Abstract
Objectives
Increased arterial wave reflection is a predictor of cardiovascular events and has been hypothesized to be a cofactor in the pathophysiology of heart failure. Whether increased wave reflection is inversely associated with left ventricular (LV) systolic function in subjects without heart failure is not clear.
Methods
Arterial wave reflection and LV systolic function were assessed in 301 participants from the Cardiovascular Abnormalities and Brain Lesions (CABL) study using 2-dimensional echocardiography and applanation tonometry of the radial artery to derive central arterial waveform by a validated transfer function. Aortic augmentation index (AIx) and wasted energy index (WEi) were used as indices of wave reflection. LV systolic function was measured by ejection fraction (LVEF) and tissue Doppler imaging (TDI). Mitral annulus peak systolic velocity (Sm), peak longitudinal strain and strain rate were measured. Participants with history of coronary artery disease, atrial fibrillation, LVEF <50% or wall motion abnormalities were excluded.
Results
Mean age of the study population was 68.3±10.2 years (64.1% women, 65% hypertensive). LV systolic function by TDI was lower with increasing wave reflection, whereas LVEF was not. In multivariate analysis, TDI parameters of LV longitudinal systolic function were significantly and inversely correlated to AIx and WEi (p values from 0.05 to 0.002).
Conclusions
In a community cohort without heart failure and with normal LVEF, an increased arterial wave reflection was associated with subclinical reduction in LV systolic function assessed by novel TDI techniques. Further studies are needed to investigate the prognostic implications of this relationship.
Keywords: wave reflection, arterial stiffness, systolic function, strain, strain rate, tissue Doppler, echocardiography
Introduction
Aging, hypertension, and other cardiovascular risk factors are associated with a global stiffening of the vascular tree [1–3]. In the presence of normal arterial elastic properties, the systolic-diastolic ventricular-vascular coupling (the Windkessel function) has an important role in reducing left ventricular (LV) afterload and improving coronary blood flow [4,5]. As a consequence of the progressive stiffening of the elastic arteries, cardiac afterload increases both directly (because of a reduction in the Windkessel function) and indirectly (because the accelerated return from periphery of the arterial wave reflection that adds to the resistance to the LV ejection effort) [6–8]. Indicators of increased arterial wave reflection are independent predictors of coronary artery disease, heart failure, cardiovascular events, and mortality [9–16], and alterations in ventriculo-vascular coupling have been advocated as possible factors involved in the pathogenesis of both systolic [17,18] and diastolic heart failure [19,20].
The echocardiographic evaluation of LV tissue velocities and tissue deformation by tissue Doppler imaging (TDI) constitutes a novel method for evaluating the LV systolic function, able to analyze different components of LV function and to detect subclinical and regional alterations even in patients with normal LV chamber function (i.e. normal or increased ejection fraction) [21]. In fact, a reduced LV systolic function, measured by TDI in the longitudinal direction, is present in patients with heart failure and normal ejection fraction when measured by TDI technique [22,23]. It is possible, therefore, that even in the setting of a normal LV ejection fraction, an increase in arterial stiffness may be associated with an initial, subclinical reduction in the longitudinal LV systolic function. The few previous studies that investigated this topic showed conflicting results [24,25]. Therefore, in this cross-sectional study we sought to assess the correlation of arterial wave reflection with LV longitudinal systolic function measured by TDI-derived techniques in a community-based cohort free of overt coronary heart disease and with normal LV ejection fraction.
Methods
Study population
The study cohort of the Cardiac Abnormalities and Brain Lesions (CABL) study was derived from the Northern Manhattan Study (NOMAS), an epidemiological study that evaluates the incidence, risk factors, and clinical outcome of stroke in the multiethnic population of Northern Manhattan. The study design and methodological details regarding NOMAS have been described previously [26]. Briefly, community subjects from Northern Manhattan were eligible if they: (1) had never been diagnosed with a stroke, (2) were age 40 or older, and (3) resided in Northern Manhattan for at least 3 months in a household with telephone. NOMAS subjects over age 50 that voluntarily agreed to undergo a brain MRI study and a more extensive echocardiographic evaluation including assessment of diastolic function were included in the CABL study. Informed consent was obtained from all study participants. The study was approved by the Institutional Review Board of Columbia University Medical Center.
Risk Factors Assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg at the time of the visit (mean of two readings), or a patient’s self-reported history of hypertension or of anti-hypertensive medications (diuretics, beta-blockers, ACE-inhibitors, AT2-blockers, or Ca++-blockers). Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or patient’s self-reported history of diabetes or of diabetes medications (insulin or oral hypoglycemic agents). Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-report of hypercholesterolemia, or of use of lipid-lowering treatment. Participants with coronary artery disease, defined as a history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention, and those with atrial fibrillation, were excluded from analysis.
Echocardiographic Assessment
Transthoracic echocardiography evaluation was performed using a commercially available system (iE 33, Philips, Andover, MA) by a trained registered cardiac sonographer according to a standardized protocol. LV end-diastolic and end-systolic diameters, interventricular septum thickness and posterior wall thickness were measured at end-diastole from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography (ASE) [27]. LV mass was calculated with a validated formula [28], and indexed by height to the power of 2.7 to account for the effect of body size on LV mass growth [29]. LV hypertrophy was defined using the ASE guidelines sex-specific cutoffs [27]. Left ventricular relative wall thickness (RWT), an index of LV geometry, was calculated with the formula: (2 × posterior wall thickness)/end-diastolic diameter [30]. LV ejection fraction (LVEF) was calculated by the biplane-modified Simpson’s rule as recommended by the ASE [27]. Subjects with an LVEF < 50% or with LV segmental wall motion abnormalities and those not in sinus rhythm at the time of examination were excluded from analysis.
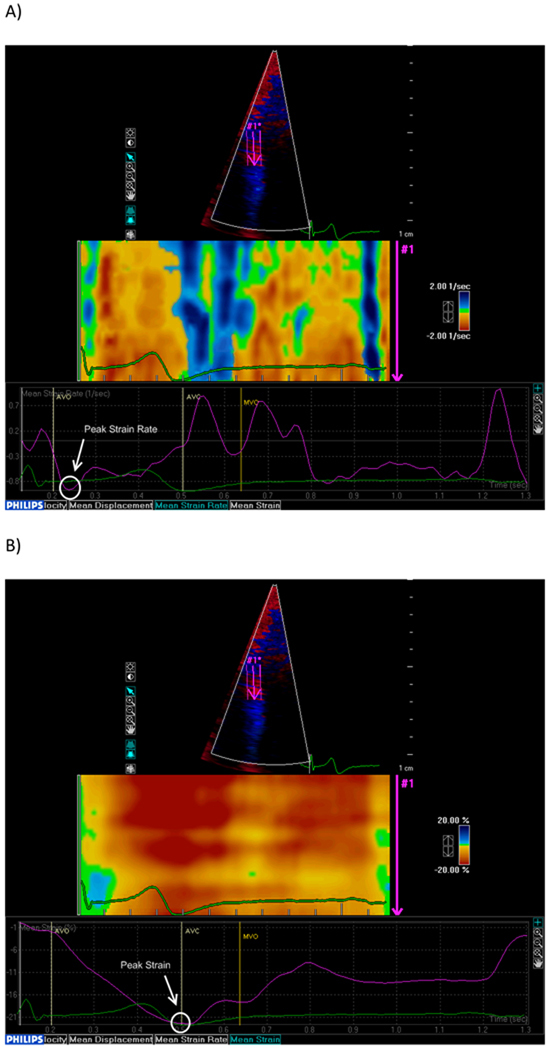
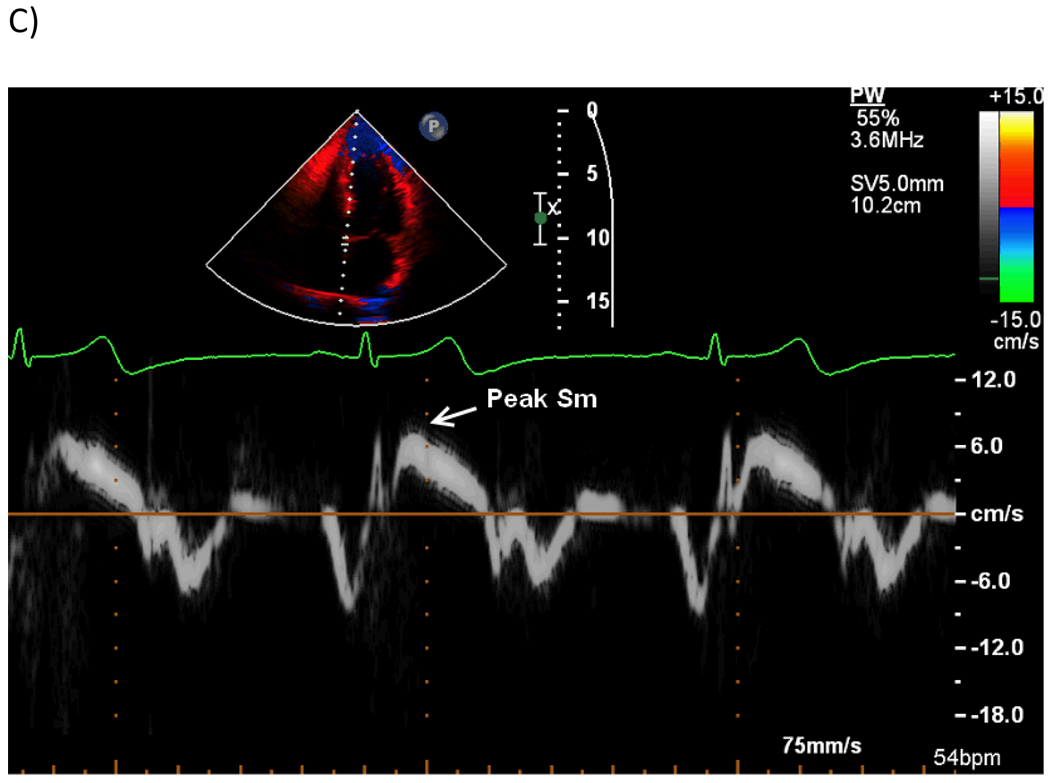
TDI-based deformation analysis was performed off-line using commercially available software (QLAB Strain Quantification, Philips, version 7.0). From a color TDI loop of an apical 4-chamber view, three consecutive beats were recorded during patient apnea and stored in digital format for off-line analysis. The sector was narrowed to include only the wall of interest (interventricular septum) in order to obtain high frame rates (>150 frames/sec). The region of interest was placed at the level of the mid interventricular septum and a frame-by-frame manual tracking was used during the whole cardiac cycle to compensate the lateral shift of the wall and maintain the region of interest on the myocardium. Visual analysis of the strain rate curved M-mode spectrum (Figure 1 A) was used to judge the quality of the TDI signal, based on a reproducible and meaningful visualization of systolic and diastolic phases [31]. Three cardiac cycles were averaged and strain rate and strain curves were derived (Figure 1A and 1B). Peak LV systolic strain and strain rate, defined as the negative strain and strain rate curve peaks during the ejection period, were measured and were used as indicators of global LV chamber systolic function in the absence of regional wall motion abnormalities [32,33]. In addition, time to peak systolic strain, measured from the onset of the QRS to the peak systolic strain, was also recorded. LV peak longitudinal systolic myocardial velocity at the septal mitral annulus (Sm) was evaluated by pulsed TDI from the apical 4-chamber view (Figure 1 C).
Figure 1. Tissue Doppler analysis of the LV longitudinal systolic function.
Peak systolic longitudinal strain rate (A) and strain (B) measured at the mid-level of the ventricular septum. AVO: aortic valve opening. AVC: aortic valve closing. MVO: mitral valve opening. (C) Pulsed tissue Doppler of the septal mitral annulus.
Pulse waveform analysis
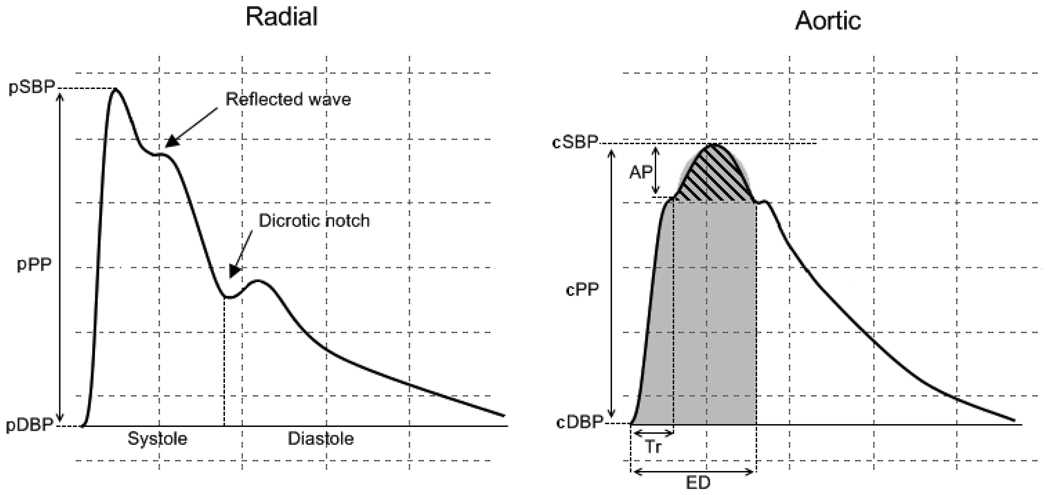
On the same day, after the performance of the echocardiogram, blood pressure was measured with the subjects still in supine position, and pulse wave analysis of the radial artery by applanation tonometry at the wrist was performed using a commercially available device (SphygmoCor, Pulse Wave Analysis System, AtCor Medical). After the acquisition of 20 to 30 reproducible sequential waveforms, the radial pulse wave was generated, and a validated generalized transfer function [34,35] was used to derive the corresponding central aortic pressure waveform and calculate central systolic, diastolic, and pulse pressures. Aortic augmented pressure (AP) from the reflected wave was measured as the difference between the peak systolic central pressure and the pressure at the onset of the reflected wave from the lower body (time to reflection, Tr) (Figure 2). The aortic augmentation index (AIx) was calculated as the ratio between the augmented pressure and the central pulse pressure and expressed as percent. In addition to the AIx, we calculated the wasted energy that the LV generates to overcome the extra afterload imposed by the wave reflection. The aortic pressure-time integral is a main determinant of myocardial oxygen consumption [36]. The portion of the systolic aortic pressure-time integral that is attributable to the reflected wave, representing the extra effort sustained by the LV (wasted energy, WE), was calculated as the area of a half ellipse: WE (sec-dyne-cm−2) = AP (mmHg) * reflected wave duration (msec) * π/2 multiplied by the conversion factor 1.33 [37]. The wasted energy is associated with LV hypertrophy in patients with hypertension, and is related to the myocardial oxygen demand [37–39]. The ratio of the WE by the total systolic pressure-time integral (wasted energy index, WEi) expressed in percent, provides an estimation of the contribution of wave reflection to the late systolic afterload, an estimation that takes into account not only the amplitude of the reflection (like AIx does) but also its duration. The WEi was therefore used as an additional index of wave reflection and arterial stiffness.
Figure 2. Arterial pulse waveform analysis by applanation tonometry.
Radial (left) and aortic (right) waveforms. Aortic waveform is generated with a transfer function from radial artery waveform. pSBP: peripheral systolic blood pressure. pDBP: peripheral diastolic blood pressure: pPP: peripheral pulse pressure. cSBP: central systolic blood pressure. cDBP: central diastolic blood pressure: cPP: central pulse pressure. AP: augmented pressure. Tr: time to the beginning of the reflected wave. ED: ejection duration. The aortic augmentation index is calculated as 100 × AP/cPP. The striped area represents the area of under the reflected wave (wasted energy, WE) due to the return of the arterial wave from the reflection sites of the lower body. The gray area is the total systolic effort (pressure-time integral, PTI) of the systolic curve. The wasted energy index (WEi) is calculated as 100*WE/PTI.
An algorithm included in the device evaluated the overall quality of the captured signal for all recordings (based on average pulse height, pulse height variation, diastolic variation, shape deviation and maximum dP/dT), and only studies with an acceptable quality score (operator index > 80%) were included in the analysis.
Statistical Analysis
Data are presented as mean ± standard deviation for continuous variables and as proportions for categorical variables. Simple correlations were evaluated by Pearson’s r correlation coefficients. Analysis of variance (ANOVA) was used to test differences between quartiles of AIx and WEi. Multivariate linear regressions were performed to assess the independent association of aortic stiffness and wave reflections variables with parameters of LV longitudinal systolic function. Reproducibility of the measurements was assessed by calculating the intraclass correlation coefficients (ICC) for absolute agreement and relative 95% confidence intervals (CI), and by Bland-Altman plots. For all statistical analyses, a two-tailed p<0.05 was considered significant. Statistical analyses were performed using SPSS software version 17.0 (SPSS inc., Chicago, IL).
Reproducibility of wave reflection measurements
In a random sample of 20 study participants, a pulse waveform recording was re-obtained on the same day of the first measurement, and test-retest reproducibility for the parameters of wave reflection was assessed. Intraobserver test-retest variability was 0.6±4.1% for AIx, and 0.3±1.5% for WEi. ICC were 0.93 for AIx (95% CI: 0.84–0.97, p<0.001) and 0.98 for WEi (95% CI: 0.94–0.99, p<0.001)
Reproducibility of TDI measurements
In a random sample of 20 study participants, TDI measurements were re-measured by the original reader, and intra-observer reproducibility was assessed. ICC were: 0.95 for Sm (95% CI: 0.88–0.98, p<0.001), 0.94 for peak strain (95% CI: 0.85–0.98, p<0.001), 0.90 for peak strain rate (95% CI: 0.76–0.96, p<0.001), 0.94 for time-to-peak strain (95% CI: 0.84–0.97, p<0.001).
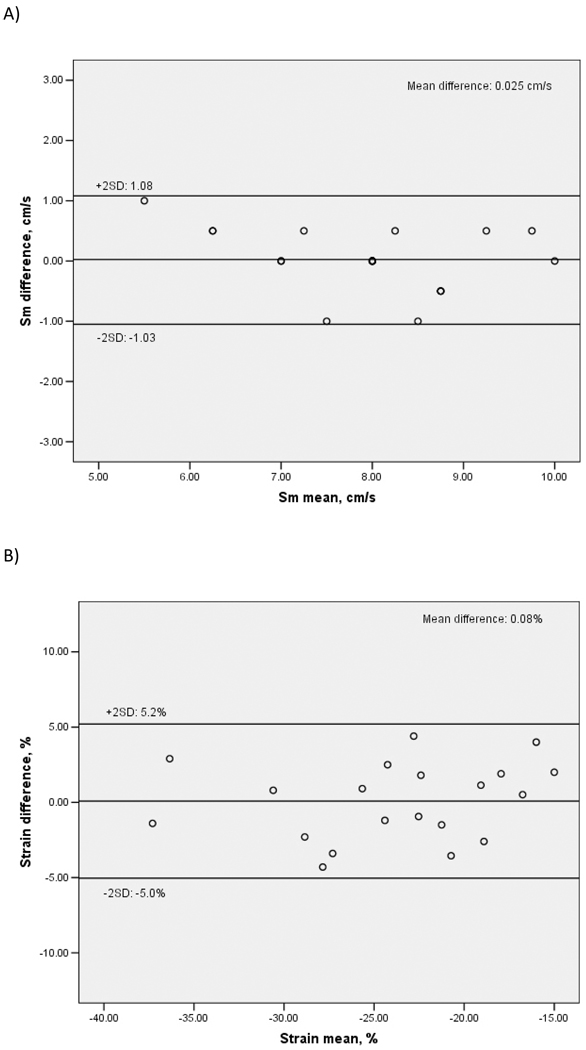
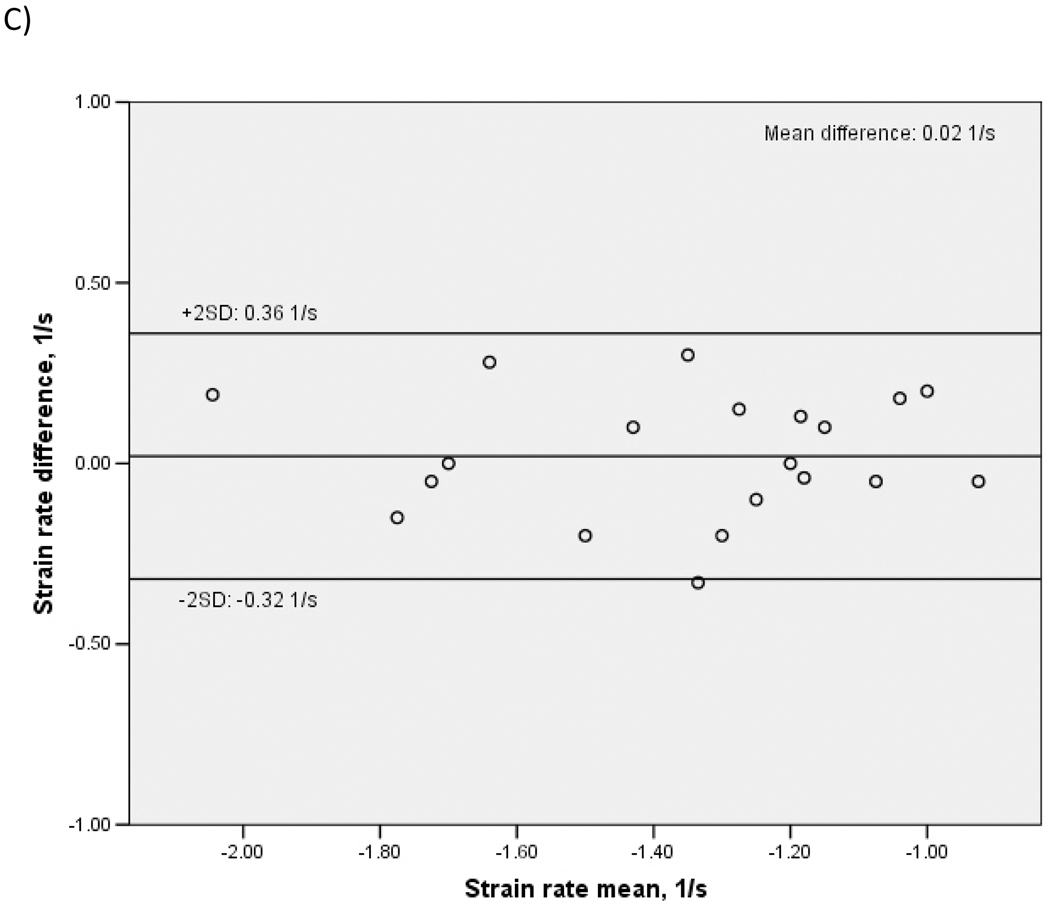
To assess inter-observer reproducibility, a different reader re-measured TDI parameters in a random subset of 20 study participants. ICC were: 0.90 for Sm (95% CI: 0.77–0.96, p<0.001), 0.92 for peak strain (95% CI: 0.80–0.97, p<0.001), 0.85 for peak strain rate (95% CI: 0.65–0.94, p<0.001), 0.93 for time-to-peak strain (95% CI: 0.86–0.98, p<0.001). Bland-Altman plots showing inter-observer variability of Sm, peak strain and peak strain rate are shown in Figure 3. Mean differences between readers were: 0.03±0.53 cm/s for Sm (p=0.83 for the comparison between the two readings), 0.08±2.56% for peak strain (p=0.88), and 0.02±0.17 1/s for peak strain rate (p=0.56).
Figure 3. Bland-Altman plots showing inter-observer reproducibility of LV systolic function by TDI.
A: Peak mitral annulus systolic velocity (Sm). B: Peak LV longitudinal systolic strain. C: Peak LV longitudinal systolic strain rate.
Results
Study population
The study population consisted of 301 subjects. Characteristics of the study sample are shown in Table 1. As per inclusion criteria, no subject had history of CAD, LV segmental wall motion abnormalities or LVEF < 50%.
Table 1.
Demographic, clinical, echocardiographic and vascular characteristics of the study population.
| Clinical/Demographic characteristics | N=301 |
|---|---|
| Age, years | 68.3 ± 10.2 |
| Body mass index, kg/m2 | 27.6 ± 4.7 |
| Women, n (%) | 193 (64.1) |
| SBP, mmHg | 130.9 ± 21.0 |
| DBP, mmHg | 72.7 ± 10.3 |
| MBP, mmHg | 93.2 ± 12.7 |
| Heart rate, bpm | 67.9 ± 10.6 |
| Hypertension, n (%) | 197 (65.4) |
| Anti-hypertensive treatment, n (%) | 125 (41.5) |
| Diabetes, n (%) | 84 (27.9) |
| Anti-diabetic treatment, n (%) | 52 (17.3) |
| Hypercholesterolemia, n (%) | 171 (56.8) |
| Lipid lowering treatment, n (%) | 37 (13.5) |
| Echocardiographic variables | |
| LV septum thickness, mm | 11.0 ± 1.8 |
| LV end-diastolic diameter, mm | 45.0 ± 4.4 |
| LV posterior wall thickness, mm | 10.8 ± 1.5 |
| LV mass index, g/m2.7 | 48.9 ± 12.9 |
| LV hypertrophy, n (%) | 162 (53.8) |
| Relative wall thickness | 0.48 ± 0.08 |
| LVEF, % | 63.5 ± 4.8 |
| Sm, cm/s | 8.0 ± 1.7 |
| Peak strain, % | −23.4 ± 7.3 |
| Peak strain rate, 1/s | −1.30 ± 0.42 |
| Time to peak strain, ms | 361.1 ± 56.8 |
| Pulse waveform | |
| Aortic augmented pressure, mmHg | 14.5 ± 8.9 |
| Aortic AIx, % | 29.2 ± 10.6 |
| LV wasted energy, sec-dyne-cm−2 | 5699.8 ± 4131.5 |
| WEi, % | 12.0 ± 6.8 |
| Systolic pressure-time integral, sec-dyne-cm−2 | 44429.4 ± 8384.4 |
| Time to reflection (Tr), msec | 135.1 ± 9.8 |
SBP: Systolic blood pressure. DBP: Diastolic blood pressure. MBP: Mean blood pressure. LVEF: LV ejection fraction. AIx: Augmentation index. WEi: Wasted energy index.
Results provided as mean ± standard deviation or n (%) when indicated.
Correlates of wave reflection parameters
Simple correlations of AIx and WEi with clinical, demographic and echocardiographic variables are shown in Table 2. AIx showed significant correlations with age, body surface area, mean BP and heart rate (all p<0.01). WEi was significantly correlated with age, body surface area, mean BP, heart rate (all p<0.01) and LV mass index (p<0.05). The presence of diabetes, hypercholesterolemia, and medical treatment for hypertension, diabetes and dyslipidemia were not correlated to wave reflection parameters. In sub-analyses adjusted for age and sex, treatment with beta-blockers was associated with lower AIx (β=−0.13, p=0.02) and lower WEi (β=−0.12, p=0.03). No significant associations were found in AIx nor in WEi associated with the use of angiotensin converting enzyme-inhibitors (β for AIx=0.02, p=0.69; β for WEi=0.01, p=0.28) and of calcium channel-blockers (β for AIx=0.08, p=0.12; β for WEi=0.09, p=0.09).
Table 2.
Univariate correlates of wave reflection parameters
| Aortic AIx | WEi | |
|---|---|---|
| Age | 0.22** | 0.42** |
| Body surface area | −0.33** | −0.32** |
| Mean BP | 0.27** | 0.28** |
| Diabetes | −0.06 | 0.04 |
| Hypercholesterolemia | −0.004 | −0.02 |
| Anti-hypertensive treatment | 0.00 | 0.01 |
| Anti-diabetic treatment | −0.11 | −0.09 |
| Lipid lowering treatment | 0.03 | 0.07 |
| LV mass index | 0.04 | 0.13* |
| Relative wall thickness | −0.09 | −0.03 |
| Heart rate | −0.54** | −0.50** |
Values in table are Pearson’s correlation coefficients
= p<0.05;
= p<0.01
Wave reflections and LV systolic function
The study population was divided into quartiles of AIx and WEi, and LV systolic function was compared among the groups (Table 3). LVEF was not different among the quartiles of AIx (p=0.22) or WEi (p=0.57). Sm showed a significant decrease with the increase in AIx and WEi (both p<0.001). Peak strain rate was lower (less negative) in higher AIx quartiles (p=0.03) and showed a borderline significant trend in the same direction in higher WEi quartiles (p=0.06). Time to peak strain was significantly higher in higher AIx and WEi quartiles (both p<0.001).
Table 3.
Left ventricular systolic function stratified by quartiles of AIx and WEi
| AIx quartiles | |||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | P* | |
| LVEF | 64.4±4.6 | 63.4±4.6 | 63.3±5.6 | 62.8±4.6 | 0.22 |
| Sm, cm/s | 8.8±1.9 | 8.1±1.6 | 7.8±1.7 | 7.5±1.4 | <0.001 |
| Peak strain, % | −23.9±7.8 | −23.1±8.1 | −23.4±6.6 | −22.9±6.5 | 0.82 |
| Time to peak strain, msec | 332.1±41.9 | 349±44.1 | 356.2±41.5 | 399.1±66.4 | <0.001 |
| Peak Strain Rate, 1/s | −1.40±0.42 | −1.27±0.47 | −1.29±0.41 | −1.20±0.34 | 0.03 |
| WEi quartiles | |||||
| 1st | 2nd | 3rd | 4th | P* | |
| LVEF | 63.9±4.9 | 63.1±4.1 | 63.9±4.7 | 63.0±5.6 | 0.57 |
| Sm, cm/s | 8.9±1.8 | 8.0±1.8 | 7.7±1.4 | 7.6±1.6 | <0.001 |
| Peak strain, % | −23.7±7.8 | −23.3±7.6 | −22.7±6.4 | −23.6±7.1 | 0.85 |
| Time to peak strain, msec | 340.3±44.4 | 341.1±45.2 | 377.4±71.9 | 384.6±54.3 | <0.001 |
| Peak Strain Rate, 1/s | −1.39±0.47 | −1.28±0.42 | −1.21±0.38 | −1.26±0.37 | 0.06 |
p value from analysis of variance (ANOVA) between quartiles
In linear regression analysis, after adjusting for covariates (Table 4), a higher AIx was associated with lower Sm (β=−0.17, p=0.03), lower (less negative) peak strain (β=0.18, p=0.008) and strain rate (β =0.12, p=0.05), and longer time to peak strain (β=0.28, p=0.002). Similarly, a higher WEi was significantly associated with lower TDI parameters of LV systolic function (β =−0.18, p=0.01 for Sm; β =0.13, p=0.04 for peak strain; β =0.14, p=0.03 for peak strain rate; β=0.22, p=0.02 for time to peak strain). The results of the analyses did not change when in the linear models the interventricular septum thickness was used as a covariate instead of the LV mass.
Table 4.
Multivariate analysis of the relationship between indices of wave reflections and LV longitudinal systolic function
| Sm | Peak strain | Peak strain rate | Time to peak strain |
|
|---|---|---|---|---|
| AIx, β | −0.17 | 0.18 | 0.12 | 0.28 |
| P value | 0.03 | 0.008 | 0.05 | 0.002 |
| WEi, β | −0.18 | 0.13 | 0.14 | 0.22 |
| P value | 0.01 | 0.04 | 0.03 | 0.02 |
Values in table are standardized correlation coefficients (β) and relative p values.
Covariates: Age, sex, body surface area, mean BP, heart rate, LV mass index
Discussion
In the present study, we demonstrated that, in a community-based cohort without overt heart disease, TDI parameters of LV systolic function correlated inversely with arterial wave reflection parameters, whereas LVEF did not. AIx and WEi are the result of the ratio between the extra pressure/effort caused by reflected waves and the total pulse pressure/systolic effort, therefore they express the amount of afterload caused by arterial wave reflection, and are considered indicators of vascular aging and vascular disease, and surrogate indicators of arterial stiffness [39]. In fact, with increasing stiffening of the arterial tree, reflected waves from periphery travel faster due to increased pulse wave velocity, returning to the heart during systole. The result is a substantial increase in late-systolic LV afterload [6,40]. Our findings may be interpreted as either a consequence of the afterload-shortening paradigm or as an indicator of impending reduction in LV contractility associated to increased arterial stiffness. The TDI parameters that we used to assess LV systolic function explore different aspects of LV mechanics. The peak systolic myocardial velocity of the mitral annulus (Sm) reaches its peak in proto-systole, and, although its afterload dependence is debated [41,42], it has been shown to be able to detect alterations of LV systolic function in different conditions [43,44]. Peak LV strain, which represents the amount of systolic deformation of the sampled myocardium in respect to the end-diastolic state, has been found to be related to the LV systolic chamber function [45]. Similarly to LVEF, LV strain reaches its peak at the end of systole, being the expression of the total deformation of the sampled LV segments. Among the TDI parameters, LV strain is probably the one most influenced by afterload. Peak LV strain rate, which is the rate of deformation of the sampled myocardium, is an early systolic index of LV function, occurring in the very early systolic phase. LV strain rate showed good correlation with LV contractility measured invasively in several studies [32,45]. Since the extra afterload generated by wave reflection occurs in late systole, the relationships between wave reflections and Sm and LV strain rate suggest that a subclinical reduction in myocardial contractility may be already present even in the absence of overt clinical LV systolic dysfunction. Although LVEF was not significantly associated with wave reflection, a non significant trend towards lower LVEF in higher quartiles of AIx was observed. It is possible that the absence of subjects with heart failure and impaired LVEF in our study affected the strength of the association between wave reflection parameters and LVEF by truncating the distribution of LVEF at 50% on the lower side. Consistent with this hypothesis, in a study in subjects with coronary artery disease in which the whole spectrum of LVEF was represented, LVEF was found to be correlated with aortic stiffness [46], although the strength of the correlation was fairly low (r2=−0.11). If the relationship between wave reflection parameters and systolic function were linear, as it is likely the case, the absence of subjects with low LVEF in our study might have also affected the magnitude of the association between TDI parameters and wave reflection. However, in our population TDI parameters were able to detect alterations in systolic function; therefore, the TDI technique might be a clinically useful tool to identify early reduction in LV systolic function when LVEF is still in the normal range, given its higher sensitivity to detect subclinical alterations of the LV systolic function [21].
The evaluation for a cause-effect relationship between higher wave reflection, reduction in LV systolic function, and development of heart failure was not an aim of our study. However, several reports have demonstrated that alterations in ventriculo-vascular coupling may be a cofactor in the pathogenesis of heart failure. Increased arterial wave reflection and stiffness have been described in patients with systolic heart failure [17,18,47,48], and brain natriuretic peptide, a marker of severity of heart failure with prognostic value, has been shown to be strongly associated with increased aortic stiffness in the setting of idiopathic dilated cardiomyopathy and in patients with CAD [49,50]. Alterations in arterial stiffness and wave reflection have also been documented in patients with heart failure and normal ejection fraction [10,19,46]. Previous studies suggest that one of the possible factors linking arterial stiffness to alterations in LV function is atherosclerotic vascular disease. Arterial stiffness is strongly associated to coronary artery disease [10,51]. In addition, diastolic pressure is reduced in stiffened arteries, thus affecting coronary perfusion and predisposing to ischemia. Data from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that subclinical atherosclerosis is associated with initial myocardial dysfunction, as measured by strain imaging, in subjects free of heart disease [52]. It is possible, therefore, that subclinical acute or chronic ischemia may result in a damage of the subendocardial fibers, which are the major determinant of longitudinal LV function, and that this event can be detected by sensitive techniques such as TDI and strain imaging, but not by the conventional assessment of LVEF.
The lack of correlation between LVEF and parameters of arterial wave reflection in our study is consistent with the results of other studies in subjects with normal LVEF. In patients undergoing coronary angiography but with normal LVEF, there was a correlation between aortic stiffness (measured by AIx) and mitral systolic velocity but not LVEF; the analysis, however, could not be adjusted for LV mass in that study [53]. In a study on 49 subjects without heart failure, Borlaug et al. found that peak mitral systolic velocity was not correlated with LVEF but was inversely correlated with carotid augmentation index in univariate analysis; however, after adjusting for covariates, this relation was no longer present [24].
In our study, the aortic AIx and the WEi were inversely associated with the LV systolic function measured by TDI and by strain/strain rate imaging after adjustment for covariates that affect LV systolic function. While the correlation between AIx and systolic function had been previously described, we are the first to report on the WEi as an index of vascular stiffness related to systolic function. WEi showed also stronger correlations than AIx with age, blood pressure, and LV mass, and could represent a better indicator of LV afterload. Our finding confirms data from a study by Hashimoto et al., in which LV wasted effort pressure was found to be associated with LV hypertrophy [38]. However, the association between aortic stiffness and LV mass has been questioned, especially when stiffness was measured by pressure-independent methods [54]. Despite the significant relationship found in our study in univariate analysis, the correlation between WEi and LV mass lost statistical significance after adjusting for age (data not shown), confirming data from previous studies [55].
Our study provides interesting and potentially important preventive implications. The assessment of LV systolic function by TDI and strain imaging might be useful in selecting patients with initial subclinical impairment in LV function that might benefit from targeted treatment interventions aimed at reducing aortic stiffness, with the potential of delaying the progression from subclinical to overt LV dysfunction. Several studies have demonstrated the beneficial effect of pharmacological treatment in reducing arterial stiffness [56,57]. However, the clinical impact of these interventions needs to be established in prospective studies.
Study limitations
The parameters derived from arterial waveforms analysis provide information about wave reflection, and are only indirect indices of arterial stiffness. The measurement of pulse wave velocity, an important method for assessing arterial stiffness, was not available in our study. Moreover, the TDI analysis of LV longitudinal function by strain and strain rate was performed on the ventricular septum, under the assumption that, in subjects with normal EF and without LV segmental wall motion abnormalities, the systolic function of the septal wall can reasonably represent the overall longitudinal LV function. However, in some patients a heterogeneity in LV function may exist that is not detected by traditional wall motion analysis. The cross-sectional design of our study prevented us from detecting cause-effect relationships between the studied variables, therefore our findings must be seen as hypothesis-generating. Finally, despite being representative of the community living in Northern Manhattan, the population sample of this study is an elderly cohort with overall high cardiovascular risk. Therefore, the results of the study may not extrapolate to populations with different demographics and risk factor distribution.
Acknowledgments
The authors wish to thank Janet De Rosa, MPH, for the coordination of the study activities; Rui Liu, M.D. for the performance and preliminary interpretation of the echocardiographic studies; Rafi Cabral, M.D. and Palma Gervasi-Franklin for their help in the collection and management of the data.
Sources of Funding: The study was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) R01 NS36286 (P.I.: Dr. Marco R. Di Tullio) and R37 NS29993 (P.I.’s: Drs. Ralph L. Sacco/Mitchell S. V. Elkind).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation disclaimer: Part of the work presented in this article has been presented in abstract form at the American College of Cardiology 2010 Annual Scientific Sessions.
Conflict of interest: None.
References
- 1.McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 2.Asmar R, Rudnichi A, Blacher J, London GM, Safar ME. Pulse pressure and aortic pulse wave are markers of cardiovascular risk in hypertensive populations. Am J Hypertens. 2001;14:91–97. doi: 10.1016/s0895-7061(00)01232-2. [DOI] [PubMed] [Google Scholar]
- 3.Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107:2089–2095. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y. Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol. 1993;21:1497–1506. doi: 10.1016/0735-1097(93)90330-4. [DOI] [PubMed] [Google Scholar]
- 5.Bader H. Importance of the gerontology of elastic arteries in the development of essential hypertension. Clin Physiol Biochem. 1983;1:36–56. [PubMed] [Google Scholar]
- 6.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 7.O'Rourke MF. Arterial hemodynamics in hypertension. Circ Res. 1970;27 Suppl [PubMed] [Google Scholar]
- 8.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 9.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 10.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Lamm G, et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 12.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 14.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 16.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 17.Curtis SL, Zambanini A, Mayet J, McG Thom SA, Foale R, Parker KH, et al. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H557–H562. doi: 10.1152/ajpheart.01095.2006. [DOI] [PubMed] [Google Scholar]
- 18.Patrianakos AP, Parthenakis FI, Karakitsos D, Nyktari E, Vardas PE. Proximal aortic stiffness is related to left ventricular function and exercise capacity in patients with dilated cardiomyopathy. Eur J Echocardiogr. 2009;10:425–432. doi: 10.1093/ejechocard/jen304. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 20.Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. doi: 10.1016/j.cardfail.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Nikitin NP, Witte KK, Clark AL, Cleland JG. Color tissue Doppler-derived long-axis left ventricular function in heart failure with preserved global systolic function. Am J Cardiol. 2002;90:1174–1177. doi: 10.1016/s0002-9149(02)02794-7. [DOI] [PubMed] [Google Scholar]
- 22.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with "isolated" diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 23.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–125. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Sharman JE, Davies JE, Jenkins C, Marwick TH. Augmentation index, left ventricular contractility, and wave reflection. Hypertension. 2009;54:1099–1105. doi: 10.1161/HYPERTENSIONAHA.109.133066. [DOI] [PubMed] [Google Scholar]
- 26.Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929–935. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 29.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 30.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 31.Voigt JU, Arnold MF, Karlsson M, Hubbert L, Kukulski T, Hatle L, et al. Assessment of regional longitudinal myocardial strain rate derived from doppler myocardial imaging indexes in normal and infarcted myocardium. J Am Soc Echocardiogr. 2000;13:588–598. doi: 10.1067/mje.2000.105631. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, et al. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002;105:99–105. doi: 10.1161/hc0102.101396. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri V, Russo C, Palmieri EA, Pezzullo S, Celentano A. Changes in components of left ventricular mechanics under selective beta-1 blockade: insight from traditional and new technologies in echocardiography. Eur J Echocardiogr. 2009;10:745–752. doi: 10.1093/ejechocard/jep055. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 35.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 36.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 37.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto J, Nichols WW, O'Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens. 2008;21:329–333. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 39.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 40.O'Rourke M. Arterial stiffening and vascular/ventricular interaction. J Hum Hypertens. 1994;8 Suppl 1:S9–S15. S9-15. [PubMed] [Google Scholar]
- 41.Yamada H, Oki T, Tabata T, Iuchi A, Ito S. Assessment of left ventricular systolic wall motion velocity with pulsed tissue Doppler imaging: comparison with peak dP/dt of the left ventricular pressure curve. J Am Soc Echocardiogr. 1998;11:442–449. doi: 10.1016/s0894-7317(98)70024-0. [DOI] [PubMed] [Google Scholar]
- 42.Palmieri V, Russo C, Arezzi E, Pezzullo S, Sabatella M, Minichiello S, et al. Relations of longitudinal left ventricular systolic function to left ventricular mass, load, and Doppler stroke volume. Eur J Echocardiogr. 2006;7:348–355. doi: 10.1016/j.euje.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104:128–130. doi: 10.1161/01.cir.104.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derumeaux G, Ovize M, Loufoua J, ndre-Fouet X, Minaire Y, Cribier A, et al. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998;97:1970–1977. doi: 10.1161/01.cir.97.19.1970. [DOI] [PubMed] [Google Scholar]
- 45.Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, et al. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283:H792–H799. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 46.Sakuragi S, Iwasaki J, Tokunaga N, Hiramatsu S, Ohe T. Aortic stiffness is an independent predictor of left ventricular function in patients with coronary heart disease. Cardiology. 2005;103:107–112. doi: 10.1159/000082472. [DOI] [PubMed] [Google Scholar]
- 47.Lage SG, Kopel L, Monachini MC, Medeiros CJ, Pileggi F, Polak JF, et al. Carotid arterial compliance in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;74:691–695. doi: 10.1016/0002-9149(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 48.Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, et al. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–1225. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 49.Patrianakos AP, Parthenakis FI, Nyktari E, Malliaraki N, Karakitsos DN, Vardas PE. Central aortic stiffness in patients with nonischemic dilated cardiomyopathy: relationship with neurohumoral activation. J Card Fail. 2009;15:665–672. doi: 10.1016/j.cardfail.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Sakuragi S, Okawa K, Iwasaki J, Tokunaga N, Kakishita M, Ohe T. Aortic stiffness is an independent determinant of B-type natriuretic peptide in patients with coronary artery disease. Cardiology. 2007;107:140–146. doi: 10.1159/000094720. [DOI] [PubMed] [Google Scholar]
- 51.Gatzka CD, Cameron JD, Kingwell BA, Dart AM. Relation between coronary artery disease, aortic stiffness, and left ventricular structure in a population sample. Hypertension. 1998;32:575–578. doi: 10.1161/01.hyp.32.3.575. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 53.Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 54.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 55.Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. doi: 10.1097/00004872-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 56.Soma J, Angelsen BA, Techn D, Aakhus S, Skjaerpe T. Sublingual nitroglycerin delays arterial wave reflections despite increased aortic "stiffness" in patients with hypertension: a Doppler echocardiography study. J Am Soc Echocardiogr. 2000;13:1100–1108. doi: 10.1067/mje.2000.109686. [DOI] [PubMed] [Google Scholar]
- 57.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension. 2001;37:1429–1433. doi: 10.1161/01.hyp.37.6.1429. [DOI] [PubMed] [Google Scholar]