Abstract
Diabetes is featured by elevated levels of blood glucose, i.e. hyperglycemia, which might be a risk factor for hepatic fibrogenesis in patients with non-alcoholic steatohepatitis. Hepatic stellate cells (HSCs) are the major effectors during hepatic fibrogenesis. This study was designed to evaluate impacts of high levels of glucose on HSC activation, assess roles of the phytochemical curcumin in attenuating the glucose impacts, and elucidate underlying mechanisms. In this report, levels of intracellular glucose were measured. Contents and gene expression of glucose transporter-2 (GLUT2) in cell fractions were examined. Levels of cellular glutathione and oxidative stress were analyzed. We observed that high levels of glucose induced cell proliferation, type I collagen production and expression of genes relevant to HSC activation, and elevated intracellular glucose levels in cultured HSCs. Curcumin eliminated the stimulatory impacts. Curcumin abrogated the membrane translocation of GLUT2 by interrupting the p38 MAPK signaling pathway. In addition, curcumin suppressed glut2 expression by stimulating the activity of peroxisome proliferator-activated receptor-gamma (PPARγ) and de novo synthesis of glutathione. In conclusion, hyperglycemia stimulated HSC activation in vitro by increasing intracellular glucose, which was eliminated by curcumin by blocking the membrane translocation of GLUT2 and suppressing glut2 expression. The latter was mediated by activating PPARγ and attenuating oxidative stress. Our results presented evidence to impacts of hyperglycemia on stimulating HSC activation and hepatic fibrogenesis, and provided novel insights into the mechanisms by which curcumin eliminated the hyperglycemia-caused HSC activation and potential therapeutic strategies for treatment of diabetes-associated hepatic fibrogenesis.
Keywords: Hyperglycemia, hepatic fibrosis, glucose transport, hepatic stellate cell, polyphenol, gene expression, oxidative stress
INTRODUCTION
Diabetes mellitus (DM) is featured by elevated levels of blood glucose, i.e. hyperglycemia. Almost 6% of the world’s adult population lives with diabetes [1]. The total number of people with diabetes worldwide has been projected to rise from 171 million in 2000 to 366 million in 2030, an increase by 213% [1]. Type 2 diabetes mellitus (T2DM) represents over 80% of all diabetics and is dramatically increasing in incidence because of changes in human behavior and increased body mass index. The liver, together with skeletal muscle and adipose tissue, is one of the principal organs involved in glucose metabolism. Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome [2]. Non-alcoholic steatohepatitis (NASH) is an advanced form of NAFLD. They are often associated with obesity, insulin resistance and T2DM. NAFLD is estimated to affect about 20–30% of the population in U. S. [2]. Approximately 15–40% of NASH patients develop hepatic fibrosis [2]. T2DM- & NASH-associated hepatic fibrosis currently is the object of significant scientific and clinical interest, and is to remain so in the future years. The correlation of hyperglycemia with the presence of liver fibrosis in NASH patients has been clinically described [3]. T2DM could be a predictor of worsening hepatic fibrosis. Hyperglycemia is suggested as a harmful prognostic factor in the evolution of NASH towards fibrosis. However, little attention has been paid to impacts of hyperglycemia on HSC activation and on NASH-associated hepatic fibrogenesis.
Glucose transport across the plasma membranes of mammalian cells is carried out by two distinct processes: facilitative transport, mediated by a family of facilitative glucose transporters (GLUT); and sodium-dependent transport, mediated by the Na+/glucose cotransporters (SGLT). GLUT are important for maintaining glucose metabolism homeostasis and are molecular targets of anti-diabetic drugs [4]. Thus far, 13 functional facilitative GLUT isoforms have been cloned, characterized, 12 of which were designated as GLUT1 GLUT12 [5]. The glucose transporters GLUT1 to GLUT5 have been extensively studied. GLUT1 has been ubiquitously detected in cells and tissues. Because of the ubiquitous distribution and cellular localization, GLUT1 is considered to be the primary transporter responsible for basal glucose uptake [5]. In the liver, glucose transporter-2 (GLUT2) and glucose transporter-4 (GLUT4) are the major GLUT responsible for glucose transportation into hepatocytes [6,7]. Due to its low affinity and high capacity, GLUT2 transports glucose in a large range of physiological concentrations of glucose, whereas GLUT4 action is extensively regulated by insulin-activated phosphoinositide 3-kinases (PI3K) [6,7]. In the liver, GLUT2 is translocated from the cytoplasm to the plasma membrane in response to high levels of plasma glucose and is the primary carrier to transport plasma glucose into hepatocytes [6,7]. An abnormally high level of intracellular glucose could be a deleterious factor to impair cellular functions and to cause problems in some types of cells [8].
Hepatic stellate cells (HSCs) are the most relevant cell type for the development of hepatic fibrosis [9,10]. During liver injury, quiescent HSCs become proliferative and fibrogenic [9,10]. This process of HSC activation is coupled with the activation of signaling pathways for pro-mitogenic platelet-derived growth factor-beta (PDGF-β) [11] and pro-fibrogenic transforming growth factor-beta (TGF-β) [12], as well as the depletion of peroxisome proliferator activated receptor-gamma (PPARγ) [13–15]. HSC activation is stimulated by elevated oxidative stress and inflammation [9]. It is important to note that culturing quiescent HSCs on plastic plates causes spontaneous activation, mimicking the process seen in vivo, which provides a good model for elucidating underlying mechanisms of HSC activation and for studying possible therapeutic intervention of the process [9,10]. It has been shown that high levels of glucose stimulate expression of connective tissue growth factor (CTGF), one of the downstream effectors of TGF-β1, in HSCs [16] and induce oxidative stress [17].
In the last two decades, advances in the understanding of genes promoting HSC activation were notable. However, few effective medicines are available for treatment and/or prevention of T2DM- & NASH-associated hepatic fibrosis [18]. It is, therefore, of high priority to identify innocuous anti-fibrotic agents. Curcumin, the yellow pigment in curry from turmeric, is one of the best-studied natural compounds [19]. Studies have demonstrated the beneficial effects of curcumin on the prevalence of diabetic pathogenesis [20,21]. Curcumin has recently received attention as a promising dietary supplement for liver protection [22]. We previously reported that curcumin inhibited HSC activation by inhibiting cell proliferation, inducing apoptosis and attenuating oxidative stress in vitro and in vivo [23–26]. In addition, curcumin dramatically induced expression of endogenous PPARγ gene and its activity in activated HSCs, which was required for curcumin to inhibit HSC activation [23–26].
This study was designed to evaluate impacts of high levels of glucose on the activation of HSCs, to assess roles of the phytochemical curcumin in attenuating the glucose impacts, and to elucidate underlying mechanisms. Results in this report supported our initial hypothesis that hyperglycemia stimulated HSC activation in vitro by elevating the level of intracellular glucose, which could be eliminated by curcumin by blocking the hyperglycemia-induced membrane translocation of GLUT2 and suppressing gene expression of GLUT2 in HSCs.
MATERIALS AND METHODS
Materials
Curcumin (purity>94%), D(+)-Glucose, N-acetyl-cysteine (NAC), L-Buthionine- sulfoximine (BSO) and 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) were purchased from Sigma (St. Louis, MO). Primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), unless otherwise noted. Rosiglitazone (BRL 49653), purchased from Cayman Chemical (Ann Arbor, MI), was dissolved in DMSO in stocking solution at 100 mM.
HSC isolation and cell culture
Male Sprague Dawley rats (200–250 g), purchased from the Harlan Laboratories, Inc. (Indianapolis, IN), were housed in a temperature-controlled animal facility (23°C) with a 12-h light, 12-h dark cycle and allowed free access to regular chew and water ad libitum. HSCs were isolated by the pronase-collagenase perfusion in situ before density gradient centrifugation, as we previously described [24]. The animal protocol for the use of rats was approved by Institutional Animal Care and Use Committee of Saint Louis University. Freshly-isolated primary HSCs were cultured in Dulbecco’s modi ed Eagle’s medium (DMEM), containing glucose at 100 mg/dl, supplemented with 20% of fetal bovine serum (FBS). Cells were cultured and passaged in DMEM with 10% of FBS and 100 mg/dl of glucose, prior to the exposure to a high level of glucose, such as a final concentration at 450 mg/dl, for an indicated period of time. Semi-confluent HSCs at 60–70% confluence with four to eight passages were used for experiments in this report. Immortalized human hepatocytes (IHH) were kindly provided by Dr. Ratna Ray (Department of Pathology, St. Louis University) [27]. IHH were cultured and passaged in DMEM supplemented with glucose at 450 mg/dl and 10% of FBS, in which the high level of glucose is necessary. Otherwise, IHH would not grow well and be dying. In some of experiments, passaged HSCs were serum-starved in serum-depleted DMEM for 24 hr, which made HSCs more sensitive to the subsequent stimulation with glucose. These cells were then cultured in serum-depleted media containing glucose at indicated concentrations with or without treatment. The culture in serum-depleted media excluded the interference from other factors in FBS [28,29].
Determination of cell proliferation in vitro
Cell growth was determined by using the CellTiter 96 aqueous nonradioactive cell proliferation assay kit (MTS assays) (Promega, Madison, WI), following the protocol provided by the manufacturer. Each group was carried out in triplicates and repeated for at least three times. Results were expressed as percentage changes in the density of viable cells.
Determination of intracellular glucose levels
Intracellular glucose concentrations were determined by using a glucose assay kit from BioVision (Mountain View, CA), following the manufacturer’s instruction. Final concentrations of intracellular glucose were expressed as nmol /mg protein.
Preparation of cellular membrane protein extracts
The preparation of cellular membrane fraction was performed as described previously [30]. In brief, after washing three times with PBS, HSCs were lysed with Buffer A [Tris, pH 8.0, 50 mM; dithiothreitol, 0.5 mM; NP-40, 0.1% (v/v); protease inhibitors (phenylmethylsulfonyl fluoride, 1 mM; leupeptin, 5 μg/ml; and aprotinin, 5 μg/ml) and phosphatase inhibitors (NaF, 10 mM and Na3VO4, 1 mM)]. The lysates were then centrifuged at 1000 ×g for 10 minutes at 4ºC. Pellets were re-suspended in NP-40 free Buffer A in ice for another 10 minutes with occasional vortex and re-centrifuged 1000 ×g for 10 minutes at 4ºC. The pellets were re-suspended in Buffer A and stood in ice for 1hr with occasional vortex and centrifuged at 16,000 ×g for 20 minutes at 4ºC. The supernatant was collected as the plasma membrane fraction and stored at −80ºC until use. The supernatants from the first and second spins at 1000 xg were combined and spun at 16,000 ×g for 20 minutes at 4ºC. The resultant supernatant was collected and used as the cytosol fraction.
Western blotting analyses
Whole cell extracts were prepared as we previously described [24]. Protein concentrations were determined using the BCA Protein Assay Kit according to the manufacturer’s instruction (Pierce, Rockford, IL). SDS-PAGE, trans-blotting and subsequent immuno-detection were conducted as we previously described [24]. β-actin was used in most of experiments as an internal invariable control for equal loading. Levels of target protein bands were densitometrically determined by using Quantity One 4.4.1 (Bio-Rad, Hercules, CA). Variations in the density were expressed as fold changes compared with the control in the blot (n = 3).
Real-time polymerase chain reactions (PCR)
Total RNA extraction and real-time PCR were carried out as we previously described [24]. Total RNA was treated with DNase I prior to the synthesis of the first strand of cDNA. mRNA levels were expressed as fold changes after normalization with endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as suggested by Schmittgen et. al. [31]. The PCR primers for GLUT2 were: (F) 5′-GTG TGA GGA TGA GCT GCC TAA A-3′; (R) 5′-TTC GAG TTA AGA GGG AGC GC-3′. Other primers were described in our previous studies [23].
Plasmids and transient transfection assays
The glut2 promoter luciferase reporter plasmid pGluT2-Luc was a gift from Dr. Yong-ho Ahn (Institute of Genetic Sciences, Yonsei University College of Medicine, Seoul, Korea) [32]. It contained a DNA fragment spanning from −732 to +189 bp of the rat glut2 promoter region subcloned into the luciferase expression vector pGL3-Basic [32]. Transient transfection assays were performed using the LipofectAMINE® reagent (Invitrogen, Carlsbad, CA), as we previously described [24]. Results were combined from multiple independent experiments (n≥6).
Analyses of levels of cellular reactive oxygen species (ROS)
Levels of ROS in HSCs were determined by analyzing dichlorofluorescein (DCF) fluorescence, as described previously [26]. The DCF fluorescence units were normalized to the DNA fluorescence of Hoechst 33342 (10 μM, Molecular Probes Inc., Eugene, OR), which stained the nuclei of living cells. Results were presented as fold changes compared with those in HSCs cultured in media with glucose at 100 mg/dl.
Analyses of lipid peroxidation (LPO)
LPO assays were performed by using the Lipid Hydroperoxide Assay Kit from Cayman Chemical (Ann Arbor, MI), following the protocol provided by the manufacturer. 13-HpODE (13-hydroperoxy- octadecadienoic acid) was used as a standard.
Determination of the levels of cellular glutathione
Levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined by using the Enzyme Immune Assay Kit GSH-400® (Cayman Chemical, Ann Arbor, MI), as we previously described [23,26]. Concentrations of total GSH were calculated according to the equation in the protocol.
Analyses of the activity of glutamate-cysteine ligase (GCL)
GCL activities were spectrophotometrically determined using a coupled assay with pyruvate kinase and lactate dehydrogenase, as we previously described [23,26].
Statistical Analysis
Differences between means were evaluated using an unpaired two-sided Student’s t-test (p<0.05 was considered significant). Where appropriate, comparisons of multiple treatment conditions with control were analyzed by ANOVA with Dunnetts’s test for post hoc analysis.
RESULTS
Hyperglycemia stimulated cell growth and expression of genes relevant to HSC activation in cultured HSCs
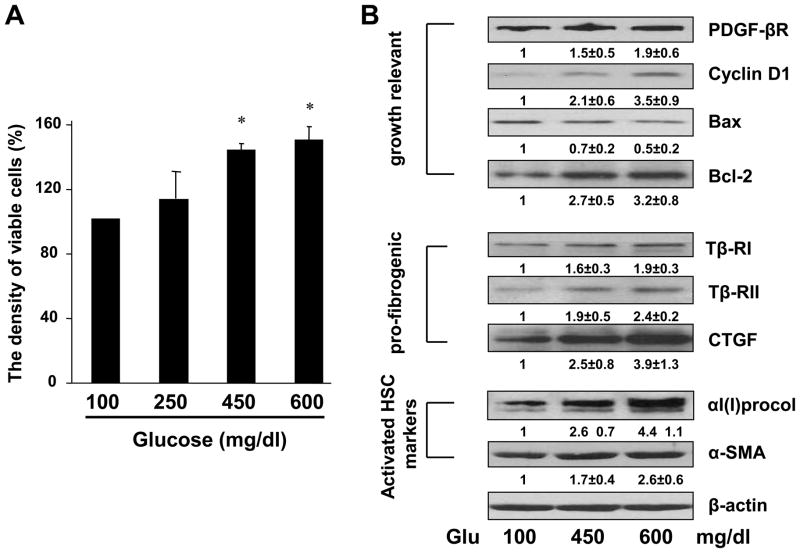
To assess impacts of hyperglycemia on HSC activation, we evaluated, first of all, the impact of high levels of glucose on stimulating HSC proliferation, a feature of activated HSCs. Glucose provides calories to cells, but it does not necessarily stimulate proliferation of cells, including neurons and quiescent HSCs. Passaged HSCs at 60–70% confluence were cultured in DMEM containing glucose at various concentrations for 24 hr. Cell growth was determined by MTS assays. As shown in Fig. 1A, compared with glucose at a normal concentration, i.e. 100 mg/dl, in the media, high levels of glucose, i.e. 250, 450, 600 mg/dl, stimulated the proliferation of HSCs in a dose-dependent manner.
Figure 1. Hyperglycemia stimulated cell growth and expression of genes relevant to HSC activation in cultured HSCs.
Passaged HSCs were cultured in DMEM with glucose at indicated concentrations for 24 hr. (A). Cell proliferation was assessed by colorimetric MTS assays. Results were expressed as percentage changes in the density of viable cells, compared with cells grown in media with glucose at 100 mg/dl (the 1st column). Values were expressed as means ± S. D. (n ≥3). *p<0.05 vs the control cells grown in media with glucose at 100 mg/dl. (B). Western blotting analyses of the abundance of proteins related to cell growth or fibrogenesis. β-actin was used as an invariant control for equal loading. Representative results were shown from three independent experiments. Italic numbers beneath blots were fold changes (means ± S.D.) in the densities of the bands compared with the control grown in media with glucose at 100 mg/dl in the blot (n=3), after normalization with the internal invariable control.
We next investigated the impact of high levels of glucose on inducing the production of type I collagen and expression of alpha-smooth muscle actin (α-SMA), two unique markers of activated HSCs. Whole cell extracts were prepared from HSCs with the aforementioned treatment. Western blotting analyses demonstrated that compared with glucose at 100 mg/dl, high levels of glucose at 450 and 600 mg/dl significantly increased the abundance of αI(I) procollagen and α-SMA in cultured HSCs (Fig. 1B). In addition, glucose at both levels in media enhanced the contents of pro-mitogenic PDGF-βR, cyclin D1 and anti-apoptotic Bcl-2, while reducing the abundance of pro-apoptotic Bax (Fig. 1B). Furthermore, the high levels of glucose elevated the contents of proteins relevant to pro-fibrogenesis, including type I and II TGF-β receptors (Tβ-RI/II) and CTGF (Fig. 1B). Glucose at 450 mg/dl in DMEM was chosen as hyperglycemia in most of the following experiments. Taken together, these results suggested that high levels of glucose dose-dependently stimulated the activation of HSCs in vitro by inducing cell proliferation and type I collagen production and altering expression of key genes relevant to the activation of HSCs.
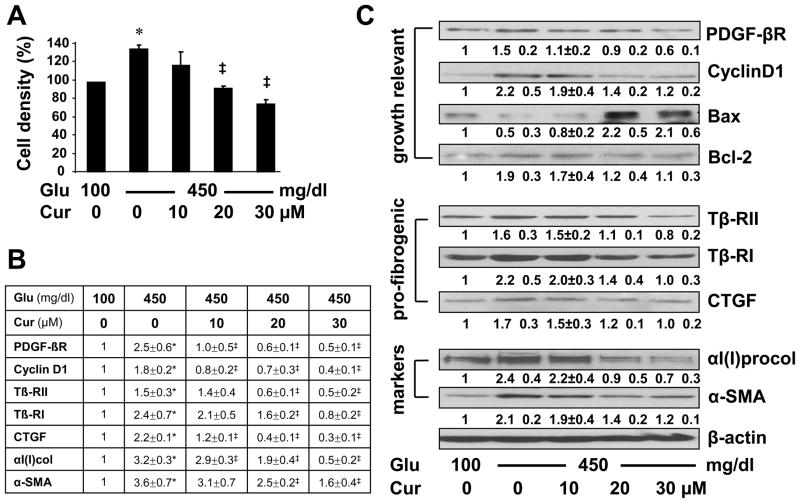
Curcumin eliminated the stimulatory impacts of hyperglycemia on HSC activation by reducing cell proliferation and suppressing expression of genes relevant to HSC activation
We previously reported that curcumin inhibited HSC activation by reducing cell proliferation and suppressing gene expression of extracellular matrix components in vitro and in vivo [23–25]. We, therefore, postulated that HSC activation induced by hyperglycemia in vitro could be inhibited by curcumin. To test the postulation, HSCs were cultured in media containing glucose at 100 or 450 mg/dl with or without the curcumin treatment (0–30 μM) for 24 hr. Cell growth was determined by MTS assays. As showed in Fig. 2A, compared with glucose at 100 mg/dl in the media, glucose at 450 mg/dl was verified to induce the proliferation of HSCs in vitro. Curcumin eliminated the stimulatory impact of hyperglycemia and reduced hyperglycemia-induced HSC proliferation in a dose-dependent manner. Further experiments were conducted to validate the observation. Total RNA and whole cell extracts were prepared from HSCs with the aforementioned treatment. Real-time PCR (Fig. 2B) and Western blotting analyses (Fig. 2C) confirmed that curcumin dose-dependently attenuated the impacts of hyperglycemia on elevating the mRNA and protein levels of genes relevant to cell growth, including PDGF-βR, cyclin D1 and Bcl-2. In addition, curcumin diminished the impacts of hyperglycemia on stimulating expression of αI(I) collagen and α-SMA, as well as pro-fibrogenic genes, including Tβ-RI/II and CTGF, in HSCs in vitro. Taken together, our results indicated that curcumin apparently eliminated the impacts of glucose on the induction of HSC activation by reducing cell proliferation and suppressing expression of genes relevant to HSC activation.
Figure 2. Curcumin eliminated the stimulatory impacts of hyperglycemia on HSC activation by reducing cell proliferation and suppressing expression of genes relevant to HSC activation.
Passaged HSCs were cultured in media with glucose at 100 mg/dl, or 450 mg/dl in the presence of curcumin (0–30 μM) for 24 hr. Values were expressed as means ± S. D. (n 3). *p<0.05 vs the control with glucose at 100 mg/dl, ‡p<0.05 vs cells cultured in media with 450 mg/dl of glucose alone. (A). Cell proliferation was assessed by colorimetric MTS assays. Results were expressed as percentage changes in the density of viable cells, compared with cells grown in media with glucose at 100 mg/dl. (B). Real-time PCR analyses of the mRNA levels of genes relevant to HSC activation. Results were expressed as fold changes (means ± S.D.), compared with cells grown in media with glucose at 100 mg/dl (n=3). (C). Western blotting analyses of the abundance of proteins related to cell growth, or fibrogenesis. β-actin was used as an invariant control for equal loading. Representative results were shown from three independent experiments. Italic numbers beneath blots were fold changes (means ± S.D.) in the densities of the bands compared with the control with glucose at 100 mg/dl in the blot (n=3), after normalization with the internal invariable control.
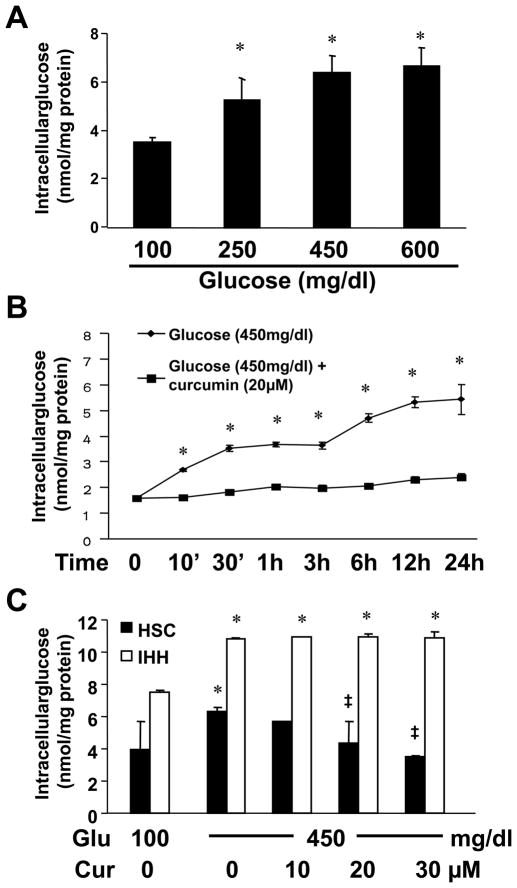
Hyperglycemia caused dose- and time-dependent biphasic increases in the level of intracellular glucose in cultured HSCs, which were abrogated by curcumin
To explore the mechanisms by which hyperglycemia stimulated HSC activation and curcumin eliminated the stimulatory impacts, we postulated that hyperglycemia elevated the level of intracellular glucose in HSCs, which was abrogated by curcumin. To test the postulation, HSCs were cultured in media with glucose at 100 mg/dl, or with glucose at 250, 450 or 600 mg/dl for 24 hr. As shown in Fig. 3A, glucose dose-dependently increased the level of intracellular glucose in cultured HSCs.
Figure 3. Hyperglycemia caused dose- and time-dependent biphasic increases in the level of intracellular glucose in cultured HSCs, which were abrogated by curcumin.
Passaged HSCs were cultured in DMEM with glucose at indicated concentrations in the presence or absence of curcumin for different hours. Levels of intracellular glucose were determined. Values were expressed as means ± S. D. (n ≥3). *p<0.05 vs the control in the 1st column, or point. ‡p <0.05 vs cells cultured in media with glucose at 450 mg/dl alone. (A). Analyses of levels of intracellular glucose in HSCs exposed to glucose at various doses for 24 hr; (B). Analyses of levels of intracellular glucose in HSCs exposed to glucose at 450 mg/dl with or without curcumin at 20 μM for 0–24 hr; (C). Analyses of levels of intracellular glucose in HSCs or in immortalized human hepatocytes (IHH) exposed to glucose at 100 mg/dl, or 450 mg/dl with curcumin (0–30 μM) for 24 hr.
To further test our postulation, passaged HSCs were pretreated with or without curcumin at 20 μM for 1 hr prior to the stimulation with glucose at 450 mg/dl for an indicated period of time (0–24 hr). The pretreatment of curcumin was used to prevent any instance action of hyperglycemia. Results in Fig. 3B showed that hyperglycemia caused time-dependent and biphasic increases in the level of intracellular glucose in cultured HSCs. The first phase occurred within the first 30 minutes after the exposure to the high level of glucose and reached its plateau. The second phase started 3 hr later after the exposure to the high level of glucose. The presence of curcumin apparently eliminated the hyperglycemia-caused biphasic increases in the level of intracellular glucose in cultured HSCs. Curcumin had no apparent impact on reducing the level of intracellular glucose when HSCs were cultured in normoglycemic media, i.e. glucose at 100 mg/dl (data not shown). Additional experiments in Fig. 3C verified the inhibitory role of curcumin and demonstrated that curcumin dose-dependently abrogated the stimulatory impact of hyperglycemia on elevating the level of intracellular glucose in cultured HSCs. However, it was of interest to observe that curcumin had no such impact on the level of intracellular glucose elevated by glucose in immortalized human hepatocytes (IHH) when IHH were in media with glucose at 450 mg/dl (Fig. 3C), suggesting that curcumin might show differential roles in regulating the level of intracellular glucose, depending on cell types. Our results collectively demonstrated that hyperglycemia caused dose-dependent and time-dependent biphasic increases in the level of intracellular glucose in cultured HSCs, which was abrogated by curcumin.
To start to elucidate the underlying mechanisms, we assumed that the early and first action of hyperglycemia in increasing the level of intracellular glucose resulted from stimulation of rapid translocation of GLUT2 from the cytoplasm to the cell membrane. The second action of hyperglycemia was caused by inducing gene expression of GLUT2. We also presumed that curcumin eliminated both actions of hyperglycemia in activated HSC respectively by blocking the membrane translocation of GLUT2 and suppressing gene expression of GLUT2.
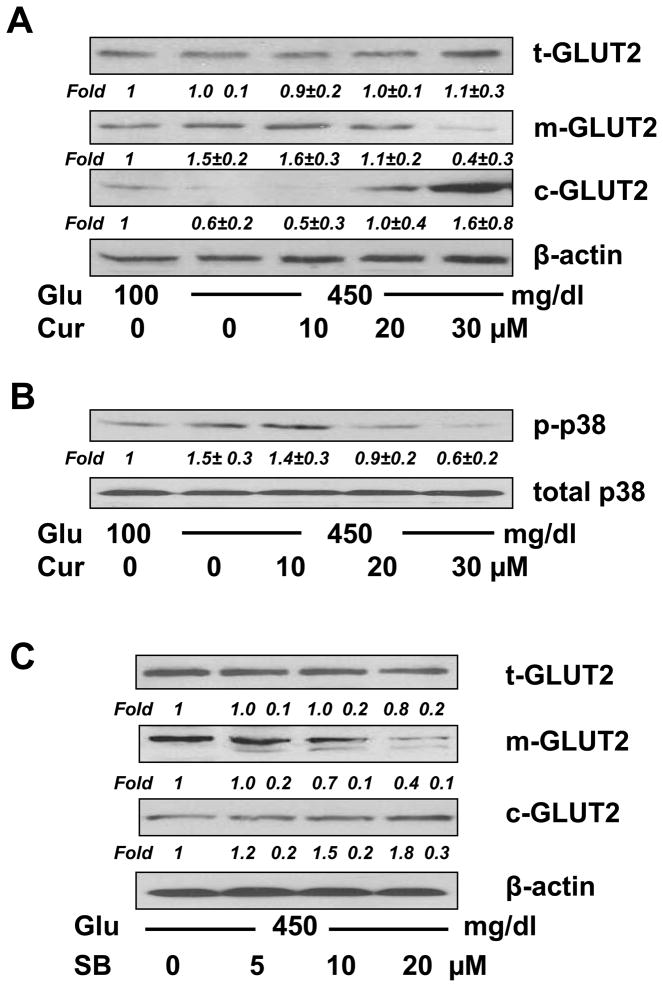
Curcumin suppressed the impact of hyperglycemia on inducing the rapid membrane translocation of GLUT2 in cultured HSCs by quickly blocking the p38 MAPK signaling pathway
To test the above assumptions, we first evaluated the impact of hyperglycemia on stimulating the membrane translocation of GLUT2 and the role of curcumin in blocking the process, and further elucidated the underlying mechanisms. Passaged HSCs were serum-starved in serum-depleted DMEM for 24 hr, which made HSCs more sensitive to the subsequent stimulation with glucose. Serum-starved HSCs were pretreated with curcumin (0–20 μM) for 1 hr prior to the exposure to a final concentration of glucose at 450 mg/dl in serum-depleted media for another 1 hr. Serum-starvation and subsequent culture in serum-depleted media excluded potential influence of factors in serum on the membrane translocation of GLUT2. Protein extracts respectively from whole cells, membrane and cytosol were prepared from these cells. Western blotting analyses in Fig. 4A demonstrated that, compared with glucose at 100 mg/dl in the media (the corresponding 1st lane), glucose at 450 mg/dl dramatically increased the abundance of membrane GLUT2 (m-GLUT2) and reduced the content of cytoplasmic GLUT2 (c-GLUT2) (the corresponding 2nd lane). Further experiments indicated that curcumin dose-dependently eliminated the impact of hyperglycemia on elevating the abundance of m-GLUT2, and increased the abundance of c-GLUT2 (the corresponding 2nd - 5th lanes). There was no apparent change in the level of total GLUT2 (t-GLUT2). Because it was a short-term treatment within a total of 2 hr, no apparent alterations in gene expression occurred. These results supported our initial assumption and suggested that curcumin abrogated the early action of hyperglycemia in elevating the level of intracellular glucose in cultured HSCs by quickly blocking the membrane translocation of GLUT2.
Figure 4. Curcumin suppressed the impact of hyperglycemia on rapidly inducing the membrane translocation of GLUT2 in cultured HSCs, likely by quickly blocking the p38 MAPK signaling pathway.
A & B. Serum-starved HSCs were pretreated with curcumin at indicated concentrations for 1 hr prior to the exposure to glucose (450 mg/dl) in serum-depleted media for additional 1 hr (A), or 30 minutes (B). Whole cell, membrane and cytosol protein extracts were respectively prepared. Contents of total GLUT2 (t-GLUT2), membrane GLUT2 (m-GLUT2) and cytoplasmic GLUT2 (c-GLUT2) (A), or phosphorylated p38 (p-p38) in whole cell extracts (B), were analyzed by Western blotting analyses. β-actin (A), or total p38 (B), was used as an invariant control for equal loading. Representatives were shown from three independent experiments. Italic numbers beneath blots were fold changes (means ± S.D.) in the densities of the bands compared with the control with glucose at 100 mg/dl in the blot (n=3), after normalization with the internal invariable control.
C. Serum-starved HSCs were pretreated with the p38 inhibitor SB202190 at various doses for 1 hr prior to the exposure to glucose (450 mg/dl) in serum-depleted media for an additional 1 hr. The abundance of t-GLUT2, m-GLUT2 and c-GLUT2 was analyzed by Western blotting analyses. β-actin was used as an invariant control for equal loading. Italic numbers beneath blots were fold changes (means ± S.D.) in the densities of the bands compared with the no treatment control (the corresponding 1st lane) in the blot (n=3), after normalization with the internal invariable control.
Prior other studies have indicated the role of the p38 MAPK signaling pathway in regulating intestinal fructose transport mediated by GLUT2 [33]. We, therefore, postulated that hyperglycemia, e.g. glucose at 450 mg/dl, might rapidly activate the p38 MAPK signaling pathway in HSCs, leading to the swift translocation of GLUT2 from the cytoplasm to the plasma membrane, and that curcumin might block the rapid GLUT2 membrane translocation by quickly interrupting the hyperglycemia-activated p38 signaling pathway. To test our postulation, serum-starved HSCs were pretreated with curcumin (0–30 μM) for 1 hr prior to the stimulation with or without glucose at a final concentration of 450 mg/dl in serum-depleted media for additional 30 minutes. The subsequent culture in the serum-depleted media excluded the influence of factors in serum on activating the p38 signaling pathway. Our pilot experiments revealed that glucose at 450 mg/dl activated the p38 signaling pathway and reached its peak within 20 to 30 minutes (data not shown here). Western blotting analyses in Fig. 4B demonstrated that compared with glucose at 100 mg/dl in the media (the 1st lane), glucose at 450 mg/dl significantly increased the level of phosphorylated p38 by approximately 50% (the 2nd lane), which was dose-dependently diminished by curcumin (the 2nd - 5th lanes). These results supported our assumption and suggested that hyperglycemia could rapidly activate the p38 MAPK signaling pathway in cultured HSCs, which might be quickly blocked by curcumin.
To further test our assumption, serum-starved HSC were pretreated with the p38 inhibitor SB202190 at various concentrations for 1 hr prior to the stimulation with glucose 450 mg/dl for additional 30 minutes. Protein extracts from fractions of whole cells, membrane and cytosol were respectively prepared. As shown in Fig. 4C by Western blotting analyses, SB202190 dose-dependently eliminated the impact of hyperglycemia on the stimulation of the GLUT2 membrane translocation, demonstrated by reducing the abundance of m-GLUT2 and increasing the level of c-GLUT2. SB202190 had no apparent impact on the level of t-GLUT2 in the cells during the short-term treatment. These results indicated that the blockade of the p38 signaling pathway by SB202190, like curcumin, dose-dependently eliminated the impact of hyperglycemia on inducing the rapid membrane translocation of GLUT2. However, the ERK inhibitor PD98059 did not show a similar role in blocking the glucose-induced membrane translocation of GLUT2 in activated HSCs (data not shown here). Taken together, these results supported our assumption and indicated that curcumin abrogated the impact of hyperglycemia on inducing the rapid membrane translocation of GLUT2 in activated HSCs in vitro by quickly blocking the p38 MAPK signaling pathway, likely leading to the abrogation of the early action of hyperglycemia in elevating the level of intracellular glucose.
Curcumin eliminated the stimulatory impact of hyperglycemia on inducing gene expression of GLUT2 in cultured HSCs
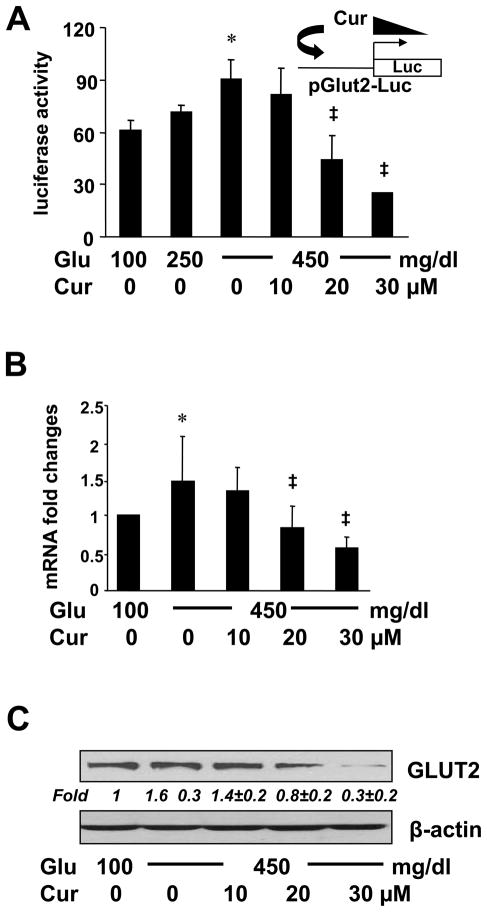
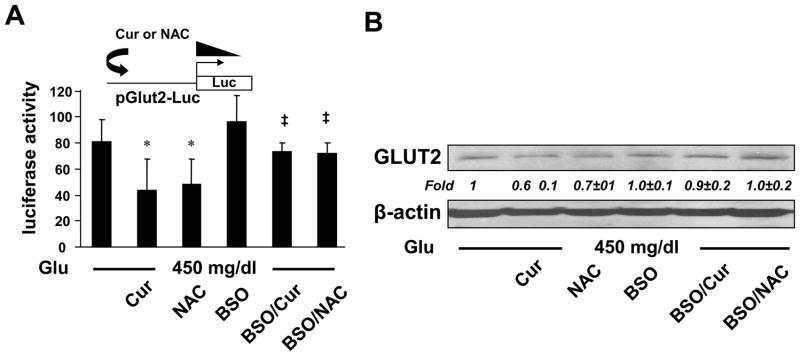
To further explore the underlying mechanism observed in Fig. 3B, we assumed that curcumin suppressed gene expression of GLUT2 in HSCs, leading to the elimination of the second action of hyperglycemia in elevating the level of intracellular glucose. To test the assumption, HSCs were transfected with the glut2 promoter luciferase reporter plasmid pGluT2-Luc, which contained a DNA fragment of the rat glut2 promoter region in the luciferase expression vector pGL3-Basic [32]. After overnight recovery, cells were stimulated with glucose at indicated doses in the presence of curcumin at 0–30 μM for 24 hr. Luciferase activity assays in Fig. 5A demonstrated that compared with glucose at 100 mg/dl (the 1st column), glucose dose-dependently increased luciferase activities in the cells (the 1st - 3rd columns). Curcumin dose-dependently eliminated the stimulatory impact of glucose (the 3rd - 6th columns), suggesting that curcumin suppressed the hyperglycemia-induced glut2 promoter activity in cultured HSCs.
Figure 5. Curcumin eliminated the stimulatory impact of hyperglycemia on inducing gene expression of GLUT2 in cultured HSCs.
Passaged HSCs were cultured in media with glucose at indicated concentrations in the presence or absence of curcumin (0–30 μM) for 24 hr. *p<0.05 vs cells in glucose at 100 mg/dl. ‡p<0.05 vs cells cultured in media with glucose at 450 mg/dl alone. (A). Luciferase activity assays of cells transfected with the glut2 promoter luciferase reporter plasmid pGluT2-Luc (n=6). The inset denoted the pGluT2-Luc construct in use and the application of curcumin to the system. (B). Real-time PCR analyses of mRNA levels of GLUT2 in the cells. Results were expressed as fold changes (means ± S.D.), compared with cells grown in media with glucose at 100 mg/dl (n=3). (C). Western blotting analyses of the abundance of total GLUT2. β-actin was used as an invariant control for equal loading. Representatives were shown from three independent experiments. Italic numbers beneath blots were fold changes (means ± S.D.) in the densities of the bands compared with the control with glucose at 100 mg/dl in the blot (n=3), after normalization with the internal invariable control.
To further evaluate the impact of hyperglycemia on inducing glut2 expression and the role of curcumin in eliminating the impact, HSCs were stimulated with glucose at 100 or 450 mg/dl with or without curcumin (0–30 μM) for 24 hr. Real-time PCR assays and Western blotting analyses verified that compared with glucose at 100 mg/dl (the 1st column in Fig. 5B and the 1st lane in Fig. 5C), glucose at 450 mg/dl significantly increased the levels of mRNA and protein of GLUT2 (the 2nd column in Fig. 5B and the 2nd lane in Fig. 5C), which were diminished by curcumin in a dose-dependent manner (the 2nd - 5th columns and lanes). The major difference between Fig. 4A and 5C was the duration of the curcumin treatment. Cells in Fig. 4A were treated with curcumin (20 μM) for a total of 2 hr, which was not long enough to show apparent changes in the transcription and translation of GLUT2 gene. However, cells in Fig. 5C were treated with curcumin (20 μM) for 24 hr, which was long enough to cause changes in glut2 expression. Taken together, these results indicated that hyperglycemia induced gene expression of GLUT2 in activated HSCs in vitro, which was eliminated by curcumin, likely leading to the abrogation of the hyperglycemia-caused second phasic increase in the level of intracellular glucose.
The activation of PPARγ played a critical role for curcumin to eliminate the impact of hyperglycemia on inducing gene expression of GLUT2 in activated HSCs in vitro
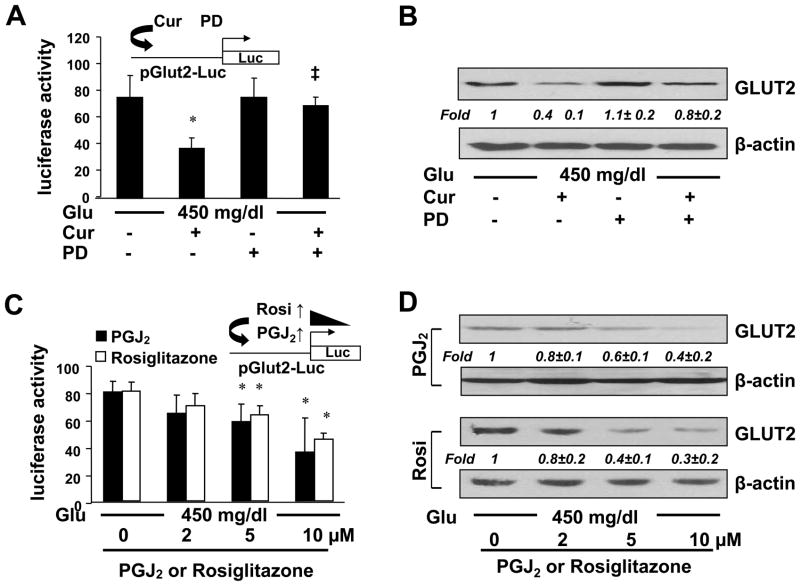
We previously demonstrated that curcumin induced gene expression of endogenous PPARγ and its activity in activated HSCs in vitro and in vivo [23,24], which was required for curcumin to inhibit HSC activation [24,25]. To explore the mechanisms by which curcumin inhibited glut2 expression in HSCs, we assumed that the activation of PPARγ might mediate the inhibitory role of curcumin in regulating the glut2 expression in HSCs. To test the assumption, HSCs were transfected with the plasmid pGluT2-Luc. After recovery, cells were pretreated with or without the PPARγ antagonist PD68235 (20 μM) for 1 hr prior to the stimulation with glucose at 450 mg/dl plus or minus curcumin (20 μM) for additional 24 hr. Luciferase activity assays in Fig. 6A showed that compared with glucose at 450 mg/dl alone (the 1st column), the addition of curcumin dramatically reduced the luciferase activity (the 2nd column), indicting that curcumin significantly reduced, as expected, the promoter activity of GLUT2 gene. It was of interest to observe that the inhibitory role of curcumin was significantly abrogated by the pretreatment with the PPARγ antagonist PD68235 (the 4th column). The PPARγ antagonist itself had no apparent impact on the luciferase activity (the 3rd column). To verify the observation, whole cell extracts from passaged HSCs with the aforementioned treatment were analyzed by Western blotting analyses. As shown in Fig. 6B, compared with glucose at 450 mg/dl (the 1st lane), curcumin dramatically reduced, as expected, the abundance of GLUT2 in the cells (the 2nd lane). The pretreatment with the PPARγ antagonist PD68235 apparently diminished the role of curcumin in reducing the abundance of GLUT2 (the 4th lane).
Figure 6. The activation of PPARγ played a critical role for curcumin to eliminate the impact of hyperglycemia on inducing gene expression of GLUT2 in activated HSCs in vitro.
A & B. Passaged HSCs were pretreated with or without the PPARγ antagonist PD68235 (20 μM) for 1 hr prior to the stimulation with glucose (450 mg/dl) plus or minus curcumin (20μM) for additional 24 hr. (A). Luciferase activity assays of cells transfected with the plasmid pGlut2-Luc. The inset denoted the pGluT2-Luc construct in use and the application of curcumin plus or minus PD68235 (PD) to the system. *p<0.05 vs cells stimulated with glucose at 450 mg/dl alone. ‡p<0.05 vs cells treated with both glucose and curcumin. (B). Western blotting analyses of the abundance of GLUT2. β-actin was used as an invariant control for equal loading. Representatives were shown from three independent experiments. Italic numbers were fold changes (means ± S.D.) in densities of the bands compared with the control with glucose at 450 mg/dl alone (the 1st lane) after normalization (n=3).
C & D. Serum-starved HSCs were pretreated with the natural PPARγ agonist PGJ2 or synthesized PPARγ agonist rosiglitazone at indicated doses for 1 hr prior to the exposure to glucose (450 mg/dl) in serum-depleted DMEM for 24 hr. (C). Luciferase activity assays of cells transfected with pGluT2-Luc. The inset denoted the pGluT2-Luc construct in use and the application of PGJ2 or rosiglitazone to the system. *p<0.05 vs cells stimulated with glucose at 450 mg/dl alone. (D). Western blotting analyses of the abundance of GLUT2. β-actin was used as an invariant control for equal loading. Representative results were shown from three independent experiments. Italic numbers were fold changes (means ± S.D.) in densities of the bands compared with the control stimulated with glucose at 450 mg/dl alone (the corresponding 1st lane) after normalization (n=3).
To further test our above assumption, HSCs were transfected with pGluT2-Luc. After recovery, cells were serum-starved for 4 hr before the subsequent treatment. Cells were then pretreated with the synthesized PPARγ agonist rosiglitazone, or the natural PPARγ agonist PGJ2, at indicated doses for 1 hr prior to the exposure to glucose at 450 mg/dl in serum-depleted media for additional 24 hr. The culture in serum-depleted media excluded the interference from other potential PPARγ agonists in serum [24,25]. Luciferase activity assays in Fig. 6C demonstrated that the activation of PPARγ by rosiglitazone or PGJ2 dose-dependently reduced luciferase activities in the cells, suggesting an inhibitory effect of the activation of PPARγ on the glut2 promoter activity. Further Western blotting analyses of whole cell extracts from cells with the aforementioned treatment validated the suggestion and indicated that the activation of PPARγ by rosiglitazone or PGJ2 reduced the abundance of total GLUT2 in HSCs in a dose-dependent manner (Fig. 6D). Taken together, our results demonstrated that the activation of PPARγ played a critical role for curcumin to eliminate the impact of hyperglycemia on inducing gene expression of GLUT2 in activated HSCs in vitro.
Hyperglycemia induced oxidative stress in HSCs by elevating the level of ROS and LPO, which was attenuated by curcumin, possibly by increasing the contents of GSH
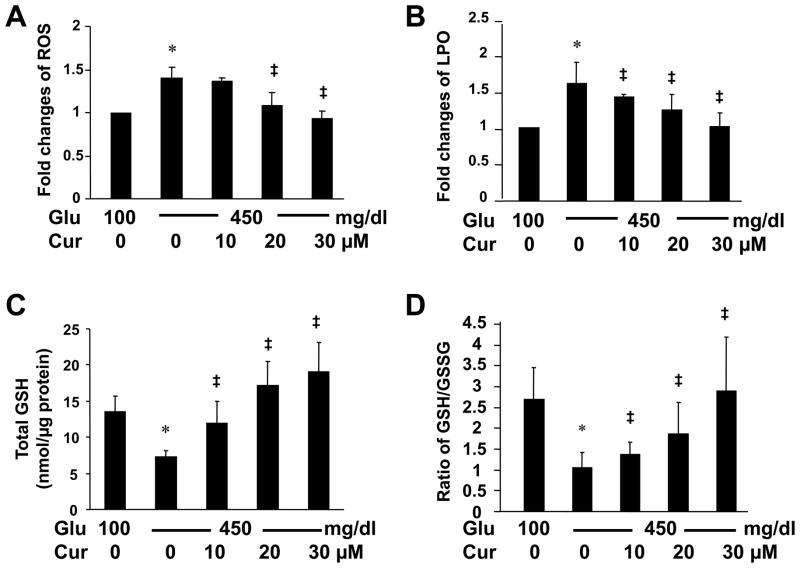
Studies have shown that hyperglycemia induces oxidative stress in diabetic complications [34]. Oxidative stress induces HSC activation and hepatic fibrogenesis [9,10]. Among the non-enzymatic molecules in mammalian cells, glutathione (GSH) is the most abundant thiol antioxidant [35]. To further elucidate the mechanisms by which curcumin eliminated the impact of hyperglycemia on the induction of HSC activation, we postulated that hyperglycemia might induce oxidative stress in HSCs, which could be attenuated by curcumin by increasing the level of cellular GSH. To test the postulation, HSCs were cultured in DMEM with glucose at 100 mg/dl, or at 450 mg/dl in the presence of curcumin (0–30 μM) for 24 hr. Levels of reactive oxygen species (ROS) and lipid peroxides (LPO) in the cells were determined. As shown in Fig. 7A and B, compared with glucose at 100 mg/dl (the corresponding 1st column), glucose at 450 mg/dl significantly increased the levels of ROS and LPO in cultured HSCs (the corresponding 2nd column). The stimulatory impact of glucose at 450 mg/dl was apparently diminished by curcumin in a dose-dependent manner (the corresponding 2nd - 5th columns). These results collectively demonstrated that hyperglycemia induced oxidative stress in cultured HSCs, which was attenuated by curcumin.
Figure 7. Hyperglycemia induced oxidative stress in HSCs by elevating the level of ROS and LPO, which was attenuated by curcumin, likely by increasing the contents of GSH.
Passaged HSCs were cultured in media with glucose at 100 mg/dl, or 450 mg/dl in the presence of curcumin (0–30 μM) for 24 hr. Levels of intracellular ROS (A), LPO (B) and GSH (C), as well as the ratio of GSH/GSSG (D) were analyzed. Values were represented as means ± S. D. (n=6). *p<0.05 vs cells cultured in media with glucose at 100 mg/dl. ‡p<0.05 vs cells stimulated with glucose at 450 mg/dl alone.
To further test our above postulation, the level of cellular GSH and the ratio of reduced GSH versus oxidized GSH (GSSG), the index for oxidative stress, were analyzed in HSCs with the aforementioned treatment. As shown in Fig. 7C and D, compared with glucose at 100 mg/dl (the corresponding 1st column), glucose at 450 mg/dl apparently reduced the level of GSH and the ratio of GSH/GSSG (the corresponding 2nd column). Curcumin dose-dependently eliminated the inhibitory impacts and increased the level of cellular GSH and the ratio of GSH/GSSG in cultured HSCs (the 2nd - 5th columns). Taken together, these results supported our initial postulation and demonstrated that hyperglycemia induced oxidative stress in HSCs by increasing the level of ROS and LPO, which was attenuated by curcumin, likely by increasing the level of cellular GSH.
Curcumin eliminated the impact of hyperglycemia on reducing the level of GSH in cultured HSCs by elevating the abundance of GCL subunits and increasing the activity of GCL
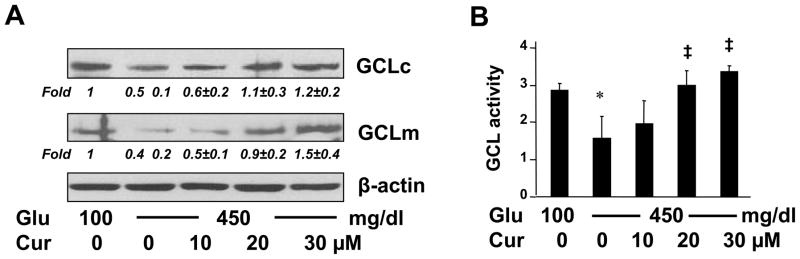
Glutamate cysteine ligase (GCL) is the rate-limiting enzyme in de novo syntheses of GSH [35], and is composed of a large catalytic subunit (GCLc, ~73 kD) and a small modulatory subunit (GCLm, ~30 kD) [36]. To answer the question how curcumin eliminated the impact of hyperglycemia on reducing the level of cellular GSH, we assumed that curcumin abrogated the inhibitory impact of hyperglycemia by inducing gene expression of GCL in HSCs. To test the assumption, HSCs were cultured in media with glucose at 100 mg/dl, or 450 mg/dl plus or minus curcumin (0–30 μM) for 24 hr. As demonstrated in Fig. 8A by Western blotting analyses, compared with glucose at 100 mg/dl (the 1st lane), glucose at 450 mg/dl significantly reduced the abundance of GCLm and GCLc in HSCs (the corresponding 2nd lane). Curcumin attenuated the inhibitory impact of hyperglycemia and dose-dependently increased the contents of GCLc and GCLm (the corresponding 2nd - 5th lanes). To verify the observation, additional GCL activity assays were performed using HSCs with the aforesaid treatment and culture conditions (Fig. 8B). Compared with glucose at 100 mg/dl (the 1st column), glucose at 450 mg/dl dramatically reduced the activity of GCL (the 2nd column), which was dose-dependently abrogated by curcumin (the 2nd - 5th columns). Taken together, these results suggested that curcumin eliminated the impact of hyperglycemia on reducing the level of GSH in cultured HSCs by elevating the abundance of GCL subunits and increasing the activity of GCL.
Figure 8. Curcumin eliminated the impact of hyperglycemia on reducing the level of GSH in cultured HSCs by elevating the abundance of GCL subunits and increasing the activity of GCL.
Passaged HSCs were cultured in media with glucose at 100 mg/dl, or 450 mg/dl in the presence of curcumin (0–30 μM) for 24 hr. (A). Western blotting analyses of the abundance of GCLc and GCLm in the cells. β-actin was used as an invariant control for equal loading. Representatives were shown from three independent experiments. Italic numbers were fold changes (means ± S. D) in densities of the bands compared with cells grown in media with glucose at 100 mg/dl (the corresponding 1st lane) after normalization (n=3). (B). Analyses of the activity of GCL in the cells. Values were represented as means ± S. D. (n=6). *p<0.05 vs cells cultured in media with glucose at 100 mg/dl. ‡p<0.05 vs cells stimulated with glucose at 450 mg/dl alone.
de novo synthesis of GSH played a pivotal role for curcumin to inhibit the hyperglycemia-induced gene expression of GLUT2 in activated HSCs in vitro
Additional experiments were carried out to answer the question whether the elevated level of GSH played a critical role for curcumin to inhibit the hyperglycemia-induced gene expression of GLUT2 in activated HSCs in vitro. In the following experiments, cellular GSH contents were altered by two well-known manipulators of GSH synthesis. N-acetyl-cysteine (NAC) is a precursor of GSH, increasing GSH contents by supplying cysteine [37]. L-buthionine-sulfoximine (BSO) is a specific inhibitor of GCL, depleting cellular GSH [38]. Cultured HSCs were transfected with the glut2 promoter luciferase reporter plasmid pGluT2-Luc. After recovery, HSCs were cultured in media with glucose at 450 mg/dl and divided into two groups. One was treated with or without curcumin (20 μM), or NAC (5 mM), for 24 hr. The other group was pretreated with BSO (0.25 mM) for 1 hr prior to the treatment with curcumin (20 μM), or NAC (5 mM), for additional 24 hr. Luciferase activity assays in Fig. 9A demonstrated that compared with the no treatment control (the 1st column), NAC (the 3rd column), mimicking curcumin (the 2nd column), significantly reduced the luciferase activity. The inhibition of GSH synthesis by the pre-exposure to BSO apparently abolished the inhibitory effect of both NAC and curcumin (the last two columns), suggesting a critical role of de novo synthesis of GSH in the inhibition of the glut2 promoter activity by curcumin, as well as by NAC. These observations were verified by Western blotting analyses in cultured HSCs with the aforesaid treatment (Fig. 9B). NAC (the 3rd lane), like curcumin (the 2nd lane), significantly reduced the abundance of total GLUT2 in HSCs. BSO apparently eliminated the inhibitory effect of both NAC and curcumin on the abundance of GLUT2 (the last two lanes). Taken together, these results indicated that de novo synthesis of GSH played a pivotal role for curcumin to inhibit the hyperglycemia-induced gene expression of GLUT2 in activated HSCs in vitro.
Figure 9. de novo synthesis of GSH played a pivotal role for curcumin to inhibit the hyperglycemia-induced gene expression of GLUT2 in activated HSCs in vitro.
Passaged HSCs were stimulated with glucose at 450 mg/dl and divided into two groups. One was treated with or without curcumin (20 μM), or NAC (5 mM), for 24 hr. The other group was pretreated with BSO (0.25 mM) for 1 hr prior to the treatment with curcumin (20 μM), or NAC (5 mM), for additional 24 hr. (A). Luciferase activity assays of cells transfected with pGluT2-Luc. The inset denoted the pGluT2-Luc construct in use and the application of curcumin or NAC to the system. *p<0.05 vs cells stimulated with glucose at 450 mg/dl alone. ‡p<0.05 vs cells treated with glucose plus curcumin or NAC. (B). Western blotting analyses of the abundance of GLUT2. Representatives were shown from three independent experiments. Italic numbers were fold changes (means ± S. D) in densities of the bands compared with the control with no treatment (the 1st lane) after normalization (n=3).
DISCUSSION
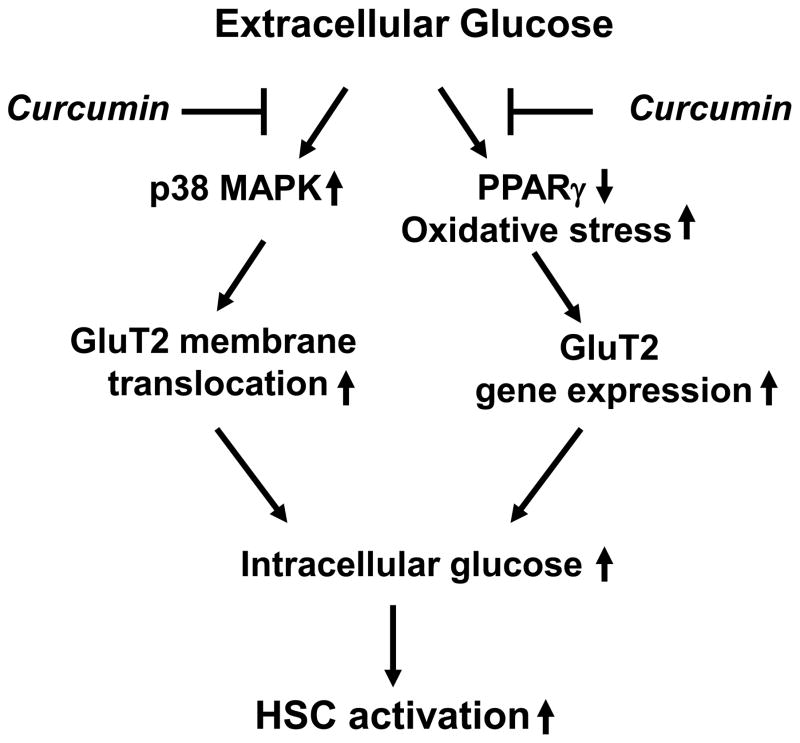
To start to define the impact of hyperglycemia on stimulating hepatic fibrogenesis, we evaluated the impact of high levels of glucose on HSC activation in vitro. Prior studies indicated that no significant differences in cell proliferation were observed between HSCs cultured in media with glucose at 100 mg/dl and with mannitol at 600 mg/dl, suggesting that the high osmolarity itself had no influence on stimulating cell proliferation in rat HSCs [17]. The present study demonstrated, as summarized in Fig. 10, that hyperglycemia, e.g. glucose at 450 mg/dl, induced HSC activation in vitro, which might be caused by elevating the level of intracellular glucose. The latter process took two-steps. The first step occurred within 30 minutes after the exposure of cells to glucose at 450 mg/dl. The second step happened 3 hr later. The early event might mainly result from the glucose-stimulated membrane translocation of GLUT2 from the cytoplasm, while the later event was suggested to be the consequence of the glucose-induced gene expression of GLUT2. Curcumin quickly blocked the membrane translocation of GLUT2 by interrupting the p38 MAPK signaling pathway and subsequently suppressed gene expression of GLUT2 by stimulating PPARγ activity and attenuating oxidative stress, collectively leading to the elimination of the two-step actions of hyperglycemia in elevating the level of intracellular glucose and to the inhibition of HSC activation in vitro.
Figure 10. A schematic diagram of the role of curcumin in inhibiting hyperglycemia-caused HSC activation.
Hyperglycemia elevates the level of intracellular glucose, leading to the activation of HSCs. Curcumin eliminates the stimulatory effects of hyperglycemia by blocking the membrane translocation of GLUT2 and suppressing expression of GLUT2 gene. The latter is mediated by activating PPARγ and attenuating oxidative stress.
The toxicity of curcumin to cultured HSCs was previously evaluated [24]. Based on results from lactate dehydrogenase release assays, trypan blue exclusion assays and a rapid recovery of cell proliferation after withdrawal of curcumin, it was concluded that curcumin up to 100 μM was not toxic to cultured HSCs. Curcumin at 20 μM was used in most of our in vitro experiments. The systemic bioavailability of curcumin is relatively low [39]. Curcumin concentrations in human plasma can reach up to 2 μM following oral intake of a large amount of curcumin [40]. In addition, we are cognizant that the concentration of glucose at 450 mg/dl used in most of our in vitro experiments might not be often found in vivo conditions. However, these concentrations are often used in vitro experiments [17,41–44]. One of explanations why in vitro experiments use a higher level of curcumin or glucose than those observed in vivo is that in vitro experiments often use one type of cells, which is a uni-factorial system. However, an in vivo system is usually multi-factorial. It has been shown that liver metabolism is regulated by intercellular communications [45]. Curcumin or hyperglycemia might simultaneously have impacts on several types of cells, among which they cross-talk, interact and show synergistic impacts. Cells might be, therefore, less sensitive to stimuli in an in vitro system than in an in vivo system. It is noteworthy that because the in vivo system is multi-factorial, directly extrapolating in vitro conditions and results, e.g. effective concentrations, to the in vivo system, or vice versa, might be misleading.
Our results suggested that curcumin might exert dual-functions in reducing the level of intracellular glucose in HSCs in vitro. Curcumin eliminated the rapid action of extracellular glucose by quickly interrupting the hyperglycemia-activated p38 signaling pathway, leading to the blockade of the membrane translocation of GLUT2 (Fig. 4A & B). Curcumin, as an inhibitor of the phosphoinositide 5-kinase PIKfyve, was also reported to inhibit insulin-induced GLUT4 translocation and glucose transport into 3T3-L1 adipocytes [46]. In addition to the quick and short-term action, curcumin also showed a slow but long-lasting action by suppressing gene expression of GLUT2 in cultured HSCs (Fig. 5), which was mediated by the activation of PPARγ (Fig. 6). We formerly reported that curcumin restored gene expression of PPARγ in activated HSCs in vitro and in vivo [23,24]. The curcumin-induced expression of PPARγ is likely activated by its agonists present in media or in blood, leading to the inhibition of HSC activation. The activation of PPARγ was required for curcumin to inhibit HSC activation [24,25]. It bears emphasis that in addition to other beneficial effects, because of its role in restoring gene expression of PPARγ in activated HSCs, curcumin could not be simply replaced by PPARγ agonists, which, though, showed inhibitory impacts on HSC activation [14,47]. PPARγ has displayed a critical role in the development of T2DM and in the treatment of this disease. Patients with a dominant-negative mutation in PPARγ gene were associated with severe insulin resistance and diabetes [48]. Adipose-specific PPARγ knockout caused insulin resistance in the adipose tissue and liver [49]. PPARγ agonists had effects on promoting insulin sensitization and improving dyslipidemia in patients with T2DM [50].
A critical concern was raised during conducting the experiments in this report whether curcumin would block the transport of plasma glucose into hepatocytes and/or muscle cells. If true, curcumin would deteriorate hyperglycemia and diabetes. We conducted additional experiments to evaluate the role of curcumin in altering the level of glucose in hepatocytes. Curcumin was observed to have no impact on the hyperglycemia-caused increase in the level of intracellular glucose in hepatocytes (Fig. 3C). This result was supported by a recent report that curcumin facilitated the maintenance of the level of pancreatic GLUT2 in STZ-treated animals [51]. Curcumin showed distinct roles in regulating expression of genes depending on cell types. Curcumin induced gene expression of LDL receptor (LDLR) in hepatoma cell line HepG2 [52], which might result in an increase in the endocytosis of plasma LDL by hepatocytes and a reduction in the level of plasma LDL. However, curcumin suppressed gene expression of LDLR in cultured HSCs [53], which attenuated the stimulatory effect of LDL on the activation of HSCs. Our results suggested that curcumin inhibited GLUT2 gene expression in HSCs mediated by activating PPARγ. However, PPARγ induced, instead, GLUT2 gene expression in rat pancreatic insulinoma beta-cells [54], in cultured primary hepatocytes and in human epithelial hepatoma cell line Alexander cells [55]. Although beyond the scope of this report, it is of interest to explore the underlying mechanisms by which curcumin shows distinct roles in different cell types, including HSCs and hepatocytes.
The level of intracellular glucose is mainly determined by glucose-supplying and glucose-consuming. In the liver, extracellular glucose could be rapidly transported into cells by the major transporter GLUT2 [6]. On the other hand, intracellular glucose could be swiftly converted to glucose-6-phosphate (G-6-P) catalyzed by hexokinase [56]. The experiments in this report focused on the effects of curcumin on glucose-supplying factors. Additional experiments are necessary to evaluate effects of curcumin on glucose-consuming factors, including on the conversion of glucose to G-6-P in activated HSCs, which might also contribute to the curcumin-caused reduction in the level of intracellular glucose in HSCs. It bears emphasis that our results in this report do not exclude any other mechanisms of curcumin in the inhibition of the hyperglycemia-induced activation of HSCs. Our results in this study present evidence to the impacts of hyperglycemia on stimulating hepatic fibrogenesis. In addition, our results provide novel insights into the roles of curcumin and its underlying mechanisms in the inhibition of hyperglycemia-induced HSC activation and its implications in the therapeutic intervention of hyperglycemia-associated hepatic fibrogenesis.
Acknowledgments
The work was supported by the grant DK 47995 from NIH/NIDDK to A. Chen.
ABBREVIATIONS
- α-SMA
alpha-smooth muscle actin
- BSO
L-buthionine sulfoximine
- CTGF
connective tissue growth factor
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GLUT2
glucose transporter-2
- GSH
glutathione
- HSCs
hepatic stellate cells
- LPO
Lipid peroxidation
- NASH
non-alcoholic steatohepatitis
- NAC
N-acetylcysteine
- PDGF-βR
platelet-derived growth factor-beta receptor
- PPARγ
peroxisome proliferator-activated receptor-gamma
- ROS
reactive oxygen species
- TGF-βR
transforming growth factor-beta receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meetoo D, McGovern P, Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16:1002–7. doi: 10.12968/bjon.2007.16.16.27079. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM. Review article: current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:2–12. doi: 10.1111/j.1365-2036.2008.03710.x. [DOI] [PubMed] [Google Scholar]
- 3.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 4.Asano T, Ogihara T, Katagiri H, Sakoda H, Ono H, Fujishiro M, Anai M, Kurihara H, Uchijima Y. Glucose transporter and Na+/glucose cotransporter as molecular targets of anti-diabetic drugs. Curr Med Chem. 2004;11:2717–24. doi: 10.2174/0929867043364360. [DOI] [PubMed] [Google Scholar]
- 5.Zhao FQ, Keating AF. Expression and regulation of glucose transporters in the bovine mammary gland. J Dairy Sci. 2007;90(Suppl 1):E76–86. doi: 10.3168/jds.2006-470. [DOI] [PubMed] [Google Scholar]
- 6.Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. The role of GLUT2 in dietary sugar handling. J Physiol Biochem. 2005;61:529–37. doi: 10.1007/BF03168378. [DOI] [PubMed] [Google Scholar]
- 7.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clin Cornerstone. 2007;8(Suppl 7):S30–42. doi: 10.1016/s1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S84–7. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 11.Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman SL, Yamasaki G, Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994;269:10551–8. [PubMed] [Google Scholar]
- 13.Galli A, Crabb D, Price D, Ceni E, Salzano R, Surrenti C, Casini A. Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology. 2000;31:101–8. doi: 10.1002/hep.510310117. [DOI] [PubMed] [Google Scholar]
- 14.Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–78. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 15.Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr, Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–22. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 16.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, Bedossa P. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto R, Enjoji M, Kohjima M, Tsuruta S, Fukushima M, Iwao M, Sonta T, Kotoh K, Inoguchi T, Nakamuta M. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int. 2005;25:1018–26. doi: 10.1111/j.1478-3231.2005.01130.x. [DOI] [PubMed] [Google Scholar]
- 18.Calamita G, Portincasa P. Present and future therapeutic strategies in non-alcoholic fatty liver disease. Expert Opin Ther Targets. 2007;11:1231–49. doi: 10.1517/14728222.11.9.1231. [DOI] [PubMed] [Google Scholar]
- 19.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 20.Pari L, Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16:257–74. doi: 10.1515/jbcpp.2005.16.4.257. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–5. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Connell MA, Rushworth SA. Curcumin: potential for hepatic fibrosis therapy? Br J Pharmacol. 2008;153:403–5. doi: 10.1038/sj.bjp.0707580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384:149–57. doi: 10.1042/BJ20040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007;43:444–53. doi: 10.1016/j.freeradbiomed.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Chen A. Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Invest. 2008;88:529–40. doi: 10.1038/labinvest.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zheng S, Lin J, Zhang QJ, Chen A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab Invest. 2007;87:488–98. doi: 10.1038/labinvest.3700532. [DOI] [PubMed] [Google Scholar]
- 30.Nishiumi S, Ashida H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci Biotechnol Biochem. 2007;71:2343–6. doi: 10.1271/bbb.70342. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 32.Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes. 2000;49:1517–24. doi: 10.2337/diabetes.49.9.1517. [DOI] [PubMed] [Google Scholar]
- 33.Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000;350(Pt 1):163–9. [PMC free article] [PubMed] [Google Scholar]
- 34.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 36.Seelig GF, Meister A. Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–90. doi: 10.1016/s0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- 37.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–27. [PubMed] [Google Scholar]
- 38.Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis. 1998;18:415–24. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- 39.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- 40.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res. 2004;94:892–901. doi: 10.1161/01.RES.0000124920.09738.26. [DOI] [PubMed] [Google Scholar]
- 42.Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Yoshii J, Yanase K, Namisaki T, Yamazaki M, Moriya K, Tsujimoto T, Kawaratani H, Akahane T, Uemura M, Fukui H. Impact of insulin resistance on the progression of chronic liver diseases. Int J Mol Med. 2008;22:801–8. [PubMed] [Google Scholar]
- 43.Liu W, Tang F, Deng Y, Li X, Lan T, Zhang X, Huang H, Liu P. Berberine reduces fibronectin and collagen accumulation in rat glomerular mesangial cells cultured under high glucose condition. Mol Cell Biochem. 2009;325:99–105. doi: 10.1007/s11010-008-0024-y. [DOI] [PubMed] [Google Scholar]
- 44.Somanath S, Barg S, Marshall C, Silwood CJ, Turner MD. High Extracellular Glucose Inhibits Exocytosis through Disruption of Syntaxin 1A-Containing Lipid Rafts. Biochem Biophys Res Commun. 2009;389:241–6. doi: 10.1016/j.bbrc.2009.08.126. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper J, Casteleyn E, Van Berkel TJ. Regulation of liver metabolism by intercellular communication. Adv Enzyme Regul. 1988;27:193–208. doi: 10.1016/0065-2571(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 46.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology. 2002;143:4742–54. doi: 10.1210/en.2002-220615. [DOI] [PubMed] [Google Scholar]
- 47.Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–40. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 48.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 49.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fogo AB. Potential for peroxisome proliferator-activated receptor-gamma agonists in progression: beyond metabolism. Curr Opin Nephrol Hypertens. 2008;17:282–5. doi: 10.1097/MNH.0b013e3282f9b1c0. [DOI] [PubMed] [Google Scholar]
- 51.Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702–13. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem. 2007;18:113–9. doi: 10.1016/j.jnutbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Kang Q, Chen A. Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol. 2009;157:1354–67. doi: 10.1111/j.1476-5381.2009.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moibi JA, Gupta D, Jetton TL, Peshavaria M, Desai R, Leahy JL. Peroxisome proliferator-activated receptor-gamma regulates expression of PDX-1 and NKX6.1 in INS-1 cells. Diabetes. 2007;56:88–95. doi: 10.2337/db06-0948. [DOI] [PubMed] [Google Scholar]
- 55.Im SS, Kim JW, Kim TH, Song XL, Kim SY, Kim HI, Ahn YH. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med. 2005;37:101–10. doi: 10.1038/emm.2005.14. [DOI] [PubMed] [Google Scholar]
- 56.Grimsby J, Berthel SJ, Sarabu R. Glucokinase activators for the potential treatment of type 2 diabetes. Curr Top Med Chem. 2008;8:1524–32. doi: 10.2174/156802608786413483. [DOI] [PubMed] [Google Scholar]