Abstract
Hexavalent chromium [Cr(VI)] compounds (e.g. chromates) are strong oxidants that readily enter cells where they are reduced to reactive Cr intermediates that can directly oxidize some cell components and can promote the generation of reactive oxygen and nitrogen species. Inhalation is a major route of exposure which directly exposes the bronchial epithelium. Previous studies with non-cancerous human bronchial epithelial cells (BEAS-2B) demonstrated that Cr(VI) treatment results in the irreversible inhibition of thioredoxin reductase (TrxR) and the oxidation of thioredoxins (Trx) and peroxiredoxins (Prx). The mitochondrial Trx/Prx system is somewhat more sensitive to Cr(VI) than the cytosolic Trx/Prx system, and other redox-sensitive mitochondrial functions are subsequently affected including electron transport complexes I and II. Studies reported here show that Cr(VI) does not cause indiscriminant thiol oxidation, and that the Trx/Prx system is among the most sensitive of cellular protein thiols. Trx/Prx oxidation is not unique to BEAS-2B cells, as it was also observed in primary human bronchial epithelial cells. Increasing the intracellular levels of ascorbate, an endogenous Cr(VI) reductant, did not alter the effects on TrxR, Trx, or Prx. The peroxynitrite scavenger MnTBAP did not protect TrxR, Trx, Prx, or the electron transport chain from the effects of Cr(VI), implying that peroxynitrite is not required for these effects. Nitration of tyrosine residues of TrxR was not observed following Cr(VI) treatment, further ruling out peroxynitrite as a significant contributor to the irreversible inhibition of TrxR. Cr(VI) treatments that disrupt the TrxR/Trx/Prx system did not cause detectable mitochondrial DNA damage. Overall, the redox stress that results from Cr(VI) exposure shows selectivity for key proteins which are known to be important for redox signaling, antioxidant defense, and cell survival.
Keywords: Chromium, Thioredoxin, Thioredoxin reductase, Peroxiredoxin, Bronchial epithelial cells
Introduction
Hexavalent chromium [Cr(VI)] exposure can occur as a result of several industrial uses including chromate (CrO42−) pigments, chromate-based corrosion inhibitors, stainless steel machining and welding, chrome plating, and others. Inhalation is a common form of Cr(VI) exposure, and results in a number of serious respiratory effects, including pulmonary fibrosis, chronic bronchitis, lung cancer, and others (Baruthio, 1992; Deschamps et al. 1995; Franchini et al. 1983; Ishikawa et al. 1994). The bronchial epithelial cells line the airways and are therefore directly exposed to inhaled Cr(VI) fumes and dusts. Because industrial uses result in the annual release of more than 105 tons of Cr to the environment, environmental exposure is also of significant concern and Cr is a significant contaminant at hundreds of sites (Environmental Protection Agency, 1999).
Cr(VI) compounds enter cells via an anion carrier, and once inside cells Cr(VI) is reduced to Cr(III), the next stable oxidation state, by a variety of chemical and enzymatic intracellular reductants (Borthiry et al. 2007; Myers et al. 2000b; Shi and Dalal, 1990; Standeven and Wetterhahn, 1992; Suzuki and Fukuda, 1990). During this reduction, the reactive species Cr(V) and/or Cr(IV) are formed, and these can directly cause oxidative damage (Sugden, 1999; Sugden et al. 2001) and generate reactive oxygen species such as hydroxyl radical (HO•) via redox cycling (Borthiry et al. 2007; Shi et al. 1999a; Shi and Dalal, 1992; Shi et al. 1999b; Standeven and Wetterhahn, 1991). Cr(VI) can also promote the generation of the highly reactive oxidant peroxynitrite (Pritchard et al. 2000).
Cellular indicators of intracellular redox status demonstrate considerable oxidative stress as a result of the reductive activation of Cr(VI). Cr(VI) treatment of human bronchial BEAS-2B cells results in the pronounced inhibition of thioredoxin reductase (TrxR)1 and the oxidation of the thioredoxins that are dependent upon TrxR for their reducing equivalents (Myers et al. 2008; Myers and Myers, 2009). Peroxiredoxins such as Prx1 and Prx3, which are directly dependent on their respective thioredoxins, are also oxidized by Cr(VI) treatments that result in significant Trx oxidation (Myers and Myers, 2009). Mitochondrial Trx2 is more susceptible than cytosolic Trx1 (Myers et al. 2008; Myers and Myers, 2009) which implies that Cr-mediated pro-oxidant effects may be greater in the mitochondria, or that the mitochondria may be more susceptible. Cr(VI) treatments that cause Trx2 oxidation in BEAS-2B cells also cause pronounced and irreversible inhibition of aconitase (Myers et al. 2010). Mitochondrial electron transport complexes I and II are also inhibited, resulting in the appearance of electron paramagnetic resonance (EPR) signals that are consistent with the disruption of electron flow through these complexes (Myers et al. 2010). The activities of complexes I and II and aconitase are known to be susceptible to a number of oxidants including reactive oxygen and nitrogen species (Gardner et al. 1994; Pearce et al. 2001; Powell and Jackson, 2003; Williams et al. 1998; Zhang et al. 1990).
The thioredoxins are normally maintained largely in the reduced state in cells (Myers et al. 2008; Nordberg and Arnér, 2001; Szadkowski and Myers, 2008; Watson et al. 2003), and the TrxR/Trx system has a critical role in maintaining many intracellular protein thiols in their reduced state (Arnér and Holmgren, 2000). The TrxR/Trx system is critical for normal cell growth and viability and inhibition or suppression of these proteins enhances oxidant sensitivity and decreases survival (Chen et al. 2006; Hansen et al. 2006; Nordberg and Arnér, 2001). The peroxiredoxins are also important for cell survival (Chang et al. 2004; Cox et al. 2008a, 2008b), and one or more peroxiredoxins may have critical roles in H2O2 signaling (Chang et al. 2004; Low et al. 2008). The effects of Cr(VI) on these proteins therefore not only serve as indicators of intracellular redox stress, but could be important contributors to Cr(VI) toxicity.
The purpose of the studies reported here was to further define the effects of Cr(VI) exposure on the TrxR/Trx/Prx system. Several new findings are reported: (a) the thiols of the Trx/Prx system are among the most sensitive of the protein thiols to Cr(VI) treatment; (b) the oxidation of the Trx/Prx system also occurs in primary human bronchial epithelial cells and is therefore not unique to the BEAS-2B cell line; (c) the effects of Cr(VI) on the TrxR/Trx/Prx system are not altered by increasing the intracellular levels of ascorbate; (d) a peroxynitrite scavenger does not protect the TrxR/Trx/Prx system or the electron transport chain from Cr(VI); (e) Cr(VI) does not cause nitration of tyrosines within TrxR; (f) Cr(VI) treatments that disrupt the TrxR/Trx/Prx system do not cause detectable mitochondrial DNA damage; and (g) Cr(VI) does not alter the levels of Trx-interacting protein (Txnip).
2. Materials and methods
2.1. Chemicals and reagents
The following were purchased from Invitrogen Corp. (Carlsbad, CA): Hank’s balanced salt solution (HBSS), 4-acetamido-4'-maleimidylstilbene-2,2'-disulfonate (AMS), and pre-cast gels (12% Bis-Tris, 16% Tris-Glycine) and matching electrophoresis and loading buffers. Phenylmethylsulfonyl fluoride and Tris were from Research Organics (Cleveland, OH). EDTA, guanidine-HCl, and trichloroacetic acid were obtained from Fisher Scientific (Hampton, NH). Sodium chromate (Na2CrO4; 99+%) was the highest purity available from Aldrich Chemical (Milwaukee, WI). Chromates are recognized carcinogens and should be handled accordingly. Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) was obtained from Axxora LLC (San Diego, CA). Primary antibodies were from the following sources: Trx1 (Abcam 16835, rabbit polyclonal to full-length human Trx1), Trx2 (Santa Cruz 50336, rabbit polyclonal to residues 92–166 of human Trx2), TrxR1 (Abcam 16840, rabbit polyclonal to full-length human TrxR1), Prx1 (Abcam 58252, mouse monoclonal to recombinant human Prx1), Prx3 (Abcam 16751, mouse monoclonal to recombinant human full-length Prx3), GAPDH (Abcam 9485, rabbit polyclonal to full-length human GAPDH), Txnip (also known as VDUP1, Santa Cruz 33098, goat polyclonal to a C-terminal epitope of human Txnip), and nitrotyrosine (Santa Cruz 32757, mouse monoclonal to 3-nitrotyrosine). Affinity purified horseradish peroxidase-conjugated secondary antibodies were goat-antirabbit IgG (Promega W401B), goat anti-mouse IgG (Promega W402B), and donkey anti-goat IgG (Santa Cruz 2020). Anti-Trx1 only detects cytosolic Trx1, not mitochondrial Trx2. Anti-Trx2 reacts with Trx2 but not Trx1. In SDS-PAGE, anti-TrxR1 detects a 55 kDa protein in human cell lysates (the expected size for the TrxR monomer) and reacts strongly with purified TrxR. In reducing SDS-PAGE gels, anti-Prx1 and anti-Prx3 detect single bands of ca. 21 and 25 kDa, respectively, consistent with their expected masses. In non-reducing gels, they detect the dimeric (oxidized) forms of these peroxiredoxins in cells under oxidative stress. Anti-nitrotyrosine reacts strongly with nitrated BSA but shows no reactivity with BSA. All other chemicals and reagents were purchased from Sigma Chemical or from the sources indicated.
2.2. Cell culture and Cr(VI) treatment
BEAS-2B cells (human bronchial epithelial cell line, ATCC CRL-9609) were grown at 37°C in humidified air containing 5% CO2 in Dulbecco's Modified Eagle's Medium with 25 mM HEPES and 4.5 g/L glucose (BioWhittaker 12-709F), supplemented with 10% LHC-9 medium (Invitrogen), 10% fetal bovine serum (Valley Biomedical, Winchester, VA), penicillin (100 U/ml), and streptomycin (100 µg/ml). The cells were fed every 48 h, and were subcultured prior to reaching confluence using the Reagent Pak system (Clonetics, CC-5034). Normal plating density was 3000 to 5000 cells/cm2.
Primary normal human bronchial epithelial (NHBE) cells (Lonza Biosciences CC-2641) were grown in BEGM® medium (Lonza CC-3171) with the recommended BulletKit growth supplements (Lonza CC-3170). They were fed every 48 h, and were subcultured as described above. Normal plating density was 3500 cells/cm2.
Both BEAS-2B and NHBE cells were grown to 70–90% of confluence (typically in T-75 or T-25 flasks), washed once in pre-warmed HBSS, and treated with Cr(VI) as Na2CrO4 in HBSS at 37°C (see specifics below). Untreated cells were exposed to HBSS without Cr(VI). Following treatment, the cells were washed in HBSS and harvested as described below for the various assays. All experiments were repeated three times, except where noted otherwise.
2.3. 2D Electrophoretic analysis of oxidant-sensitive thiol proteins
The analysis of oxidized protein thiols in control and Cr(VI)-treated BEAS-2B cells was done using published methods (Baty et al. 2005; Cox et al. 2008b). BEAS-2B cells were washed once in pre-warmed HBSS, and treated with 0 or 25 µM Cr(VI) as Na2CrO4 in HBSS at 37°C for 90 min. Following treatment, the cells were washed and harvested into HBSS, and pelleted by centrifugation. The cell pellet was suspended in buffer (40 mM HEPES pH 7.4, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, and Roche complete protease inhibitor cocktail) containing 0.1 M N-ethylmaleimide (NEM) to alkylate thiols. After a 15 min incubation at room temperature, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) was added to a final concentration of 1% (w/v), followed by vortexing and another 15 min incubation. Excess NEM was removed using BioRad Micro Bio-Spin 6 chromatography columns. The eluate was treated with 1 mM dithiothreitol for 10 min to reduce oxidized thiols, followed by 0.2 mM 5-iodoacetamidofluorescein (IAF) for 10 min to label the resulting thiols. Unreacted IAF was removed using microspin chromatography columns, and the samples were analyzed by 2D electrophoresis using the ZOOM IPGRunner™ system (Invitrogen). The first dimension was a nonlinear pH 3–10 gradient (pH 4–7 occupies nearly all of the area) and the second dimension was SDS-PAGE in 4–12% Bis-Tris gels with MOPS SDS running buffer. Images of the gels were captured and analyzed using a Typhoon 9400 gel imager (GE Healthcare).
2.4. Redox blots for thioredoxins and peroxiredoxins
BEAS-2B cells were washed once in pre-warmed HBSS, and treated for 3 hr with 0, 25 or 50 µM Cr(VI) as Na2CrO4 in HBSS at 37°C. NHBE cells were treated similarly except that they were treated for 3 and 6 hr. Given that the NHBE cells had not been studied in this regard, the use of both exposure times provided for analysis of the effects of both concentration and time. In some experiments with BEAS-2B cells, just prior to Cr(VI) exposure, the cells were pretreated with vehicle or 0.2 mM MnTBAP for 2 hr, or with vehicle or 1 mM DHA for 90 min. Following Cr treatment, the cells were washed once in HBSS and immediately processed for redox western blots for Trx1, Trx2, Prx1, or Prx3 as previously described (Myers and Myers, 2009) based on the principles in the original protocols (Chen et al. 2006; Cox et al. 2008b; Halvey et al. 2005). Mitochondria were isolated from BEAS-2B cells and used as standards for oxidized and reduced Trx2 as previously described (Myers et al. 2008; Szadkowski and Myers, 2008).
2.5. TrxR activity
BEAS-2B cells were washed once in pre-warmed HBSS, and treated for 3 hr with 0, 25 or 50 µM Cr(VI) as Na2CrO4 in HBSS at 37°C. In some experiments, just prior to Cr(VI) exposure, BEAS-2B cells were pretreated with vehicle or 0.2 mM MnTBAP for 2 hr, or with vehicle or 1 mM DHA for 90 min. Following Cr treatment, the cells were washed twice in HBSS, scraped into 0.5 ml HBSS, pelleted by centrifugation (800 × g, 5 min) and frozen (−80°C). The pellets were thawed, suspended in 0.1 M sodium phosphate pH 7.4/5 mM EDTA and sonicated twice (15 sec each) on ice. Following centrifugation (30 min at 12000 × g), the supernatants were analyzed for TrxR activity at 37°C, measured as the NADPH-dependent reduction of DTNB (5,5'-dithiobis(2-nitrobenzoic) acid) (Holmgren, 1977). Incubation with DTNB (3 mM) during the first 5 min consumes non-specific thiols, after which NADPH (0.2 mM) was added to the sample cuvet and the reduction of DTNB was followed at 412 nm. The amount of NADPH-dependent activity that was inhibited by auranofin (4 µM) is attributed to TrxR (Gromer et al. 1998).
2.6. Electron Paramagnetic Resonance
BEAS-2B cells were pre-treated with 0.2 mM MnTBAP or vehicle for 2 hr, and then 25 µM Cr(VI) was added for 3 hr. The cells were then washed and harvested into HBSS, pelleted by centrifugation (5 min, 100 × g), and the final cell suspensions (ca. 8 × 106 cells in 0.3 ml HBSS in a 4-mm quartz EPR tube) were immediately frozen in liquid nitrogen (77 K) and stored, typically for less than one week. EPR spectra were obtained at liquid helium temperature (10 K) using a Bruker E500 ELEXSYS spectrometer (Silberstreifen, Germany) with an Oxford Instruments ESR-9 helium flow cryostat (Oxfordshire, UK) and a Bruker DM0101 cavity. Instrument settings are indicated in the results. EPR spectra were confirmed in replicate experiments. The g values were determined by comparison to the 2,2-diphenyl-1-picrylhydrazyl radical which has a g value of 2.0036.
2.7. Nitration of TrxR
BEAS-2B cells were washed once in pre-warmed HBSS, and treated with 0, 25 or 50 µM Cr(VI) as Na2CrO4 in HBSS at 37°C for 3 hr. Following treatment, the cells were washed twice in HBSS, scraped into 0.5 ml HBSS, and pelleted by centrifugation (800 × g, 5 min). The pellets were resuspended in cold lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 1 mM Na3VO4, 10 mM NaF, 12 mM β-glycerophosphate, 5 mM EGTA, 1 mM phenylmethanesulfonylfluoride, Roche complete protease inhibitor) and rocked at 4°C for 30 min. After passage several times through a 22-ga needle, the lysates were clarified by centrifugation (15000 × g, 10 min, 4°C). The supernates were incubated with 2 µg anti-TrxR (Abcam 16840) for 1 hr and then with protein A/G agarose overnight (4°C). The agarose was pelleted (1000 × g, 5 min, 4°C), washed three times in lysis buffer, mixed with loading buffer and reducing agent, boiled for 2–3 min, and aliquots of the supernate were run on SDS-PAGE. The lanes to be probed with anti-nitrotyrosine antibody (Santa Cruz sc-32757) were loaded with 25% of the total supernate, whereas those to be probed with anti-TrxR1 were loaded with 10-fold less (2.5% of the supernate). Nitrated albumin (Sigma N8159) was used as a positive control for the anti-nitrotyrosine antibody, whereas bovine serum albumin (BSA) was used as a negative control.
2.8. Mitochondrial DNA damage
BEAS-2B cells were washed once in pre-warmed HBSS, and treated for 3 hr with 0, 25 or 50 µM Cr(VI) as Na2CrO4 in HBSS. Following treatment, the cells were washed twice in HBSS, scraped into 0.5 ml HBSS, and pelleted by centrifugation (800 × g, 5 min). High molecular weight DNA was isolated from these cells using the QIAamp DNA Mini kit (Qiagen), which provides DNA that is suitable for PCR amplification of long products (Yakes and Van Houten, 1997). PCR was done using a constant amount of DNA template per reaction. To verify equal template loading, PCR was done using the Expand PCR System (Roche) with primers that amplify <250-bp products of mitochondrial DNA and nuclear-encoded β-globin DNA (Godley et al. 2005). Such short DNA fragments are unlikely to contain DNA damage and their amplification is therefore dependent on the amount of template.
To detect possible DNA damage, PCR was done to amplify long templates using the Expand Long Template PCR System (Roche). The primers used to amplify a 16.2-kb region of mitochondrial DNA were 5'-TGAGGCCAAATATCATTCTGAGGGGC-3' and 5'-TTTCATCATGCGGAGATGTTGGATGG-3' (Yakes and Van Houten, 1997). Primers for the amplification of a 13.5-kb region of the nuclear-encoded β-globin gene were 5'-CGAGTAAGAGACCATTGTGGCAG-3' and 5'-GCACTGGCTTAGGAGTTGGACT-3' (Chen et al. 2007). The thermal cycling protocol for the 16.2-kb mitochondrial DNA included denaturation at 94°C for 2 min, followed by 11 cycles of denaturation at 94°C (10 sec), annealing at 52°C (30 sec), and elongation at 68°C (13.5 min). This was followed by another 18 cycles of the same protocol, except that 20 sec was added to the elongation step for each additional cycle. A final elongation at 68°C (7 min) was followed by a rapid cool-down to 4°C. The thermal cycling protocol for the 13.5 kb β-globin product was the same except that the annealing temperature was 62°C, and the initial elongation was 12 min, which was increased by 20 sec for each additional cycle after the first 11 cycles. Aliquots of the reactions were then loaded onto 0.6% agarose gels (in Tris-acetate-EDTA buffer) and electrophoresed for 45 min at 120 V. The DNA was visualized with the dye ethidium bromide. The relative abundance of PCR product in each sample was determined by densitometric analysis of the gel images using UN-SCAN-IT software (ver. 6.1, Silk Scientific, Orem, UT).
2.9. Miscellaneous
The activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined spectrophotometrically in cell lysates according to (Tao et al. 1994). Protein was determined by a modified Lowry method.
2.10. Statistical analysis
Differences between multiple treatments with a single variable were assessed using one-way ANOVA and the Tukey-Kramer post test (Prism software, Graphpad). Experiments with two variables were assessed by two-way ANOVA using the same software. Significance was assumed at p < 0.05.
3. Results
3.1. Relative sensitivity of protein thiols
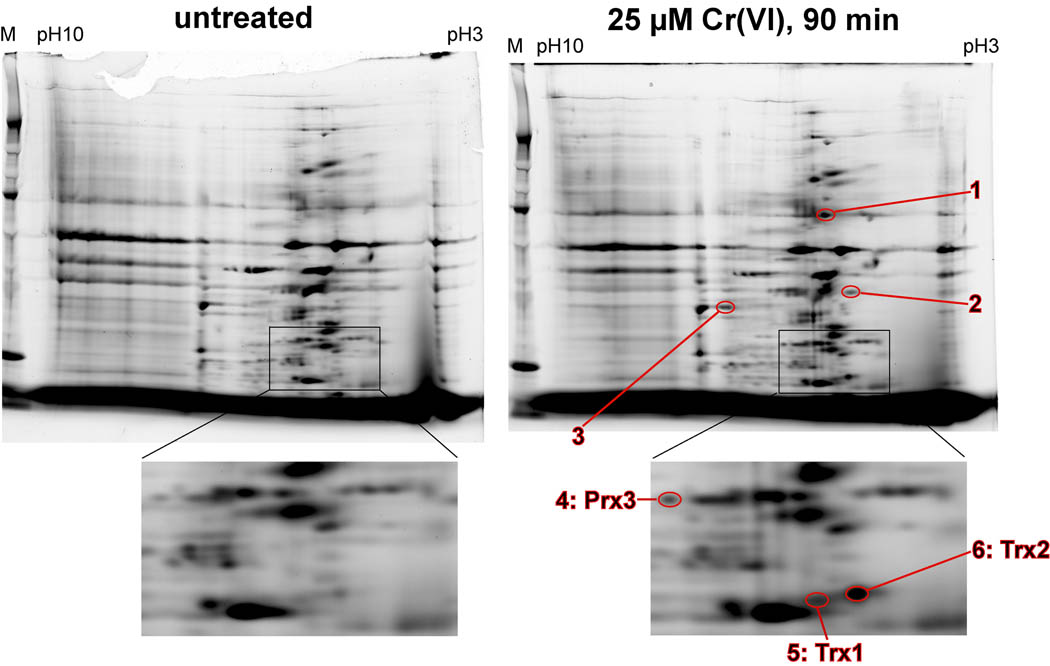
Previous studies have demonstrated that Cr(VI) treatment of human bronchial epithelial cells results in the oxidation of Trx1, Trx2, and Prx3 (Myers et al. 2008; Myers and Myers, 2009). However, Cr(VI) treatment does not change GSH levels (Myers, J.M. et al. 2008) suggesting that it does not result in the indiscriminate oxidation of cellular thiols. To further elucidate the relative susceptibility of the Trx system relative to other protein thiols, 2D electrophoresis was done to assess protein thiol oxidation in BEAS-2B cells. Oxidant treatments that result in complete oxidation of the Trx's could result in the oxidation of many proteins whose thiols are maintained by Trx, so such treatments were avoided. Instead, we examined a 90 min Cr(VI) treatment with 25 µM Cr(VI) that causes only partial oxidation of Trx1 (37%) and Trx2 (73%), (Myers et al. 2008). With this treatment, only six proteins were consistently more oxidized than in untreated cells (Fig. 1). Among these six were Trx2, Trx1, and Prx3 (Prx3 is directly dependent on Trx2) that were previously shown by redox western blots to show increased oxidation following Cr(VI) treatment (Myers et al. 2008; Myers and Myers, 2009). Therefore, this Cr(VI) treatment did not cause indiscriminant thiol oxidation, and the Trx/Prx system is among the most sensitive of the protein thiols in BEAS-2B cells. The identity of the other three proteins that were oxidized remains to be determined, and it is unknown if their redox state is controlled by Trx1 or Trx2.
Fig. 1.
Representative 2D electrophoresis of oxidized protein thiols in untreated (left) vs. Cr(VI)-treated (25 µM, 90 min) (right) BEAS-2B cells. M = marker lane at left of each gel. An expanded view of the regions containing Trx and Prx are shown at the bottom. Increased fluorescence following Cr(VI) treatment of the cells indicates greater oxidation of that protein (the 6 circled spots). After image capture, the gels were transferred to nitrocellulose and sequentially probed with different antibodies. Spots 4, 5, and 6 exactly correspond to the positions of Prx3, Trx1, and Trx2, respectively. Spots 1–3 have are have not been identified.
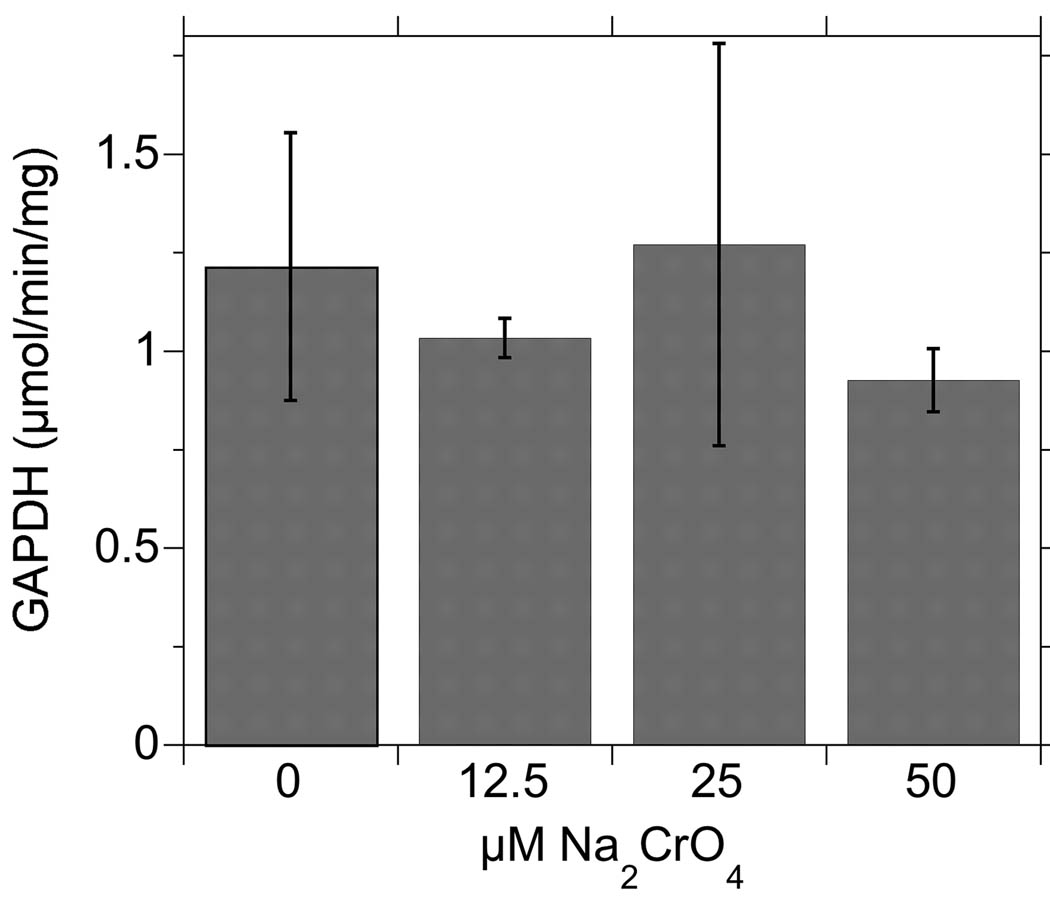
Since the active site thiol in GAPDH has proven to be very sensitive to redox modification (Baty et al. 2005; Schuppe-Koistinen et al. 1994), we examined GAPDH activity in Cr(VI)-treated BEAS-2B cells (Fig. 2). To determine if GAPDH was as sensitive as the Trx/Prx proteins, we used the 90 min exposure as in Fig. 1, but included a range of Cr concentrations (0, 12.5, 25, and 50 µM) that bracketed the 25 µM that was used in Fig. 1. These Cr(VI) treatments did not cause a detectable change in GAPDH activity indicating that the active site thiol in GAPDH was not significantly affected.
Fig. 2.
GAPDH activity (mean ± S.D., n = 3) in BEAS-2B cells treated with Cr(VI) for 90 min. One-way ANOVA indicated that the values are not significantly different (p > 0.05). There was no change in GAPDH protein level by western blot (not shown).
3.2. Normal human bronchial epithelial cells
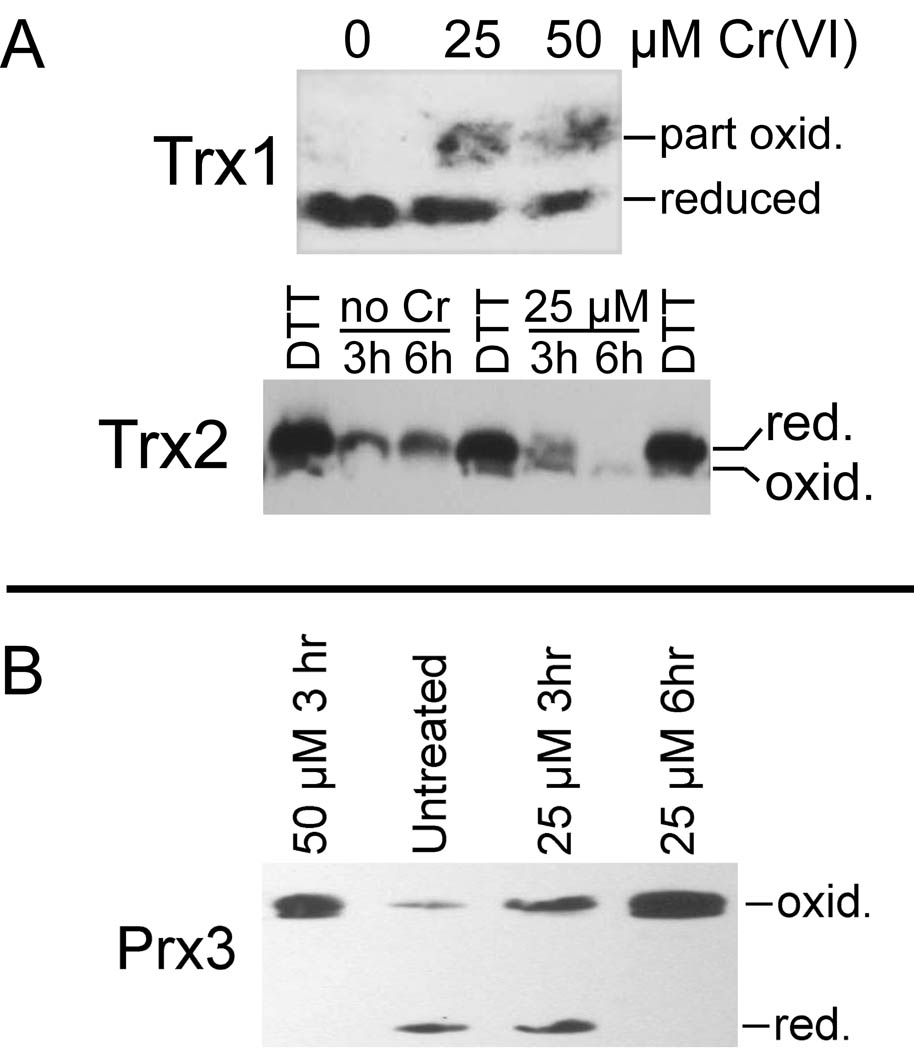
The studies to date showing Cr(VI)-mediated Trx/Prx oxidation in human bronchial epithelial cells were conducted using the BEAS-2B cell line (Myers et al. 2008; Myers and Myers, 2009). In order to validate that these findings are not unique to BEAS-2B cells, experiments were conducted with primary human bronchial epithelial (NHBE) cells. NHBE cells were treated with 0, 25, or 50 µM Cr(VI) for 3 or 6 hr as indicated, and examples of results are shown. The two different exposure times provided for assessment of the contributions of time and concentration on the results. While essentially all Trx1 is in the reduced state in control cells, both 25 and 50 µM Cr(VI) generated partially oxidized Trx1 (Fig. 3A) with 47–54% in the oxidized state. In Fig. 3A, reduced Trx1 refers to the form in which both dithiols are reduced and represents the active form. Partially oxidized Trx1 represents oxidation of one of the two dithiols, of which the active site (C32/C35) is more easily oxidized (Watson et al. 2003). Trx2 has only one dithiol which is the active site. In control cells, Trx2 was in the fully reduced state. After 3 hr with 25 µM Cr(VI), Trx2 was about a 50:50 mix of reduced and oxidized forms, whereas it was all converted to the oxidized form after 6 hr (Fig. 3A). 50 µM Cr(VI) also resulted in the complete oxidation of Trx2 after 3 hr and 6 hr (not shown). Prx3, which is directly dependent on Trx2, became more oxidized after 3 hr with 25 µM Cr(VI) (Fig. 3B), a treatment which causes partial but incomplete oxidation of Trx2 (Fig. 3A). Prx3 became completely oxidized after 6 hr with 25 µM Cr(VI), and after 3 hr with 50 µM Cr(VI) (Fig. 3B); both of these treatments resulted in the complete oxidation of Trx2. These effects on Trx1, Trx2, and Prx3 in NHBE cells are similar to those previously noted in BEAS-2B cells (Myers and Myers, 2009). Since these two cells respond similarly, and since the NHBE cells are cost-prohibitive for routine use, the remaining studies were conducted with BEAS-2B cells.
Fig. 3.
Representative redox western blots examining the Cr(VI)-mediated oxidation of Trx1, Trx2, and Prx3 in NHBE cells. A, redox status of cytosolic Trx1 after 6hr Cr(VI) treatment, and of mitochondrial Trx2 after 3 and 6 hr of treatment. For Trx2, the lanes marked DTT are isolated mitochondria that had been pre-treated with DTT which reduces Trx2 (Myers et al. 2008); these serve as a reference for the migration of reduced Trx2. B, redox status of mitochondrial Prx3 after 3 hr and 6 hr of treatment.
3.3. Does ascorbic acid protect the TrxR/Trx system from inhibition or oxidation
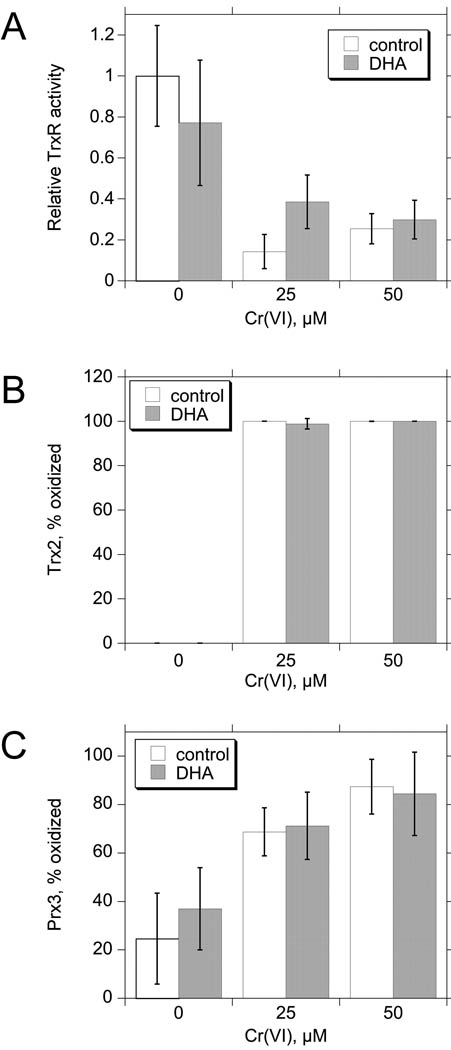
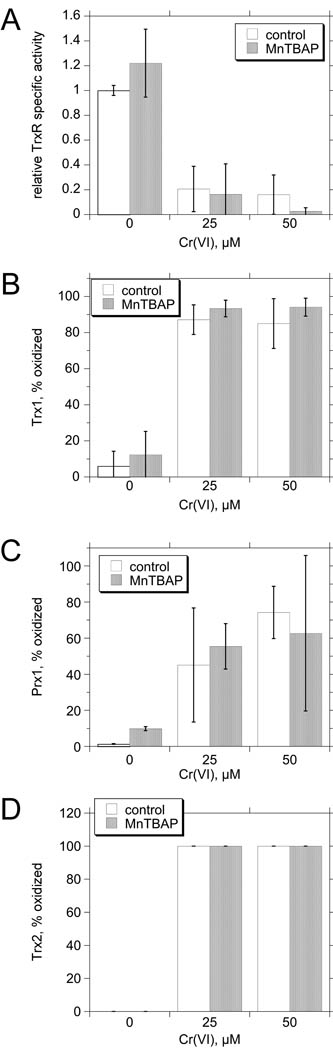
Ascorbic acid is one of several intracellular Cr(VI) reductants. Human cells cannot synthesize their own ascorbic acid, and fetal bovine serum provides ascorbate for cell culture. Because 10% FBS is routinely used, intracellular ascorbate levels are predicted to be below those observed in vivo (Reynolds et al. 2007). Dehydroascorbate (DHA) is the form taken up by cells, so supplementation of culture media with DHA can quickly restore ascorbate levels, e.g. loading human bronchial epithelial cells with 0.5 mM DHA for 90 min results in ca. 3 mM intracellular ascorbate (Reynolds et al. 2007), which is considerably higher than in vivo levels. Experiments were conducted to determine if increased intracellular ascorbate levels might alter the effects of Cr(VI) on the TrxR/Trx/Prx system. BEAS-2B cells were pre-treated with 1 mM DHA or vehicle for 90 min, after which they were treated with Cr(VI) (0, 25, or 50 µM) for 3 hr. These Cr treatments (used in subsequent experiments also) were chosen because they cause significant effects on the TrxR/Trx/Prx system (Myers and Myers, 2009) and mitochondrial electron transport (Myers et al. 2010), but they do not cause cell detachment or other signs of stress after 3 or 6 hr (Myers and Myers, 2009). Exposure to 25 or 50 µM Cr(VI) for 3 hr caused a 73–81% inhibition of TrxR activity, a shift in Trx2 oxidation from completely reduced to ≥98% oxidized, and an increase in oxidized Prx3 from 35–45% in control cells to 75–91% oxidized in Cr(VI)-treated cells (Fig. 4). Pre-loading cells with DHA had no significant effect on the activity of TrxR, or on the oxidation of Trx2 or Prx3 in control or Cr(VI)-treated cells (Fig. 4). DHA pre-loading also did not affect the redox state of Trx1 in control cells or the partial oxidation of Trx1 that results from these Cr(VI) treatments (not shown). Overall, the effects of Cr(VI) on the TrxR/Trx/Prx system are not altered by pre-loading the cells with DHA.
Fig. 4.
The effects of pre-loading BEAS-2B cells with 1 mM DHA for 90 min vs. vehicle control. analyzed for TrxR specific activity (A), or by redox western blots for Trx2 (B) or Prx3 (C). Data are the mean ± S.D. for independent experiments (n = 5 for TrxR activity, n = 4 for Trx2 and Prx3). For all assays, two-way ANOVA indicates that there were no significant differences between the presence and absence of DHA at each Cr(VI) concentration (p > 0.05).
3.4. Does MnTBAP protect the TrxR/Trx system from oxidation/inhibition
The reduction of Cr(VI) leads to several oxidants, including superoxide (O2•−), H2O2, HO•, Cr(V) and Cr(IV), and peroxynitrite (Borthiry et al. 2007, 2008; Leonard et al. 2000; Myers et al. 2000b; Pritchard et al. 2000; Sugden, 1999; Sugden et al. 2001). Some of these oxidants have been detected in Cr(VI)-treated cells, and one or more of these oxidants could therefore be directly or indirectly involved in the inhibition of TrxR and the oxidation of Trx's and Prx's.
MnTBAP is a cell-permeable antioxidant that can protect against redox cycling agents such as paraquat that promote the generation of large amounts of O2•− (Day et al. 1995). While MnTBAP is often cited as a mimetic of superoxide dismutase, its effectiveness as a peroxynitrite scavenger may largely account for its protective effects (Batinic-Haberle et al. 2009; Szabo et al. 1996). Exposure to 25 or 50 µM Cr(VI) for 3 hr caused a 79–84% inhibition of TrxR activity, a shift in Trx1 oxidation from <10% oxidized to 85–87% oxidized, an increase in Prx1 oxidation from <10% oxidized to 45–74% oxidized, and a shift in Trx2 oxidation from completely reduced to completely oxidized (Fig. 5). While peroxynitrite generation can be increased by Cr(VI) exposure (Pritchard et al. 2000), pre-loading BEAS-2B cells with 0.2 mM MnTBAP for 2 hr had no significant effect on the activity of TrxR, or on the oxidation of Trx1, Prx1, or Trx2 in control or Cr(VI)-treated cells (Fig. 5). Pre-loading cells with DHA MnTBAP also did not change the basal redox state of Prx3 or protect Prx3 from oxidation induced by 25 or 50 µM Cr(VI) (not shown).
Fig. 5.
MnTBAP does not protect the TrxR/Trx system from Cr(VI) exposure. BEAS-2B cells were pre-treated with 0.2 mM MnTBAP or vehicle for 2 hr and then Cr(VI) was added at the indicated concentrations for 3 hr. The cells were analyzed for TrxR specific activity (A), or by redox western blots for Trx1 (B) Prx1 (C), or Trx2. Data are the mean ± S.D. for independent experiments (n = 4 for TrxR activity except n = 3 for 25 µM; n = 5 for Trx1; n = 3 for Prx1 and Trx2). For all assays, two-way ANOVA indicates that there were no significant differences between the presence and absence of MnTBAP at each Cr(VI) concentration (p > 0.05).
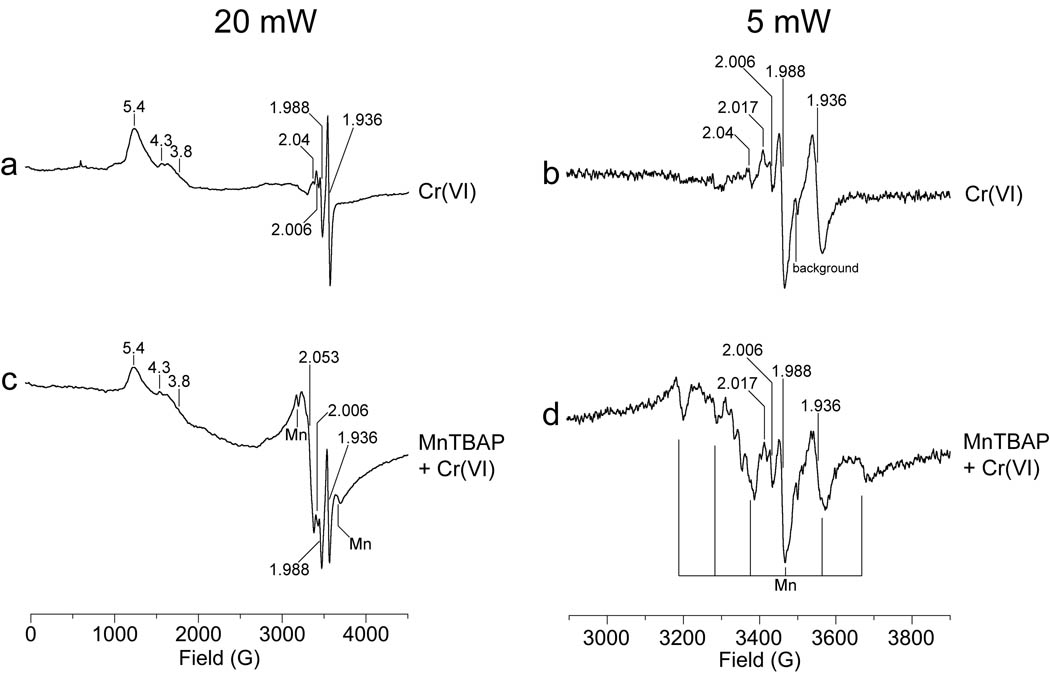
It was previously shown that treatment of BEAS-2B cells with 25 µM Cr(VI) for 3 hr also results in the inhibition of mitochondrial complex I and II activities (Myers et al. 2010). These effects were reflected in characteristic EPR signals for mitochondrial iron-sulfur (Fe-S) proteins when analyzed at liquid helium temperature (Myers et al. 2010). Peroxynitrite can cause marked inhibition of mitochondrial electron transport, and MnTBAP protects against this inhibition (Szabo et al. 1996). Peroxynitrite must therefore be considered as a potential contributor to the Cr(VI)-initiated inhibition of complex I and II. EPR was used to determine if MnTBAP could protect these centers from Cr(VI)-mediated damage. Each sample was run at two different microwave powers: 20 mW to observe the full spectrum, and 5 mW to obtain finer resolution of the region in which the Fe-S and Cr(V) signals are observed. Similar signals for both Cr(V) and Cr(III) species were observed in Cr(VI)-treated cells both with and without MnTBAP, indicating that Cr(VI) uptake and its intracellular reduction were similar in cells regardless of MnTBAP (Fig. 6). The signal at g = 1.988 is consistent with a mixture of various Cr(V) species which could include Cr(V)-thiol, Cr(V)-GSH like species, or Cr(V)-diol-thiol species (Levina et al. 2010). Signals for Cr(III) were observed at geff = 5.4 and 4.3 (Fig. 6). The lines in the g = 5 region are characteristic of Cr(III) complexes with a large ZFS (zero field splitting), signifying a distorted geometry. There is also a broad signal in the g = 2 region assigned to unresolved Cr(III) complexes with a weak ZFS. While the exact composition of these Cr(III) species is unknown, the spectral features suggest complex(es) with ligands from cellular components. Overall, these Cr signals demonstrate that some of Cr(VI) entered the BEAS-2B cells and was reduced to Cr(V) and Cr(III). Cr(V) is a short-lived reactive intermediate and its signal intensity therefore represents the relative level of Cr(V) formation near the time the sample was collected (Myers et al. 2000a). In contrast, Cr(III) is a stable redox state and therefore accumulates over time as additional Cr(VI) is reduced via the reactive intermediates to eventually generate Cr(III).
Fig. 6.
Representative EPR spectra of BEAS-2B cells showing that MnTBAP does not protect mitochondrial electron transport centers from Cr(VI) treatment. Cells were pre-treated with 0.2 mM MnTBAP (c, d) or vehicle (a, b) for 2 hr, and then 25 µM Cr(VI) was added for 3 hr, after which the cells were washed and harvested as described in the Methods. The final cell suspension (ca. 8 × 106 cells in 0.3 ml HBSS in a 4-mm quartz EPR tube) was immediately frozen in liquid nitrogen. Each sample was analyzed at liquid helium temperature (10 K), and each spectrum was corrected for background. The samples were analyzed using a microwave power of 20 mW (a, c), or 5 mW (b, d). Other instrument settings were: 5 G modulation amplitude, 60 dB receiver gain, 82 msec time constant, 9.633 GHz microwave frequency, modulation frequency = 100 kHz, scan time = 83.9 sec; number of scans, 9.
Cr(VI)-treated BEAS-2B cells also showed an intense EPR signal at g = 1.936 (Fig. 6). This signal was previously reported in these cells but it is not seen in cells treated with vehicle alone (Myers et al. 2010). This g = 1.936 signal (often referred to as the g = 1.94 signal) is consistent with reduced [2Fe-2S]1+ centers in mitochondrial electron transport complexes I and II (Yakovlev et al. 2007). Since this g = 1.936 signal is not seen in untreated cells (Myers et al. 2010), the 2Fe-2S centers of complexes I and II are normally in the oxidized [2Fe-2S]2+ state which is EPR silent. The disruption of electron flow through these complexes results in a back-up of electrons, thereby generating the reduced [2Fe-2S]1+ forms and thus the g = 1.936 signal. This signal therefore indicates diminished electron flow through complex I and/or II in Cr(VI)-treated BEAS-2B cells. While the g = 1.936 signal is a little smaller in the MnTBAP sample in the example shown (Fig. 6), replicate experiments showed that there was not a significant difference in the intensity of this signal between cells that were pre-loaded with MnTBAP versus those that received Cr(VI) only. There is, however, greater complexity to the EPR signals in cells with MnTBAP, which is attributed to the multiline signal for manganese (Mn). The six major lines for the Mn signal are indicated in Fig 6, spectrum d, and there are two lesser Mn signals between each of these major lines. These Mn signals verify the entry of MnTBAP into BEAS-2B cells, but they interfere with the ability to interpret potential changes to some other signals in this region, including those at g = 2.017 and g = 2.04. The signal at g = 2.017 (2.02) has been previously noted to increase markedly as a result of Cr(VI) exposure, and temperature and power studies indicate that it largely results from an inactive [3Fe-4S]1+ form of aconitase, although there is some contribution from the low field line of reduced mitochondrial [2Fe-2S]1+ centers (Myers et al. 2010). While a g = 2.017 signal was also noted in the cells treated with Cr(VI) plus MnTBAP (Fig. 6), the Mn signals made it difficult to accurately assess potential changes in its intensity. A small signal at g = 2.04 was also noted in the Cr(VI)-treated BEAS-2B cells (Fig. 6). This g = 2.04 signal is consistent with center N3 of complex I (Svistunenko et al. 2006), but the Mn signals interfered with its detection in those cells pre-treated with MnTBAP. The intensity of the g = 2.006 signal was not significantly changed by MnTBAP (Fig. 6). This signal is assigned to a free radical, probably ubisemiquinone (Ohnishi, 1998; Pearce et al. 2009), and has been previously shown to be enhanced by Cr(VI) exposure (Myers et al. 2010).
3.5. Is TrxR nitrated as a result of Cr(VI) exposure
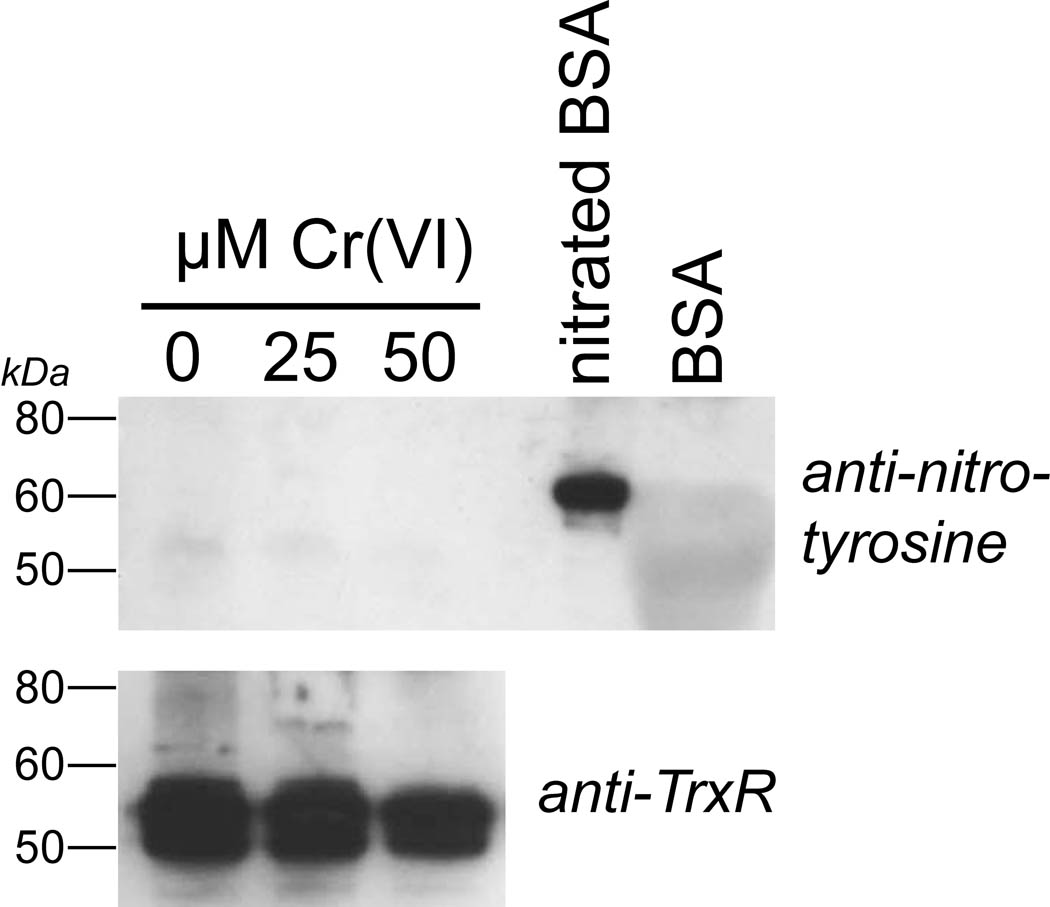
Prior studies have shown that the Cr(VI)-mediated inhibition of TrxR in BEAS-2B cells continues after Cr(VI) is removed (Myers and Myers, 2009), suggesting a modification to TrxR that is not easily reversed. Cr(VI) causes increased nitrotyrosine modifications to proteins in human endothelial cells implying that peroxynitrite generation is increased by Cr(VI) (Pritchard et al. 2000). While MnTBAP did not protect TrxR from inactivation (above), it is still possible that some nitration of TrxR may have occurred as a result of Cr(VI) treatment. Experiments were therefore conducted to determine if nitration of tyrosine residues in TrxR could be detected in Cr(VI)-treated BEAS-2B cells. Following Cr(VI) treatment, cells were washed and lysed, and TrxR was concentrated by immunoprecipitation and then analyzed by western blot using anti-nitrotyrosine antibody. While nitrotyrosine was readily detected in the nitrated albumin control protein, there was at best a faint signal for nitrotyrosine in TrxR, and this signal did not increase in Cr(VI)-treated cells (Fig. 7).
Fig. 7.
Representative western blot to determine if TrxR nitration occurs as a result of Cr(VI) treatment. BEAS-2B cells were treated for 3 hr with the indicated concentrations of Cr(VI). The cells (ca. 1 ×107) were washed, harvested, and lysed and TrxR was immunoprecipitated from the lysates using anti-TrxR and protein A/G agarose as described in the methods. The immunoprecipitates were run on SDS-PAGE. The lanes that were probed with anti-nitrotyrosine (top) were loaded with 10-fold more of the immunoprecipitate than those that were probed with anti-TrxR (bottom). The top gel also includes nitrated BSA (1 µg) as a positive control, and BSA as a negative control.
3.6. Assessment of potential DNA damage
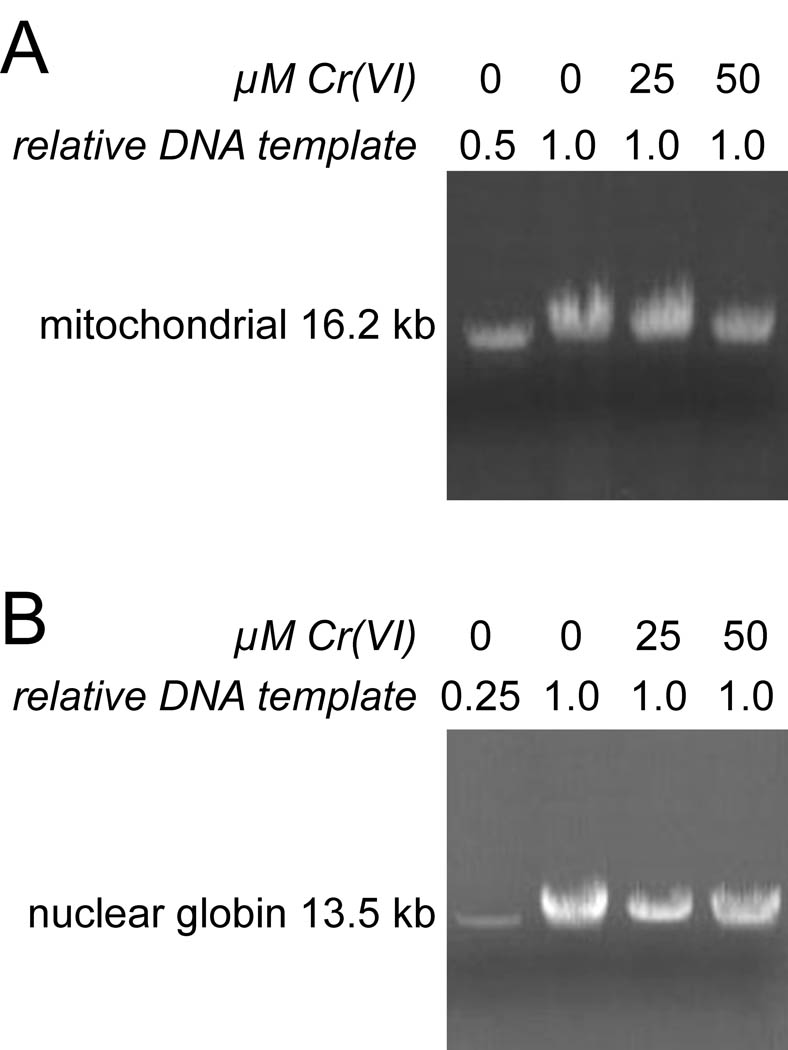
Some types of oxidant stress can result in DNA damage, particularly to mitochondrial DNA (Godley et al. 2005; Milano and Day, 2000; Yakes and Van Houten, 1997). Experiments were done to determine if Cr(VI) treatments (25 and 50 µM for 3 hr) that caused significant effects on Trx2 and Prx3 were sufficient to cause DNA damage in BEAS-2B cells, PCR was done to amplify long targets (16.2 kb mitochondrial and 13.5 kb nuclear). Several types of DNA lesions (e.g. strand breaks, several base modifications, and apurinic sites) will block amplification of these long targets. Single-stranded breaks cause complete blocks, whereas about half of the total base modifications caused by H2O2 cause strong blocks (Jaruga and Dizdaroglu, 1996). The amplification of both mitochondrial and nuclear fragments allows one to distinguish differential DNA damage in these two compartments. An example of these experiments is shown in Fig. 8A (mitochondrial) and 8B (nuclear-encoded globin gene). Controls with 50% less and 75% less template showed the expected declines in band intensity, indicating that the PCR conditions were within the exponential range and that less available template could be readily detected. In replicate experiments, we could not detect a significant decline in PCR product yield of either the mitochondrial or nuclear DNA product, even with 50 µM Cr(VI) for 3 hr (Fig. 8). While the mitochondrial band for the 50 µM Cr example shown in Fig. 8A is less intense than control (79% of 0 µM Cr), there was not a significant difference from control, i.e. 50 µM Cr was 89 ± 36% of control.
Fig. 8.
Cr(VI) treatment does not cause detectable DNA damage in the mitochondria or nucleus of BEAS-2B cells. Representative PCR of total DNA using primers that amplify 16.2 kb of mitochondrial DNA (A), or a 13.5-kb region of nuclear DNA encoding the β-globin gene (B). Total DNA was isolated from cells that had been treated with the indicated concentrations of Cr(VI) for 3 hr. The relative amount of DNA template that was used in each PCR reaction is indicated. The leftmost lane in each panel shows a decreased template control with 50% less (A) or 75% less (B) DNA template from control cells used for the PCR reaction.
3.7. Assessment of Txnip
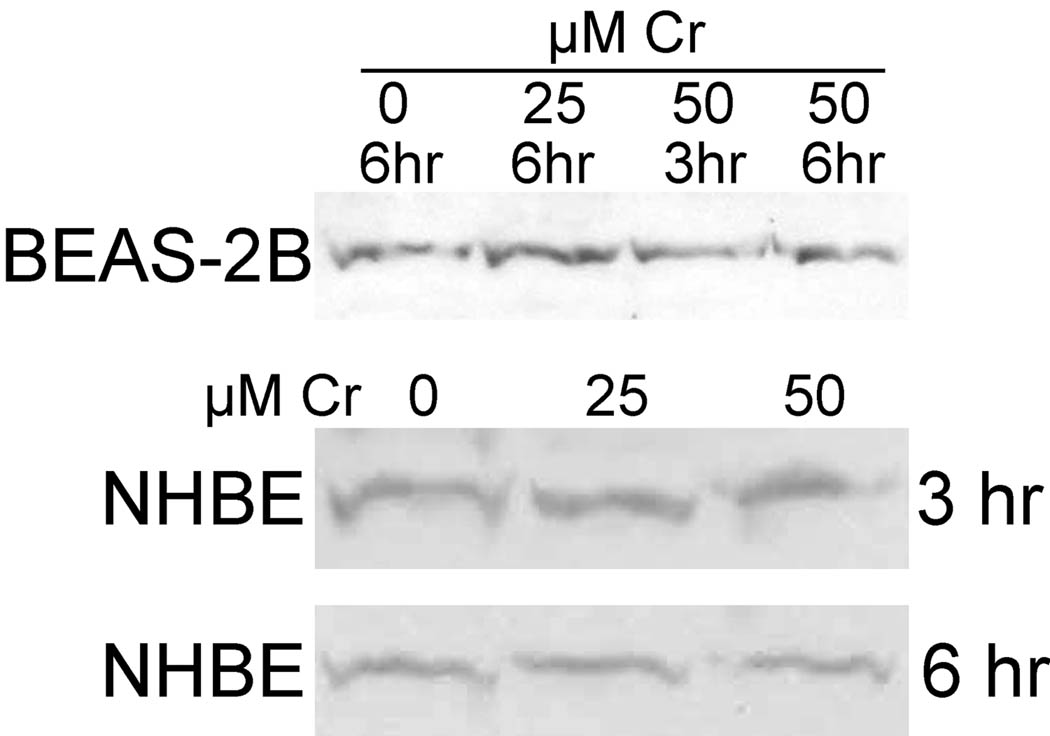
In unstressed cells, reduced Trx1 and Trx2 negatively regulate apoptosis-signaling kinase (ASK1) by binding to an N-terminal domain, whereas Trx1 and 2 oxidation results in their dissociation from ASK1 facilitating ASK1 activation and thereby promoting apoptosis (Nordberg and Arnér, 2001; Saitoh et al. 1998; Saxena et al. 2010). An increase in Txnip (Trx-interacting protein) (Patwari et al. 2006) can promote Trx1 and Trx2 oxidation and the resulting activation of ASK1 (Junn et al. 2000; Saxena et al. 2010; Yamawaki et al. 2005). Decreased Txnip would have the opposite effect (Saxena et al. 2010; Yamawaki et al. 2005). We observed that Txnip levels do not change in BEAS-2B or NHBE cells as a result of treatment with 25 or 50 µM Cr(VI) (Fig. 9). This was true for both the 3-hr treatments used for most of the other experiments, and for 6-hr treatments which cause maximal effects on Trx and Prx redox state. Therefore, the Cr(VI)-mediated oxidation of Trx1 and Trx2 cannot be explained by altered Txnip levels.
Fig. 9.
Representative western blots of whole cell lysates showing Txnip levels in NHBE or BEAS-2B cells treated for 3 or 6 hr with 0, 25, or 50 µM Cr(VI) as indicated. There was no change in GAPDH protein level in these cells as shown by western blot (not shown).
Discussion
The sum total of the 2D thiol oxidation studies (Fig. 1), GAPDH activity (Fig. 2), and the prior data on Trx and Prx redox state and on GSH levels (Myers et al. 2008; Myers and Myers, 2009), illustrate that the Trx/Prx proteins are among the most sensitive proteins in BEAS-2B cells to Cr(VI)-mediated oxidation. This is consistent with the previous results that Cr(VI) does not cause significant GSH oxidation in these cells (Myers et al. 2008; Myers and Myers, 2009). Together, the studies imply that Cr(VI) does not cause indiscriminant thiol oxidation. The enhanced sensitivity of the TrxR/Trx system is, in fact, consistent with the properties of their active sites. Most oxidants react with thiolates (−S−), not thiols (−SH) (Winterbourn and Hampton, 2008). Since a typical protein thiol has a pKa of ca. 8.5, and GSH of ca. 8.8 (Winterbourn and Hampton, 2008), most thiols are not predicted to be very reactive at pH 7.4. In contrast, the active sites of Trx (pKa of ca. 6.5) and TrxR (pKa estimated at 5.2) (Carvalho et al. 2006; Jacob et al. 2003; Winterbourn and Hampton, 2008), would be expected to be largely ionized and therefore very reactive at pH 7.4. The selenocysteine of the active site of TrxR is exposed on the surface of the enzyme (Sandalova et al. 2001), and is a stronger nucleophile than cysteine which should further enhance its reactivity (Johansson et al. 2005). While Prxs are readily oxidized by peroxides, their thiols may be somewhat protected from other species (Peskin et al. 2007). A previous study that examined the relationship between Trx and Prx oxidation in Cr(VI)-treated BEAS-2B cells indicated that Prx oxidation may largely result from lack of reducing equivalents from the respective Trxs (Myers and Myers, 2009). Similarly, the inhibition of TrxR may be a significant contributor to Trx oxidation (Myers and Myers, 2009). The 2-D thiol oxidation studies primarily assess reversible oxidation events, so TrxR inactivation would not necessarily be expected to be seen in these gels given that its inactivation in Cr(VI)-treated cells is irreversible or difficult to reverse (Myers and Myers, 2009).
For the treatments used in these studies including the most aggressive (25 and 50 µM Cr(VI) for 3 or 6 hr), the cells showed no signs of detachment or other signs of cell stress at the end of Cr(VI) exposure. It has previously been determined that when they are returned to Cr(VI)-free medium after these treatments, they will detach and show other signs of cell stress about a day later (Myers and Myers, 2009). Clonogenic survival studies have shown that 3 hr (not shown) or 6 hr (Myers and Myers, 2009) treatment with 25 or 50 µM Cr(VI) is sufficient to essentially completely prevent long-term survival and replication. Thus, while these Cr(VI) treatments are toxic to the cells from the perspective of long-term survival, rapid cell death during the period of Cr(VI) treatment does not occur and does not therefore contribute to the effects we report here.
Since peroxynitrite is known to inactivate some proteins, and can be generated in Cr(VI)-treated endothelial cells (Pritchard et al. 2000), it was considered a potential candidate for the effects we observed. However, there was no evidence for Cr-mediated nitration of TrxR in BEAS-2B cells, so nitration is therefore not a likely mechanism for TrxR inhibition. The peroxynitrite scavenger MnTBAP also had no protective effects on the TrxR/Trx/Prx system or on the mitochondrial electron transport chain as shown by the strong g = 1.936 signal in Cr(VI)-treated samples both with and without MnTBAP. Overall, the results suggest that peroxynitrite is not a significant contributor to the mitochondrial effects associated with Cr(VI) exposure. While MnTBAP is readily cell-permeable, its relative distribution into various cell subcompartments is not well-defined. However, several lines of evidence indicate that MnTBAP can enter mitochondria and serves as an important mitochondrial antioxidant. MnTBAP prevents the kainate-induced inactivation of mitochondrial aconitase in rodent hippocampi, and its effects were similar to the overexpression of SOD2 (mitochondrial) in this regard (Liang et al. 2000). MnTBAP can protect mouse fibroblast mitochondrial DNA from oxidant damage (Milano and Day, 2000), and it can rescue animals from the lethal effects of the homozygous knockout of SOD2 (Melov et al. 1998). MnTBAP also protects against Fas-induced mitochondrial events in mouse hepatocytes (Malassagne et al. 2001). Thus, it is likely that at least some of the MnTBAP entered the mitochondria in our experiments, so its inability to protect against Cr(VI) cannot be explained by exclusion from the mitochondria. Since MnTBAP also did not afford protection to the cytosolic proteins Trx1 and Prx1 (Fig. 5), it can be reasonably concluded that it is unable to block the Cr(VI)-mediated effects analyzed in these studies. Since MnTBAP is an efficient scavenger of peroxynitrite (Batinic-Haberle et al. 2009; Szabo et al. 1996), it is unlikely that peroxynitrite accounts for a significant portion of the Cr(VI)-mediated effects in BEAS-2B cells. Commercial preparations of MnTBAP also exhibit SOD activity, and it is possible that MnTBAP therefore also reduced O2•− levels in these cells. However, given the limited efficiency of MnTBAP as an SOD mimetic, it is premature to conclude that O2•− did not contribute to one or more of the Cr(VI)-mediated effects. TrxR is not inhibited by H2O2, which is in fact a substrate of TrxR (Cheng et al. 2010; Zhong and Holmgren, 2000), so H2O2 does not likely contribute to TrxR inhibition in cells.
A number of other oxidants are generated from the reductive activation of Cr(VI), including Cr(V), Cr(IV), and HO• (Borthiry et al. 2007, 2008; Sugden, 1999; Sugden et al. 2001). One or more of these species may contribute to the Cr(VI)-mediated effects we observed. Previous data suggest that a combination of TrxR inhibition and increased oxidant production results in the oxidation of Trx and Prx following Cr(VI) treatment of BEAS-2B cells (Myers and Myers, 2009). The oxidation of Trx and Prx are reversible in vitro by strong chemical disulfide reducing agents, whereas the effects on TrxR are irreversible or difficult to reverse (Myers and Myers, 2009). This prolonged inactivation of TrxR is therefore likely a critical event that has long-term implications for the disruption of normal thiol redox control. A number of divalent metals are known to inhibit the activity of TrxR including Fe2+ (Cheng et al. 2010), Mn2+ and Zn2+ (Rigobello et al. 1998). However, 50 µM Cr(III) as CrCl3 caused no inhibition of purified TrxR (data not shown). It is therefore unlikely that Cr(III) directly causes TrxR inhibition in cells, even though this Cr species accumulates as the stable final product of Cr(VI) reduction. The reactive intermediates Cr(V) and Cr(IV), as well as HO•, remain potential candidates for causing TrxR inactivation. There are multiple redox-active sites within TrxR, including the flavin (FAD), the C-terminal active site Cys-SeCys, and the N-terminal domain dithiol (-CVNVGC-) (Arnér, 2009). All of these sites are necessary for its activity, and disruption of any one of these could theoretically inactivate the enzyme.
Chromates and resulting reduced Cr species are known to induce several types of DNA damage, including strand breaks, DNA crosslinks, polymerase arrest, and base modifications (Gao et al. 1992; O'Brien et al. 2001; Sugden, 1999; Sugden et al. 2001). Many of these can impair DNA replication. With some oxidants including peroxides, mitochondrial DNA sustains greater damage than nuclear DNA (Milano and Day, 2000; Yakes and Van Houten, 1997). The enhanced susceptibility of mitochondrial DNA likely reflects several differences including the lack of histones and a complex chromatin organization, less overall DNA repair activity, and enhanced or more localized reactive species generation in mitochondria. The previously reported enhanced oxidant stress in the mitochondria of Cr(VI)-treated BEAS-2B cells (Myers and Myers, 2009) suggested the potential for mitochondrial DNA damage. However, the PCR method used here did not detect any significant damage to mitochondrial or nuclear DNA templates in BEAS-2B cells that were treated with 25 or 50 µM Cr(VI) for 3 hr. This assay is able to detect DNA damage that prevents normal extension by DNA polymerase, including strand breaks and a number of base modifications, adducts, and oxidative lesions including about half of those caused by H2O2 (Jaruga and Dizdaroglu, 1996). Since the assay samples a very small portion of the total nuclear genome, preferential damage at other sites in nuclear DNA would not be detected. In contrast, the assay accounted for more than 90% of the total mitochondrial genome (ca. 17 kb), so lesions almost anywhere within this DNA should have decreased amplification efficiency. Since there are typically dozens to hundreds of mitochondria per cell, however, lesions occurring in only a small percentage of mitochondria would not be detected. However, these Cr(VI) treatments (25 or 50 µM for 3 hr) caused complete oxidation of Trx2 indicating that essentially all mitochondria experienced marked oxidant stress. Some types of DNA modifications, such as 8-hydroxydeoxyguanosine, do not efficiently stall the polymerase, so these types of DNA modifications would have been missed. Overall, while the assay does not detect all types of DNA damage, the data suggest that the changes we observe in the TrxR/Trx/Prx system and mitochondrial electron transport of BEAS-2B cells occur prior to, or without, extensive DNA damage such as strand breaks, crosslinks, and apurinic modifications. In other reports noting Cr-mediated DNA damage, Cr(VI) exposures were typically much longer than those used here, so such events may occur well after the effects on the TrxR/Trx/Prx system.
The results with the primary NHBE cells (Figs. 3, 9) are similar to those that we have previously noted in BEAS-2B cells (Myers et al. 2008; Myers and Myers, 2009) in several ways: (a) both Trx1 and Trx2 are oxidized following Cr(VI) exposure, but the effects were greater on Trx2; (b) the oxidation of Prx3 correlates with that of Trx2, i.e. some treatments cause partial oxidation of both, whereas others cause complete oxidation of both; (3) the magnitude of the effects on the Trx/Prx proteins are dependent on both the Cr(VI) concentration and time of exposure; and (4) Txnip levels do not change as a result of Cr(VI) treatment. Overall, the effects of Cr(VI) on the Trx/Prx system are remarkably similar in these two cell types, indicating that the findings are not unique to the BEAS-2B cells.
The studies reported here used a soluble source of Cr(VI), specifically Na2CrO4. Less soluble forms of Cr(VI) such as ZnCrO4 are also strongly cytotoxic (Borthiry et al. 2008), and previous studies demonstrated that ZnCrO4 similarly disrupts the thioredoxin system and generates EPR signals for Fe-S centers as were noted here. These effects are therefore not unique to just soluble forms of Cr(VI).
In summary, the Trx/Prx system in BEAS-2B cells is among the most sensitive of cellular protein thiols to Cr(VI) exposure. While the mitochondrial Trx/Prx system is somewhat more sensitive to the effects of Cr(VI), Cr(VI) treatments that disrupted its Trx/Prx system did not cause detectable mitochondrial or nuclear DNA damage. Increasing the intracellular levels of ascorbate, an antioxidant and Cr(VI) reductant, did not alter the effects on TrxR, Trx, or Prx. Peroxynitrite does not likely contribute to the effects on the TrxR/Trx/Prx system, or to the effects on the electron transport chain. Overall, the redox stress generated by Cr(VI) treatment is quite selective for a limited number of proteins which have important roles in redox signaling, antioxidant defense, and cell survival.
Acknowledgments
This project was supported by grant number ES012707 to C. R. M. from the National Institute of Environmental Health Sciences (NIEHS), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. The EPR facilities of the Department of Biophysics are supported by National Biomedical EPR Center Grant EB001980 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AMS, 4-acetamido-4'-maleimidylstilbene-2,2'-disulfonate; BSA, bovine serum albumin; DHA, dehydroascorbate; DTNB, 5,5'-dithiobis(2-nitrobenzoic) acid; EPR, electron paramagnetic resonance; Fe-S, iron-sulfur; HBSS, Hank’s balanced salt solution; IAF, 5-iodoacetamidofluorescein; MnTBAP, Mn(III)tetrakis(4-benzoic acid)porphyrin chloride; NEM, N-ethylmaleimide; Prx, peroxiredoxin; Prx1, peroxiredoxin-1; Prx3, peroxiredoxin-3; Trx, thioredoxin; Trx1, thioredoxin-1; Trx2, thioredoxin-2; TrxR, thioredoxin reductase; Txnip, thioredoxin-interacting protein; ZFS, zero field splitting.
References
- Arnér ES. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Baruthio F. Toxic effects of chromium and its compounds. Biol. Trace Element Res. 1992;32:145–153. doi: 10.1007/BF02784599. [DOI] [PubMed] [Google Scholar]
- Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty JW, Hampton MB, Winterbourn CC. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem. J. 2005;389:785–795. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthiry GR, Antholine WE, Kalyanaraman B, Myers JM, Myers CR. Reduction of hexavalent chromium by human cytochrome b5: Generation of hydroxyl radical and superoxide. Free Radic. Biol. Med. 2007;42:738–755. doi: 10.1016/j.freeradbiomed.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthiry GR, Antholine WE, Myers JM, Myers CR. Reductive activation of hexavalent chromium by human lung epithelial cells: generation of Cr(V) and Cr(V)-thiol species. J. Inorg. Biochem. 2008;102:1449–1462. doi: 10.1016/j.jinorgbio.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AT, Fernandes PA, Ramos MJ. Determination of the ΔpKa between the active site cysteines of thioredoxin and DsbA. J. Comput. Chem. 2006;27:966–975. doi: 10.1002/jcc.20404. [DOI] [PubMed] [Google Scholar]
- Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- Chen J, Kadlubar FF, Chen JZ. DNA supercoiling suppresses real-time PCR: a new approach to the quantification of mitochondrial DNA damage and repair. Nucleic Acids Res. 2007;35:1377–1388. doi: 10.1093/nar/gkm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Antholine WE, Myers JM, Kalyanaraman B, Arnér ES, Myers CR. The selenium-independent inherent pro-oxidant NADPH oxidase activity of mammalian thioredoxin reductase and its selenium-dependent direct peroxidase activities. J. Biol. Chem. 2010;285:21708–21723. doi: 10.1074/jbc.M110.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Brown KK, Arner ES, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem. Pharmacol. 2008a;76:1097–1109. doi: 10.1016/j.bcp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Cox AG, Pullar JM, Hughes G, Ledgerwood EC, Hampton MB. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic. Biol. Med. 2008b;44:1001–1009. doi: 10.1016/j.freeradbiomed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- Deschamps F, Moulin JJ, Wild P, Labriffe H, Haguenoer JM. Mortality study among workers producing chromate pigments in France. Int. Arch. Occup. Environ. Health. 1995;67:147–152. doi: 10.1007/BF00626345. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. Integrated Risk Information System. Washington, D.C: Office of Health and Environmental Assessment, U.S. EPA; 1999. Chromium. [Google Scholar]
- Franchini I, Magnani F, Mutti A. Mortality experience among chromplating workers. Scand. J. Work Environ. Health. 1983;9:247–252. doi: 10.5271/sjweh.2413. [DOI] [PubMed] [Google Scholar]
- Gao M, Binks SP, Chipman JK, Levy LS, Braithwaite RA, Brown SS. Induction of DNA strand breaks in peripheral lymphocytes by soluble chromium compounds. Human Exp. Toxicol. 1992;11:77–82. doi: 10.1177/096032719201100203. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem. J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu. Rev. Pharmacol. Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J. Biol. Chem. 1977;252:4600–4606. [PubMed] [Google Scholar]
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers' cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br. J. Cancer. 1994;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C, Giles GI, Giles NM, Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- Jaruga P, Dizdaroglu M. Repair of products of oxidative DNA base damage in human cells. Nucleic Acids Res. 1996;24:1389–1394. doi: 10.1093/nar/24.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Gafvelin G, Arner ES. Selenocysteine in proteins-properties and biotechnological use. Biochim. Biophys. Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Leonard S, Wang S, Zang L, Castranova V, Vallyathan V, Shi X. Role of molecular oxygen in the generation of hydroxyl and superoxide anion radicals during enzymatic Cr(VI) reduction and its implication to Cr(VI)-induced carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2000;19:49–60. [PubMed] [Google Scholar]
- Levina A, Zhang L, Lay PA. Formation and reactivity of chromium(V)-thiolato complexes: a model for the intracellular reactions of carcinogenic chromium(VI) with biological thiols. J. Am. Chem. Soc. 2010;132:8720–8731. doi: 10.1021/ja101675w. [DOI] [PubMed] [Google Scholar]
- Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2008;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- Malassagne B, Ferret PJ, Hammoud R, Tulliez M, Bedda S, Trebeden H, Jaffray P, Calmus Y, Weill B, Batteux F. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121:1451–1459. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- Milano J, Day BJ. A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide-induced mitochondrial DNA damage. Nucleic Acids Res. 2000;28:968–973. doi: 10.1093/nar/28.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Antholine WE, Myers JM. The pro-oxidant chromium(VI) inhibits mitochondrial complex I, complex II, and aconitase in the bronchial epithelium: EPR markers for Fe-S proteins. Free Radic. Biol. Med. 2010;49:1903–1915. doi: 10.1016/j.freeradbiomed.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Carstens BP, Antholine WE, Myers JM. Chromium(VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Appl. Microbiol. 2000a;88:98–106. doi: 10.1046/j.1365-2672.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM, Carstens BP, Antholine WE. Reduction of chromium(VI) to chromium(V) by human microsomal enzymes: effects of iron and quinones. Toxic Subst. Mech. 2000b;19:25–51. [Google Scholar]
- Myers JM, Antholine WE, Myers CR. Hexavalent chromium causes the oxidation of thioredoxin in human bronchial epithelial cells. Toxicology. 2008;246:222–233. doi: 10.1016/j.tox.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JM, Myers CR. The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 2009;47:1477–1485. doi: 10.1016/j.freeradbiomed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- O'Brien T, Xu J, Patierno SR. Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol. Cell. Biochem. 2001;222:173–182. [PubMed] [Google Scholar]
- Ohnishi T. Iron-sulfur clusters/semiquinones in complex I. Biochim. Biophys. Acta. 1998;1364:186–206. doi: 10.1016/s0005-2728(98)00027-9. [DOI] [PubMed] [Google Scholar]
- Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LL, Epperly MW, Greenberger JS, Pitt BR, Peterson J. Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide. Nitric Oxide. 2001;5:128–136. doi: 10.1006/niox.2001.0338. [DOI] [PubMed] [Google Scholar]
- Pearce LL, Martinez-Bosch S, Manzano EL, Winnica DE, Epperly MW, Peterson J. The resistance of electron-transport chain Fe-S clusters to oxidative damage during the reaction of peroxynitrite with mitochondrial complex II and rat-heart pericardium. Nitric Oxide. 2009;20:135–142. doi: 10.1016/j.niox.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L189–L198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- Pritchard KA, Jr, Ackerman A, Kalyanaraman B. Chromium (VI) increases endothelial cell expression of ICAM-1 and decreases nitric oxide activity. J. Environ. Pathol. Toxicol. Oncol. 2000;19:251–260. [PubMed] [Google Scholar]
- Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigobello MP, Callegaro MT, Barzon E, Benetti M, Bindoli A. Purification of mitochondrial thioredoxin reductase and its involvement in the redox regulation of membrane permeability. Free Radic. Biol. Med. 1998;24:370–376. doi: 10.1016/s0891-5849(97)00216-5. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G. Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc. Natl. Acad. Sci. USA. 2001;98:9533–9538. doi: 10.1073/pnas.171178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppe-Koistinen I, Moldeus P, Bergman T, Cotgreave IA. S-thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur. J. Biochem. 1994;221:1033–1037. doi: 10.1111/j.1432-1033.1994.tb18821.x. [DOI] [PubMed] [Google Scholar]
- Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health, Pt. B. 1999a;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Shi X, Dalal NS. The role of superoxide radical in chromium(VI)-generated hydroxyl radical: the Cr(VI) Haber-Weiss cycle. Arch. Biochem. Biophys. 1992;292:323–327. doi: 10.1016/0003-9861(92)90085-b. [DOI] [PubMed] [Google Scholar]
- Shi X, Ding M, Ye J, Wang S, Leonard SS, Zang L, Castranova V, Vallyathan V, Chiu A, Dalal N, Liu K. Cr(IV) causes activation of nuclear transcription factor-kB, DNA strand breaks and dG hydroxylation via free radical reactions. J. Inorg. Biochem. 1999b;75:37–44. doi: 10.1016/S0162-0134(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. One-electron reduction of chromate by NADPH-dependent glutathione reductase. J. Inorg. Biochem. 1990;40:1–12. doi: 10.1016/0162-0134(90)80034-u. [DOI] [PubMed] [Google Scholar]
- Standeven AM, Wetterhahn KE. Is there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chem. Res. Toxicol. 1991;4:616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- Sugden KD. Formation of modified cleavage termini from the reaction of chromium(V) with DNA. J. Inorg. Biochem. 1999;77:177–183. doi: 10.1016/s0162-0134(99)00189-0. [DOI] [PubMed] [Google Scholar]
- Sugden KD, Campo CK, Martin BD. Direct oxidation of guanine and 7,8-dihydro-8-oxoguanine in DNA by a high-valent chromium complex: a possible mechanism for chromate genotoxicity. Chem. Res. Toxicol. 2001;14:1315–1322. doi: 10.1021/tx010088+. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch. Toxicol. 1990;64:169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- Svistunenko DA, Davies N, Brealey D, Singer M, Cooper CE. Mitochondrial dysfunction in patients with severe sepsis: an EPR interrogation of individual respiratory chain components. Biochim. Biophys. Acta. 2006;1757:262–272. doi: 10.1016/j.bbabio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- Szadkowski A, Myers CR. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicology. 2008;243:164–176. doi: 10.1016/j.tox.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Howlett A, Klein C. Nitric oxide regulation of glyceraldehyde-3-phosphate dehydrogenase activity in Dictyostelium discoideum cells and lysates. Eur. J. Biochem. 1994;224:447–454. doi: 10.1111/j.1432-1033.1994.00447.x. [DOI] [PubMed] [Google Scholar]
- Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev G, Reda T, Hirst J. Reevaluating the relationship between EPR spectra and enzyme structure for the iron sulfur clusters in NADH:quinone oxidoreductase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12720–12725. doi: 10.1073/pnas.0705593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J. Clin. Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- Zhong L, Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J. Biol. Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]