure and asked to speak to the target individual(s) aged 70 or older. Individuals who provided oral consent participated in a 10–15 minute telephone cognitive screening evaluation adapted from the Johns Hopkins Alzheimer Disease Research Center that included collection of demographic data and the use of standardized quantitative measures. Of those screened, 8 did not have a record of oral consent in their file and are excluded from these analyses.
The screening measures included the Telephone Interview for Cognitive Status (TICS) (Brandt et al., 1988), a telephone validated global cognition assessment, with scores ranging from 0 to 41, and the Informant Questionnaire for Cognitive Disorders in the Elderly (IQCODE) (Jorm et al, 1989), a proxy rated questionnaire, with scores ranging from 16 to 80. Both measures have good validity and reliability (Ferruci et al, 1998, Jorm et al, 1989). Using previously reported cut-off scores (Brandt et al., 1988; Jorm et al, 2004,) a positive screen was defined as a TICS total score <31 or an IQCODE total score >52. All participants testing positive on telephone screen were invited to take part in a comprehensive in-home assessment to determine the presence of dementia along with an assessment of dementia-related needs across multiple domains. A subset of individuals whose telephone screen was normal were also asked to participate in the home assessment. This resulted in a sample of 33 “screen positives” and 10 “screen negatives” who were willing to undergo an in home evaluation. Individuals who screened negative were included to determine the telephone screening methodology s diagnostic parameters for detecting dementia cases in this population. When available, a knowledgeable family member or friend was asked to participate in the in-home assessment to serve as a proxy informant.
The in-home assessment was led by a Johns Hopkins clinician specializing in memory disorders (DJ, AM) and included collection of detailed clinical data, medical and mental health history, medication review, mental status and neurological examination, and standardized cognitive, functional and behavioral measures. Cognitive quantitative measures included the Mini-Mental State Examination (Folstein et al., 1975) and the Mental Alternation (Billick et al, 2001). Functional measures included the Functional Activities Questionnaire (Pfeiffer et al, 1982) for instrumental and basic activities of daily living. Neuropsychiatric symptoms related to dementia were assessed using the Neuropsychiatric Inventory-Questionnaire (Cummings et al, 1994), an informant based measure that assesses the severity of 10 domains of neuropsychiatric symptoms common in dementia and the associated caregiver distress. Caregiver burden was rated with the Zarit Burden Inventory (Zarit et al, 1980).
All information was then reviewed by an interdisciplinary consensus panel to determine whether the individual met DSM-IV-TR criteria for dementia. Individuals that met DSM-IV-TR criteria for Cognitive Disorder NOS are referred to here as having Mild Cognitive Impairment (MCI).
The Johns Hopkins Dementia Care Needs Assessment (JHDCNA) (Black et al., 2008) was completed for each individual identified as having either MCI or dementia. The JHDCNA, developed for use by clinicians and health care professionals, is a multidimensional tool that systematically assesses 19 common domains involved in dementia care to identify patients and caregivers needs and to document the extent to which those needs are met, based on established practices and guidelines. The domains include safety, management of cognitive and noncognitive symptoms as well as medical comorbidities, daily activities, and a range of caregiver education and support needs. Each domain contains multiple items that identify needs and each need is rated as unmet, partially met, or fully met. Here, we define unmet if (1) a need has not been addressed at all and potentially beneficial interventions are available, or (2) it has been or is being addressed but the potential benefits of available interventions have not yet been achieved. Fully met is defined as a need that is being addressed and the potential benefits of available interventions have been achieved to the extent possible for the individual.
Analyses
Descriptive summary statistics were calculated using SPSS 17.0 to describe the disposition of the telephone screening sample and to describe participants and subgroups stratified by screening status and dementia status. The significance of group differences (two-tailed) was evaluated by t-test and non-parametric tests (Pearson s chi-square and Fisher s exact test). Stacked cluster bar graphs were created to summarize the most frequent types of needs among individuals of differing diagnostic strata and the extent to which the needs were met.
Results
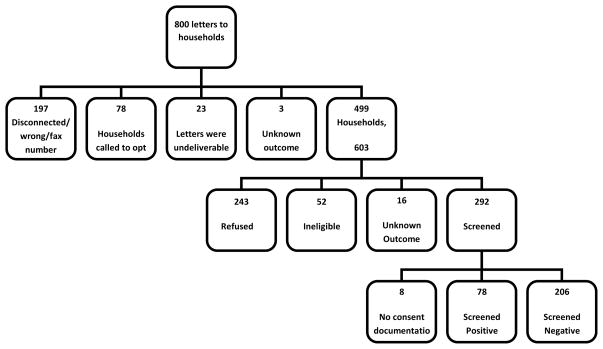
Outcomes of the telephone screening process are shown in Figure 1. Telephone contact was made with 62% (499/800) of households that included a total of 603 individuals. Evaluators made a total of 1,674 calls and spent approximately 350 total hours making contacts. Of the households contacted, 52 individuals were ineligible due to (1) lack of fluency in English (n=22), (2) being too impaired to speak on the telephone (n=8), (3) being younger than age 70 (n=3), (4) working fulltime (n=14), or (5) being deceased (n=5). Another 16 were contacted, but the outcome was not available, leaving a total of 535 individuals who were reached successfully by telephone. Of these, 292 (54.6%) were screened and 243(45.4%) declined. Of those screened and for whom there was a consent form on file (n=284), 78 (27.5%) screened positive.
Figure 1.
Sample Phone Screen Disposition
Demographic and cognitive status characteristics of the 284 individuals screened are shown in Table 1. On average, individuals who screened positive for cognitive impairment were significantly older (p<0.001) and had fewer years of education (<0.001), but were not more likely to be female, white or married.
Table 1.
Summary of Demographics and Cognitive Screening Measures for Telephone Screen Participants
| All Screened (n=284) | Screened Positive (n=78) | Screened Negative (n=206) | Test | P-value | |
|---|---|---|---|---|---|
| Variables† | Mean (SD) or N (% of total) | ||||
| Sociodemographics | |||||
| Age, years | 81.9 (6.3) | 85.0 (5.7) | 80.8 (6.1) | t= −5.2 | <0.001 |
| Female, n (%) | 168 (59.2) | 48 (61.51) | 120 (58.3) | χ2=0.3 | 0.620 |
| Education, years | 14.5 (2.9) | 13.2 (3.3) | 15.0 (2.6) | t= 4.4 | <0.001 |
| White, n (%) | 280 (99.6) | 78 (100) | 202 (99.5) | Fishers | 0.722 |
| Married, n (%) | 155 (70.1) | 43(67.2) | 112 (71.3) | χ2=0.4 | 0.541 |
| Cognitive Measures | |||||
| IQ Code total score | 53.2 (8.6) | 60.8 (10.3) | 48.7 (1.6) | t= −7.4 | <0.001 |
| TICS total score | 32.4 (4.5) | 27.1 (5.6) | 34.2 (2.1) | t= 10.1 | <0.001 |
Notes: TICS = Telephone Interview for Cognition Scale
Missing data: Age (n=5); Female (n=0); White (n=3); Education (n=12); IQ Code (n=177); TICS (n=15); White (n=3); Married (n=63)
Diagnostic data for the 43 individuals who participated in a home visit (33 screened positive, 10 screened negative) are in Table 2. Thirteen individuals met criteria for dementia, which included 36% of those who screened positive and 10% of those who screened negative. Table 3 compares demographic and clinical characteristics by diagnostic status. For 19 participants, a proxy informant was available, and of these, 18 met the definition of a caregiver (i.e. a person with whom the participant had regular contact and on whom the participant depended on for assistance with daily activities). There were no statistically significant differences in demographic variables among groups.
Table 2.
Telephone Screening Status by Diagnostic Status
| Screening status | Diagnostic Status | Total | ||
|---|---|---|---|---|
| Dementia | MCI† | No Dementia | ||
| Positive Screen | 12 | 14 | 7 | 33 |
| Row % | 36.4% | 42.4% | 21.2% | |
| Column % | 92.3% | 87.5% | 50.0% | |
| Negative Screen | 1 | 2 | 7 | 10 |
| Row% | 10.0% | 20.0% | 70.0% | |
| Column % | 7.7% | 12.5% | 50.0% | |
| Total | 13 | 16 | 14 | 43 |
Includes 1 individual with non-amnestic type MCI
Table 3.
Characteristics of Participants Randomly selected for Home Visit Assessment by Diagnostic Status (n=43)
| Total (n=43) | Dementia (n=13) | MCI (n=16) | Normal (n=14) | Test | p-value | |
|---|---|---|---|---|---|---|
| Variables† | Mean (SD) or N (% of total) | |||||
| Age, years | 84.3 (6.3) | 85.4 (5.2) | 83.4 (7.2) | 84.4 (6.4) | F(2,39)= 0.3 | 0.724 |
| Female, n (%) | 29.0 (67.4) | 7 (53.9) | 13 (81.3) | 9 (64.3) | Fishers Exact | 0.329 |
| Education, years | 13.4 (3.0) | 11.8 (4.4) | 13.9 (2.2) | 14.2 (1.9) | F(2,38)= 2.7 | 0.082 |
| White, n (%) | 43 (100) | 13 (100) | 16 (100) | 14 (100) | na | na |
| Married, n (%) | 22 (56.4) | 7 (53.9) | 9 (60.0) | 6 (54.6) | χ2=0.13 | 0.9838 |
| Had Informant, n (%) | 19 (44.2) | 8 (61.5) | 8 (50.0) | 3 (21.4) | Fishers Exact | 0.086 |
| Caregiver Present, n (%) | 18 (41.9) | 8 (61.5) | 7 (43.8) | 3 (21.4) | Fishers Exact | 0.113 |
| Physician Aware of Memory Problem, n (%) | 12 (50.0) | 6 (75.0) | 4 (40.0) | 2 (33.3) | Fishers Exact | 0.301 |
| Other Measures | ||||||
| IQ Code Total Score | 57.8 (9.4) | 62.4 (9.8) | 56.8 (9.5) | 52.0 (5.2) | F(2,18)=1.8 | 0.193 |
| TICS Total Score | 29.8 (8.7) | 27.0 (5.8) | 29.8 (3.5) | 32.1 (4.2) | F(2,38)=4.2 | 0.023 |
| Phone Screen Positive | 33 (76.7) | 12 (92.3) | 14 (87.5) | 7 (50.0) | Fishers Exact | 0.023 |
| MMSE | 26.6 (3.2) | 23.1 (3.7) | 27.6 (1.4) | 28.6 (1.2) | F(2,40)=23.0 | <0.001 |
| Mental Alternation | 22.0 (8.7) | 15.8 (7.7) | 22.4 (8.0) | 27.1 (7.0) | F(2,40)=7.5 | 0.002 |
| ADL-Instrumental | 12.3 (4.9) | 16.5 (5.4) | 11.3 (4.0) | 9.3 (1.7) | F(2,29)=8.7 | 0.001 |
| ADL-Basic | 6.8 (1.9) | 7.9 (2.9) | 6.5 (0.9) | 6.0 (0.0) | F(2,35)=3.9 | 0.030 |
| NPI-Q Severity | 6.0 (7.2) | 9.1 (7.2) | 4.9 (7.4) | 0.3 (0.6) | F(2,15)=2.0 | 0.171 |
| NPI-Q Distress | 7.9 (9.1) | 12.1 (9.0) | 6.3 (9.2) | 0.3 (0.6 | F(2,15)=2.3 | 0.131 |
| ZBI | 3.2 (2.9) | 4.4 (2.9) | 3.0 (2.9) | 0.3 (0.6) | F(2,14)=2.5 | 0.118 |
| General Health | 3.0 (2.9) | 2.5 (0.9) | 3.1 (1.0) | 3.1 (0.9) | F(2,38)=2.2 | 0.126 |
Notes: TICS = Telephone Interview for Cognition Scale; MMSE= Mini-Mental State Exam; NPI-Q= Neuropsychiatric Inventory-Questionnaire; ZBI= Zarit Burden Interview.
Missing data from total sample: Age (n=1); Education (n=1); Married (n=4); Physician Aware of Memory Problems (19); Lived alone (n=27); IQ Code (n=22); TICS (n=2); ADL-Instrumental (n=11); ADL-Basic (n=5); NPI-Q Severity (n=25); NPI-Q Distress (n=25); ZBI (n=26); General Health (n=2).
The groups differed significantly on all cognitive measures assessed except the IQCODE (p= 0.189), and also differed significantly on both instrumental ADLs (p=0.001) and basic ADLs (p=0.030). There were no significant differences between groups on self-reported general health, neuropsychiatric symptom severity, or caregiver burden.
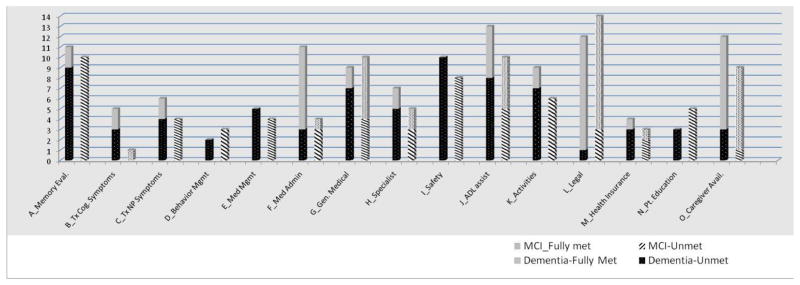
Needs related to dementia care as assessed by the JHDCNA are shown in Figure 2 for participants found to have cognitive disorders (i.e. dementia or MCI). On average, those with dementia had 6.6 (SD 3.5) unmet needs and nearly all (12/13) had at least one unmet need. The most common unmet needs of those with dementia were need for a dementia workup (9/11), general medical care (7/9), environmental safety (10/10), assistance with ADL impairments (8/13), and access to meaningful activities (7/9). Among the 8 caregivers of participants with dementia, 7 had at least one unmet dementia care related need; the average number of caregiver unmet needs was 5.0 (SD 3.7). Caregivers greatest unmet needs were for education about dementia (5/6), knowledge of community resources (3/3), and caregiver mental health care (5/5).
Figure 2.
Distribution of JHDCNA Domains by Diagnostic status and Unmet Needs
Among those with MCI, the average number of unmet needs was 3.4 (4.2) with over two-thirds (12/16) having at least one unmet dementia care related need. The primary unmet needs of those with MCI focused on memory evaluation (10/10), safety (8/8), and meaningful activities (6/6). Among the 7 caregivers for individuals in the MCI group, 4 had at least one unmet need. The primary unmet needs for caregivers were for education about memory disorders (4/6), knowledge of community resources (3/3), and caregiver mental health care (3/3). Where urgent needs were identified, participants and/or caregivers were given direct feedback and information about resources at the time of the home assessment. As this was a cross-sectional pilot study, no prospective data are available as to the outcomes.
Discussion
This pilot study underscores the challenges that arise in identifying community-residing individuals with dementia, not the least of which is the unwillingness of some to be screened for memory problems or reluctance of at risk persons to obtain an evaluation. Out of 800 households selected for age of residents in the targeted zip code areas, only 499 households (603 individuals) could be reached and of those, only 292 consented to be screened. This process involved a substantial commitment of staff time to contact the identified households and conduct screening interviews with eligible residents. A more targeted approach to identifying individuals in the community with dementia may be more efficient. Fear of receiving a diagnosis, or the cognitive symptoms characteristic of the disease itself, may have prevented some from taking part in the telephone screen.
Of the 33 home visits completed with those who screened positive, the majority (79%) had either MCI or dementia. This suggests that when persons identified as being at-risk agree to be screened, a memory disorder can be identified before the person has presented in crisis.
In addition to detecting the presence of MCI or dementia, this study also assessed participants memory disorder-related needs across multiple domains in order to develop a needs assessment instrument for future use in persons with dementia living at home. The highest prevalence of unmet needs were in the areas of safety, dementia work-up, activities of daily living, and access to meaningful activities. Caregivers also had unmet needs, particularly for caregiver education and mental health care. Interventions to address these needs may reduce the likelihood of adverse outcomes and improve quality of life for the individuals with memory disorder and their caregivers.
As this was a small sample that focused on a specific cultural group, the Jewish community of Northwest Baltimore, it may not be representative of the broader population of at-risk persons over age 70. Also, mailing only to the targeted community may miss many community dwelling persons with dementia. Our data show that some individuals are unwilling to be screened for memory disorders. We are also aware of the reluctance of persons with dementia to be told of the diagnosis, with its attendant potential for stigma and the therapeutic nihilism that often goes along with the diagnosis. However, some of these perceptions may be due to the inevitability of poor outcomes in the absence of data showing what can be done to identify and prevent or mitigate the occurrence of complications in community-dwelling persons with dementia.
Based on these results, we are currently undertaking a larger scale study of a multi-pronged case-finding approach that includes: (1) training members of high-contact community agencies (e.g., Meals on Wheels) to recognize and refer persons with memory disorders, (2) targeted mailings to individuals in the broader community aimed at reaching family members of persons with dementia, (3) raising the public s awareness of memory disorders via the media, and (4) collaborating with primary care practices and other health care providers. This case-finding approach will be combined with a randomized controlled trial of a multidimensional patient and caregiver centered intervention provided by dementia care coordinators aimed at maintaining function and independence and averting crises in community-dwelling persons with memory disorders.
One of the main goals of MIND at Home Phase 1 was to develop a needs assessment instrument which can be used as the basis for a multidimensional, patient-specific intervention based on established practices and guidelines. Such an instrument needs to encapsulate a broad range of needs across a multidimensional spectrum, while documenting those needs precisely for each individual participant and caregiver if present, along with easily accessible, updatable, information relevant to those needs. It will also capture, longitudinally, patient responses to delivered interventions and subsequent adjustments to the treatment plan. The Johns Hopkins Dementia Care Needs Assessment (JHDCNA) instrument created based on MIND at Home Phase 1 data and experience, will provide a framework around which an individualized intervention care plan can be organized and evaluated for feasibility and outcomes in MIND at Home Phase 2 in combination with the enhanced casefinding methods described above.
Acknowledgments
The MIND at Home team thanks the Associated for their generous support of MIND at Home Phase 1.
References
- Balardy L, Voisin T, Cantet C, Vellas B REAL.FR Group. Predictive factors of emergency hospitalisation in Alzheimer’s patients: results of one-year follow-up in the REAL.FR Cohort. J Nutr Health Aging. 2005;9(2):112–6. [PubMed] [Google Scholar]
- Beck RA, Arizmendi A, Purnell C, Fultz BA, Callahan CM. MODELS OF GERIATRIC CARE, QUALITY OF IMPROVEMENT, AND PROGRAM DISSEMINATION House Calls for Seniors: Building and Sustaining a Model of Care for Homebound Seniors. Journal of the American Geriatrics Society. 2009;57:1103–1109. doi: 10.1111/j.1532-5415.2009.02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billick SB, Siedenburg E, Burgert W, 3rd, Bruni-Solhkhah SM. Validation of the Mental Alternation Test with the Mini-Mental State Examination in geriatric psychiatric inpatients and normal controls. Comp Psychiatry. 2001;42(3):202–205. doi: 10.1053/comp.2001.23146. [DOI] [PubMed] [Google Scholar]
- Boustani M, Perkins AJ, Fox C, Unverzagt F, Austrom MG, Fultz B, Hui S, Callahan CM, Hendrie HC. Who refuses the diagnostic assessment for dementia in primary care? Int J Geriatr Psychiatry. 2006 Jun;21(6):556–63. doi: 10.1002/gps.1524. [DOI] [PubMed] [Google Scholar]
- Boustani M, Peterson B, Hanson L, Harris R, Lohr KN U.S. Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003 Jun 3;138(11):927–37. doi: 10.7326/0003-4819-138-11-200306030-00015. Review. [DOI] [PubMed] [Google Scholar]
- Bradford A, Kunik MEMD, Schulz P, Williams SP, Singh H. Missed and Delayed Diagnosis of Dementia in Primary Care Prevalence and Contributing Factors. Alzheimer Dis Assoc Disord. 2009 Jun 29;00:000–000. doi: 10.1097/WAD.0b013e3181a6bebc. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiat Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, Fultz BA, Hui SL, Counsell SR, Hendrie HC. Effectiveness of Collaborative Care for Older Adults With Alzheimer Disease in Primary Care. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- Cummings J, Mega M, Gray K, Thompson S, Carusi D, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;(44):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Del Lungo I, Guralnik JM, Bandinelli S, Benvenuti E, Salani B, Lamponi M, Ubezio C, Benvenuti F, Baroni A. Is the telephone interview for cognitive status a valid alternative in persons who cannot be evaluated by the Mini Mental State Examination? Aging (Milano) 1998 Aug;10(4):332–8. doi: 10.1007/BF03339796. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. JOURNAL OF PSYCHIATRIC RESEARCH. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, Ashley P. DeNDRoN Primary Care Clinical Studies Group Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009 Sep;24(9):895–901. doi: 10.1002/gps.2204. [DOI] [PubMed] [Google Scholar]
- Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. International Psychogeriatrics. 2004;16(3):275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychological Medicine. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Davidoff DA, Katt-Lloyd D, Connell A, Berlow YA, Savoie JA. Is Large-Scale Community Memory Screening Feasible? Experience from a Regional Memory-Screening Day. Journal of the American Geriatrics Society. 2003;51(8):1072–1078. doi: 10.1046/j.1532-5415.2003.51354.x. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, Kalunian DA, Yaffe K. Position Statement of the American Association for Geriatric PsychiatryRegarding Principles of Care for Patients With Dementia Resulting From Alzheimer Disease. American Journal of Geriatric Psychiatry. 2006 Jul;14(7):561–72. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;(37):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heering SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;(29):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CC, Boustani M, Callahan CM, Perkins AJ, Hui S, Hendrie HC. Acute care utilization by dementia caregivers within urban primary care practices. Journal of General Internal Medicine. 2008 Nov;23(11):1736–40. doi: 10.1007/s11606-008-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton P, Schraeder C, Dworak D, Fraser C, Sager MA. Caregivers’ utilization of health services: results from the Medicare Alzheimer’s Disease Demonstration, Illinois site. Journal of the American Geriatrics Society. 2001;49(12):1600–5. [PubMed] [Google Scholar]
- Trujillo M. Multicultural Aspects of Mental Health. Primary Psychiatry. 2008 Apr;15(4):65–84. [Google Scholar]
- Ukeles JB, Miller R. [Accessed 10/28/2009];Aging in Place in the Baltimore Jewish Community: A Briefing Book. 2001 Available at http://www.associated.org/local_includes/downloads/33237.pdf.
- U.S. Department of Health and Human Services Agency for Healthcare Research and Quality United States Government. [Accessed Nov 11, 2009];2005 National Healthcare Disparities Report. 2005 http://www.ahrq.gov/qual/nhdr05/nhdr05.pdf.
- U.S. Preventive Task Force. Screening for dementia: recommendation and rationale. Ann Intern Med. 2003;138:925–926. doi: 10.7326/0003-4819-138-11-200306030-00014. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]