Abstract
Before this study, the human norepinephrine transporter (hNET) was the only member of the biogenic amine neurotransmitter transporter family that had not been demonstrated to be a functional homo-oligomer. Here, using two forms of the transporter, I155C and hNET-myc, with distinct antigenicity and inhibitor sensitivity, we demonstrated that hNET exists as a homo-oligomer. hNET I155C is a functional mutant and is sensitive to inactivation by the sulfhydryl reagent [2-(trimethylammonium)ethyl]methanethiosulfonate, while hNET-myc is resistant to inactivation by this reagent. Coimmunoprecipitation of these two forms demonstrated that a physical interaction exists between norepinephrine transporter monomers. Further characterization of this physical interaction has revealed that the activity of norepinephrine transporters depends on interactions between monomers. Because norepinephrine transporters and serotonin transporters are the only two members of the neurotransmitter transporter family endogenously expressed in the cell membrane of the same cells, placental syncytiotrophoblasts, we tested the ability of norepinephrine transporters and serotonin transporters to associate and function in a hetero-oligomeric form. Similarly, coexpression of hNET-myc with serotonin transporter-FLAG showed a physical interaction in coimmunoprecipitation assays. However, coexpression of serotonin and norepinephrine transporters did not sensitize norepinephrine transporter activity to inhibition by citalopram, a selective serotonin transport inhibitor. Thus, the norepinephrine transporter–serotonin transporter physical association did not produce functional consequences. Based on this, we propose that the transporters for biogenic amine neurotransmitters interact functionally in homo- but not hetero-oligomeric forms.
Keywords: citalopram, dopamine transporter, functional and physical association, MTS-reagent, norepinephrine transporter, serotonin transporter
Human norepinephrine transporter (hNET) is a member of the neurotransmitter sodium symporter (NSS) family of transporters (Saier 1999), a family that also includes dopamine and serotonin transporters (DAT and SERT, respectively). Norepinephrine transporter, DAT and SERT are among many proteins in the NSS family that transport neurotransmitters into neurons and glia near sites of transmitter release (Amara and Kuhar 1993; Lester et al. 1994; Nelson 1998). As such, these proteins maintain a low concentration of transmitters in the region of pre- and post-synaptic receptors. Compounds that interfere with the action of these transporters exert powerful behavioral effects and are used therapeutically as antidepressants and recreationally as stimulants (Leonard 1999; Fleckenstein et al. 2000). Among the clinically effective antidepressant drugs are desipramine and reboxetine, which selectively inhibit hNET (Leonard 1999).
The high degree of sequence similarity between NET and other members of the NSS family suggests a common structure and mechanism. These proteins were predicted to contain 12 transmembrane domains and to have intracellular NH2- and COOH-termini (Guastella et al. 1990; Blakely et al. 1991a; Pacholczyk et al. 1991; Shimada et al. 1991). Studies using site-specific antibodies and chemical modification have substantially verified this topology for hNET, DAT and SERT (Androutsellis-Theotokis and Rudnick 2002; Bruss et al. 1995; Hersch et al. 1997; Chen et al. 1998; Ferrer and Javitch 1998). Moreover, modification of a cysteine residue inserted in the corresponding position of SERT (I179C) and hNET (I155C) leads to inactivation of both transporters (Chen and Rudnick 2000; Chen et al. 1997). This position was proposed to function as part of the gate that regulates access from the external medium to the substrate-binding site (Chen and Rudnick 2000).
It has become apparent that these transporters for biogenic amine neurotransmitters can exist as homo-oligomers. The preponderance of evidence relates to SERT, for which cross-linking and mutagenesis studies suggested the possibility of multimers (Jess et al. 1996; Chang et al. 1998). This was firmly established using coprecipitation and inactivation studies (Kilic and Rudnick 2000) and further confirmed using fluorescence resonance energy transfer (Schmid et al. 2001). Subsequently, site-directed cross-linking studies provided evidence for homo-oligomerization of DAT (Hastrup et al. 2001; Torres et al. 2003). On the basis of these observations and the high homology between SERT, DAT and hNET, we hypothesize that homo-oligomerization could be a common phenomenon between transporters of biogenic amine neurotransmitters.
In this work, the physical and functional self association of hNET monomers was studied using two forms of the transporter, I155C and hNET-myc, with distinct antigenicity and inhibitor sensitivity. As was previously reported (Chen et al. 1997), I155C is a functional mutant and is sensitive to inactivation by [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET), while hNET-myc is resistant to inactivation by this reagent. Our results are consistent with a dimeric form of hNET with functional interactions between subunits.
The SERT and NET are the only members of the transporter family endogenously expressed in the same cell line, the placental syncytiotrophoblast (Balkovetz et al. 1989; Ramamoorthy et al. 1993). Given the ability of SERT and DAT (Kilic and Rudnick 2000; Hastrup et al. 2001; Torres et al. 2003) to form functional homo-oligomers, there may be a structural basis for the process embedded in the domains common to SERT, DAT and NET. Thus, we tested for the possibility that these common domains may allow association of SERT and hNET in a hetero-oligomeric form. When transiently coexpressed in HeLa cells, hNET was physically but not functionally associated with SERT proteins. Our data presented here support the proposal that homo-oligomerization, but not hetero-oligomerization, is a functional common phenomenon between the transporters for the biogenic amine neurotransmitters.
Experimental procedures
Construction of human norepinephrine transporter-myc
An oligonucleotide encoding a myc tag fused to the COOH-terminal region of hNET (TATATAAGCTTCTATCATTACAGATCCTCTTCTGAAATGAGTTTTTGTTCGTGTTGCAACTGGAACTGTCTG) was used together with an oligonucleotide complementary to the T7 promoter region of pBluescript II SK1 to amplify the hNET insert from hNET plasmid (phNET) (Chen and Rudnick 2000) using Pfu polymerase. The product was subcloned into HindIII/KpnI sites of Bluescript. We confirmed the subcloning process by sequencing the genes. The modified COOH-terminal amino acid sequence of hNET-myc is shown in Fig. 1.
Fig. 1.
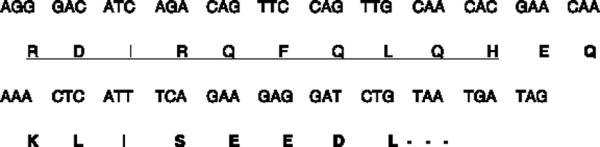
Modified COOH-terminal amino acid sequence of hNETmyc. hNET amino acid residues are underlined and the c-myc tag is in bold.
Expression of human norepinephrine and serotonin transporter constructs
Human cervical epithelioid carcinoma cells (HeLa; American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 incubator. There is no endogenous SERT or NET expression in HeLa cells. Transporters were transiently transfected using the vaccinia-T7 transient expression system, as previously described (Blakely et al. 1991b). Briefly, 50% confluent cells were incubated for 30 min at 37°C with the vaccinia virus strain (VTF-7) which encodes T7 RNA polymerase. The cells were then transiently transfected with plasmids containing a promoter for T7 RNA polymerase upstream from human NET or rat SERT cDNA in a 1 : 3 ratio of plasmid DNA : Lipofectin (Invitrogen, Grand Island, NY, USA) per well in 100 μL Dulbecco's modified Eagle's medium without serum. Cells with no plasmid (mocktransfected) were used as negative controls. Transfected cells were incubated for 16–20 h at 37°C before being used for the transport experiment. The protein concentration was obtained by means of the Micro BCA Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL, USA).
Transport assay
At 16 h post-transfection, cells were washed and incubated for 10 min with phosphate-buffered saline (PBS) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS/CM). Transport functions were measured by incubating the cells with either 20.5 nM [3H]serotonin (3500 cpm/pmol; NEN Life Science Products, Boston, MA, USA) or 28.7 nM [3H]dopamine (DA; 1619 cpm/pmol; NEN Life Science Products) in PBS/CM for 10 min at room temperature, an interval previously determined to include only the initial linear phase of transport. The intact cells were quickly washed with ice-cold PBS, lyzed in sodium dodecyl sulfate (SDS), transferred to scintillation vials and counted as described previously (Blakely et al. 1991b; Kilic and Rudnick 2000). The effects of MTSET or citalopram were tested by including the inhibitors in the 250 μL of PBS/CM during a 10-min pre-incubation step before addition of substrate. Results are from triplicate samples and were repeated in two to three separate experiments.
Western blotting
Cells were collected in PBS containing 1 mM phenylmethylsulfonyl fluoride [PMSF; freshly prepared in acetone : ethanol (1 : 1)] and 2 mM EDTA, washed with the same buffer and re-suspended in 400 μL of PBS containing 0.44% SDS, 2 μg/mL DNase I, 1 mM PMSF and 2 μL of protease inhibitor mixture. The suspension was mixed with 200 μL of Laemmli 3 × sample buffer containing 0.7 M β-mercaptoethanol and separated by 9% SDS–polyacrylamide gel electrophoresis (PAGE) (Laemmli 1970). The separated proteins were transferred to a nitrocellulose membrane using the procedure of Towbin et al. (1979). The transfer was probed first with a rabbit polyclonal anti-hNET antibody (a generous gift from Dr R. Blakely, Vanderbilt University, Nashville, TN, USA) at a dilution of 1 : 2500 (Melikian et al. 1994) and then with a secondary donkey anti-rabbit antibody coupled to horseradish peroxidase (HRP) at a dilution of 1 : 5000. The hNET-myc was detected using a mouse monoclonal anti-myc antibody (catalog no. CBL430; Chemicon, Temecula, CA, USA) at a 1 : 1000 dilution and a secondary rabbit anti-mouse antibody coupled to HRP. In all experiments, signals were developed using the SuperSignal Western Blotting Detection Kit (Pierce Biotechnology). Immunoblots were quantitated using an Alpha Innotech (San Leandro, CA, USA) IS-1000 system.
Quantitation of human norepinephrine transporter expression
Standard curves were prepared by plotting the integrated density values of known quantities of protein extract from cells expressing hNET-myc or I155C, as previously described (Kilic and Rudnick 2000). Using these standard curves, the relative amounts of I155C and hNET-myc were determined in extracts of cells transfected with a mixture of the two cDNAs in a 1 : 1 ratio.
Coimmunoprecipitation
To demonstrate the physical association between hNET-myc/I155C or between the three neurotransmitter transporters, HeLa cells were cotransfected with their constructs in a 1 : 1 ratio. The next day, cells (2.5 × 106) were treated with 10 mM N-ethylmaleimide (NEM) for 30 min (to prevent formation of non-specific disulfide bonds), harvested, washed and resuspended in 400 μL of immunoprecipitation buffer (55 mM triethylamine, pH 7.5, 111 mM NaCl, 2.2 mM EDTA and 0.44% SDS + 1% Triton X-100, containing 1 mM PMSF) and 0.35% (v/v) protease inhibitor mixture (consisting of 5 mg/mL final concentration each of leupeptin, pepstatin A, chymostatin, bestatin, antipain and aprotinin). Next, cells were lyzed by sonication and pre-cleared to reduce non-specific binding to rat anti-mouse protein A sepharose (RAM-PAS) beads (Kilic and Rudnick 2000). The pre-cleared cell lysate (400 μL) was mixed with 10 μL of mouse monoclonal anti-myc antibody (1 : 1000 dilution) and an equal volume of a 1 : 1 slurry of RAM-PAS beads and incubated and washed as previously described (Kilic and Rudnick 2000). Proteins bound to the RAM-PAS beads were eluted with 100 μL of SDS sample buffer (Laemmli 1970) containing 0.7 M β-mercaptoethanol, resolved on a 9% SDS–PAGE and transferred to nitrocellulose. The transfer was probed with biotinylated anti-FLAG monoclonal antibody at a dilution of 1 : 4000 and visualized using streptavidin coupled to HRP at a dilution of 1 : 5000. Signals were developed using the SuperSignal Western Blotting Detection Kit.
Cell surface biotinylation
Cell surface expression of the transporters was determined using the membrane-impermeant biotinylation reagent NHS-SS-biotin (Pierce Biotechnology) by a modification of the procedure used by Gottardi et al. (1995) as previously described (Kilic and Rudnick 2000). Briefly, cells were labeled with NHS-SS-biotin, the excess reagent was quenched and the cells were solubilized. Cell surface proteins were isolated from the cell extract with immobilized streptavidin and the transporter was detected in the pool of surface proteins by gel electrophoresis and western blotting using either an anti-hNET antibody or a myc antibody. Immunoblots were quantitated using an IS-1000 (Alpha Innotech). Experiments were performed in triplicate and repeated in two to three separate assays.
Results
Characterization of human norepinephrine transporter-myc and human norepinephrine transporter-I155C
To test for hNET oligomerization, we generated two forms of the transporter with distinct antigenicity and inhibitor sensitivity. One of these forms was hNET-I155C, which was shown to be sensitive to inactivation by MTSET (Chen and Rudnick 2000) and which reacts with antibodies directed against the COOH-terminus of hNET (Schroeter et al. 2000). The other form was generated from hNET, which is resistant to inactivation by MTSET (Chen and Rudnick 2000), by replacing the last four residues of hNET with c-myc (see Experimental procedures) to provide a distinct epitope tag. This replacement masked the COOH-terminal epitope recognized by the anti-COOH-terminal antibody.
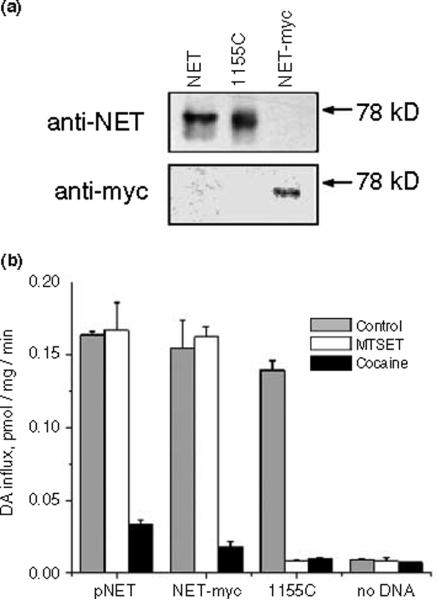
To verify that these forms of NET had the appropriate antigenic and transport characteristics, we expressed them in HeLa cells using the vaccinia-T7 system (Fuerst et al. 1986; Blakely et al. 1991b). The results are shown in Fig. 2. Figure 2(a) shows that the anti-hNET antibody reacted with native hNET and the I155C mutant, but not with the COOH-terminally modified hNET-myc. An antibody against the c-myc epitope reacted with hNET-myc, but not with hNET or I155C. Figure 2(b) shows the sensitivity of DA transport by these hNET constructs to inhibition by 2.5 mM MTSET. Both hNET-myc and I155C are fully functional and transport DA at a rate indistinguishable from that of wild-type hNET. MTSET inhibited the activity of I155C to the level of the 0.1 mM cocaine-treated control, but had no significant effect on the activity of hNET or hNET-myc.
Fig. 2.
Characterization of human norepinephrine transporter (hNET)-myc and I155C. (a) Antibody specificity. Each panel represents a western blot of HeLa cells expressing either hNET, I155C or hNETmyc. The upper blot was visualized with anti-NET antibody and the lower blot with antibody against c-myc. (b) Sensitivity to inactivation. HeLa cells expressing either hNET (hNET wild-type), hNET-myc or I155C were incubated with either 100 μM cocaine or 2.5 mM [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET) for 10 min and then assayed for transport activity. All data plotted represent means ± SE, are based on triplicate determinations and are representative of three separate experiments. DA, Dopamine.
The differences between hNET-myc and I155C are restricted to only one residue in TM3 and 10 residues at the COOH-terminus. The characterization studies also showed that, functionally, these two forms behave similarly to unmodified hNET. Neither epitope tag nor single mutation changed its transport function or expression (Table 1).
Table 1.
Fraction of human norepinephrine transporter (hNET)-myc and I155C in surface biotinylated pool
| Relative abundance in each pool (μg of cell protein) | ||
|---|---|---|
| Construct | ||
| I155C | hNET-myc | |
| Surface hNET | 3.50 | 4.23 |
| Total hNET | 23.6 | 21.1 |
HeLa cells expressing either hNET-I155C or hNET-myc were treated with Sulfo-NHS-SS-biotin (Pierce Biotechnology) to label surface proteins as previously described (Chen et al. 1998). The cells were then solubilized and either directly assessed for hNET by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and quantitative western blotting as described in Experimental procedures' (Total hNET) or treated with streptavidin beads to adsorb biotinylated surface proteins. After washing the beads, bound hNET was eluted and assessed by SDS-PAGE and quantitative western blotting (Surface hNET) as previously described (Chen et al. 1998). Results from a single representative experiment are shown.
Physical interaction between human norepinephrine transporter/human norepinephrine transporter and human norepinephrine transporter/serotonin transporter
The ability to separately detect each construct with specific antibodies allows an assessment of their interaction by coimmunoprecipitation. Figure 3 shows that not only do the two NET constructs interact (Fig. 3a) but that NET also interacts with FLAG-tagged SERT (Fig. 3b) (Kilic and Rudnick 2000). Cells expressing hNET-myc and either hNET I155C or FLAG-SERT were collected, lyzed, solubilized and incubated with protein A beads coated with anti-myc antibodies. To prevent the formation of possible non-specific disulfide bonds, the cells and extracts were treated with 10 mM NEM beginning 30 min prior to immunoprecipitation. After washing the beads, hNET-myc and any proteins that remained associated with it during solubilization were eluted with SDS–PAGE sample buffer and separated by gel electrophoresis. Transfers of the gels were tested for the presence of I155C (Fig. 3a) or SERT-FLAG (Fig. 3b). In both cases, precipitation was detected only if hNET-myc was coexpressed. In the absence of anti-myc, controls were also negative indicating that, in the presence of 10 mM NEM, the transporters did not aggregate non-specifically.
Fig. 3.
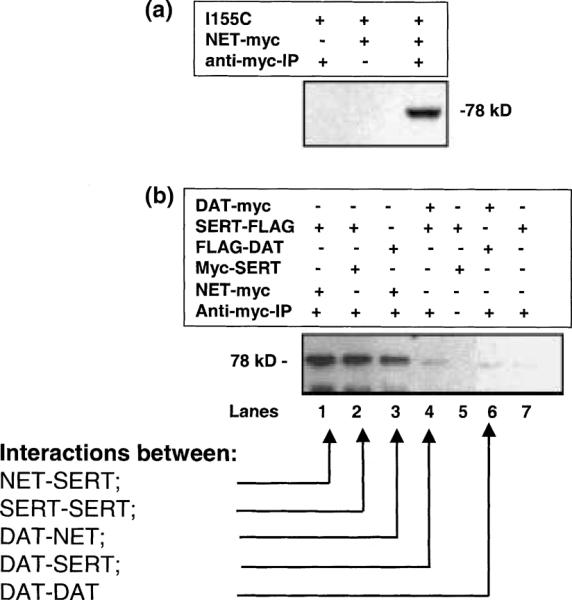
Physical association between human norepinephrine transporter (hNET) monomers, serotonin transporter (SERT) and dopamine transporter (DAT). (a) Coprecipitation of hNET-myc with I155C. HeLa cells transfected with I155C, hNET-myc or both constructs were solubilized in the presence of 10 mM N-ethylmaleimide (NEM), treated with rat anti-mouse protein A sepharose beads and, where indicated, antibody against c-myc. The immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) and blotted with anti-NET antibody as described in Experimental procedures. (b) Physical association between three neurotransmitter transporters. HeLa cells transiently cotransfected with equal amounts of hNET-myc/SERT-FLAG (lane 1), myc-SERT/SERT-FLAG (lanes 2 and 5), FLAG-DAT/hNET-myc (lane 3), DAT-myc/SERT-FLAG (lane 4), DAT-myc/FLAG-DAT (lane 6) or only with SERT-FLAG (lane 7) were treated with 10 mM NEM and harvested, solubilized and treated with protein A beads and, where indicated, antibody against c-myc. The immunoprecipitates were separated by SDS-PAGE and were blotted with biotinylated anti-FLAG antibody as described in Experimental procedures.
These experiments were performed using a lysis buffer containing 0.44% SDS and 1% Triton X-100. However, similar results (data not shown) were obtained in experiments using 1% digitonin, which is known to preserve the imipramine-binding activity of the transporter (Talvenheimo and Rudnick 1980). In our previous study with SERT under the same experimental conditions, we clearly demonstrated that the two monomers found to be associated in coimmunoprecipitation assays were functionally active (Kilic and Rudnick 2000). Here we followed up these findings by testing whether hNET associates with itself or SERT to form an oligomer. Our data (Fig. 3b, lane 1) clearly demonstrate that NET and SERT proteins physically associate and lane 2 demonstrates the oligomerization of SERT. We also tested the ability of NET and SERT to associate with the third member of this transporter family, DAT, which is highly homologous to NET and SERT. Under our experimental conditions, we saw minimal association of DAT-myc with FLAG-DAT (lane 6) or DAT-myc with SERT-FLAG (lane 4). As shown in Fig. 3, all three transporters resolve on SDS–PAGE at approximately the same molecular weight. However, we observed significant association between hNET-myc and FLAG-DAT (lane 3). Thus, the strongest associations were between NET and NET (Fig. 3a), SERT and SERT (Fig. 3b, lane 2), NET and SERT (lane 1) and NET and DAT (lane 3).
Our controls for coimmunoprecipitation assays were either the absence of myc antibody (lane 5) or using HeLa cells expressing only SERT-FLAG (lane 7). Interaction of DAT with DAT (lane 6) or DAT with SERT (lane 4) gave intensities only slightly greater than the SERT-FLAG control. However, in the light of recent reports of DAT oligomerization (Hastrup et al. 2001; Torres et al. 2003), these bands may represent weakoligomerization of DAT and its association with SERT.
Relative levels of total and surface expression
Although the similarity between hNET-myc and I155C suggested that the two constructs would be expressed and delivered to the cell surface equally well, we tested the possibility that either the myc tag or the mutation at position 155 (I155C) would alter the efficiency with which hNET was delivered to the cell surface. We transfected HeLa cells with a 1 : 1 mixture of hNET-myc and I155C plasmids and treated the cells with NHS-SS-biotin. NHS-SS-biotin reacts preferentially with lysine residues and both forms of hNET should have equal numbers of extracellular lysines. Subsequent to surface biotinylation, cells were solubilized and biotinylated proteins were extracted using streptavidin-agarose (see Experimental procedures). Using SDS–PAGE and quantitative immunoblotting, we determined the total expression and cell surface expression for each hNET construct. The results indicated that approximately 24% of hNET-I155C and 21% of hNET-myc was expressed on the cell surface (Table 1). Moreover, when HeLa cells were transfected with mixtures of I155C and hNET-myc cDNAs, the amount of each protein expressed, as determined by quantitative immunoblotting, was directly proportional to the amount of input cDNA. These results, shown in Fig. 4, demonstrate that the expression of each form of hNET was not significantly affected by expression of the other form, at least under conditions where the total cDNA level was constant.
Fig. 4.
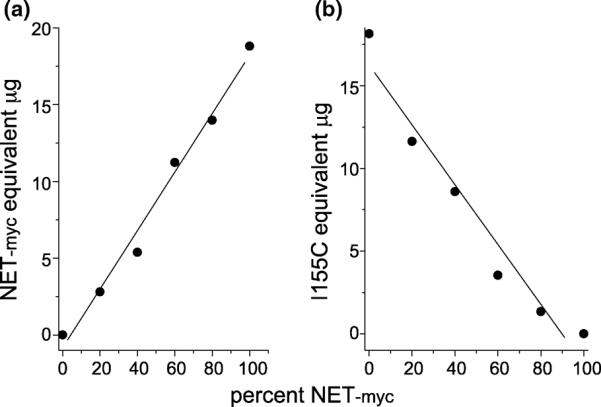
Quantitation of expressed human norepinephrine transporter (hNET) constructs. HeLa cells were transfected with mixtures of hNET-myc and I155C cDNA such that the total DNA per well was constant; however, the percent of hNET-myc in the mixture varied from 0 to 100% as indicated. Extracts of the cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were visualized by western blot analysis using (a) anti-myc and (b) anti-NET antibodies. The relative amount of each hNET construct was estimated by comparison with standard curves using cells expressing either hNET-myc or I155C. The values are expressed in terms of the amount of cell protein required in the standard curve to give an equivalent signal.
Functional effect of coexpression
The transport activity of cells expressing mixtures of hNETI155C and hNET-myc was also constant and independent of the mixture ratio (Fig. 5, ●). This is a consequence of the similar activities of the two forms and the fact that the expression of each form does not affect the other. As expected, transport into cells expressing 100% hNET-myc was unaffected by MTSET treatment and transport by cells expressing 100% I155C was almost completely inactivated (Fig. 5, ◯). If the activity of the two forms was completely independent, as expected for a monomeric transporter, cells expressing intermediate mixtures would retain intermediate levels of transport after MTSET treatment, proportional to the amount of hNET-myc expressed. The resulting activities should fall on the straight short-dashed line in Fig. 5; however, the experimental points deviate noticeably from this line (open circles). Also shown in Fig. 5 are predictions for cases where hNET exists as a dimer and inactivation requires modification of either one (lower dash-dot line) or both subunits (upper long-dashed line) of the dimer. It is clear that the experimental points coincide with the predic-tion for a two-hit inactivation process in which both subunits need to be modified for inactivation to occur.
Fig. 5.

Inactivation of human norepinephrine transporter (hNET)-myc and I155C hNET mixtures by [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET). HeLa cells were transfected with mixtures of hNET-myc and I155C, as described in the legend to Fig. 3, and were assayed for dopamine (DA) transport. The expression of I155C relative to total hNET was determined by quantitative immunoblotting and is indicated on the x-axis. The normalized mean of three separate experiments is shown, with error bars indicating SD. Transport rates before (●) and after (◯) treatment with 2.5 mM MTSET for 10 min. The solid line is the best fit to the control transport rates. The shortdashed line is the activity expected if no interaction occurred between resistant and sensitive forms and the amount of inactivation was equal to the amount of activity contributed by I155C. The long-dashed line represents the calculated activity if hNET was a dimer and modification of both subunits was required for inactivation of activity in that dimer. The dot-dashed line represents the calculated activity if modification of one subunit in a dimer inactivated all the activity of that dimer. All calculations assumed random dimer formation. Mean nonspecific uptake was determined in HeLa cells transfected with the parent vector pBluescript SK II(–) alone and was subtracted from total uptake to yield specific DA uptake.
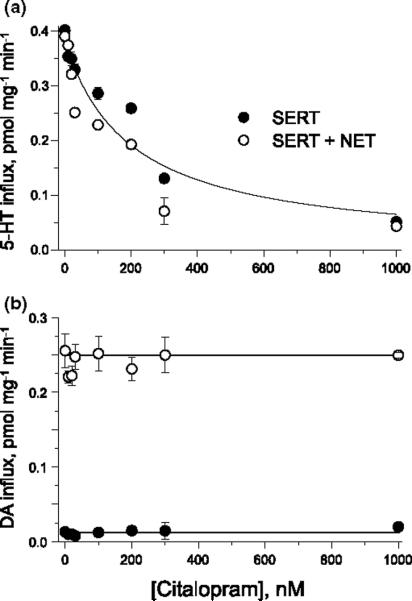
To examine the possibility that inhibitor binding by one subunit affects the function of the other, we tookadvantage of the association between SERT and hNET demonstrated in Fig. 3. Citalopram is a SERT inhibitor with little affinity for hNET. We measured the ability of citalopram to inhibit SERT and hNET activity in cells expressing either SERT alone or coexpressing SERT and hNET. The results, shown in Fig. 6, suggest that the two transporters are functionally independent despite their association, as measured by coprecipitation. Figure 6(a) shows that citalopram blocked SERT activity with half-maximal inhibition occurring at approximately 200 nM (closed circles). There was no change in the citalopram sensitivity when SERT was coexpressed with hNET. Furthermore, there was no effect of citalopram on hNET-mediated DA transport when coexpressed with SERT (Fig. 6b).
Fig. 6.
The association between serotonin transporter (SERT) and human norepinephrine transporter (hNET) does not lead to functional coupling. HeLa cells were transiently transfected with hNET or a mixture of hNET and SERT cDNA as described in Experimental procedures. Cells were incubated with the indicated concentrations of citalopram for 10 min before the addition of (a) 20.5 nM [3H]serotonin (5-HT) or (b) 28.7 nM dopamine (DA). Influx was determined after an additional 10 min incubation as described in Experimental procedures.
Discussion
The results described here demonstrate the homo-oligomeric nature of hNET and establish that oligomerization is a common phenomenon between the three members of the Na+/Cl--dependent transporters of biogenic amine neuro-transmitters. These findings raise the possibility that, func-tionally, all these proteins are also similar in structure due to the high degree of sequence identity between members of this gene family. Both immunoprecipitation and functional assays show that a myc-tagged construct of hNET associates with an hNET mutant sensitive to inactivation by cysteine reagents. These studies extend previous workfrom our and other laboratories showing that SERT and DAT form oligomers in the plasma membrane (Kilic and Rudnick 2000; Hastrup et al. 2001; Torres et al. 2003).
These experiments used a form of NET with a COOH-terminal epitope tag in which we replaced the last four amino acids with a c-myc epitope tag to mask the anti-NET antibody recognition region. Other workers have reported that the COOH-terminus of NET is important for proper transporter function. In one study (Bauman and Blakely 2002), it was suggested that the 31 residues from the COOH-terminus of NET might be required for the folding, oligomerization and exit of the transporter from the endoplasmic reticulum. In another study, Burton et al. (1998) found that a variant NET cDNA in which the 31 COOH-terminal residues were replaced with a different 18-residue sequence was not properly glycosylated or delivered to the cell surface. Apparently, the small changes in the COOH-terminus of the NET-myc transporter used here were not critical for expression. From the fact that we observed robust association of NET-myc with NET I155C, we suggest that the sequence of the extreme COOH-terminus was not critical for oligomerization, but this question could be answered definitively only with constructs using an epitope tag in a different location.
A detergent-solubilized preparation can be helpful in evaluating oligomerization but is not sufficient to judge whether an interaction between transport proteins occurs in the membrane. Aggregation of transporters can be induced by detergents and may lead to the incorrect conclusion that a monomeric transporter is present in an oligomer. Detergents can also disrupt interactions between proteins leading to monomeric behavior of a transporter that is physiologically present as an oligomer. For these reasons, it is significant that evidence for hNET oligomerization comes from both coimmunoprecipitation (Fig. 3) and functional studies (Fig. 5). Apparently, the association between hNET-myc and hNET-I155C is strong enough to withstand detergent extraction from the membrane. Although others have shown that association of DAT monomers requires coexpression in the same cell and does not occur between solubilized transporters mixed after expression in different cells (Torres et al. 2003), we have not performed similar experiments with NET or SERT.
The data in Fig. 5 indicate a two-hit sensitivity for inactivation of hNET by MTSET. The results are consistent with a process in which an hNET dimer is inactivated only when both subunits are modified. This is because, in mixtures of sensitive and resistant subunits that associate into dimers, there will be a significant fraction of hetero-dimers composed of one sensitive and one resistant subunit. Inactivation was less than expected for independent, monomeric subunits, presumably because of the presence of such hetero-oligomers that were resistant to inactivation (Fig. 5, ◯).
At least two possibilities would lead to two-hit inactivation. If either subunit in a dimer could be functional for transport at any time, but not both at the same time, then modification of one subunit might force the other subunit to remain functional, with no measurable change in activity for a singly modified dimer. Another possibility is that modification at a residue such as Cys155 partially blocks a pathway through which substrates must pass. If Cys155 residues on two subunits face the same pathway, then modification of both may be required to prevent substrate movement through the transporter. At present, we have no experimental evidence on which to base a preference for one or other of these possibilities.
An interesting side of hNET oligomerization is the apparent association between SERT and hNET in immuno-precipitation experiments (Fig. 3). The physiological consequences of this association are not yet understood. Thus, we tested their association by two approaches: (i) performing immunoprecipitation assays under similar conditions for hNET and DAT and DAT and SERT and (ii) testing to determine if hNET and SERT associate functionally by measuring transport in the presence of citalopram, the SERT-specific inhibitor. In coimmunoprecipitation assays, DAT appears to physically associate with hNET but not with SERT. Because we did not see DAT self association under the same conditions as described by Ferrer and Javitch (1998), we tested the physical association of hNET and SERT to determine if they associate functionally in hetero-oligomeric form. Of the transporters studied here NET and SERT are the only ones known to be expressed endogenously in the same cells (placental syncytiotrophoblasts). Clearly, inhibition of SERT has no effect on the activity of coexpressed hNET (Fig. 6b) and coexpressed hNET does not alter citalopram inhibition of SERT. The association observed by coimmunoprecipitation may be an artifact of solubilization, although SERT does not randomly associate with other membrane proteins (Kilic and Rudnick 2000). It is also possible that the structural basis for SERT and hNET homo-oligomerization is sufficiently similar that the two transporters recognize each other or that they both recognize another protein with the capacity to bind more than one transporter monomer. Because placental syncytiotrophoblast expresses both SERT and hNET, there may be a functional consequence to their association of which we are currently unaware.
Acknowledgements
We thank Dr J. Javitch (Columbia University Medical School, New York, NY, USA) for providing DAT-myc and FLAG-DAT. This work was supported by grants from the Rockefeller Brothers Fund, the National Alliance for Research on Schizophrenia and Depression (F K) and the National Institute on Drug Abuse (G R).
Abbreviations used
- DA
dopamine
- DAT
dopamine transporter
- hNET
human norepinephrine transporter
- HRP
horseradish peroxidase
- MTSET
[2-(trimethylammonium)ethyl]methanethiosulfonate
- NEM
N-ethylmaleimide
- NSS
neurotransmitter sodium symporter
- PBS
phosphate-buffered saline
- PBS/CM
PBS containing 0.1 mM CaCl2 and 1 mM MgCl2
- PMSF
phenylmethylsulfonyl fluoride
- RAM-PAS
rat anti-mouse protein A sepharose
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SERT
serotonin transporter
References
- Amara S, Kuhar M. Neurotransmitter transporters — recent progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rudnick G. Accessibility and conformational coupling in serotonin transporter predicted internal domains. J. Neurosci. 2002;22:8370–8378. doi: 10.1523/JNEUROSCI.22-19-08370.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkovetz DF, Tirruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J. Biol. Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- Bauman PA, Blakely RD. Determinants within the C-terminus of the human norepinephrine transporter dictate transporter trafficking, stability, and activity. Arch. Biochem. Biophys. 2002;404:80–91. doi: 10.1016/s0003-9861(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Blakely R, Berson H, Fremeau R, Caron M, Peek M, Prince H, Bradely C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991a;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Clark JA, Rudnick G, Amara SG. Vaccinia-T7 RNA polymerase expression system. Evaluation for expression cloning plasma membrane transporters. Anal. Biochem. 1991b;194:302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- Bruss M, Hammermann R, Brimijoin S, Bonisch H. Anti-peptide antibodies confirm the topology of the human norepi-nephrine transporter. J. Biol. Chem. 1995;270:9197–9201. doi: 10.1074/jbc.270.16.9197. [DOI] [PubMed] [Google Scholar]
- Burton LD, Kippenberger AG, Lingen B, Bruss M, Bonisch H, Christie DL. A variant of the bovine noradrenaline transporter reveals the importance of the C-terminal region for correct targeting to the membrane and functional expression. Bio-chem. J. 1998;330:909–914. doi: 10.1042/bj3300909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AS, Starnes DM, Chang SM. Possible existence of quaternary structure in the high-affinity serotonin transport complex. Biochem. Biophys. Res. Commun. 1998;249:416–421. doi: 10.1006/bbrc.1998.9158. [DOI] [PubMed] [Google Scholar]
- Chen JG, Rudnick G. Permeation and gating residues in serotonin transporter. Proc. Natl Acad. Sci. USA. 2000;97:1044–1049. doi: 10.1073/pnas.97.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Sachpatzidis A, Rudnick G. The third transmembrane domain of the serotonin transporter contains residues associated with substrate and cocaine binding. J. Biol. Chem. 1997;272(28):321–28. 327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- Chen JG, Liu-Chen S, Rudnick G. Determination of external loop topology in the serotonin transporter by site-directed chemical labeling. J. Biol. Chem. 1998;273(12):675–12. 681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- Ferrer JV, Javitch JA. Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc. Natl Acad. Sci. USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implications for neurotoxicity. Eur. J. Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl Acad. Sci. USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi C, Dunbar L, Caplan M. Biotinylation and assessment of membrane polarity — caveats and methodological concerns. Am. J. Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel M, Davidson N, Lester H, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hastrup H, Karlin A, Javitch JA. Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc. Natl Acad. Sci. USA. 2001;98(10):055–10. 060. doi: 10.1073/pnas.181344298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J. Comp. Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- Jess U, Betz H, Schloss P. The membrane-bound rat serotonin transporter, Sert1, is an oligomeric protein. FEBS. Lett. 1996;394:44–46. doi: 10.1016/0014-5793(96)00916-7. [DOI] [PubMed] [Google Scholar]
- Kilic F, Rudnick G. Oligomerization of serotonin transporter and its functional consequences. Proc. Natl Acad. Sci. USA. 2000;97:3106–3111. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Neuropharmacology of antidepressants that modify central noradrenergic and serotonergic function: A short review. Human Psychopharmacology. 1999;14:75–81. [Google Scholar]
- Lester HA, Mager S, Quick MW, Corey JL. Permeation properties of neurotransmitter transporters. Annu. Rev. Pharmacol. Toxicol. 1994;34:219–249. doi: 10.1146/annurev.pa.34.040194.001251. [DOI] [PubMed] [Google Scholar]
- Melikian H, McDonald J, Gu H, Rudnick G, Moore K, Blakely R. Human norepinephrine transporter: Biosynthetic studies using a site-directed polyclonal antibody. J. Biol. Chem. 1994;269(12):290–12. 297. [PubMed] [Google Scholar]
- Nelson N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely R, Amara S. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Prasad P, Kulanthaivel P, Leibach FH, Blakely RD, Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochem. J. 1993;32:1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- Saier MH. A functional-phylogenetic system for the classification of transport proteins. J. Cell. Biochem. Suppl. 1999;32–33:84–94. doi: 10.1002/(sici)1097-4644(1999)75:32+<84::aid-jcb11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human serotonin transporter and of the rat GABA transporter 1 visualized by fluorescence resonance energy transfer microscopy in living cells. J. Biol. Chem. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive 1-norepinephrine transporter. J. Comp. Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin C, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocainesensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Talvenheimo J, Rudnick G. Solubilization of the platelet plasma membrane the serotonin transporter in an active form. J. Biol. Chem. 1980;255:8606–8611. [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 2003;278:2731–2739. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]