Abstract
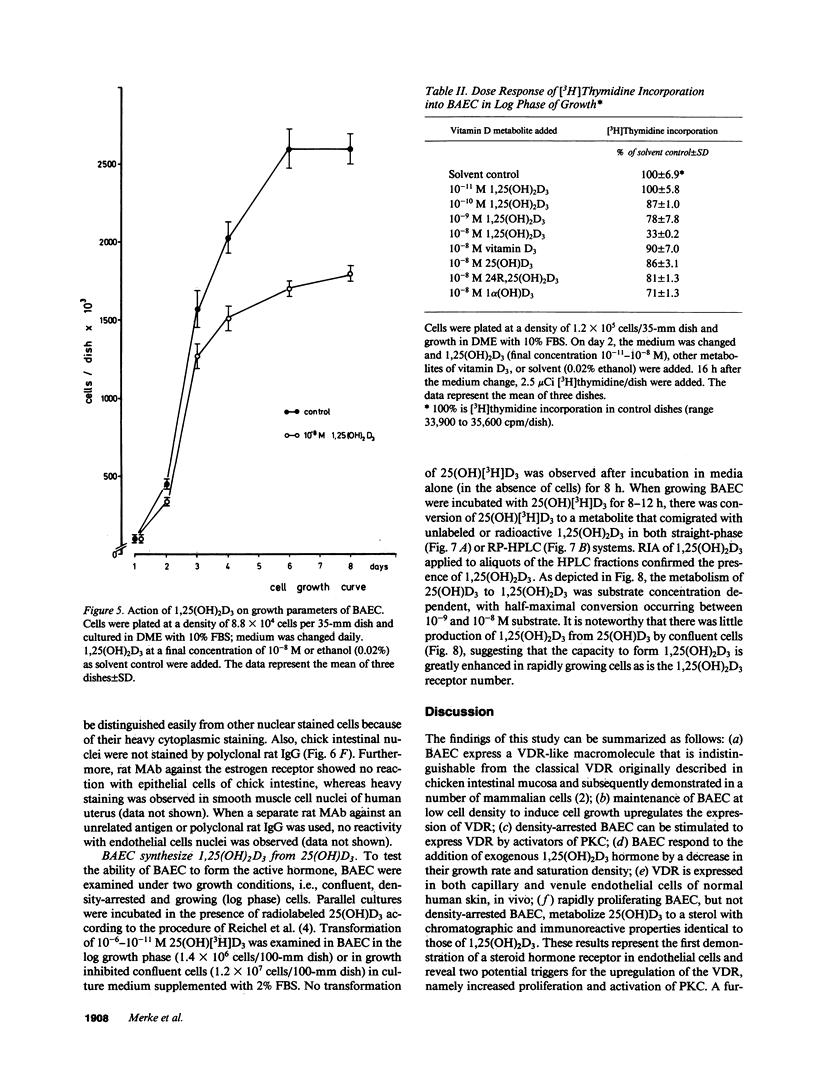
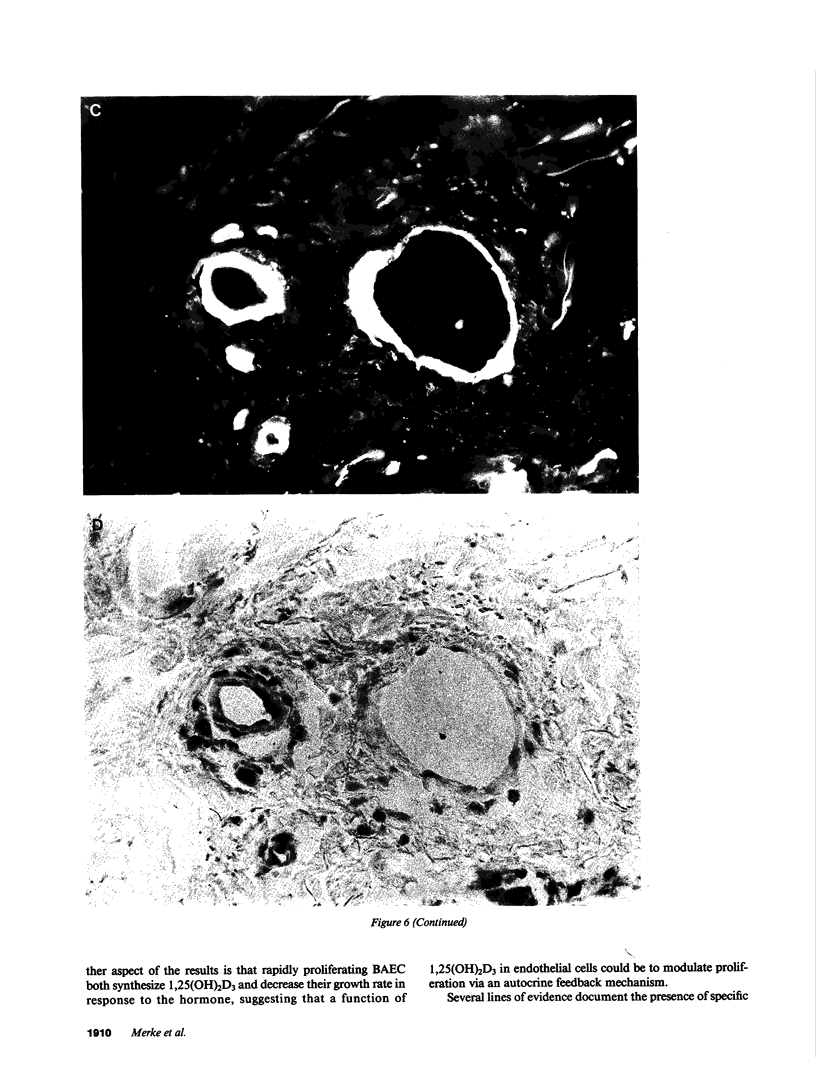
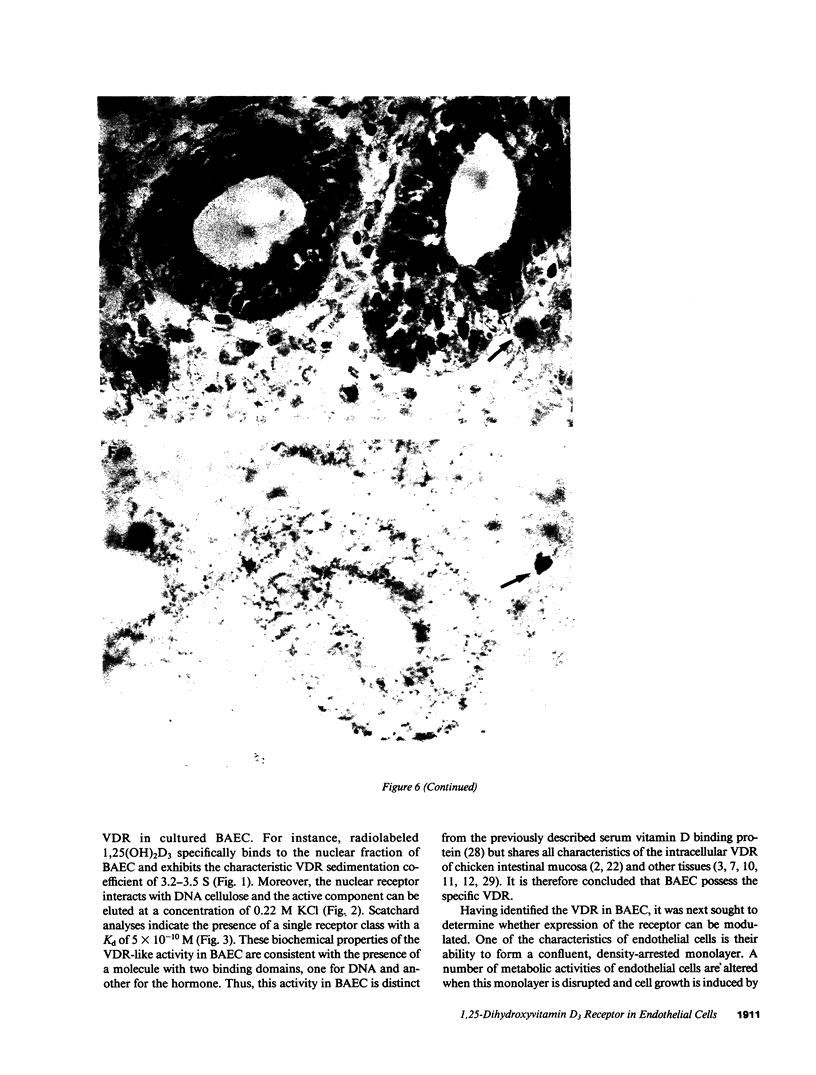
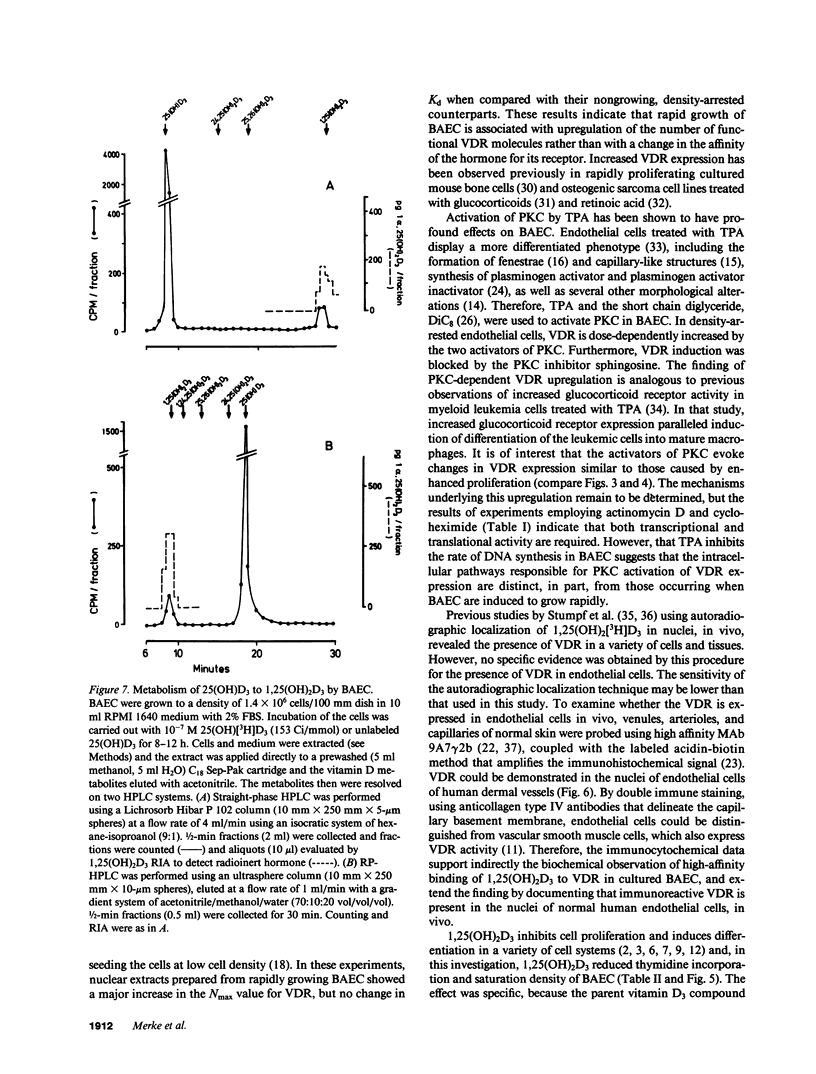
Because 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) has been shown to play roles in both proliferation and differentiation of novel target cells, the potential expression of 1,25(OH)2D3 receptor (VDR) activity was investigated in cultured bovine aortic endothelial cells (BAEC). Receptor binding assays performed on nuclear extracts of BAEC revealed a single class of specific, high-affinity VDR that displayed a 4.5-fold increase in maximal ligand binding (Nmax) in rapidly proliferating BAEC compared with confluent, density-arrested cells. When confluent BAEC were incubated with activators of protein kinase C (PKC), Nmax increased 2.5-fold within 6-24 h and this upregulation was prevented by sphingosine, an inhibitor of PKC, as well as by actinomycin D or cycloheximide. Immunohistochemical visualization using a specific MAb disclosed nuclear localized VDR in venular and capillary endothelial cells of human skin biopsies, documenting the expression of VDR, in vivo, and validating the BAEC model. Finally, additional experiments indicated that BAEC formed the 1,25(OH)2D3 hormonal metabolite from 25(OH)D3 substrate, in vitro, and growth curves of BAEC maintained in the presence of 10(-8) M 1,25(OH)2D3 showed a 36% decrease in saturation density. These data provide evidence for the presence of a vitamin D microendocrine system in endothelial cells, consisting of the VDR and a 1 alpha-hydroxylase enzyme capable of producing 1,25(OH)2D3. That both components of this system are coordinately regulated, and that BAEC respond to the 1,25(OH)2D3 hormone by modulating growth kinetics, suggests the existence of a vitamin D autocrine loop in endothelium that may play a role in the development and/or functions of this pathophysiologically significant cell population.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983 Nov;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov A. S., Lukashev M. E., Romanov Y. A., Tkachuk V. A., Repin V. S., Smirnov V. N. Morphological alterations in endothelial cells from human aorta and umbilical vein induced by forskolin and phorbol 12-myristate 13-acetate: a synergistic action of adenylate cyclase and protein kinase C activators. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9704–9708. doi: 10.1073/pnas.83.24.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R., De Moor P., Baggiolini E. G., Uskokovic M. R. A radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin Chem. 1980 Apr;26(5):562–567. [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Regulation of 1,25-dihydroxyvitamin D3 receptors in cultured mouse bone cells. Correlation of receptor concentration with the rate of cell division. J Biol Chem. 1981 Jun 10;256(11):5561–5566. [PubMed] [Google Scholar]
- Clemens T. L., Garrett K. P., Zhou X. Y., Pike J. W., Haussler M. R., Dempster D. W. Immunocytochemical localization of the 1,25-dihydroxyvitamin D3 receptor in target cells. Endocrinology. 1988 Apr;122(4):1224–1230. doi: 10.1210/endo-122-4-1224. [DOI] [PubMed] [Google Scholar]
- Doctrow S. R., Folkman J. Protein kinase C activators suppress stimulation of capillary endothelial cell growth by angiogenic endothelial mitogens. J Cell Biol. 1987 Mar;104(3):679–687. doi: 10.1083/jcb.104.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Heitz R., Zeh W., Katus H., Kommerell B., Ziegler R., Glomset J. A. sn-1,2-Diacylglycerols and phorbol diesters stimulate thromboxane synthesis by de novo synthesis of prostaglandin H synthase in human promyelocytic leukemia cells. J Clin Invest. 1987 Mar;79(3):903–911. doi: 10.1172/JCI112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Jaffe E. A., Rifkin D. B. Plasminogen activator and collagenase production by cultured capillary endothelial cells. J Cell Biol. 1982 Dec;95(3):974–981. doi: 10.1083/jcb.95.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler C. A., Marion S. L., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3 inhibits the clonogenic growth of transformed cells via its receptor. Biochem Biophys Res Commun. 1986 Aug 29;139(1):136–143. doi: 10.1016/s0006-291x(86)80090-0. [DOI] [PubMed] [Google Scholar]
- Haussler M. R. Vitamin D receptors: nature and function. Annu Rev Nutr. 1986;6:527–562. doi: 10.1146/annurev.nu.06.070186.002523. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979 Apr 25;254(8):2722–2729. [PubMed] [Google Scholar]
- Hirai H., Murakami T., Urabe A., Takaku F. Increased glucocorticoid receptor concentration in macrophage differentiation of myeloid leukemia cells with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1985 Jun;45(6):2456–2461. [PubMed] [Google Scholar]
- Howard G. A., Turner R. T., Sherrard D. J., Baylink D. J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J Biol Chem. 1981 Aug 10;256(15):7738–7740. [PubMed] [Google Scholar]
- Kream B. E., DeLuca H. F., Moriarity D. M., Kendrick N. C., Ghazarian J. G. Origin of 25-hydroxyvitamin D3 binding protein from tissue cytosol preparations. Arch Biochem Biophys. 1979 Jan;192(1):318–323. doi: 10.1016/0003-9861(79)90098-5. [DOI] [PubMed] [Google Scholar]
- Lombardi T., Montesano R., Furie M. B., Silverstein S. C., Orci L. Endothelial diaphragmed fenestrae: in vitro modulation by phorbol myristate acetate. J Cell Biol. 1986 May;102(5):1965–1970. doi: 10.1083/jcb.102.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Koeffler H. P., Donaldson C. A., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984 Feb;98(2):391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas S. C., Abare J., Deftos L. J. Glucocorticoids increase the 1,25(OH)2D3 receptor concentration in rat osteogenic sarcoma cells. Calcif Tissue Int. 1984 Mar;36(2):153–157. doi: 10.1007/BF02405311. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Merke J., Hofmann W., Goldschmidt D., Ritz E. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int. 1987 Aug;41(2):112–114. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- Merke J., Hügel U., Ritz E. Nuclear testicular 1,25-dihydroxyvitamin D3 receptors in Sertoli cells and seminiferous tubules of adult rodents. Biochem Biophys Res Commun. 1985 Feb 28;127(1):303–309. doi: 10.1016/s0006-291x(85)80159-5. [DOI] [PubMed] [Google Scholar]
- Merke J., Hügel U., Zlotkowski A., Szabó A., Bommer J., Mall G., Ritz E. Diminished parathyroid 1,25(OH)2D3 receptors in experimental uremia. Kidney Int. 1987 Sep;32(3):350–353. doi: 10.1038/ki.1987.216. [DOI] [PubMed] [Google Scholar]
- Merke J., Klaus G., Hügel U., Waldherr R., Ritz E. No 1,25-dihydroxyvitamin D3 receptors on osteoclasts of calcium-deficient chicken despite demonstrable receptors on circulating monocytes. J Clin Invest. 1986 Jan;77(1):312–314. doi: 10.1172/JCI112292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merke J., Schwittay D., Fürstenberger G., Gross M., Marks F., Ritz E. Demonstration and characterization of 1,25-dihydroxyvitamin D3 receptors in basal cells of epidermis of neonatal and adult mice. Calcif Tissue Int. 1985 May;37(3):257–267. doi: 10.1007/BF02554872. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Sereni A. M., Stevens V. L., Hannun Y. A., Bell R. M., Kinkade J. M., Jr Inhibition of phorbol ester-dependent differentiation of human promyelocytic leukemic (HL-60) cells by sphinganine and other long-chain bases. J Biol Chem. 1986 Sep 25;261(27):12610–12615. [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pawlowski N. A., Abraham E. L., Pontier S., Scott W. A., Cohn Z. A. Human monocyte-endothelial cell interaction in vitro. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8208–8212. doi: 10.1073/pnas.82.23.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich P. M., Heersche J. N., Tinker D. O., Jones G. Retinoic acid stimulates 1,25-dihydroxyvitamin D3 binding in rat osteosarcoma cells. J Biol Chem. 1984 Jul 10;259(13):8274–8280. [PubMed] [Google Scholar]
- Pike J. W., Donaldson C. A., Marion S. L., Haussler M. R. Development of hybridomas secreting monoclonal antibodies to the chicken intestinal 1 alpha,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7719–7723. doi: 10.1073/pnas.79.24.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J. W., Marion S. L., Donaldson C. A., Haussler M. R. Serum and monoclonal antibodies against the chick intestinal receptor for 1,25-dihydroxyvitamin D3. Generation by a preparation enriched in a 64,000-dalton protein. J Biol Chem. 1983 Jan 25;258(2):1289–1296. [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Elias P. M. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988 Apr 15;263(11):5390–5395. [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Bishop J. E., Norman A. W. 25-Hydroxyvitamin D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987 Jan;64(1):1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Tobler A., Norman A. W. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E., Clark S. A., Sar M., DeLuca H. F. Topographical and developmental studies on target sites of 1,25 (OH)2 vitamin D3 in skin. Cell Tissue Res. 1984;238(3):489–496. doi: 10.1007/BF00219863. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Reid F. A., Tanaka Y., DeLuca H. F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979 Dec 7;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Harlan J. M. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988 Mar;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar R. E., Simpson R. U. Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am J Physiol. 1987 Dec;253(6 Pt 1):E675–E683. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Ho M., Tsang R. C., Norman E. J., Adams K. G. Synthesis of 1,25-dihydroxyvitamin D3 by human placenta in vitro. J Clin Endocrinol Metab. 1981 Sep;53(3):484–488. doi: 10.1210/jcem-53-3-484. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Young E. W., Bukoski R. D., McCarron D. A. Calcium metabolism in experimental hypertension. Proc Soc Exp Biol Med. 1988 Feb;187(2):123–141. doi: 10.3181/00379727-187-42646. [DOI] [PubMed] [Google Scholar]