Abstract
The pleiotropic cytokine, interleukin-6 (IL-6), has emerged as a key factor in the biology of aging and the physiology of inflammation. Yet much of what we know about the normal functioning of IL-6 has been generated primarily from research on European populations and Americans of European descent. Our analyses compared IL-6 levels in 382 middle-aged and older Japanese to the values found in 1209 Caucasian- and African-Americans from the Midlife in the United States survey (MIDUS). Across the life span from 30–80 years of age, mean IL-6 levels were strikingly lower in Japanese individuals. Significantly lower levels of C-reactive protein (CRP) and fibrinogen (FBG) provided confirmatory evidence for a population difference in proinflammatory activity. Because IL-6 release has been associated with obesity, differences in body mass index (BMI) were taken into consideration. Japanese had the lowest, and African-Americans had the highest overall BMIs, but significant group differences in IL-6 persisted even after BMI was included as a covariate in the analyses. Additional support for distinct variation in IL-6 biology was generated when systemic levels of the soluble receptor for IL-6 (sIL-6r) were evaluated. Serum sIL-6r was higher in Japanese than Americans, but was most notably low in African-Americans. Our cytokine data concur with national differences in the prevalence of age-related illnesses linked to inflammatory physiology, including cardiovascular disease. The findings also highlight the importance of broadening the diversity of people included in population studies of health and aging, especially given the relative paucity of information for some Asian countries and on individuals of Asian heritage living in the US.
Keywords: interleukin-6, soluble interleukin-6 receptor, aging, inflammation, Japanese, African-American, race, C-reactive protein, fibrinogen, body mass index, obesity
1. Introduction
Many studies have now documented that old age is associated with a progressive decline in immune competence, which is often first manifest by signs in peripheral circulation of the impending dysregulation in cytokine activity (Bruunsgaard et al., 2001; Papanicoloau et al., 1998; Straub et al., 2000; Sauerwein-Teissi et al., 2000). Specifically, beginning in middle-aged adults, there may be an incremental rise in several of the proinflammatory cytokines, especially the ubiquitous IL-6 (Ershler and Keller, 2000; Harris et al., 1999; Wei et al., 1993). Increases in IL-6 have been shown to be a risk factor for a number of age-related illnesses associated with inflammation, including cardiovascular disease, diabetes, and osteoporosis (Loucks et al., 2006; Kristiansen and Mandrup-Poulsen, 2005; Pradham et al., 2001; Ridker et al., 2000; Tamura et al., 1993; Whooley et al., 2007). An augmentation of IL-6 in middle-aged and older adults has also been associated with low socioeconomic status (SES), stressful life events, depression, and obesity (Black, 2003; Koster et al., 2006; Petersen et al., 2008; Pollitt et al., 2008). Thus, it is perhaps not surprising that several studies have reported that IL-6 levels tend to be higher in African-Americans than in Caucasian-Americans, which is then posited as one likely explanation for the racial differences in morbidity and mortality (Gruenewald et al., 2009; Ranjit et al., 2007; Slopen et al., 2010). However, our knowledge about the extent of population differences in cytokine biology is actually quite limited. The primary goal of the following analyses was to extend this comparative perspective: by assessing IL-6 levels in Japanese adults and comparing the results to the typical ranges found in Americans who had participated in a representative national survey (Midlife in the United States [MIDUS], Radler and Ryff, 2010).
The rising epidemic of obesity in industrialized countries is one of the more common reasons offered for elevated IL-6 levels in otherwise healthy middle-aged adults (Fried et al., 1998; McLaren, 2007; Xu et al., 2003). In addition to being produced by lymphoid cells, we now know that adipocytes are a major source of the IL-6 in circulation and further that activated monocytes present in fat tissue also release cytokines into the blood stream (Mohamed-Ali et al., 1997; Yamashita et al., 2007). The many different cellular sources of IL-6, which include hepatocytes, fibrobasts, and endothelial cells, help to explain why it has been linked to the risk for the Metabolic Syndrome. Because Japanese are traditionally less likely to be overweight than Americans, a secondary aim of our study was to investigate the contribution of body mass index (BMI) to any national differences observed in cytokine biology. In addition, we examined the correlation between IL-6 and two other hematological markers of inflammation, C-reactive protein (CRP) and fibrinogen (FBG) (Deepa et al., 2006; Friedlander et al., 2006; Gabay and Kushner, 1999; Yamaguchi et al., 1998). Prior research on MIDUS participants and on other healthy and patient populations had demonstrated that there is usually a positive association between IL-6 and CRP as well as between IL-6 and FBG (Friedman and Herd, 2010; Howren et al., 2009; Tracy et al., 1995). IL-6 is known to be a primary stimulator of the hepatic production and release of both CRP and FBG, especially during the acute phase response to infection and injury (Heinrich et al., 1990; Kopf et al., 1994; Moshage, 1997).
Although most investigators interested in IL-6 assay only the protein levels in circulation, the functional activity of IL-6 can be strongly influenced by its receptor, which is comprised of a transmembrane ligand-binding subunit and signaling subunit on cell surfaces. The receptor is also found as a soluble form in the blood stream (Jones et al., 2001; Montero-Julian, 2001; Mulberg et al., 1999). High circulating levels of soluble receptors, which in the case of sIL6r are1000-fold higher than IL-6 in the nanogram/mL range, may act as a buffer, attenuating acute over-reactions (May et al., 1992; Rose-John and Heinrich, 1994). It has been hypothesized that cells will shed the membrane-bound receptor when activated or over-stimulated by IL-6 for sustained periods of time (Yokoyama et al., 1997). However, the function of the soluble receptor varies across different types of tissue, and it can also act as an agonist. The receptor/ligand complex can facilitate uptake of the bound IL-6 into cells that don’t endogenously express IL6r (i.e., a process described as transsignaling, Jones et al., 2001; Kallen, 2002). Of more immediate relevance to our aim to investigate population differences in cytokine activity, prior research has shown that the genetic regulation of IL-6 and its receptor is distinct (Galicia et al., 2004). Each factor is controlled somewhat independently by actions of different single nucleotide polymorphisms (SNPs). Thus, one cannot assume that an individual with low or high IL-6 levels in systemic circulation will necessarily have a similar receptor profile. The polymorphisms, as well as both IL-6 and sIL-6r, have been used as unique prognostic predictors of disease progression and poor outcomes in different patient populations (Galicia et al., 2006; Lin et al., 2006; Mehra et al., 2006; Nakjima et al., 1999; Velez et al., 2008; Yeh et al., 2010)
Japanese immunologists, such as Dr. Tadamitsu Kishimoto, were pioneers in the discovery of IL-6, and continue to generate seminal findings about its many functions (Kishimoto, 2005, 2010), but there has not previously been a normative study comparing IL-6 levels in healthy Japanese individuals to other populations. Most articles on IL-6 from Japan instead tend to focus on clinical abnormalities in patients (e.g., Komatsu et al., 2004; Yamaguchi et al., 1998). A population-level comparison across countries is logistically challenging because it requires that samples be analyzed at the same laboratories to preclude differences in assay methods. In addition to meeting this quality control criterion for the cytokine measures, the CRP and FBG assays comparing Japanese and Americans were also conducted by only one laboratory. Thus, it was possible to directly contrast the test results across the two countries, and to use the CRP and FBG values to validate conclusions about IL-6. Beyond determining whether there were overall population differences in cytokine biology, we were interested in discovering if the age-related changes in proinflammatory activity would be similar, especially if both elderly Japanese and Americans progress toward higher cytokine levels with age.
2. Methods
2.1. Participants
The biological measures were obtained from randomly selected adult men and women who were participants in surveys of health and aging in Japan or the United States, Midlife in Japan (MIDJA) and Midlife in the US (MIDUS), respectively. The 382 MIDJA subjects were a subset of 1027 adults between 30–79 years of age who had been recruited and stratified by age and gender to proportionately reflect the 23 neighborhood wards in Tokyo. Blood and urine were obtained from 37.2% of these MIDJA participants (168 men, 214 women) during visits to a medical clinic near the University of Tokyo. Over 95% were obtained between 0900–1145, with most of the remainder by 1330, and just 8 in the afternoon by 1530. Specimens were frozen in an ultracold freezer and shipped on dry ice by overnight courier for analysis in laboratories in the United States. The mean age of the MIDJA participants was 55.5 years (+/−14.0 years); approximately half (55%) were married.
The comparison data for the United States were generated from the second wave of a longitudinal, national study (Radler and Ryff, 2010). Because the methods for these biomarker analyses have been described in detail elsewhere, they are repeated only briefly here (Love et al., in press). MIDUS was begun in 1995–1996 as a survey of Americans who were recruited through random digit dialing and included individuals between 25–74 years of age across 48 states. Subsequently, following a second survey begun in 2004, biological samples were obtained from a subset of the original participants who consented to an overnight hospital stay at one of 3 General Clinical Research Centers (GCRC), either in Madison, WI, Los Angeles, CA, or Washington DC. Love et al. (in press) have already demonstrated that subjects agreeing to the biological assessment were sociodemographically similar to the larger survey with respect to age, gender, and marital status, but likely to be somewhat more educated (although 25% still had attained only a high school degree, while 50% had attended some college). In order to increase the representation of African-American participants during this second assessment, a city-specific sample was added from Milwaukee, WI. The current biological data on Americans are based on 1209 adults between 35–86 years of age with complete data (976 Caucasian-American and 233 African-American, mean age 58.4 and 53.6, respectively). Some analyses pertaining to racial differences in IL-6 between white and black participants have been reported previously, first on IL-6 levels in 721 and then on 999 individuals as the data accrued (Friedman and Herd, 2010; Slopen et al., 2010). Males comprised a smaller proportion of the African-American participants, 32.6% as compared to 45% for Caucasian-Americans and 45% for the Japanese adults, although still included a large enough number of African-American men (n=76) for the statistical evaluation of ethnicity effects.
The biological measures for American participants were determined from fasted blood samples obtained between 0500–0700, with a modal time of 0605. Specimens were aliquoted and shipped frozen on dry ice to the same two laboratories that conducted the cytokine, CRP and FBG assays on the MIDJA samples. All sample collections and analyses were approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison, as well as by the IRBs at UCLA and Georgetown University, and the comparable review panel at the University of Tokyo for the MIDJA project. All participants provided informed consent.
2.2. IL-6 and sIL6r
Serum IL-6 levels were determined by high-sensitivity enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN), with a lower sensitivity of detection at 0.16 pg/mL. All values were quantified in duplicate; any value over 10 pg/mL was re-run in diluted sera to fall on the standard curve. The laboratory intra-assay coefficient of variance (CV) was 4.1% and the inter-assay CV was 12.9% (generated by inclusion of a low and high IL-6 serum pool in each assay). Sandwich ELISA kits were also employed to quantify sIL-6r levels (Quantikine, R&D Systems). Sera were diluted 1:100 so values would fall on the standard reference curve from 7–2000 pg/mL. Thus, the effective assay range was 0.7–200 ng/mL. The intra-assay and inter-assay CVs were 2.0% and 6.9%, respectively.
2.3. CRP and FBG
Briefly, high sensitivity CRP was assessed at Dr. Tracy’s laboratory using a particle-enhanced immunonepholometric assay (Macy et al., 1997). Polystyrene particles are coated with monoclonal antibodies to CRP, which in the presence of CRP results in an antigen agglutinate and increased light intensity that can be measured on a BNII nephelometer (Seimans Healthcare Diagnostics, Deerfield, IL). The assay range is 0.16–1100; the intra-assay CV is 2.3–4.4%, and the inter-assay CV ranged from 2.1–5.7%. FBG was also determined with a BNII nephelometer using a semi-automated modification of the traditional Clauss method (Tracy et al., 1995). The amount of FBG in the specimen is quantitatively determined by an immunochemical reaction using antisera to human FBG. The intensity of the scattered light is proportional to the FBG concentration. The mean monthly intra-assay CV is 3.2–5.3%, and inter-assay CV is 2.6%.
2.4. Statistical analyses
The data were analyzed using SPSS PASW (Predictive Analytics Software, version 18). First, descriptive statistics were generated for all variables (means and SDs for continuous variables, proportions for discrete variables). Demographic factors, including age, gender representation and BMI, were compared across the 3 populations using t tests and tests of significant differences in proportions. Univariate analyses of variance were then employed to examine the influence of ethnicity, age, and gender on each of the 4 biological outcome measures (IL-6, sIL-6r, CRP, and FBG). Age was analyzed as a categorical variable (younger adult: 30–49 yr, older adult: 50–65 yr, old: 66+ yr). Because BMI differed so dramatically between Japanese and American participants, full factorial models analyzing for significant effects of ethnicity, age, and gender were run twice, both with and without BMI as a covariate. Estimated marginal means were computed for the biological measures by holding BMI at its mean value (although unadjusted means and variance estimates are shown in the figures to illustrate actual assay results). The influence of ethnicity on IL-6 levels was also tested in one analysis by excluding all obese participants with a BMI over 30. Post hoc testing of significant main effects or interaction terms relied on Tukey’s HSD. Correlations between IL6, CRP, and FBG were determined with the Pearson statistic.
3. Results
3.1. Demographic information
The demographic composition of the Japanese and American samples is presented in Table 1. As can be seen, the mean ages averaged between 53.6 and 58.4 years across the 3 groups, with broad coverage spanning adulthood from 30–84 years of age. African-American participants tended to be 2 years younger on average than the other two groups (p<.001), although there was extensive overlap in the age distributions. In general, there were more female than male participants in all 3 groups, and this gender difference was more pronounced among African-Americans (p<.01), but with adequate numbers of men and women for the analyses. The most dramatic difference between the 3 population groups was in the BMIs, which were significantly smaller in the Japanese than Americans (F=191.85, p<.001). The mean BMI for MIDJA participants was only 22.6, as compared to 29.1 and 32.9 for Caucasian-American and African-American participants, respectively. American women also tended to have larger BMIs than men, whereas the reverse trend was true for Japanese, resulting in a significant interaction between Ethnicity and Gender (F=10.93, p<.001). Because of the large differences in BMI, all statistical models were run twice, with and without BMI as a covariate, in order to determine the degree to which obesity contributed to the influence of ethnicity, age, and gender on the biological measures.
Table 1.
Demographic characteristics and mean biomarker values for Japanese and American participants (MIDJA and MIDUS).
| Japanese (n = 382) | Cau-American (n = 976) | Afr-American (n = 233) | |
|---|---|---|---|
| Gender1 | |||
| Male | 168 (44.0%) | 445 (45.6%) | 76 (32.6%) |
| Female | 214 (56.0%) | 531 (54.4%) | 157 (67.4%) |
| Mean Age2 (at clinic visit) | 55.5 (14.0) | 58.4 (11.7) | 53.6 (10.4) |
| Mean BMI3 (SD) | 22.58 (2.96) | 29.06 (5.84) | 32.88 (8.57) |
| Mean IL-64 (SD) (pg/mL) | 1.70 (1.99) | 2.79 (2.30) | 4.16 (3.72) |
| Mean sIL6r5 (SD) (ng/mL) | 37.91 (9.55) | 36.66 (10.27) | 28.48 (7.93) |
| Mean CRP6 (SD) (mg/L) | 0.75 (2.00) | 2.71 (4.36) | 4.50 (6.31) |
| Mean FBG7 (SD) (mg/dL) | 319.1 (64.1) | 339.5 (83.1) | 388.0 (96.7) |
Afr-Amer sample had proportionately more women and fewer men than the Cau-Amer and Japanese (p<.01). For all 3 groups, there were more female participants.
Although age ranges were similar, Cau-Americans were slightly older and the mean age of Afr-Americans was 2 years younger than Japanese (p<.001)
Japanese had significantly smaller BMIs (p<.001), and Cau-Americans were less overweight than the Afr-Americans (p<.001)
Japanese had significantly lower IL-6, and Afr-Amer were significantly above Cau-Americans (p<.01)
sIL-6r levels in Japanese were above Afr-Amer (p<.001), but not significantly above Cau-Amer (p=.52)
CRP in Japanese was dramatically below American values (p<.001); CRP in Afr-Americans was the highest (p<.001)
FBG in Japanese was below Americans (p<.001), and the levels in Afr-Americans were significantly above Cau-Americans (p=.026)
3.2. IL-6 and sIL-6r
IL-6 levels differed significantly between the 3 populations, with markedly lower values found in Japanese participants (F=40.97, p<.001). Even with BMI included as a covariate in the model, this effect of ethnic group on IL-6 remained highly significant (F=15.92, p<.001). As can be seen in Fig. 1, African-Americans clearly had the highest IL-6 levels, above both the Japanese and Caucasian-Americans. In both statistical models, including with BMI as a covariate, there was also a significant main effect for age, with higher IL-6 levels evident in older participants (F=7.46, p=.001). Notwithstanding these age-related changes in IL-6, many younger African-American adults between 30–49 years of age already had surprisingly high levels of IL-6. If one considers a criterion of 10 pg/mL to be a particularly high IL-6 value (Yeh et al., 2010), it was notable that 38 American participants (3%) exceeded than this level. In contrast only 4 MIDJA individuals in the old category had an IL-6 value over 10 pg/mL (<1% of participants).
Fig. 1.
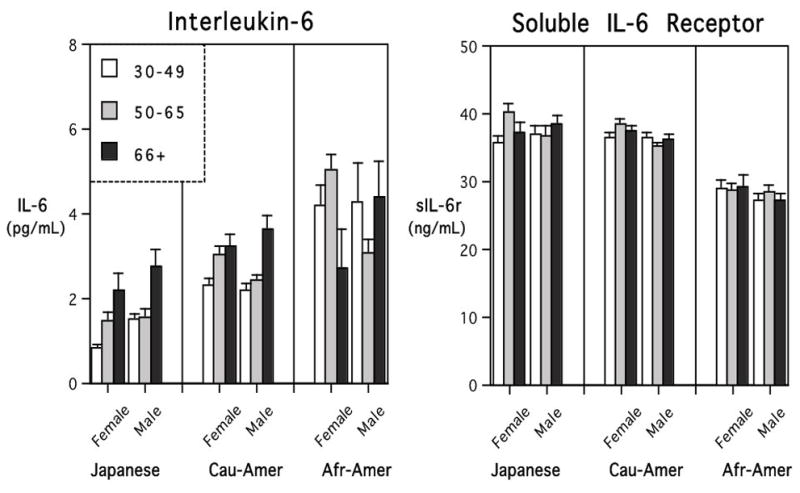
Mean (+SE) levels of interleukin-6 (IL-6) and soluble interleukin-6 receptor (sIL-6r) for MIDJA and MIDUS participants. Portrayed for 3 age categories: 30–49, 50–65, and older than 66 years). Japanese had significantly lower IL-6 levels than Americans. IL-6 was highest in African-Americans, already evident in younger adults in the context of the lowest systemic levels of sIL-6r.
There was also a main effect of ethnic group on sIL-6r levels, which was not lessened by considering BMI as a covariate (F=51.77, p<.001). However, the direction of this difference was opposite to the group effects for IL-6. African-American participants had the lowest sIL-6r levels, below both Caucasian-Americans and Japanese participants. The levels of sIL-6r in systemic circulation did not appear to be consistently affected either by the age of participant or gender, nor were they overtly associated with group differences in BMI.
3.3. IL-6 and obesity
Because 44% of American participants were sufficiently overweight to be considered obese, whereas only 5 of the MIDJA subjects were in this category, the influence of ethnicity was analyzed after excluding all individuals with a BMI over 30. As can be seen in Figure 2, Japanese continued to have significantly lower IL-6 levels, even after removing all of the obese American participants (F=15.59, p<.001). This analysis also indicated a significant interaction between Gender and Ethnicity, because Japanese females had the lowest IL-6 values, whereas when African-American females had a BMI >30, they had the highest IL-6, above even the African-American men who typically had the highest values (Fig. 2B).
Fig. 2.
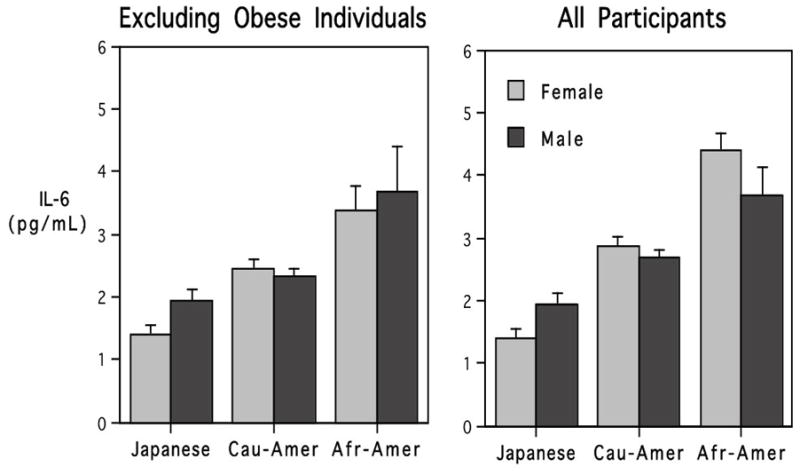
The influence of ethnicity and gender on IL-6 values before and after excluding individuals with a BMI >30. Significant differences between Japanese and Americans were evident in both analyses, although average IL-6 levels for Americans were higher when obese individuals were included.
3.4. CRP and FBG
The analyses of the CRP and FBG data concurred with the IL-6 findings (Fig. 3). Japanese participants had the lowest CRP values, significantly below Americans (F=38.62, p<.001). When BMI was included as a covariate in the model, the magnitude of these group differences was reduced, but remained statistically significant (F=4.90, p<.01). There was also a main effect of gender on CRP levels, with women having higher overall values than men, but a significant interaction between Gender and Ethnicity indicated this difference was more pronounced in American than Japanese participants. The influence of gender on CRP was also less evident in the statistical model with BMI included as a covariate (F=3.51, p=03).
Fig. 3.
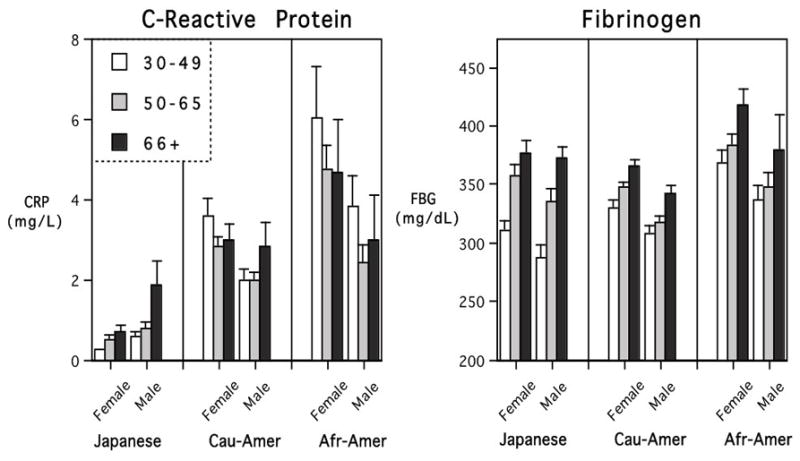
Mean (+SE) levels of CRP and FBG across adulthood. Japanese participants had remarkably low CRP, whereas African-Americans had the highest CRP and FBG values. High CRP in many younger African-Americans obscured a simple age-related increase that was more evident and linear in Japan.
When BMI was not included in the model, the occurrence of relatively high CRP values in so many of the younger, overweight American adults obscured a simple linear effect of age (see Fig 3). However, when BMI was taken into consideration, the age-related trend for higher CRP levels in the oldest adults became somewhat clearer (F=2.73, p=.066). Post hoc tests of the CRP levels in African-American participants confirmed the common occurrence of elevated CRP values in middle-aged adults. Their high CRP levels mitigated a simple, linear influence of age, which was more evident in the Japanese participants. Approximately 34% of Japanese participants had such low CRP that their levels were below the lower sensitivity of detection for our assay (<0.18 mg/L). On the upper end of the distribution, only 12 (3%) of MIDJA participants had a CRP value over 3 mg/L. In contrast, many younger adult Americans had CRP levels over 3.0 mg/L, which is typically considered to be a criterion of health concern (38% of MIDUS adults in the 30–49 age category were above 3.0 mg/L).
The statistical models for FBG were largely confirmatory of the conclusions based on IL-6 and CRP (Fig. 3). Even after taking BMI into consideration, there was a highly significant difference in FBG between Japanese and American participants (F=13.14, p<.001). Women tended to have higher FBG than men, but the difference became nonsignificant after including BMI as a covariate. The more notable finding was a strong influence of increasing age on FBG. A main effect of age was evident in Japanese and American participants, even when BMI was included in the model (F=21.61, p<.001). Further, the younger Japanese adults had relatively lower FBG levels when compared to equivalently aged Americans, which resulted in a significant interaction between Age and Ethnicity (F=3.92, p=.004). Higher FBG values were more typically found only in older Japanese, although at this point, they were just reaching the average levels seen in younger adult African-American participants (see Fig 3).
3.5. Correlation between IL-6, CRP, and FBG
The consistent differences in IL-6, CRP, and FBG levels between Japanese and Americans, and the congruent effect of age, were reinforced by examining the correlations between these 3 biomarkers. As can be seen in Table 2, IL-6 and CRP levels were significantly correlated, both across the whole sample and when considered separately for each ethnic group. Similarly, IL-6 and FBG values were significantly correlated, both when analyzing MIDJA and MIDUS together, and when examining the data separately for men and women in each population. Although some associations between the soluble receptor and FBG reached statistical significance, overall these correlations were less consistent and weaker (Table 2). The importance of IL-6, and the likely direction of its effect on CRP and FBG, was indicated further by considering the ratio of IL-6/sIL-6r. Across all participants, and within each subgroup, the IL-6/sIL-6r ratio was significantly associated with CRP and FBG (r=0.41, p<.001; r=0.32, p<.001, respectively). That is, high IL-6 in the context of lower sIL-6r levels in circulation was associated with higher CRP and FBG. In African-American males, the correlation between IL-6/sIL-6r and CRP reached 0.63 (p<.0001). Even among Japanese males who generally tended to have low IL-6 levels, the correlation between the IL-6/sIL-6r ratio and CRP was 0.74 (p<.0001).
Table 2.
Associations between IL-6 and sIL-6r and the two other hematological measures. IL-6 was significantly correlated with both CRP and FBG. Relationships with sIL-6r tended to be weaker and less consistent.
| IL-6/CRP | p* | IL-6/FBG | p | sIL6r/CRP | p | sIL-6r/FBG | p | |
|---|---|---|---|---|---|---|---|---|
| All Participants | .43 | <.001 | .36 | <.001 | −.01 | NS | .03 | NS |
| Japanese | .54 | <.001 | .21 | <.001 | −.03 | NS | .10 | NS |
| Female | .28 | <.001 | .16 | <.020 | .08 | NS | .08 | NS |
| Male | .69 | <.001 | .28 | <.001 | −.09 | NS | .12 | NS |
| Caucasian-Amer | .40 | <.001 | .33 | <.001 | .05 | NS | .09 | NS |
| Female | .41 | <.001 | .32 | <.001 | .03 | NS | .11 | <.01 |
| Male | .39 | <.001 | .34 | <.001 | .07 | NS | .03 | NS |
| African-Amer | .31 | <.001 | .35 | <.001 | .13 | <.06 | .21 | <.001 |
| Female | .59 | <.001 | .33 | <.001 | .15 | <.07 | .27 | <.001 |
| Male | .25 | <.003 | .34 | <.001 | −.06 | NS | .01 | NS |
Statistical significance of the r value; NS = non-significant
4. Discussion
This survey of cytokine biology and two other hematological markers in Japanese adults living in Tokyo has revealed dramatic differences when compared to the typical values seen in Americans. Systemic levels of IL-6 in MIDJA participants were significantly below the average levels for MIDUS, which was comprised of representative participants from a national survey of middle-aged and elderly Americans. Moreover, the low serum IL-6 values in Japanese adults occurred in the context of relatively high levels of the soluble receptor. In contrast, African-American participants in MIDUS were found to have particularly high levels of IL-6 circulating in the presence of lower sIL-6r, which may not be a healthy profile (Ueda et al., 1999). High levels of IL-6 in middle-aged and elderly people, whether they are Caucasian, African-American, or Asian, have been reported to be predictive of subsequent frailty, cardiovascular disease, poorer cognitive functioning, and ultimate mortality in longitudinal studies (Ferruci et al., 1999; Harris et al., 1999; Ridker et al., 2000; Yaffe et al., 2003). Moreover, even among Japanese, allele polymorphisms that are associated with greater IL-6 activity appear to predict the occurrence of lower bone mineral density and osteoporosis in older individuals (Yamada et al., 2003). The current findings on higher IL-6 in African-American replicate and extend two prior reports on smaller subsets of the MIDUS sample, which had focused primarily on the long-term influence of SES factors and early childhood adversity (Friedman and Herd, 2010; Slopen et al., 2010). The racial differences in IL-6 from MIDUS also concur with other large population studies of white and black Americans (Gruenewald et al., 2009).
While a growing number of papers have documented differences in IL-6 and the regulatory alleles for cytokines in Caucasian- and African-Americans (e.g., Upperman et al., 2005; Watson et al., 2007), to our knowledge this is the first comparison that extends the evaluation to simultaneously include an Asian population. Confirmatory evidence to support that these population differences in IL-6 are biologically meaningful was obtained by examining CRP and FBG levels, which are well-established predictors of disease risk (Friedlander et al., 2006; Kakafika et al., 2007; Tracy et al., 1995). It is known that IL-6 can be a potent stimulator of CRP and FBG both in healthy individuals and in patients (O’Donovan et al., 2010; Kanabrocki et al., 1999; Yamaguchi et al., 1998). The significant correlations shown in Table 2 are in keeping with this view (also, see Howren et al., 2009). However, the equally important message is that the CRP and FBG levels differed so markedly between the Japanese and American participants. The low levels evinced by the MIDJA participants would normally be interpreted as indicative of a better physiological profile (Ernst, 1990). Thus, both the cytokine and the CRP and FBG results would appear to concur with the lower prevalence of cardiovascular disease in Japan as compared to the United States and European countries.
It is possible that variation in the blood collection times--early morning for MIDUS and mostly mid-morning for MIDJA—may have contributed to the magnitude of the observed differences (Hermann et al., 2006). Some studies have found that IL-6 is highest during the sleep phase before awakening (Vgontzas et al., 1999, 2005), although others have failed to replicate the finding of large diurnal fluctuations in IL-6 levels, except in the context of sleep disruption, clinical depression, and autoimmune disease (Alesci et al., 2005; Arvidson et al., 1994, Haack et al., 2002). The extent of the variation from day to night in a healthy person may be only on the order of 1 pg/mL (Irwin, 2002, p. 508). Conversely, the later sample collection for MIDJA likely underestimated the extent of population differences in sIL-6r, because their soluble receptor values would have been even higher at awakening (Dimitrov et al., 2006). Moreover, temporal effects would not explain the large population differences in CRP levels, which don’t vary across the day (Meier-Ewert et al., 2001), although timing could have influenced the magnitude of the differences in FBG. FBG levels are highest in the morning and tend to decline in the afternoon (Kanabrocki et al., 1999). With regard specifically to the values for Americans, however, it should be reiterated that age, gender and race differences, well as the influence of BMI, could not have been affected by collection time because the nurses consistently obtained all samples at the same time of day.
Notwithstanding this important methodological issue that is germane to the full extent of the differences in these biological measures, it is probably more parsimonious to attribute the salubrious physiology found in Japanese participants to a number of life style factors related to diet and body weight. For example, it is known that foods that contain phytoestrogens, such as soy products, can reduce IL-6 levels, and the more frequent inclusion of plant isoflavones in the Japanese diet may even have anti-microbial actions (Dijsselbloem et al., 2004). The likely contribution of diet is probably even more evident in terms of overall food consumption. The BMI values of Japanese were very low when compared to the average scores for Americans, which included 36% in the overweight and 44% in the obese ranges. It is known that obesity is associated with proinflammatory physiology, both because adipocytes are a source of IL-6 and because fat stimulates monocytes and macrophages to become activated and release cytokines (Beasley et al., 2009; La Cava and Matarese, 2004). Further, western-style diets that are high in fats and carbohydrates have been shown to enhance inflammatory responses in animal models (Rivera et al., 2010). Given the very significant differences in BMI, we included it as a covariate in each of the statistical models when assessing the influence of ethnicity, age and gender. BMI did contribute some to the variation in each biological measure, but significant differences between Japanese and Americans persisted even after taking weight into consideration and excluding values for obese individuals (as others have found, e.g., Miles et al., 2008).
In fact, for the analysis of FBG, inclusion of BMI as a covariate actually served to clarify the trend for increased FBG in older participants. The propensity for many younger Americans to be overweight appeared to have led them to produce higher levels of FBG sooner in adulthood. These high levels of FBG and CRP in many Americans are definitely a concern because both are considered to be risk factors for cardiovascular disease (Ernst, 1990; Tracy et al., 1995; Whooley et al., 2007). The specific clinical concern for Type 2 diabetes may differ somewhat across racial groups because there is now evidence that the risk for insulin resistance in Japanese and some other South Asian populations can occur at a much lower BMI than in Americans (Mandavilli and Cyranoski, 2004; Rush et al., 2007). Moreover, based on a study of non-obese Japanese diabetics, IL-6 levels may not contribute as prominently to insulin-resistance (Taniguchi et al., 2006) as it does in overweight Americans (Fernandez-Real et al., 2000; Kern et al., 2001).
It is probable that genetic factors accounted for some of these differences in IL-6 between Japanese and Americans. There is a growing literature on the SNPs that regulate both IL-6 and sIL-6r, demonstrating how they influence the levels found in circulation (Cole et al., 2010; Watson et al., 2007). The SNPs that promote IL-6 and its receptor are distinct, which may help to explain why we didn’t see a simple congruence between cytokine level and the concentration of the receptor across the 3 ethnic groups. If the presence of high IL-6 in the context of low sIL-6r proves to be prognostic of poor health, then this would be of particular significance for many African-Americans (Anuurad et al, 2008). Although speculative, some have argued that a more proinflammatory profile could have been advantageous in other settings and time periods, especially in regions of the world where malaria was endemic (Upperman et al., 2005). The potential for a dissociation between IL-6 and sIL-6r levels was also clearly evident when examining the influence of age in all 3 groups. Neither in Japan nor in the US were the age-related increases in IL-6 offset by a commensurate rise in the receptor levels, which remained relatively constant across the life span in these middle-aged and older adults.
Given that the SNPs for both IL-6 and sIL6r have now been implicated in disease progression, including for diabetes and certain forms of cancer, and contribute to risk for adverse pregnancy outcomes and periodontal gum conditions, a better understanding of population variation is a high priority (Galicia et al., 2004). Several genetic surveys have already documented that the prevalence of these cytokine-related alleles varies among African, Asian, Indian, and European ethnic groups (Gadelha et al., 2005). Moreover, the G/C polymorphism at the promoter region for IL-6 can even affect prognostic risk for poor outcomes in a differential manner across ethnic groups (Beasley et al., 2009; Berger, 2004).
Finally, it should also be acknowledged that cytokines and inflammatory biomarkers have been linked to other types of demographic factors, such as educational attainment and income (Alley et al., 2006; Koster et al., 2006; Morozink et al., 2010; Petersen et al., 2008), to social integration and alienation (Cole et al., 2007) and to many emotional and stress-related processes (Cole et al., 2010; Kiecolt-Glaser et al., 2003; Stewart et al., 2009). Thus, it is tempting to speculate that the differences observed between Japanese and Americans could reflect some additional cultural and national characteristics. For example, with regard to other important indices of health, such as cholesterol, the influence of SES tends to be less pronounced in many Japanese studies than typically found in the US and European countries (Koster et al., 2005; Loucks et al., 2006; Martikainen et al., 2001). As compared to the US, Japan tends to be a more homogenous society with a less stratified middle-class, which could lessen some downstream effects of social factors that promote inflammatory physiology. Differences in physiology between Japan and the US were already evident in middle-aged adults, but the persistence of large differences in the oldest participants over 65 years of age may prove to be more clinically important. The healthier physiological profiles captured by our MIDJA survey may help to explain why the citizens of Japan have the longest life expectancy worldwide. Therefore, Japan is a country of special interest for investigating the influence of psychosocial and cultural factors on the biology of aging.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (5R37 AG027343) to conduct the study on Midlife in Japan (MIDJA) for comparative analysis with MIDUS (Midlife in the U.S., P01 AG020166). The original MIDUS study was supported by the MacArthur Foundation Research Network on Successful Midlife Development. The specimen collection was also facilitated by the General Clinical Research Centers program (M01-RR023942 [Georgetown], M01-RR00865 [UCLA]), and at UW from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (1UL1RR025011). The contributions of Ms. D. Brar in specimen processing and cytokine assays are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alesci S, Martinez PE, Kelkar S, Ilias S, Ioannis I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its diurnal rhythm, and loss of physiological complexity in its secretion: Clinical implications. J Clin Endocr Metabol. 2005;90(5):2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Ki Kim J, Karlamangla A, Hu P, Crimmins EM. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2006;20:498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Anuurad E, Rubin J, Chiem A, Tracy RP, Perason TA, Berglund L. High levels of inflammatory biomarkers are associated with increased allele-specific apolipoproteins in African Americans. J Clin Endocrinol Metab. 2008;93:1482–1488. doi: 10.1210/jc.2007-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson NG, Gudbjornsson B, Elfman L, Ryden AC, Totterman TH, Hallgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheumat Dis. 1994;53:521–524. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley LE, Koster A, Neweman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, Rubin SM, Goodpaster BH, Harris TB. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity. 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger FG. The interleukin-6 gene: a susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treat. 2004;88(3):281–285. doi: 10.1007/s10549-004-0726-0. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes, and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hemato. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene social environment interaction at the human IL-6 locus. Proc Nat Acad Sci. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sun CY, Rose RM, Cacioppo CT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189, 1–13. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa R, Velmurugan K, Arvind K, Sivaram P, Sientay C, Uday S, Mohan V. Serum levels of interleukin-6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein in relation to insulin resistance and glucose intolerance – the Chennai Urban Rural Epidemiology Study (Cures) Metabolism: Clin Exp. 2006;55(9):1232–1238. doi: 10.1016/j.metabol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF- alpha, TGF-β, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Reports of the ASHI minority Workshops: part IV. Human Immunol. 2004;65:1413–1419. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- Dijsselbloem N, Vanden Berghe W, De Naeyer A, Haegeman G. Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections. Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer, and anti- inflammatory therapy. Biochem Pharmacol. 2004;68:1171–1185. doi: 10.1016/j.bcp.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller, Rose-John, Born J. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:E-1599–1609. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Ernst E. Plasma fibrinogen- an independent cardiovascular risk factor. J Intern Med. 1990;227:365–372. doi: 10.1111/j.1365-2796.1990.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Ann Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Vendrell J, Richart C, Ricart W. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab. 2000;85(3):1334–1339. doi: 10.1210/jcem.85.3.6555. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunk DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocr Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Friedlander Y, Kark JD, Sinnreich R, Tracy RP, Siscovick DS. Fibrinogen and CRP in Israeli families: Genetic and environmental sources of concentrations and longitudinal changes. Atherosclerosis. 2006;189:169–177. doi: 10.1016/j.atherosclerosis.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national sample (the MIDUS study) Psychosomat Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gadelha SR, Alcantara LCJ, Costa GCS. Ethnic differences in the distribution of interleukin-6 polymorphisms among three Brazilian ethnic groups. Human Biol. 2005;771(4):509–514. doi: 10.1353/hub.2005.0061. [DOI] [PubMed] [Google Scholar]
- Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong: evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immunity. 2004;5:513–516. doi: 10.1038/sj.gene.6364120. [DOI] [PubMed] [Google Scholar]
- Galicia JC, Tai H, Komatsu Y, Shimada Y, Ikezawa I, Yoshie H. Interleukin-6 receptor gene polymorphisms and periodontitis in a non-smoking Japanese population. J Clin Periodontal. 2006;33:704–709. doi: 10.1111/j.1600-051X.2006.00978.x. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy RP, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinol. 2002;27(8):921–931. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, von Aulock S, Dehus O, Keller M, Okigami H, Gantner F, Wendel A, Hartung T. Endogenous cortisol determines the circadian rhythm of lipopolysaccharide- but not lipoteichoic acid-inducible cytokine release. Eur J Immunol. 2006;36:371–379. doi: 10.1002/eji.200535470. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomat Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Beh Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15(1):43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Kanabrocki EL, Sothern RB, Messmore HL, Roitman-Johnson, McCormick JB, Dawson S, Bremner FW, Third JLHC, Nemchausky BA. Circadian interrelationships among levels of plasma fibrinogen, blood platelets, and serum interleukin-6. Clin Appl Thromb Hemost. 1999;5(1):37–42. doi: 10.1177/107602969900500108. [DOI] [PubMed] [Google Scholar]
- Kallen KJ. The role of transignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- Kakafika AI, Liberopoulos EN, Mikhailidi DP. Fibrinogen: A predictor of vascular disease. Curr Pharm Design. 2007;13(16):1647–1659. doi: 10.2174/138161207780831310. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Nat Acad Sci. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Tai H, Galicia JC, Shimada Y, Endo M, Akazawa K, Yamazaki K, Yoshie H. Interleukin-6 (IL-6)-373 A9T11 allele is associated with reduced susceptibility to chronic periodontitis in Japanese subjects and decreased serum IL-6 level. Tissue Antigens. 2004;65:110–114. doi: 10.1111/j.1399-0039.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Koster A, Bosma H, Penninx BWJH, Newman AB, Harris TB, van Eijk JTM, Kempen GIJM, Simonsick EM, Johnson KC, Rooks RN, Ayonayon HN, Rubin SM, Kritchevsky SB for the Health ABC study. Association of inflammatory markers with socioeconomic status. J Gerontol: Med Sci. 2006;61A(3):284–290. doi: 10.1093/gerona/61.3.284. [DOI] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Pouslsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent. Diabetes. 2005;54(Suppl 2):114–124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nature Rev: Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berthier-Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, Smith MW, Coresh J. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol. 2006;17:3158–66. doi: 10.1681/ASN.2005050465. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, Hayes LJ, D’Agostino RB, Sr, Larson MG, Vasan RS, Benjamin EJ, Berkman LF. Association of education level with inflammatory markers in the Framingham Offspring study. Am J Epidemiol. 2006;163:622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample and comparative context. J Aging Health. 2010 doi: 10.1177/089826431037355. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: Implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- May LT, Viguet H, Kenney JS, Ida N, Allsion AC, Sehgal PB. High levels of ‘complexed’ interleukin-6 in human blood. J Biol Chem. 1992;267:19698–19704. [PubMed] [Google Scholar]
- Madan AK, Tichansky DS, Coday M, Fain JN. Comparison of IL-8, IL-6, and PGE2 formation by visceral (omental) adipose tissue of obese Caucasian compared to African-American women. Obesity Surg. 2009;16(10):1342–1350. doi: 10.1381/096089206778663652. [DOI] [PubMed] [Google Scholar]
- Mandavilli A, Cyranoski D. Asia’s big problem. Nature Med. 2004;10:325–327. doi: 10.1038/nm0404-325. [DOI] [PubMed] [Google Scholar]
- Martikainen P, Ishizaki M, Marmot MG, Nakagawa H, Kagamimori S. Socioeconomic differences in behavioral and biological risk factors: a comparison of a Japanese and an English cohort of employed men. Intern J Epidemiol. 2001;30:833–838. doi: 10.1093/ije/30.4.833. [DOI] [PubMed] [Google Scholar]
- Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, Redline S. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep- related breathing disorder. Arch Intern Med. 2006;166:1725–1731. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47(4):426–430. [PubMed] [Google Scholar]
- Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, Wahle KW, Calder PC. Age-related increases in circulating inflammatory markers are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocr Metabol. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Montero-Julian FA. The soluble IL-6 receptors: serum levels and biological function. Cell Mol Biol. 2001;47(4):583–597. [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psych. 2010 doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mulberg J, Vollmer P, Marz P, Rose-John S. Generation and function of the soluble interleukin-6 receptor. Biochem Soc Trans. 1999;27:211–219. doi: 10.1042/bst0270211. [DOI] [PubMed] [Google Scholar]
- Nakjima T, Ota N, Yoshida H, Watanabe S, Suzuki T, Emi M. Allelic variants in the interleukin-6 gene and essential hypertension in Japanese women. Genes Immun. 1999;1(2):115–119. doi: 10.1038/sj.gene.6363642. [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psych. doi: 10.1037/a0021360. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly CL, Malone KM. Clinical anxiety, cortisol and interleukin-6. Evidence for specificity in emotion-biology relationships. Brain Beh Immun. 2010;24(7):1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C- reactive protein. Psychosomat Med. 2008;70:646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Comm Health. 2008;62:484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- Pradham AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health. 2010;22(3):307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- Rao KMK, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and cross-linked fibrin dimmers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Gaskin L, Singer G, Houghton J, Allman M. Western diet enhances hepatic inflammation in mice exposed to cecal ligation and puncture. BMC Physiol. 2010;10(20) doi: 10.1186/1472-6793-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Part 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush EC, Plank LD, Yajnik CS. Interleukin-6, tumour necrosis factor-alpha, and Insulin relationships to body composition, metabolism and resting energy expenditure in a migrant Asian Indian population. Clin Endocrinol. 2007;66:684–690. doi: 10.1111/j.1365-2265.2007.02801.x. [DOI] [PubMed] [Google Scholar]
- Sauerwein-Teissl M, Blasko I, Zisterer K, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine. 2000;12(8):1160–1161. doi: 10.1006/cyto.2000.0679. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomat Med. 2010:72. doi: 10.1097/PSY.0b013e3181e9c16f. (ePub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Beh Immun. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Miller LE, Scholmerich J, Zietz B. Cytokines and hormones as possible links between endocrinosenescence and immunosenescence. J Neuroimmunol. 2000;109(1):10–15. doi: 10.1016/s0165-5728(00)00296-4. [DOI] [PubMed] [Google Scholar]
- Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumki K, Taga T, Kishimoto T, Suda T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin-6. Proc Nat Acad Sci. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi A, Fukushima M, Ohya M, Nakai Y, Yohii S, Nagasak S, Matsumoto K, Taki Y, Kuroe A, Nishimura F, Seino Y. Interleukin-6, leptin, and insulin resistance in nonobese Japanese type 2 diabetic patients. Metabol Clin Exp. 2006;55:258–262. doi: 10.1016/j.metabol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Tiermeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MMB. Inflammatory proteins and depression in the elderly. Epidemiol. 2003;14(1):103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Tracy RP, Bovill EG, Yanez D, Psaaty BM, Fried LP, Heiss G, Lee M, Polak JF, Savage PJ. Fibrinogen and factor VIII, but not factor VII, are associated with measures of subclinical cardiovascular disease in the elderly. Results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1995;15:1269–1279. doi: 10.1161/01.atv.15.9.1269. [DOI] [PubMed] [Google Scholar]
- Ueda K, Takahashi M, Ozawa K, Kinoshita M. Decreased soluble interleukin-6 receptor in patients with acute myocardial infarction. Am Heart J. 1999;138:908–915. doi: 10.1016/s0002-8703(99)70016-5. [DOI] [PubMed] [Google Scholar]
- Upperman JS, Pillage g, Siddiqi MS, Zeevi A, Kelly N, Ford HR, Kammerer C, Spolarics Z. Dominance of high-producing interleukin 6 and low producing interleukin 10 and interferon gamma alleles in glucose-6-phosphate dehydrogenase-deficient trauma patients. Shock. 2005;23(3):197–201. [PubMed] [Google Scholar]
- Van Mark A, Weiler SW, Schroder M, Otto A, Jauch-Chara K, Groneberg DA, Spallek M, Kessel R, Kalsdorf B. The impact of shift work induced circadian disruption on IL-6 and TNF-alpha immune responses. J Occup Med Toxicol. 2010;5(18) doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez DR, Fortunato SJ, Williams SM, Menon R. Interleukin-6 (IL-6) and receptor (IL-6R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum Mol Genet. 2008;17(11):1629–1630. doi: 10.1093/hmg/ddn049. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotskas A, Zachman K, Kales A, Prolo P, Wong M-L, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocr Metab. 1999;84(8):2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Watson JD, Fallin MD, Cushman M, Lange L, Psaty B, Jenny N, Browner W, Tracy RP, Durda P, Reiner A. IL-6 gene variation is associated with IL-6 and C-reactive protein levels but not cardiovascular outcomes in the Cardiovascular Health study. Human Genet. 2007;122:485–494. doi: 10.1007/s00439-007-0428-x. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1993;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: Findings from the Heart and Soul Study. Biol Psychiat. 2007;62:314–3. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Krichevsky S, Launer L, Kuller L, Harris T. Inflammatory markers and cognition in well- functioning African-American and white elders. Neurol. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ando F, Nino N, Shimokata H. Association of polymorphisms of interleukin-6, osteocalcin, and vitamin D receptor genes, alone or in combination, with bone mineral density in community dwelling Japanese women and men. J Clin Endocr Metab. 2003;88(7):3372–3378. doi: 10.1210/jc.2002-021449. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamamoto Y, Yokota S, Nakgawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Japan J Clin Oncol. 1998;28(12):740–744. doi: 10.1093/jjco/28.12.740. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Soga Y, Iwamoto Y, Yoshizawa S, Iwata H, Kokeguchi S, Takashiba S, Nishmimura F. Macrophage-adipocyte interaction: Marked interleukin-6 production by lipopolysaccharide. Obesity. 2007;15:2549–2552. doi: 10.1038/oby.2007.305. [DOI] [PubMed] [Google Scholar]
- Yeh K-Y, Li Y-Y, Hsieh L-L, Lu C-H, Chou W-C, Liaw C-C, Tang R-P, Liao S-K. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Japanese J Clin Oncol. 2010;40(6):580–587. doi: 10.1093/jjco/hyq010. [DOI] [PubMed] [Google Scholar]
- Yokoyma A, Kohno N, Sakai K, Kondo KI, Hirasawa Y, Hiwada K. Circulating levels of soluble interleukin-6 receptors in patients with bronchial asthma. Am J Respirat Crit Care Med. 1997;156(5):1688–1691. doi: 10.1164/ajrccm.156.5.9610070. [DOI] [PubMed] [Google Scholar]


