Abstract
Naturally occurring regulatory T cells (nTregs) suppress the development of GVHD and may spare graft-versus-leukemia (GVL) effect. Because nTreg is a rare population in a healthy individual, the limited source and the non-selective suppression are major hurdles towards the application of nTregs in the control of clinical GVHD after allogeneic HCT. An alternative approach is to generate induced Tregs (iTregs) from naïve CD4 precursors, but the effectiveness of iTregs in the control of GVHD is highly controversial and requires further investigation. The other critical but unsolved issue on Treg therapy is how to achieve antigen (Ag)-specific tolerance that distinguishes GVHD and GVL effect. To address the important issues on the effectiveness of iTregs and Ag-specificity of Tregs, we generated Ag-specific iTregs and tested their potential in the prevention of GVHD in pre-clinical BMT model. CD4+CD25+Foxp3+ iTregs generated from OT-II TCR transgenic T cells specific for OVA target Ag efficiently prevented GVHD induced by polyclonal T effector cells (Teffs) only in the allogeneic recipients that express OVA protein but not in OVA− recipients. The efficacy of these Ag-specific iTregs was significantly higher than polyclonal iTregs. As controls, OT-II CD4+Foxp3− cells had no effect on GVHD development in OVA− recipients and exacerbated GVHD in OVA+ recipients when transplanted together with polyclonal Teffs. Because the iTregs recognize OVA whereas Teffs recognize alloAg bm12, our data reveal for the first time that Tregs prevent GVHD through a linked suppression. Mechanistically, OT-II iTregs expanded extensively, and significantly suppressed expansion and infiltration of Teffs in OVA+ but not in OVA− recipients. These results demonstrate that Ag-specific iTregs can prevent GVHD efficiently and selectively, providing a proof of principle that Ag-specific iTregs may represent a promising cell therapy for their specificity and higher efficacy in allogeneic HCT.
Introduction
Allogeneic BMT or HCT offers great promise for the treatment of a variety of diseases including cancer, autoimmunity, aplastic anemia, and other hematopoietic diseases. However, GVHD remains the major complication following this therapeutic procedure because it leads to high morbidity and mortality in patients (1, 2). Despite the magnitude of this complication and the extensive efforts to overcome this problem, no clinical strategy has been established to efficiently prevent GVHD without producing a broad immune suppression. Recent evidence indicates that the use of Tregs (CD4+Foxp3+) is one of the promising approaches to control GVHD in numerous mouse models (3–8) in addition to early clinical trials (9).
Although it is widely accepted that natural CD4+Foxp3+ Tregs are developed in the thymus (termed nTregs), accumulating evidence suggests that T cells with regulatory function may also arise in the periphery under certain conditions and are termed induced Tregs (iTregs). The full extent of differences and similarities between iTregs and nTregs has not yet been defined (10). Due to the infrequency of nTregs in the peripheral blood and the difficulty in isolating sufficient nTregs with adequate purity, much attention has been placed on the use of in vitro-expanded nTregs with emphasis on retaining their regulatory capabilities. Other studies have focused on iTregs generated from naive CD4+CD25− cells to obtain a regulatory cell population to suppress immune responses in vitro and in vivo. However, the use of iTregs as an immunotherapy is still controversial concerning their stability in Foxp3 expression (11–15).
Because Tregs need to be activated by their specific antigen (Ag) to exert their suppressive function, it is understood that polyclonal populations of Tregs will only have limited efficacy on a per cell basis to regulate allogeneic responses due to the low frequency of alloantigen-reactive Tregs within the whole population. Although large numbers of polyclonal Tregs are capable of preventing GVHD in rodents, broad polyclonal suppression is expected. Therefore, Ag specificity of Tregs is critical to selective suppression mediated by these cells. In experimental autoimmune disease models, Ag-specific Tregs are highly effective in controlling autoimmune diabetes, gastritis and encephalomyelitis (16–18). However, the advantage of using Ag-specific Tregs in the prevention of GVHD has not yet been investigated.
We previously generated Ag-specific iTregs by foxp3 transduction and demonstrated that they persist long-term in vivo and suppress GVHD in a non-myeloablative BMT model when activated by the cognate Ag; either constitutively expressed or introduced via immunization (11). In our previous study, however, a non-myeloablative BMT model was used that is not representative of clinical HCT, and iTregs were generated through gene transfection. In the current study, we addressed these two important issues and demonstrate that TGFβ-induced, Ag-specific iTregs efficiently and selectively prevent GVHD in a murine model of myeloablative BMT.
Material and methods
Mice
C57BL/6 (B6, H-2b), B6 that express congenic Ly5.1 or Thy1.1, B6 bm12 and OT-II TCR transgenic (Tg) strains were purchased from the Jackson Laboratory (Bar Harbor, ME). Foxp3gfp knock-in (KI) strain was obtained from Rudensky’s laboratory at University of Washington (Seattle, WA) (19, 20). Luciferase-transgenic (Luc-Tg) strain on B6 background was kindly provided by Dr. R. Negrin (Stanford Univ., CA) (21). B6 OVA Tg under β-actin strain was kindly provided by S. Schoenberger (La Jolla Institute for Allergy and Immunology, San Diego, CA). OT-II Foxp3gfp KI and (B6.OVA × bm12)F1 strains were produced by cross-breeding. All the mice were housed in a pathogen-free condition at H. Lee Moffitt Cancer Center. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
T-cell purification and iTreg generation
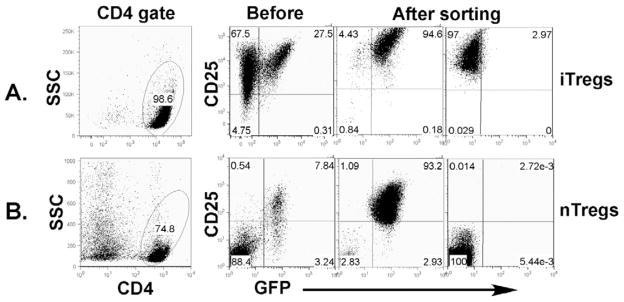
CD4+CD25− T cells were purified through negative selection as described in our previous work (22). The purity of CD4+CD25− cells ranged from 85 to 95%, but CD4+CD25+ cells was always less than 1% among total CD4+ cells. To generate polyclonal iTregs, CD4+CD25− T cells from B6 Foxp3gfp KI mice were seeded at 2.5 × 105/ml and stimulated with 0.5 μg/ml anti-CD3 mAb in the presence of 1.25 × 106/ml irradiated syngeneic T-cell depleted (TCD)-splenoctyes as APCs with TGF-β1 and IL-2 both at 2 ng/ml. OT-II Tg iTregs were generated in the same way, except that 0.5 μg/ml OVA peptide was used instead of anti-CD3 mAb. After incubation for 4–6 days, cells were harvested for measuring GFP, CD4 and CD25 expression. Percentage of CD25+GFP+ cells ranged from 20% to 60% among CD4+ cells after 4–6 day culture. CD4+CD25+GFP+ cells were purified by FACS sorting and used as iTregs, whereas CD4+GFP− cells were used as controls.
Immuno-fluorescence analysis
Two-, 3- or 4 -color flow cytometry was performed to measure the expression of surface molecules according to standard techniques. Intracellular Foxp3 expression was measured with a Foxp3 detection kit from eBioscience (San Diego, CA), according to manufacturer’s instruction. Intracellular cytokines were measured after stimulation with PMA + ionomycin in vitro for 4–5 h with the addition of GolgiStop for the last 2h. The cells were then stained for surface expression of CD4, Ly5.1 and Thy1.1, and for intracellular expression of IFN-γ and IL-17. Analysis was performed using a FACScan or FACS Calibur and CellQuest software (Becton Dickinson, San Jose, CA). Fluorescence conjugated-Abs were purchased from BD-Pharmingen (San Diego, CA) or eBioscience (San Diego, CA).
BMT and bioluminescent imaging (BLI)
(B6 × bm12)F1 mice were exposed to 1200 – 1300 cGy (split does) of total body irradiation. TCD-BM cells alone or in combination with purified CD4+CD25− T cells from B6 donors were injected via the tail vein into recipients within 24 hrs after irradiation. Recipient mice were monitored every other day for clinical signs of GVHD, such as ruffled fur, hunched back, lethargy or diarrhea, and mortality. Animals judged to be moribund were sacrificed and counted as GVHD lethality as described in our previous work (23, 24). In vivo BLI of BALB/c recipients transplanted with T cells from Luc-Tg B6 donors and BM from non-Tg B6 donors was performed as described previously (23, 24), using an IVIS200 charge-coupled device imaging system (Xenogen).
Statistical analysis
The log-rank test was used to detect statistical differences in recipient survival in GVHD experiments. Student’s t test was used to compare percentages or numbers of donor T cells.
Results
TGFβ-induced, Ag-specific iTregs prevent GHVD in an Ag-dependent manner
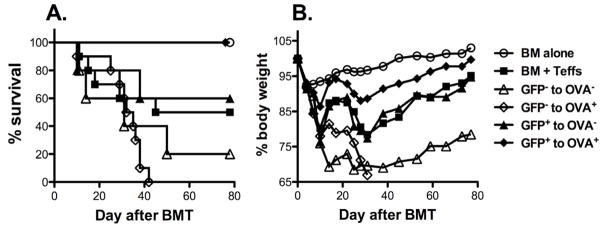
Recent progress made by many groups including ours indicates that iTregs can be generated from naïve CD4 T cells upon TCR stimulation in the presence of TGFβ (22, 25, 26). iTregs are effective in suppressing autoimmune diseases, but their effect in controlling GVHD is controversial and remains to be further investigated. For this reason, we generated OT-II TCR Tg and foxp3/gfp KI mice by cross-breeding. OVA-specific iTregs were then generated from OT-II Tg and foxp3/gfp KI CD4+CD25− T cells by stimulating them with OVA peptide in the presence of TGFβ (Fig. 1). We then tested whether OVA-specific iTregs (CD4+CD25+GFP+) were able to prevent GVHD induced by polyclonal T cells in a B6 → (B6 × bm12)F1 BMT model, in which donor CD4+ T cells (Teffs) recognize mismatched recipient MHC II alloAg (H2bm12). To specifically activate iTregs, (B6.OVA × bm12)F1 mice were used as recipients that ubiquitously express OVA. The bm12 mutation can present OVA peptide, but OT-II T cells cannot recognize this MHC/peptide complex. In this setting, Teffs at indicated dose induced 50% GVHD lethality. Similar numbers of OVA+ and OVA− recipients were used for the Teff alone group, but the same results was observed in survival or weight loss regardless of OVA expression (data not shown). Additional iTregs completely prevented GVHD lethality in OVA+ (p = 0.01) but not in OVA− recipients (p = 0.8) (Fig. 2), indicating that activation of iTregs was required for their suppressive function. CD4+GFP− control cells had no effect on GVHD in OVA− recipients, or even accelerated GVHD in OVA+ recipients as Teffs (Fig. 2). These results demonstrate that Ag-specific iTregs are potent in suppressing GVHD in an activation-dependent manner. Because the iTregs recognize OVA whereas Teffs recognize alloAg bm12, these data reveal that Tregs prevent GVHD through a linked suppression.
Fig. 1. Isolation of iTregs and nTregs.
A, generation and purification of iTregs. CD4+CD25− cells were purified from spleen and lymph node of OT-II TCR Tg and foxp3/gfp KI mice. These purified T cells were stimulated with OVA peptide at 0.5 μM in the presence of irradiated TCD-splenocytes. TGFβ was added in the culture at 2 ng/ml for Treg generation. Four to six days after culture, cells were harvested and stained for CD4, CD25 and GFP expression. The phenotype of cultured cells is shown on gated live cells (2 left panels). CD4+ CD25+GFP+ and CD25+GFP− cells were separated by FACS sorting (2 right panels). B, purification of nTregs. CD4+ cells were isolated through negative selection from spleen and lymph node of B6 foxp3/gfp KI mice. These CD4+ enriched cells were stained for CD4 and CD25 expression. The phenotype of these cells is shown on gated live cells (2 left panels). CD4+ CD25+GFP+ and CD25-GFP− cells were separated by FACS sorting (2 right panels).
Fig. 2. The effect of TGFβ-induced Tregs in GVHD.
OVA+ or OVA− (B6 × bm12)F1 mice were lethally irradiated and transferred with 5 × 106 TCD-BM alone or plus 1 × 106 CD4+ T cells (Teffs) from B6 donors. OVA-specific iTregs (CD4+CD25+GFP+) were generated and purified by FACS sorting as shown in figure 1. OVA-specific iTregs or controls at 0.5 × 106/mouse each were added into donor graft. Recipient survival (A) and body weight changes (B) are shown. Ten recipients were included in each group except that 5 mice were used in GFP+ or GFP− cells to OVA− groups. The data are pooled from 2 replicate experiments using a similar setting.
TGFβ-induced, Ag-specific iTregs are significantly more effective in the prevention of GVHD then polyclonal iTregs
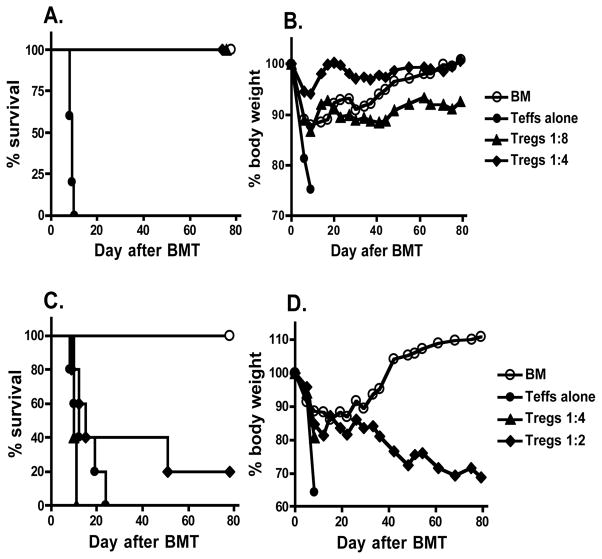
To further evaluate the potency of OVA-specific iTregs in the prevention of GVHD, these iTregs were used at 1:4 or 1:8 ratio of Treg:Teff. We found that GVHD lethality was completely prevented at either cell dose (Fig. 3A and B). To compare the potency of Ag-specific versus non Ag-specific iTregs, polyclonal iTregs were generated from CD4+CD25− cells of B6 foxp3/gfp KI mice by stimulating with anti-CD3 mAb in the presence of TGFβ as shown in our previous work (22). In contrast to Ag-specific iTregs, the polyclonal iTregs had a partial effect only at 1:2 ratio of Treg:Teff in suppressing GVHD (Fig. 3C and D). These data indicate that Ag-specific iTregs are ~ 8-fold more effective than polyclonal iTregs in GVHD prevention.
Fig. 3. The potency of Ag-specific and polyclonal iTregs in suppressing GVHD.
A and B, OVA+ (B6 × bm12)F1 mice were lethally irradiated and transferred with TCD-BM alone or plus 1.6 × 106 CD4+CD25− T cells (Teffs alone) from B6 donors. OVA-specific iTregs (CD4+CD25+GFP+) were generated from OT-II T cells and were added at 0.2 or 0.4 × 106 each into donor graft. C and D, OVA− (B6 × bm12)F1 mice were lethally irradiated and transferred with TCD-BM alone or plus 1 × 106 CD4+CD25− T cells (Teffs alone) from B6 donors. Polyclonal iTregs (CD4+CD25+GFP+) generated from WT B6 T cells with anti-CD3 stimulation plus TGFβ were added at 0.25 or 0.5 × 106 each into donor graft. Recipient survival (A and C) and body weight changes (B and D) are shown. Five or six recipients were included in each group for both experiments.
Ag-specific iTregs suppress the expansion, activation and infiltration of Teffs in vivo
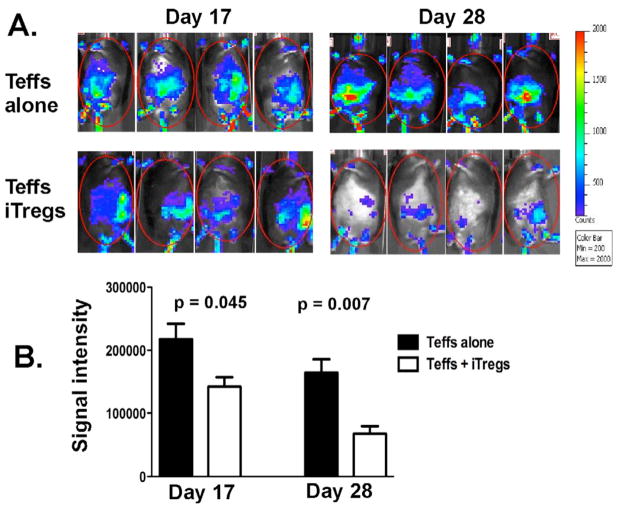
We next assessed the suppressive effects of Ag-specific iTregs on Teffs in vivo. Taking advantage of Luc-Tg mice, the expansion and infiltration of Luc-Tg Teffs can be measured in vivo over time using BLI assay. Because low dose of Teffs (5 × 105/mouse) was transferred into B6 mice (black) that are less sensitive for signal detection, no significant BLI signal was detected on day 7. The BLI detected on day 17 and 28 demonstrate that additional OT-II iTregs significantly reduced Teff expansion in OVA-expressing recipients (Fig. 4A and B). The distribution of the BLI signal suggests that the Teffs infiltrated more broadly to liver and gut without iTregs whereas Teffs were more constrained in spleen with iTregs (Fig. 4A).
Fig. 4. The effect of Ag-specific iTregs on expansion and infiltration of Teffs.
Lethally irradiated OVA+ (B6 × bm12)F1 mice were transplanted with B6 TCD-BM plus 0.5 × 106/mouse Teffs (CD4+CD25−) isolated from Luc-Tg mice on B6 background. One group of recipients was also transferred with additional 0.25 × 106/mouse OT-II iTregs (CD4+CD25+GFP+). Donor Teffs were monitored in recipient mice 17 and 28 days after BMT. A, animals were imaged from the ventral position for quantification of donor T cells. B, the average of relative signal intensity of 4 mice per group, and the data represent one of 2 replicate experiments.
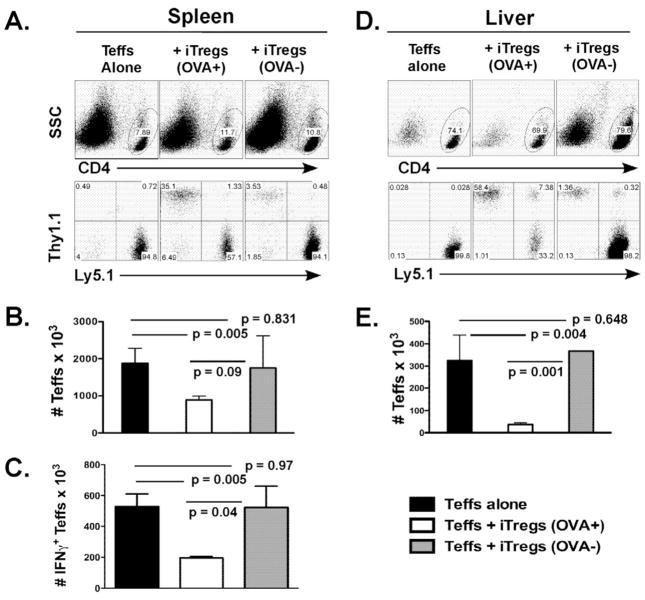
To further evaluate the effect of iTregs on expansion and activation of Teffs, we transferred Teffs isolated from B6 Ly5.1+ mice and iTregs generated from Thy1.1+ OT-II CD4 precursor (1:2 ratio of Treg:Teff) along with TCD-BM isolated from normal B6 donors into OVA+ or OVA− (B6 × bm12)F1 recipients. Seven days after BMT, we measured Teffs (CD4+Ly5.1+) in recipient spleen and liver (Fig. 5A and D). There was an average of 1.9 ± 0.4 ×106/mouse Teffs in the spleen of the recipients transferred with Teffs alone, 0.9 ± 0.1 ×106 in the OVA+ recipients transferred with Teffs plus iTregs, and1.8 ± 0.8 ×106 in the OVA− recipients transferred with Teffs plus iTregs, respectively (Fig. 5B). The data indicate that iTregs significantly reduced Teff expansion in the OVA+ (p = 0.005) but not the OVA− recipients (p = 0.8). In the liver, the number of Teffs was also significantly lower in the OVA+ recipients transferred with Teffs plus iTregs than those with Teffs alone (Fig. 5E, p = 0.004), suggesting that iTregs reduced Teff expansion and/or infiltration in recipient liver, a major GVHD target organ. Because peripheral lymphoid organs are important for T cell activation, we examined the migration of iTregs to recipient lymph nodes and spleen relevant to antigen stimulation in vivo. In a separate experiment, we observed that the percentages of iTregs among CD4+ T cells were 36.3 ± 5.3% vs. 17.1 ± 3.1% in lymph nodes and spleen of OVA+ recipients, respectively (n = 4, p = 0.0007). However, the percentages of iTregs among CD4+ T cells were similar and less than 1% in lymph nodes and spleen of OVA− recipients (n = 4, p = 0.08). These results suggest that Tregs preferentially reside in lymph nodes upon Ag stimulation.
Fig. 5. Effects of Ag-specific iTregs on expansion and activation of Teffs.
Teffs cells (CD4+CD25−) were isolated from WT Ly5.1+ donors and transferred at 1 × 106/mouse together with TCD-BM into lethally irradiated OVA− or OVA+ (B6 × bm12)F1 mice. The other 2 groups were transferred with OT-II Thy1.1+ iTregs at 0.5 × 106/mouse into OVA− or OVA+ (B6 × bm12)F1 recipients. Seven days after BMT, recipient spleen (A-C) and liver (D and E) were harvested for measuring expansion and activation of donor Teffs. A, top panels show percentages of CD4+ cells in live cells, and bottom panels show expression of Ly5.1 (Teffs’ maker) and Thy1.1 (iTregs’ marker) on gated CD4+ live cells in recipient spleen. B, absolute numbers of Teffs (CD4+Ly5.1+) are shown in average ± 1 SD. C, spleen cells were also measured for intracellular expression of IFNγ, and absolute numbers of IFNγ+ Teffs (CD4+Ly5.1+) are shown in average ± 1 SD. D, top panels show percentages of CD4+ cells in live cells, and bottom panels show expression of Ly5.1 (Teffs’ marker) and Thy1.1 (iTregs’ marker) on gated CD4+ live cells in recipient liver. E, absolute numbers of Teffs (CD4+Ly5.1+) in the liver are shown in average ± 1 SD. Each group includes 3 or 4 mice, and the data represent 1 of 3 replicate experiments.
To evaluate the activation of Teffs, we measured intracellular expression of IFNγ and IL-17, and calculated the numbers of IFNγ- and IL-17-producing Teffs in the recipient spleen. The number of IFNγ-producing Teffs in the OVA+ recipients transferred with Teffs plus iTregs was significantly lower than that in the recipients of Teffs alone (p = 0.005), whereas there was no difference between the recipients with Teffs alone and those OVA− recipients with Teffs plus iTregs (p = 0.9) (Fig. 5C). There were very few Teffs that produced IL-17 (< 2%) and no significant difference among those groups (data not shown). These results indicate that iTregs also reduced Teff activation when iTregs were activated by specific Ags.
After adoptive transfer in vivo, iTregs expanded to higher numbers while nTregs had more stable expression of Foxp3
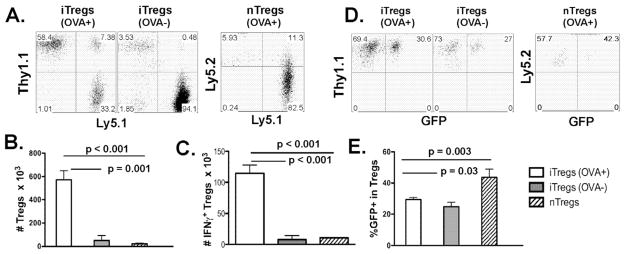
We assessed the expansion and stability of iTregs in vivo. In experiments with the same setting as in figure 5, OT-II iTregs (CD4+Thy1.1+) expanded extensively in OVA+ but not OVA− recipients (Fig. 6A and B, p < 0.001). To further compare the expanding potential between iTregs and nTregs, we isolated polyclonal nTregs (CD4+CD25+GFP+) from naïve B6 foxp3/gfp KI mice (Ly5.2+) as standard controls (Fig. 1B). The expansion levels for OT-II iTregs in OVA+ recipients were significantly higher than that of nTregs (Fig. 6A and B, p = 0.001), indicating that Treg expansion depended on Ag-stimulation in vivo.
Fig. 6. Expansion and stability of Tregs in the recipients after allogeneic BMT.
Experimental setting is the same as described in figure 5. One additional group of recipients was transferred with 0.5 × 106/mouse nTregs isolated from naïve B6 foxp3/gfp KI mice (Ly5.2+Ly5.1−). A, percentages of Thy1.1+Ly5.1− (iTregs) or Ly5.2+Ly5.1− cells (nTregs) on gated CD4+ live cells in recipient spleen. B, absolute numbers of iTregs or nTregs are shown. C, absolute numbers of IFNγ+ iTregs or nTregs are presented per spleen. D, GFP expression on gated iTregs or nTregs in recipient spleen. E, percentages of GFP+ cells among gated iTregs or nTregs are shown in average ± 1 SD. Each group includes 3 or 4 mice, and the data represent 1 of 3 replicate experiments.
Recent publications suggest that iTregs are less stable than nTregs in maintaining Foxp3 expression. To address this concern, we gated on CD4+Thy1.1+ Tregs and analyzed their Foxp3/GFP expression. Because Tregs were highly purified through FACS sorting for GFP expression (Fig. 1A), the percentage of GFP− cells in gated CD4+Thy1.1+ cells would reflect the loss of Foxp3 expression. Polyclonal nTregs (CD4+CD25+GFP+) from naïve B6 foxp3/gfp KI mice were also used as standard controls. Under myeloablative allogeneic BMT, average of 43.6 ± 5.4% nTregs kept their GFP expression 7 days after cell transfer, whereas 29.4 ± 2.8% and 24.8 ± 2.8% iTregs kept their GFP in OVA+ and OVA− recipients, respectively (Fig. 6D and E). Foxp3 expression was less stable in iTregs than nTregs (p = 0.003), whereas the stability of iTregs was similar in the recipients regardless of OVA expression (Fig. 6C and D). To measure activation of Tregs, intracellular IFNγ and IL-10 were measured. We found that 7 days after BMT there was an average of 20.0 ± 3.4% and 4.1 ± 0.5% IFNγ+ cells among Ag-specific iTregs and polyclonal nTregs, respectively. Furthermore, the number of IFNγ+ Ag-specific iTregs was significantly more in the OVA+ than OVA− recipients and significantly more than that of nTregs in recipient spleen (Fig. 6C, p < 0.001). In conclusion, Treg expansion depended on Ag-stimulation and iTregs were activated and expanded more extensively than nTregs, but iTregs were less stable than nTregs in Foxp3 expansion upon Ag-stimulation under myeloablative allogeneic BMT.
Discussion
Besides regulating autoimmunity, CD4+CD25+ Tregs also control allogeneic responses. Therefore, research on understanding and applying Tregs in the setting of HCT has been an active field in recent years (27). Due to low frequency of nTregs, current approaches in attempt to apply Tregs in clinical HCT are focused on adoptive transfer of polyclonal, ex vivo expanded, nTregs into transplant recipients before or after stem cell transplantation. Isolating and expanding polyclonal nTregs is feasible (28, 29); however, questions remain about their efficacy and the consequences of broad immune suppression in vivo. E.g. these polyclonal nTregs may have a low potency in controlling GVHD and produce non-selective immune suppression without discriminating for GVH and GVL reactions.
The current study is aimed at increasing the potency and selectivity of Treg therapy. By using TGFβ-induced Ag-specifc iTregs, we showed that Ag-specific iTregs were highly effective in preventing GVHD in a clinically relevant murine model of allogeneic BMT in an Ag-dependent manner (Fig. 2). The current study substantially extended the previous work by us and others showing that in vitro generated iTregs were effective in suppressing allogeneic responses in bone marrow or solid organ transplantation (11–14). However, our result is in contrast to a recent report by Konencke et al. that TGFβ-induced polyclonal iTregs were not effective in preventing GVHD presumably due to the instability of Foxp3 expression (15). We interpret that the differences in the protocol of generating iTregs, the specificity of iTregs and GVHD model may contribute to the distinct outcome in these two studies. Higher levels in expression of Ag-specific iTregs were likely resulted from higher levels of Ag-driven proliferation and less dependent on Ag and cytokine signals in recipients of pre-activated and dividing iTregs versus resting nTregs.
A potential concern is that iTregs may not have stable Foxp3 expression due to their status of epigenetic modification and lose their suppressive activity in vivo (30). In fact, some studies have showed that in vitro generated iTregs were less suppressive than nTregs (31, 32). However, there is also substantial evidence in the literature supporting that iTregs were as or more effective than nTregs in suppressing immune responses in vivo (16, 18, 25, 33–37). To address this concern on iTreg stability, we directly compared Foxp3 stability of iTregs and nTregs and observed that iTregs were less stable than nTregs in Foxp3 expression under allogeneic BMT (Fig. 6C and D). However, iTregs underwent substantially higher levels of Ag-driven expansion than nTregs (Fig. 6B), which may compensate for their inferior stability relative to that of nTregs. Remarkably, we showed that Ag-specific iTregs were able to prevent GVHD in 100% recipients at 1:8 ratio of Tregs to Teffs (Fig. 3A). In contrast, using the same murine BMT model where BM plus CD4+ T cells were transplanted into lethally irradiated bm12 recipients, Taylor et al. indicated that in vitro activated and expanded, polyclonal CD62Lhigh nTregs could prevent GVHD in nearly 100% at 3:1 ratio of Tregs to Teffs (7). Taken together, these data suggest that Ag-specific iTregs can be ~24-fold more effective than the most potent polyclonal nTregs tested so far. Considering the frequency of alloreactive T cells, we observed that significantly more Ag-specific iTregs produced IFNγ after activation by cognate Ag than polyclonal nTregs after activation by alloantigens (20% vs. 4%), confirming that Ag-specific iTregs were more activated than polyclonal nTregs. Because IFNγ production by Tregs is critical for their suppressive function in vivo (38), high level of IFNγ production by Ag-specific iTregs also correlated with their superior suppressive activity to polyclonal nTregs.
A fundamental issue regarding Treg-mediated suppression not yet being addressed is whether Tregs execute their regulatory function through Ag-specific, Ag-linked or bystander suppression in vivo. The current study made it clear that iTregs must be activated by their cognate Ag in vivo in order for them to exert their suppressive function and to control GVHD (Fig. 2 and 3). Because iTregs recognize nominal Ag (OVA) whereas Teffs recognize allo-Ags (bm12), the results indicate that iTregs do not have to recognize the same Ag as Teffs for Tregs to suppress the responses elicited by the Teffs in vivo and strongly support the notion that linked suppression is operational under allogeneic BMT settings. Our data are consistent with the results reported by Tang et al. that monoclonal Tregs (BDC2.5 TCR Tg) specific for an islet Ag are highly effective in controlling experimental diabetes induced by polyclonal diabetogenic Teffs (17). These studies indicate that Treg-mediated immunosuppression does not have to be exclusively Ag-specific, which seems contradictory with the results observed by Joffre et al (39) or those by Zhang et al (18). Using BM rejection model, Joffre et al. showed that Tregs specific for donor alloAgs selectively prevent rejection of donor BM but not third-party BM, both of which were transplanted into the same recipient (39), suggesting that Treg-mediated suppression is Ag-specific. Likewise, using an EAE model, Zhang et al. showed that myelin proteolipid protein (PLP)139–151-specific iTregs were effective at suppressing EAE induced by the cognate (PLP)139–151 peptide, but not by (PLP)178–191 peptide or even a mixture of the 2 peptides (18). It is not clear why Tregs mediated suppression with exquisite Ag-specificity in some studies but not the others. What is clear is that Tregs can induce Ag-specific or Ag-linked suppression but not bystander suppression in vivo. No bystander suppression in vivo is also evident in which the generation of donor-reactive iTregs prevents graft rejection without compromising immunity to a viral pathogen (40).
Isolating and expanding polyclonal nTregs has been shown to be feasible (28, 29); however, questions remain about their efficacy and Ag specificity in vivo. E.g. although they can be expanded multi-fold in vitro, generating the absolute number of Tregs needed to treat a patient successfully may still be a challenge (27). We want to emphasize that, unlike polyclonal alloreactive Tregs expanded with allogeneic APCs in vitro (41–43) or induced in vivo (44–46), the Ag-specific Tregs investigated in the current study are monoclonal and each of them specifically recognizes the cognate Ag, which likely contributes to the high efficacy of these cells in suppressing GVHD. In this proof-of-concept study the iTregs are monoclonal and uniformly recognize the cognized antigen with high affinity, thus caution should be noted from a translational perspective, as the results could be different with a population of polyclonal Ag-specific iTregs. Our current effort focuses on evaluating the effects of polyclonal iTregs specific for MHC or miHA Ags for better translational potential. The current study also provides evidence that iTregs prevent GVHD through linked suppression in an Ag-activation dependent manner, which likely has a broad impact in understanding how Tregs execute their suppressive function under biological or pathological situations. In clinical application, this finding indicates that iTregs specific for a miHA restricted on parenchymal tissues can distinguish GVHD versus GVL. Although creating Ag-specific Tregs is facilitated by the use of TCR Tg cells in mice, this approach will be more challenging in humans. However, the approach can be applied in the clinic to treat hematological tumors by generating and using iTregs specific for restricted miHAs on GVHD target tissues, because human T cells can be primed by miHAs in vitro. In conclusion, this study provides a proof of principle that Ag-specific iTregs may represent a promising Treg therapy for their specificity and higher efficacy in allogeneic HCT.
Acknowledgments
We thank Drs. R. Negrin for providing Luc-Tg, S. Schoenberger for OVA Tg, and A. Rudensky for Foxp3gfp KI strain. We are grateful for the technical assistance provided by the Flow Cytometry and Mouse Core Facility at the Moffitt Cancer Center.
This research was supported in part by NIH grants CA 116118, CA143812 (to X.-Z. Y.) and AI 51693 (to C.A.). X.-Z. Yu was a recipient of New Investigator Award supported by ASBMT.
Abbreviations used in this paper
- Ag
antigen
- TCD
T-cell depleted
- Tg
transgenic
- WT
wild type
- KI
knock-in
- Luc
luciferase
- BLI
bioluminescent imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik WD. Graft-versus-host disease. Nat Rev. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) mmunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 5.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) egulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 8.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Ianni M, Falzetti F, Carotti A, et al. Adoptive Immunotherapy with Tregs Prevents GvHD and Favours Immune Reconstitution After HLA Haploidentical Transplants for Hematological Malignancies. Blood (ASH Meeting Abstracts) 2009;114:4. [Google Scholar]
- 10.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Albert MH, Liu Y, Anasetti C, Yu XZ. Antigen-dependent suppression of alloresponses by Foxp3-induced regulatory T cells in transplantation. Eur J Immunol. 2005;35:2598–2607. doi: 10.1002/eji.200526077. [DOI] [PubMed] [Google Scholar]
- 12.Chai JG, Xue SA, Coe D, et al. Regulatory T cells, derived from naive CD4+CD25− T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplant. 2005;79:1310–1316. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 13.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenecke C, Czeloth N, Bubke A, et al. Alloantigen-specific de novo-induced Foxp3(+) Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 16.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Podojil JR, Chang J, Luo X, Miller SD. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:6629–6636. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity/ 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3− expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 21.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 22.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iclozan C, Yu Y, Liu C, et al. Th17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Y, Liu C, Djeu JY, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111:954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 27.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golovina TN, Mikheeva T, Suhoski MM, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 30.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 31.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill JA, Hall JA, Sun CM, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo X, Tarbell KV, Yang H, et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;179:11. p following 1390. [PubMed] [Google Scholar]
- 36.Zang W, Lin M, Kalache S, et al. Inhibition of the alloimmune response through the generation of regulatory T cells by a MHC class II-derived peptide. J Immunol. 2008;181:7499–7506. doi: 10.4049/jimmunol.181.11.7499. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 38.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bushell A, Jones E, Gallimore A, Wood K. The generation of CD25+ CD4+ regulatory T cells that prevent allograft rejection does not compromise immunity to a viral pathogen. J Immunol. 2005;174:3290–3297. doi: 10.4049/jimmunol.174.6.3290. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 42.Trenado A, Sudres M, Tang Q, et al. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol. 2006;176:1266–1273. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki S, Patel M, Harper A, et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci USA. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 46.Zhao D, Zhang C, Yi T, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]