Abstract
There have been several attempts to use gene microarrays from peripheral blood mononuclear cells to identify new biological pathways or targets for therapy in Posttraumatic Stress Disorder (PTSD). The few studies conducted to date have yielded an unclear pattern of findings, perhaps reflecting the use of heterogeneous samples of circulating immune cells for analysis. We used gene microarrays on a homogeneous sample of circulating monocytes to test the hypothesis that chronic PTSD would be associated with elevated inflammatory activity and to identify new pathways dysregulated in the disorder. Forty-nine men (24 PTSD+ and 25 age-matched trauma-exposed PTSD− controls) and 18 women (10 PTSD+ and 8 age-matched PTSD− controls) were recruited. Gene expression microarray analysis was performed on CD14+ monocytes, immune cells that initiate and respond to inflammatory signaling. Male subjects with PTSD had an overall pattern of under-expression of genes on monocytes (47 under-expressed versus 4 over-expressed genes). A rigorous correction for multiple comparisons and verification with q-PCR showed that of only 3 genes that were differentially expressed, all were under-expressed. There was no transcriptional evidence of chronic inflammation in male PTSD+ subjects. In contrast, preliminary data from our pilot female PTSD+ subjects showed a relatively balanced pattern of increased and decreased expression of genes and an increase in activity of pathways related to immune activation. The results indicate differential patterns of monocyte gene expression in PTSD, and the preliminary data from our female pilot subjects are suggestive of gender dimorphism in biologic pathways activated in PTSD. Changes in immune cell gene expression may contribute to medical morbidity in PTSD.
Keywords: Posttraumatic Stress Disorder, cDNA microarray, cytokines, monocyte
Introduction
Posttraumatic Stress Disorder (PTSD) is highly prevalent in both military and civilian populations, is chronic and often accompanied by substance abuse, depression, difficulties with interpersonal relationships, poorer occupational functioning and increased physical health problems (Kulka et al., 1990). A number of studies have demonstrated that PTSD is associated with alterations in immune function (Hoge et al., 2009). Specifically, PTSD is associated with enhanced cellular immune responses (Altemus et al., 2003; Laudenslager et al., 1998; Watson et al., 1993), activated T lymphocytes (Wilson et al., 1999) and impaired humoral immunity, as indexed by higher antibody responses to cytomegalovirus levels (Uddin et al., 2010). PTSD has also been shown to be associated with increased levels of some inflammatory cytokines (Maes et al., 1999a; Spivak et al., 1997; Sutherland et al., 2003). Finally, PTSD is associated with increased levels of C-reactive protein (Miller et al., 2001), an index of systemic inflammatory activity, though this has not been found in all studies (Sondergaard et al., 2004). These findings of altered immune functioning in PTSD have important implications for understanding the high rates of cardiovascular (Cohen et al., 2009; Kubzansky et al., 2007), musculoskeletal, and neurocognitive disorders associated with PTSD (Reviewed in (Schnurr et al., 2000)). Chronic inflammation is well known to be associated with a broad spectrum of neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and possibly neuropsychiatric disorders.
Gene expression microarray analysis has facilitated the discovery of genes/pathways/proteins associated with biological and pathological processes. Only in the past few years has this powerful tool been used to elucidate mechanisms of action for psychiatric disorders. Microarray studies in peripheral immune cells in PTSD have the potential to test hypotheses about immune function as well as to explore biological pathways that are expressed in both the brain and periphery (e.g. glucocorticoid signaling). For example, recent investigations using microarrays and peripheral blood mononuclear cells (PBMC) or lymphocytes have shown that major psychiatric disorders including depression, anxiety, schizophrenia and Alzheimer’s disease as well as chronic stress perturb multiple biological pathways measurable in peripheral immune cells (Irwin, 1999; Maes et al., 1998; Maes et al., 1999b; Ohmori et al., 2005; Schwarz et al., 2001a; Schwarz et al., 2001b).
The first study of gene expression in peripheral blood mononuclear cells (PBMCs) in PTSD came from a study conducted in Israel (Segman et al., 2005). This study examined gene expression patterns from 24 trauma victims, men and women, who were treated in the hospital emergency department in Jerusalem within hours of a traumatic event and 4 months later. Only 13 met the clinical criteria for PTSD; however, the signature profile did correlate with the severity of the PTSD symptom clusters and several differentially expressed genes were described as having a role in stress. A second study recently published by Yehuda and colleagues (Yehuda et al., 2009) examined gene expression from whole blood in 35 subjects exposed to the World Trade Center attack. The 15 subjects with PTSD showed significant differences from the controls in a number of genes related to immune function, HPA activity, and signal transduction. In particular, this study found decreased expression of the glucocorticoid receptor chaperone protein gene, FKBP5, which extended a previous study showing that specific polymorphisms of the FKBP5 gene interact with severity of child abuse to predict risk for adult PTSD (Binder, 2008). It is very likely that differences in findings from different studies may in part be related to the heterogeneity of different cell types used for microarrays. One important study suggests that not only age and sex can cause variation in microarray data but also cell composition (Whitney et al., 2003). For example, lymphocytes are a diverse group of cells in the periphery that produce a number of hormones such as ACTH, endorphins, insulin-like growth factor and somatostatin as well as cytokines. In a study of acute stress using lymphocytes in a microarray panel in 10 graduate students before and after an entrance examination, 70 genes were significantly responsive, in spite of individually different baseline activity (Rokutan et al., 2005).
We examined gene expression profiles in monocytes in healthy men and women with and without chronic PTSD. Monocytes are peripheral immune cells that can respond to foreign antigens as well as modulate the immune system by producing inflammatory cytokines that can additionally induce sickness behavior (Dantzer et al., 2008; Middle et al., 2000; Watkins and Maier, 2005). We chose to use CD14+ monocytes for gene expression profiling in PTSD for several reasons. The primary reason is that we wished to limit sources of heterogeneity by restricting our analyses to a homogeneous cell type. Secondly, CD14+ monocytes are relatively less differentiated than most immune cells and have a high degree of plasticity (Kuwana et al., 2003). Furthermore, monocytes (Gidron et al., 2003; Maes et al., 1999b) have demonstrated reactivity, in vivo, to psychological challenges. Our overall hypothesis was that subjects with chronic PTSD will show evidence of chronic inflammation. Given that gender is a well-established risk factor for autoimmune disorders (Fairweather et al., 2008) and that women may have greater inflammatory responses to endotoxin and psychological stress compared with men (Rohleder et al., 2001; van Eijk et al., 2007), we examined a pilot sample of healthy women with and without PTSD to examine gender differences in immune activation. Our second hypothesis was that gene expression for FKBP5 would be decreased in PTSD subjects similar to previously published results (Binder, 2008; Yehuda et al., 2009). Finally, given the lack of an established biological marker for PTSD, we tested the hypothesis that PTSD would be associated with an altered gene expression profile that would be expressed and detectable in peripheral monocytes.
Methods
Subjects
cDNA microarray gene expressions were analyzed in monocytes collected from a sample of 49 men (PTSD+ N= 24, PTSD− Control N= 25) and 18 women (PTSD+ N= 10, PTSD− Control N= 8). Medically healthy male and female subjects were recruited from the community by internet and newspaper advertisements and from the San Francisco Veterans Affairs Medical Center (SFVAMC) PTSD Outpatient Program by fliers and brochures. As such, the study subjects should be considered a convenience sample. All subjects were medication free (apart from three female participants who were taking the oral contraceptive pill), medically healthy, with no current infection, negative serology for HIV and hepatitis, no elevation in body temperature or white blood cell count, no current substance or alcohol use disorders, and no exposure to a traumatic event in the past 3 months. Baseline laboratory tests included a serum chemistry panel, liver function tests, thyroid functions, complete blood count and differential serology for Hepatitis B & C, urine toxicology screen, and urine pregnancy test (if appropriate). Self-report questionnaires were used to determine demographics, lifetime exposure to traumatic stressors, PTSD symptoms, general psychiatric symptoms, and drug and alcohol use. A structured clinical interview was used to assess lifetime and current anxiety and mood disorders, an in-depth assessment of PTSD and history of stressful life events. The interview assessments included the Structured Clinical Interview for DSM-IV, Patient edition (SCID-P) (First et al., 1996) to assess mood disorders, substance use disorders, and anxiety disorders, as well as the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995). All subjects were given details of the study and asked to sign a written informed consent form if they wished to participate. The study protocol and consent form were approved by the Committee on Human Research at the University of California, San Francisco (UCSF).
Monocyte gene expression analysis
To identify genes differentially expressed in PTSD subjects, we performed genomewide expression analysis of CD14+ monocytes using CodeLink Human Whole Genome BioArrays and methods described in detail in our previous study (Pulliam et al., 2004). To summarize, the BioArrays have a total of 54,841 probes of which 83% of the represented genes are clearly annotated (based on unique UniGene IDs), 39% well annotated (categorized to a gene ontology) and 53% have been mapped to specific chromosome locations. For monocyte gene expression analysis, sixty milliliters of blood were drawn from each subject into CPT tubes (Becton Dickinson) and immediately centrifuged at 4 C to enrich for peripheral blood mononuclear cells (PBMC). CD14+ monocytes were subsequently isolated within 20 minutes from the PBMC fraction by positive separation using MACS CD14 Microbeads (Miltenyi Biotech, Auburn, CA). Total RNA was isolated from CD14+ monocytes using Qiagen RNeasy Micro Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol and stored at −70C. All samples, control and PTSD, were handled identically and the technicians were blind to study group. The purity and concentration of the RNA were determined with spectrophotometry. Bacteria mRNAs were spiked in at different concentrations ranging from 1:1,000 mass ratio to 1:1,024,000 mass ratio as internal controls. The integrity of the RNA was determined with an RNA picochip on an Agilent Bioanalyzer 2100 (Agilent Technologies, Inc, Palo Alto, CA). Gene expression was evaluated according to the manufacturer’s instructions. One half microgram of total RNA isolated from CD14+ monocytes was amplified and labeled with biotin-11-UTP (Perkin Elmer, Boston, MA) using the CodeLink iExpress iAmplify Kit (AMI). Ten micrograms of fragmented cRNA were hybridized to microarrays using the procedures suggested by the manufacturer. The arrays were scanned on an Axon GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA). Image analysis and data extraction were performed by CodeLink Expression Software Kit v4.1 (AMI).
Validation of microarray gene expression
In significant differentially expressed targets, validation was performed using Applied Biosystem (ABI, Foster City, CA) qPCR protocols. cDNAs were generated by reverse transcription (Superscript III; Invitrogen, Carlsbad, CA). Gene-specific primers were identified using the ABI Primer Express (v2.0) software, commercially synthesized and used for RT-PCR to amplify the target sequence with the SYBR Green PCR kit (ABI). Fluorescent product was quantified and analyzed on the ABI Prism 7900HT Sequence Detection System with ABI Prism SDS software (v2.1). Relative expression of target RNA was calculated in relation to GAPDH beta-actin and 18S rRNA (Pulliam et al., 2004).
Statistical Analysis
We used the GeneSpring GX 7.3 software package (Agilent, Santa Clara, CA) together with the Bioconductor (Gentleman et al., 2004) suite of R packages (Team, 2009) as main tools for the microarray data analysis. Following data import, additional quality checks were performed. Subsequent pre-processing steps included application of background correction, standardization, normalization and summarization of the data. A laboratory adapted analytic procedure was used to adjust for multiple testing (Pulliam et al., 2004). The raw data were normalized using loess normalization in R/BioConductor. In total, 53,423 gene-mapping probes were evaluated. After normalization, the intensities of known defect spots were assigned 0.001. The full set of probes was then filtered to exclude non-responding probes defined as normalized gene expression intensities below 200 in 75% of the PTSD and Control samples. Following this screening procedure, the males had 19,046 probes and the females had 17,455 probes left. We applied a second filter to exclude probes below 1.5 fold change between PTSD and controls. A comparison between PTSD and control was made for each probe for male or female groups respectively. At 1.5 fold change level, the males had 200 probes and the females had 528 which passed the two filters. The differences were then calculated using Student’s t-test with the Benjamini and Hochberg multiple comparison correction (Benjamini and Hochberg, 1995). The p-values of each probe were rank ordered from smallest to the largest. The largest p-value (least significant) remained uncorrected. The next largest p-value was multiplied by the total number of probes (n) in the list divided by its rank (n-1). Thus, the corrected p-value = p-value multiplied by (n/n-1). The third largest p-value was corrected with the same procedure: corrected p-value = p-value multiplied by (n/n-2). The correction becomes more stringent as the p-value decreases. Genes with fold change greater than 1.5 either up or down regulated and adjusted (as above) Student’s t-test p < 0.05 were considered differentially expressed genes. The differentially expressed genes were further analyzed to identify gene ontology groups using the GeneSpring GX software package to look for possible functional or structurally related genes. Then, gene network analyses were conducted using VisAnt (Hu et al., 2009). Because the male and female data were analyzed separately, the multiple comparison correction was not applied between groups.
Results
Male Sample
Demographic and clinical details are presented in Table 1. Male PTSD+ participants were not significantly different from male PTSD− controls in age, marital status, or trauma exposure. Approximate duration of time since exposure to the traumatic event ranged from 6 months to 28 years. However, male PTSD+ participants had significantly less education and were significantly more likely to have current MDD or a lifetime history of MDD. No participants fulfilled criteria for alcohol abuse or dependence or substance abuse or dependence at the time of participation and there were no group differences in past alcohol or substance use disorders.
TABLE 1.
Demographic characteristics of men and women with and without PTSD
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| PTSD+ (N = 24) |
PTSD− (N = 25) |
PTSD+ (N = 10) |
PTSD− (N = 8) |
|||
| N (%) | N (%) | p | N (%) | N (%) | p | |
| Age M (SD) | 30 (6) | 30 (6) | .85 | 30 (7) | 27 (6) | .33 |
| Education M (SD) | 14 (2) | 15 (2) | .01* | 15 (2) | 16 (1) | .34 |
| Marital Status | ||||||
| Single | 12 (50) | 14 (56) | 7 (70) | 6 (75) | ||
| Married | 6 (25) | 8 (32) | 2 (20) | 2 (25) | ||
| Divorced | 6 (25) | 3 (12) | .32 | 1 (10) | 0 | .57 |
| Ethnicity | ||||||
| African American | 3 (12) | 2 (8) | 2 (20) | 0 | ||
| Asian | 1 (4) | 3 (12) | 2 (20) | 2 (25) | ||
| Caucasian | 12 (50) | 14 (56) | 6 (60) | 6 (75) | ||
| Hispanic | 5 (20) | 3 (12) | 0 | 0 | ||
| Pacific Islander | 1 (4) | 1 (4) | 0 | 0 | ||
| Middle Eastern | 1 (4) | 1 (4) | 0 | 0 | ||
| Mixed Race | 1 (4) | 1 (4) | .68 | 0 | 0 | .67 |
| MDD | 0 | |||||
| Current | 6 (25) | 0 | .008* | 3 (30) | 0 | .10 |
| Past | 20 (83) | 0 | .000* | 10 (100) | .000* | |
| Past Alcohol Use | 0 | |||||
| Abuse | 3 (12) | 5 (20) | 2 (20) | 0 0 | ||
| Dependence | 4 (17) | 2 (8) | 6 (60) | |||
| Abuse + Dependence | 4 (17) | 0 | .16 | 0 | .001* | |
| Past Substance Use | 0 | |||||
| Abuse | 2 (8) | 1 (4) | 4 (40) | 0 | ||
| Dependence | 2 (8) | 1 (4) | .37 | 2 (20) | .01* | |
| Trauma Exposure | 0 | |||||
| Combat | 16 (67) | 12 (48) | 0 | 0 | ||
| Physical abuse/assault | 6 (25) | 5 (20) | 6 (60) | 0 | ||
| Sexual abuse/assault | 1 (4) | 0 | 4 (40) | 1 (12) | ||
| Accident | 0 | 2 (8) | 0 | 1 (12) | ||
| Robbery | 1 (4) | 2 (8) | 0 | 0 | ||
| Other | 0 | 4 (16) | .25 | 0 | 0 | .04* |
| Current CAPS M (SD) | 57 (15) | 4 (6) | 64 (18) | 2 (3) | ||
| Intrusion | 15 (5) | 1 (3) | 16 (6) | 0 | ||
| Avoidance | 19 (7) | 1 (2) | 26 (11) | 0 | ||
| Hyperarousal | 22 (7) | 2 (3) | .000* | 21 (4) | 2 (3) | .001* |
| Lifetime CAPS M (SD) | n/a | 8 (10) | n/a | n/a | 13 (18) | n/a |
| Intrusion | 4 (6) | 9 (13) | ||||
| Avoidance | 1 (2) | 0 | ||||
| Hyperarousal | 3 (1) | 4 (4) | ||||
Numbers refer to N (%) unless otherwise specified, n/a indicates not applicable
denotes statistical significance at p < .05. P values are based on Student’s t-tests for continuous data and on Mann Whitney U tests for categorical data. The category “married” includes those who are living with a domestic partner (n = 3 females and n = 5 males). The mean current and lifetime CAPS scores for females are based on only two participants who had experienced a traumatic event.
The microarray results in the male sample showed that four genes were increased ≥1.5 fold and 47 genes were decreased ≥1.5 fold (p <0.05) suggesting an overall pattern of suppressed gene expression on monocytes in subjects with PTSD versus controls. Functional annotation analyses showed that male PTSD subjects showed decreased activation in several classes within the gene ontology including immune response, cytokine-cytokine interactions, and chemotaxis (Table 2). Overall there was no evidence for a pro-inflammatory profile in male PTSD subjects. After correction for multiple comparisons, 5 genes were identified and after validation with q-PCR, 3 genes were differentially expressed in male PTSD versus controls (Table 3). In each of the three genes, the PTSD group showed reduced expression. The first was serum deprivation response protein (SDPR) which is a calcium-independent phospholipid binding protein. The second was platelet factor 4 (PF4). The third was a histone protein (HIST1H2AC).
TABLE 2.
Functional annotation analysis for male PTSD+ versus control participants
| Functional Term | q-value | GenBank | Fold | Symbol |
|---|---|---|---|---|
| Acetylation (posttranslationally ) | 0.001 | |||
| AW237066 | −1.7 | HIST1H2BJ | ||
| AA036997 | −2.1 | HIST1H2AC | ||
| NM_001828 | −3.6 | CLC | ||
| NM_033082 | −2.6 | CIP29 | ||
| NM_003509 | −1.5 | HIST1H2AI | ||
| NM_004657 | −2.8 | SDPR | ||
| NM_020992 | −1.7 | PDLIM1 | ||
| Chemotaxis | 0.009 | |||
| NM_002619 | −4.1 | PF4 | ||
| NM_002704 | −3.2 | PPBP | ||
| NM_002985 | −2.0 | CCL5 | ||
| Cell activation | 0.010 | |||
| NM_003037 | −2.9 | SLAMF1 | ||
| NM_002619 | −4.1 | PF4 | ||
| NM_007360 | −3.8 | KLRK1 | ||
| CD244080 | −1.6 | SWAP70 | ||
| Chromatin assembly | 0.016 | |||
| AW237066 | −1.7 | HIST1H2BJ | ||
| AA036997 | −2.1 | HIST1H2AC | ||
| NM_003509 | −1.5 | HIST1H2AI | ||
| Cytokine-cytokine receptor interaction | 0.017 | |||
| NM_002619 | −4.1 | PF4 | ||
| NM_002704 | −3.2 | PPBP | ||
| NM_002985 | −2.0 | CCL5 | ||
| NM_014452 | −1.6 | TNFRSF21 | ||
| NM_002183 | −1.9 | IL3RA | ||
| Behavior | 0.023 | |||
| NM_002619 | −4.1 | PF4 | ||
| NM_002704 | −3.2 | PPBP | ||
| NM_002985 | −2.0 | CCL5 | ||
| NM_000954 | −2.5 | PTGDS | ||
| Immune response | 0.029 | |||
| NM_002619 | −4.1 | PF4 | ||
| NM_002704 | −3.2 | PPBP | ||
| NM_007360 | −3.8 | KLRK1 | ||
| NM_002985 | −2.0 | CCL5 | ||
| AF533922 | −4.0 | HLA-DQA1 | ||
| CD244080 | −1.6 | SWAP70 | ||
| Protein-DNA complex assembly | 0.034 | |||
| AW237066 | −1.7 | HIST1H2BJ | ||
| AA036997 | −2.1 | HIST1H2AC | ||
| NM_003509 | −1.5 | HIST1H2AI | ||
| Lymphocyte activation | 0.047 | |||
| NM_003037 | −2.9 | SLAMF1 | ||
| NM_007360 | −3.8 | KLRK1 | ||
| CD244080 | −1.6 | SWAP70 | ||
| Cell death | 0.070 | |||
| NM_014288 | −1.6 | ITGB3BP | ||
| NM_007360 | −3.8 | KLRK1 | ||
| AF010238 | 1.6 | VHL | ||
| NM_014452 | −1.6 | TNFRSF21 | ||
| NM_012329 | −1.7 | MMD | ||
| Cell adhesion molecules (CAMs) | 0.093 | |||
| AF533922 | −4.0 | HLA-DQA1 | ||
| NM_032801 | −1.8 | JAM3 | ||
| NM_138961 | −1.9 | ESAM |
Genes shown are significantly expressed for PTSD versus control with Student’s t-test p<0.05. The q-value is the probability that a random subset of genes drawn from the total set of genes will have one or more genes containing the functional term. For each functional term, it reflects the enrichment in frequency of that term in the input gene list relative to the entire genes of the term.
TABLE 3.
Differentially expressed genes from microarray with qPCR validation for male PTSD+ versus control participants
| Microarray |
QPCR |
||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Genbank | Control M (SE) |
PTSD M (SE) |
Fold (p) |
Control M (SE) |
PTSD M (SE) |
Fold (p) |
| PF4 | NM_002619 | 586.60 (35.1) |
161.90 (9.5) |
−3.62 (0.03) |
0.36 (0.03) |
0.061 (0.003) |
−5.88 (0.03) |
|
| |||||||
| SDPR | NM_004657 | 607.50 (44.6) |
114.90 (4.4) |
−5.29 (0.04) |
0.018 (0.001) |
0.0037 (<0.001) |
−4.98 (<0.05) |
|
| |||||||
| HIST1H2AC | AA036997 | 1548.30 (46.7) |
650.20 (17.4) |
−2.38 (<0.001) |
0.10 (0.004) |
0.05 (0.001) |
−2.02 (0.01) |
Notes. All genes shown passed multiple comparison correction for microarray data.
Denotes Student’s t-test p < 0.05.
Female Pilot Sample
Female PTSD+ participants were not significantly different from female PTSD− controls in age, years of education, marital status or current presence of MDD. However, PTSD+ participants were significantly more likely to have a lifetime history of MDD. Further, only two of the eight female control participants had experienced a traumatic event fulfilling Criterion A for PTSD, leading to a significant group difference in trauma exposure. Approximate duration of time since exposure to the traumatic event ranged from 6 months to 26 years. PTSD+ females were also significantly more likely to have past alcohol abuse or past alcohol dependence.
Female PTSD+ subjects showed 11 genes increased ≥1.5 fold and 13 genes decreased ≥1.5 fold (Adjusted p <0.05) suggesting a gender-specific differential pattern of monocyte gene expression in PTSD versus controls. The female sample demonstrated a relative balance of increased versus decreased gene expression. Of note, functional annotation analysis in the female PTSD subjects relative to controls showed a pattern of expression of several gene ontologies suggestive of increased inflammatory response including regulation of phagocytosis, and leukocyte activation (Table 4). For example, there was increased expression of orosomucoid 1 (ORM1), transforming growth factor beta 1 (TGFB1), interleukin 2 receptor, gamma (IL2RG), and azurocidin 1 (ASU1).
TABLE 4.
Functional annotation analysis for female PTSD+ versus control participants
| Functional Term | q-value | Genbank | Fold | Symbol |
|---|---|---|---|---|
| Immune system process | 0.0054 | |||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_000206 | 1.53 | IL2RG | ||
| NM_001700 | 1.50 | AZU1 | ||
| NM_006435 | −1.51 | IFITM2 | ||
| NM_000057 | −1.63 | BLM | ||
| NM_006332 | −1.77 | IFI30 | ||
| Regulation of phagocytosis | 0.0104 | |||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_001700 | 1.50 | AZU1 | ||
| Leukocyte activation | 0.0197 | |||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_001700 | 1.50 | AZU1 | ||
| NM_000057 | −1.63 | BLM | ||
| Inflammatory response | 0.0383 | |||
| NM_000607 | 2.72 | ORM1 | ||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_001700 | 1.50 | AZU1 | ||
| Proteolysis | 0.0484 | |||
| NM_004851 | 2.22 | NAPSA | ||
| NM_014224 | 2.20 | PGA5 | ||
| NM_001700 | 1.50 | AZU1 | ||
| NM_019029 | −1.55 | CPVL | ||
| Immune response | 0.0701 | |||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_000206 | 1.53 | IL2RG | ||
| NM_006435 | −1.51 | IFITM2 | ||
| NM_006332 | −1.77 | IFI30 | ||
| Response to stimulus | 0.0809 | |||
| NM_000607 | 2.72 | ORM1 | ||
| NM_000660 | 1.54 | TGFB1 | ||
| NM_000206 | 1.53 | IL2RG | ||
| NM_001700 | 1.50 | AZU1 | ||
| NM_006435 | −1.51 | IFITM2 | ||
| NM_000057 | −1.63 | BLM | ||
| NM_006332 | −1.77 | IFI30 | ||
| Response to stress | 0.0980 | |||
| M_000607 | 2.72 | ORM1 | ||
| NM_000660 | 1.54 | TGFB1 | ||
| M_001700 | 1.50 | AZU1 | ||
| M_000057 | −1.63 | BLM |
Genes shown are significantly expressed for PTSD versus control with Student’s t-test p<0.05. The q-value is the probability that a random subset of genes drawn from the total set of genes will have one or more genes containing the functional term. For each functional term, it reflects the enrichment in frequency of that term in the input gene list relative to the entire genes of the term.
We did not find any differences in expression of FKBP5 in either of our male or female PTSD subjects, and there were no genes commonly over- or under-expressed in the male and female PTSD+ samples (Figure 1).
Figure 1. Differentially expressed monocyte genes in males and females with PTSD.
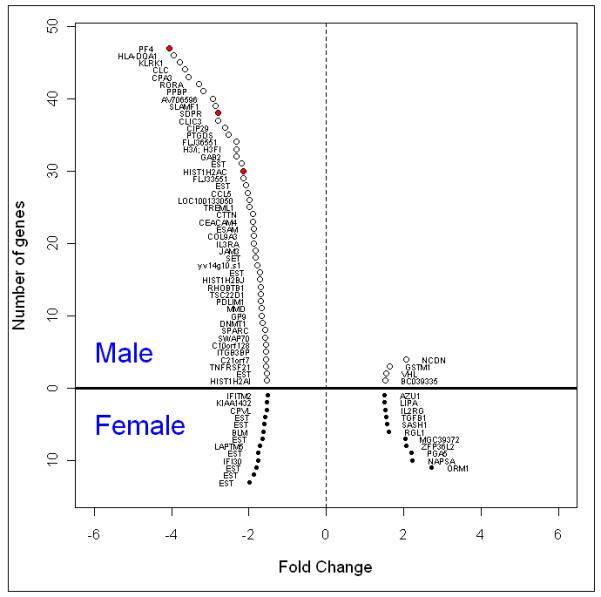
Hollow circles denote male and solid circles denote female. Red dots show the differentially expressed genes that passed multiple comparison correction and remained significant for qPCR (Table 3). EST = expressed sequence tag; probes without an official gene name
Discussion
The primary finding of the present research was that monocytes in healthy male PTSD subjects showed a predominant pattern of decreased gene expression. These results are consistent with the finding of diminished gene expression of transcription factors reported by Segman et al (Segman et al., 2005). Notable categories of decreased expression pertain to cytokine/chemokine signaling, platelet function, and histone activity. Thus, there was no evidence for chronic inflammation in our male PTSD subjects. In contrast, preliminary data from our pilot sample of women subjects with PTSD showed a relatively balanced pattern of increased and decreased expression of genes with ontology suggestive of increased activity of pathways related to immune activation. We did not replicate the published finding of decreased expression of FKBP5 in either our male or female samples. Overall, our results indicate altered monocyte gene expression profiles in males and females with PTSD, and are suggestive of gender dimorphism in biologic pathways activated in PTSD, particularly in the domain of immune function.
Diminished gene activation in immune cells in PTSD has been reported in both published studies (Segman et al., 2005; Yehuda et al., 2009) and a third unpublished study (Kerry Ressler, personal communication). In the Segman el al study (Segman et al., 2005), PTSD subjects at 4 months post trauma showed a similar pattern of diminished transcription in PBMCs relative to controls. In our secondary analysis of the publically available data from this study, we found that with a cut-off of 50% difference in expression levels and using p values less than 0.05 (without corrections for multiple testing), 386 transcripts were downregulated and only 56 were upregulated. Using Ingenuity Pathways Analysis software suite (Ingenuity Systems) to annotate gene function in this data set, we found 28 known transcription regulators were under-expressed versus 2 that were over-expressed. Yehuda and colleagues found that PTSD subjects differed from controls in 17 transcription factors, 15 of which were under-expressed (Yehuda et al., 2009) in whole blood. In addition, Miller et al found in a sample of chronically stressed caregivers, that monocyte genes that were differentially expressed by at least 50%, were predominantly in the direction of under-expression (79% vs. 21 %) (Miller et al., 2008). Recently, Segman and colleagues found in a study using microarrays of PBMCs sampled from postpartum women, that women with post partum depression had marked diminished gene expression in a broad range of markers (Segman et al., 2010). It is possible that PTSD, chronic stress, and postpartum depression each have broad general effects on overall gene transcription in circulating immune cells, which by function are supposed to exhibit a high degree of plasticity. It suggests that these disorders may involve adaptations that reduce cellular reactivity to homeostatic challenges, and if proven, may account for the high rates of comorbid medical illness associated with these conditions (Cohen et al., 2007; Evans et al., 2005; Schnurr et al., 2000).
Our results in the male PTSD subjects did not show a pro-inflammatory pattern as was previously reported by Miller and colleagues in chronically stressed caregivers (Miller et al., 2008). This difference may be related to two issues. The first is that biological aspects of chronic PTSD maybe fundamentally different from those associated with chronic stress exposure as has been emphasized by several investigators (e.g. (Yehuda and McFarlane, 1995)). Second, the sample of chronic caregivers in the Miller study was predominantly women. Our results suggest that gender maybe a crucial factor for examining the role of inflammation in the disease burden associated with PTSD.
In contrast with findings in our male sample with PTSD, we found that our pilot sample of women with PTSD had gene expression patterns indicative of greater immune activation compared with women without PTSD. Previous research has shown differences in stress-related inflammatory responses between males and females, but the pattern of findings has been mixed and apparently related to the menstrual phase and menopausal status of the female participants (Prather et al., 2009; Rohleder et al., 2001). Sex hormones may mediate these effects because immune cells bear receptors for the hormones estrogen and progesterone, and levels of these hormones have been found to modulate inflammatory activity and immune cell gene expression (Burger and Dayer, 2002; Kramer and Wray, 2002). Female gender is a well-established risk factor for both PTSD (Kessler et al., 1995) and autoimmune disorders (Fairweather et al., 2008). Further research, dedicated to understanding the transcriptional control pathways mediating observed patterns of gene expression in women and men with and without PTSD, will be necessary to begin examining potential mechanisms of the sex differences in gene expression observed in the present study.
This study has a number of limitations including a limited sample size, cross-sectional design, and genetically heterogeneous population. Further, our small female PTSD− control sample included a mix of trauma exposed and trauma unexposed women meaning that our findings of menstrual phase gender differences must be taken with caution until replicated. Further, we did not control for in our female sample. Another limitation relates to the presence of comorbid major depressive disorder (MDD) in approximately a quarter of our male and female subjects. PTSD and MDD have common overlapping criterion symptoms, and hence are by definition are intricately intertwined. Covarying for the presence of MDD is not a satisfactory statistical approach to this problem because presence of MDD increases with PTSD symptom severity. PTSD symptoms residualized for presence of MDD represents variance attributable to milder severity of PTSD. In the absence of a robust biomarker which distinguishes PTSD from MDD, comborbid MDD remains a limitation of this study. Significant strengths of this study include examination of subjects on the day of blood draw, rigorous screening for chronic illness, leukocytosis screening and the selection of subjects who were afebrile and medication free. Moreover, our selection of CD14+ monocytes for analysis as a homogenous population of immune cells limits sources of variability not related to group assignment and thereby extends previous research that has focused on heterogeneous populations of cells. It is possible that biological pathways relevant to PTSD that are co-expressed in the brain and circulating immune cells may be discernible only in specific cell types. In order to discern these cell-specific patterns, it is necessary to analyze homogenous cell populations as we have done.
Differential gene expression in male subjects with PTSD was disproportionately in the direction of under-expression of genes across functional classes (within the Gene Ontology). There was no evidence for chronic inflammation in male PTSD subjects. In contrast, preliminary data from our pilot sample of female subjects with PTSD showed a different profile of gene expression, particularly in pathways related to immune activation. The results are suggestive of gender dimorphism in biologic pathways altered in PTSD particularly in the domain of immune function. In sum, our data indicate that monocyte gene expression patterns are altered in PTSD, supporting the idea that changes in monocyte gene expression could contribute to the increased medical morbidity observed in PTSD.
Research Highlights.
Male subjects with PTSD had an overall pattern of under-expression of genes on monocytes (47 under-expressed versus 4 over-expressed genes).
There was no transcriptional evidence of chronic inflammation in male PTSD+ subjects.
In contrast, preliminary data from our pilot sample of female PTSD+ subjects showed a relatively balanced pattern of increased and decreased expression of genes and an increase in activity of pathways related to immune activation.
The results indicate differential patterns of monocyte gene expression in PTSD, and are suggestive of gender dimorphism in biologic pathways activated in PTSD.
Acknowledgments
We thank Cyrus Calosing and Thomas Metzler for excellent technical assistance. This research was supported in part by grants from the Department of Defense (W81XWH-05-2-0094), the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration, and the National Institute for Mental Health (TCN: R01 MH73978). This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center, San Francisco California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. Am J Psychiatry. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 Polymorphisms and Childhood Abuse With Risk of Posttraumatic Stress disorder Symptoms in Adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann N Y Acad Sci. 2002;966:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Marmar CR, Neylan TC, Schiller NB, Ali S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2009;66:1214–1220. doi: 10.1001/archgenpsychiatry.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr., Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. The American journal of pathology. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV. Patient Version ed. New York State Psychiatric Institute, Biometrics Research; New York: 1996. SCID-I. [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidron Y, Armon T, Gilutz H, Huleihel M. Psychological factors correlate meaningfully with percent-monocytes among acute coronary syndrome patients. Brain Behav Immun. 2003;17:310–315. doi: 10.1016/s0889-1591(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Hu Z, Hung JH, Wang Y, Chang YC, Huang CL, Huyck M, DeLisi C. VisANT 3.5: multi-scale network visualization, analysis and inference based on the gene ontology. Nucleic acids research. 2009;37:W115–121. doi: 10.1093/nar/gkp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. Immune correlates of depression. Adv Exp Med Biol. 1999;461:1–24. doi: 10.1007/978-0-585-37970-8_1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Wray S. 17-Beta-estradiol regulates expression of genes that function in macrophage activation and cholesterol homeostasis. The Journal of steroid biochemistry and molecular biology. 2002;81:203–216. doi: 10.1016/s0960-0760(02)00065-1. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Trauma and the Vietnam war generation: Report of the findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. Journal of leukocyte biology. 2003;74:833–845. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Aasal R, Adler L, Berger CL, Montgomery PT, Sandberg E, Wahlberg LJ, Wilkins RT, Zweig L, Reite ML. Elevated cytotoxicity in combat veterans with long-term post-traumatic stress disorder: preliminary observations. Brain, behavior, and immunity. 1998;12:74–79. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999a;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Maes M, Van Bockstaele DR, Gastel A, Song C, Schotte C, Neels H, DeMeester I, Scharpe S, Janca A. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999b;39:1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- Middle F, Jones I, Robertson E, Lendon C, Craddock N. Tumour necrosis factor alpha and bipolar affective puerperal psychosis. Psychiatr Genet. 2000;10:195–198. doi: 10.1097/00041444-200010040-00008. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Morita K, Saito T, Ohta M, Ueno S, Rokutan K. Assessment of human stress and depression by DNA microarray analysis. J Med Invest. 2005;52(Suppl):266–271. doi: 10.2152/jmi.52.266. [DOI] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav Immun. 2009;23:622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Rokutan K, Morita K, Masuda K, Tominaga K, Shikishima M, Teshima-Kondo S, Omori T, Sekiyama A. Gene expression profiling in peripheral blood leukocytes as a new approach for assessment of human stress response. J Med Invest. 2005;52:137–144. doi: 10.2152/jmi.52.137. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Spiro A, 3rd, Paris AH. Physician-diagnosed medical disorders in relation to PTSD symptoms in older male military veterans. Health Psychol. 2000;19:91–97. doi: 10.1037//0278-6133.19.1.91. [DOI] [PubMed] [Google Scholar]
- Schwarz MJ, Chiang S, Muller N, Ackenheil M. T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behav Immun. 2001a;15:340–370. doi: 10.1006/brbi.2001.0647. [DOI] [PubMed] [Google Scholar]
- Schwarz MJ, Muller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001b;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, Pablov V, Friedman N, Hochner-Celnikier D. Blood mononuclear cell gene expression signature of postpartum depression. Mol Psychiatry. 2010;15:93–100. doi: 10.1038/mp.2009.65. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:425. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342:93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Sutherland AG, Alexander DA, Hutchison JD. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine. 2003;24:219–225. doi: 10.1016/j.cyto.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Team, R.D.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Watson IP, Muller HK, Jones IH, Bradley AJ. Cell-mediated immunity in combat veterans with post-traumatic stress disorder. Medical Journal of Australia. 1993;159:513–516. doi: 10.5694/j.1326-5377.1993.tb138003.x. see comments. [DOI] [PubMed] [Google Scholar]
- Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci U S A. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SN, van der Kolk B, Burbridge J, Fisler R, Kradin R. Phenotype of blood lymphocytes in PTSD suggests chronic immune activation. Psychosomatics. 1999;40:222–225. doi: 10.1016/S0033-3182(99)71238-7. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. American Journal of Psychiatry. 1995;152:1705–1713. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]


