Abstract
Rapid shifts of the point of visual fixation between equidistant targets require equal-sized saccades of each eye. The brainstem medial longitudinal fasciculus (MLF) plays a cardinal role in ensuring that horizontal saccades between equidistant targets are tightly yoked. Lesions of the MLF—internuclear ophthalmoparesis (INO)—cause horizontal saccades to become disjunctive: adducting saccades are slow, small, or absent. However, in INO, convergence movements may remain intact. We studied horizontal gaze shifts between equidistant targets and between far and near targets aligned on the visual axis of one eye (Müller test paradigm) in five cases of INO and five control subjects. We estimated the saccadic component of each movement by measuring peak velocity and peak acceleration. We tested whether the ratio of the saccadic component of the adducting/abducting eyes stayed constant or changed for the two types of saccades. For saccades made by control subjects between equidistant targets, the group mean ratio (±SD) of adducting/abducting peak velocity was 0.96 ± 0.07 and adducting/abducting peak acceleration was 0.94 ± 0.09. Corresponding ratios for INO cases were 0.45 ± 0.10 for peak velocity and 0.27 ± 0.11 for peak acceleration, reflecting reduced saccadic pulses for adduction. For control subjects, during the Müller paradigm, the adducting/abducting ratio was 1.25 ± 0.14 for peak velocity and 1.03 ± 0.12 for peak acceleration. Corresponding ratios for INO cases were 0.82 ± 0.18 for peak velocity and 0.48 ± 0.13 for peak acceleration. When adducting/abducting ratios during Müller versus equidistant targets paradigms were compared, INO cases showed larger relative increases for both peak velocity and peak acceleration compared with control subjects. Comparison of similar-sized movements during the two test paradigms indicated that whereas INO patients could decrease peak velocity of their abducting eye during the Müller paradigm, they were unable to modulate adducting velocity in response to viewing conditions. However, the initial component of each eye’s movement was similar in both cases, possibly reflecting activation of saccadic burst neurons. These findings support the hypothesis that horizontal saccades are governed by disjunctive signals, preceded by an initial, high-acceleration conjugate transient and followed by a slower vergence component.
Keywords: Eye movements, Saccades, Vergence, Medial longitudinal fasciculus, Hering’s law, Multiple sclerosis, Internuclear ophthalmoplegia
Introduction
Clinicians depend on the fact that the eyes move together during saccades made between two horizontally separated equidistant points (Leigh and Zee 2006). Loss of conjugacy of horizontal saccades under these test conditions is a common and useful clinical sign of horizontal rectus muscle palsy or internuclear ophthalmoplegia (INO) (Gamlin et al. 1989b; Zee 1992). Under natural conditions, most shifts of the point of visual fixation, which corresponds to binocular foveal viewing, are between features in the environment lying in different directions and at different distances; such gaze shifts require a combination of conjugate and vergence eye movements (Collewijn et al. 1995). Traditionally, saccades are viewed as fast conjugate eye movements, which change the direction of gaze. Vergence movements have been thought of as slower movements of the eyes in different directions, which make it possible to point the fovea of each eye at a single target. During combined saccade-vergence movements, vergence speeds up and saccades are slowed down, but the interaction of these two components is complex (Zee et al. 1992; Kumar et al. 2004).
Figure 1a summarizes some traditional concepts of the neural circuitry responsible for horizontal conjugate gaze shifts. Abducens motoneurons and internuclear neurons in the abducens nucleus receive a saccadic command (pulse of innervation) from burst neurons in the paramedian pontine reticular formation (PPRF) (Van Gisbergen et al. 1981; Horn et al. 1997). In Fig. 1, if the signal encoded by left-eye bursters (LEB), which project to abducens internuclear neurons, and right-eye bursters (REB), which project to abducens motoneurons, is the same, this will cause the eyes to move together during horizontal saccades between equidistant targets. It should be noted, however, projections of REB and LEB are unlikely to be sharply separated, as shown in Fig. 1a, and that some overlap of projections seems probable (Zhou and King 1998; Sylvestre and Cullen 2002). In this simplified scheme, vergence burst neurons (VBN) (Mays et al. 1986) project directly to medial rectus motoneurons (Zhang et al. 1991), where vergence commands can be superimposed on saccadic signals during combined saccade-vergence movements. Although there is evidence that vergence drives also project to the abducens nucleus (Gamlin et al. 1989a), this influence seems less important than the strong projections to medial rectus motoneurons, and its basis is open to several interpretations (King and Zhou 2000); therefore, it will be ignored in the current considerations.
Fig. 1.
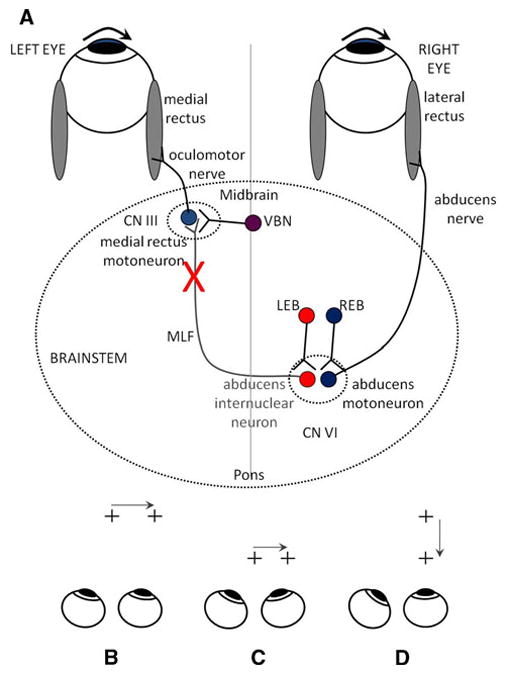
a Summary of simple models for generating horizontal gaze shifts in INO. Premotor excitatory burst neurons (right-eye burster: REB; left-eye burster: LEB), lying in the paramedian pontine reticular formation, project a pulse of innervation to the abducens nucleus (CN VI). Abducens motoneurons project the pulse of innervation via the sixth nerve to the right lateral rectus, which contracts rapidly to generate an abducting saccade of the right eye. Abducens internuclear neurons project the pulse of innervation, via the medial longitudinal fasciculus (MLF, internuclear pathway) to medial rectus motoneurons that, in turn, innervate the left medial rectus via the third nerve, to generate a fast adducting saccade of the left eye. If the MLF is demyelinated (indicate by X), signals are low-pass filtered, thereby reducing the size of the pulse and causing the adducting saccade of the left eye to be slow. Vergence burst neurons (VBN) project a fast vergence command directly to medial rectus motoneurons and are unaffected by INO. Bottom Schematic of test paradigms. b saccades between equidistant targets at 1 m. c saccades between equidistant targets at 35 cm. d Müller paradigm, with far and near targets aligned on the visual axis of the right eye
An interesting case occurs when subjects are required to shift gaze between two targets aligned on one eye (the Müller paradigm–Fig. 1d); in this case, it appears that there are large differences between the saccades made by each eye (for an example, see Fig. 2c). It is worth noting, though, that when subjects are required to shift gaze between the two aligned targets in the Müller paradigm, both eyes produce a saccadic eye movement, transiently deviating the aligned eye from the target straight ahead. In contrast, when foveal stabilization eye movements, such as smooth pursuit or linear vestibulo-ocular responses, are evoked using the same target arrangement, the ocular motor system produces eye movements that closely follow the geometrical requirements of the setting: the aligned eye remains fixed in space while the non-aligned eye converges or diverges appropriately (King and Zhou 1995; Ramat and Zee 2005).
Fig. 2.
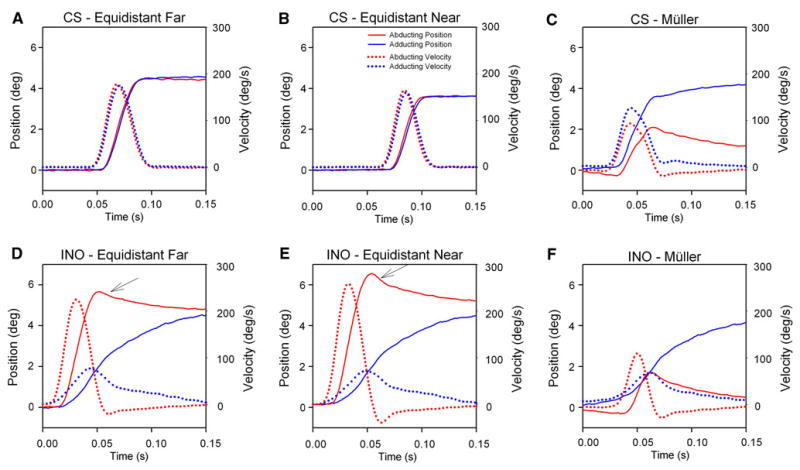
Representative time plots. Top panels show responses of a control subject (CS) to target jumps (a) between equidistant locations at 1.0 m; (b) between equidistant locations at 35 cm; and (c) during the Müller paradigm. Bottom panels (d–f) show corresponding plots from a patient with INO. Displacement implies eye position that has been offset to a value of zero at the onset of each response; thus, initial vergence angle is not shown. Note that the normal subject makes similar-sized movements of each eye between equidistant targets (a and b), but adduction is larger and faster than abduction during the Müller paradigm (c). Also note the overshooting abducting eye movements made by the INO patient during movements between equidistant targets (arrows in d and e) but the much smaller abducting movement during the Müller paradigm (f). In contrast, the adduction movement remains similar during all test conditions (d–f). Following the saccadic response to the Müller paradigm, both the CS and INO patient show slow vergence movements
A traditional explanation for different-sized saccades in responses to the Müller paradigm, which we refer to as Hypothesis 1, accounts for differences between the movements of the two eyes by superposition of an equal saccadic command and an equal vergence command to each eye: this is an extension of Hering’s law to account for combined conjugate and vergence movements (Leigh and Zee 2006). But is this scheme correct? An alternative explanation (Hypothesis 2) is that REB and LEB project different-sized saccadic pulses to abducens motoneurons and internuclear neurons and thereby independently control the saccades made by each eye. Electrophysiological studies of burst neurons in the PPRF (Zhou and King 1998), abducens nucleus neurons (Zhou and King 1998; Sylvestre and Cullen 2002), neurons contributing to the eye velocity-to-position neural integrator (Zhou and King 1998; McConville et al. 1994; Sylvestre et al. 2003), and medial rectus neurons (Van Horn and Cullen 2009) all support the view that horizontal saccades are programmed monocularly. Since the size of the saccadic pulse to each eye would differ, VBN would not be needed for the eyes to move rapidly by different amounts. However, the tenets of Hypothesis 2 have been debated (Mays 1998; King and Zhou 2000, 2002). Furthermore, studies of disjunctive saccades made by human subjects during the Müller test paradigm demonstrated that although the size of movements made by each eye is different, their peak velocity and peak acceleration are more similar (Ramat et al. 1999a). This result provided support for the view that similar-sized saccadic pulses are sent to abducens motoneurons and internuclear neurons during the Müller paradigm. Thus, further experimental tests of these two hypotheses seem justified.
In the present study, we examined the dynamics of adducting and abducting eyes of individuals with INO when they made conjugate and Müller saccades and compared their responses to those of normal subjects. The subjects that we studied suffered from multiple sclerosis (MS), which causes INO due to demyelination of axons (Davis et al. 2008; Leigh and Serra 2008). Demyelination usually does not stop neural transmission in axons but, rather, it filters higher frequencies out of the “pulse” signal, so that the saccade is slowed in the adducting eye. To estimate the size of the saccadic pulse delivered to the horizontal rectus muscle of each eye, we measured peak velocity and peak acceleration. By comparing the ratio of adducting/abducting peak velocities or peak accelerations for each movement, we tested the two hypotheses. Specifically, we asked whether adducting Müller saccades are either a combination of saccadic and vergence commands (Hypothesis 1) or are monocularly programmed (Hypothesis 2). In INO, Hypothesis 1 predicts that the adduction/abduction ratio during the Müller paradigm can be adjusted by changing either the adduction command (generated by VBN) or the abduction pulse; Hypothesis 2 predicts that only the abduction pulse can be modulated. Our findings are more supportive of Hypothesis 2.
Methods
Subjects
We examined 4 patients with MS (age range 46–74 years, mean = 58.75; disease duration 6–23 years, mean = 16.75); we tested eye movements corresponding to unilateral INO in 3 patients and bilateral INO in one (total of 5 cases of INO). Clinically, all patients were able to converge in response to a near target. The patients’ MS diagnoses, medications, and other characteristics are listed in Table 1. We also tested 5 healthy control subjects (age range 22–63 years, mean = 44). Those individuals requiring a refractive correction greater than six diopters wore their corrective lenses during testing. Testing took place in a dark room; the principal investigator remained in the room and encouraged all subjects during the session. All individuals gave signed, informed consent in accordance with our Institutional Review Board and the Declaration of Helsinki.
Table 1.
Summary of INO patients studied
| Patient No. | Agea | Sex | Durationb | MS type | INO | Visual acuityb | Medicines with CNS effects |
|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 23 | SP | B | 20/20;20/20 | Baclofen; donepezil |
| 2 | 74 | M | 19 | RR | R | 20/25;20/30 | Alprazolam |
| 3 | 53 | F | 10 | SP | R | 20/50;20/30 | Gabapentin; memantine |
| 4 | 46 | M | 6 | PP | R | 20/20;20/30 | Gabapentin; amantidine |
Duration is in years
Sex: M male, F female
MS type: SP secondary progressive, RR relapsing-remitting, PP primary progressive
Age is in years
Visual acuity is right eye; left eye, measured at near with correction
Eye movement measurements
We measured horizontal and vertical movements of each eye using the magnetic field/search coil technique; technical details of calibration, data collection, and analysis have been described previously (Liao et al. 2008). Subjects’ eyes were topically anesthetized before the pre-calibrated coils were placed on them. Subjects were seated in a stationary chair with a headrest to restrain movement. To detect any small head movements that occurred, they also wore a coil attached to their foreheads.
Experimental stimuli
For all experiments, the near target was a red laser spot projected onto a horizontal board placed just below the subject’s eye level. The far target was a red laser spot projected onto a tangent screen at a viewing distance of 1 m. Before the Müller test paradigm, patients and subjects aligned near and far targets on one eye by slightly turning their heads such that both targets were superimposed.
Experimental paradigms
Three types of binocular saccades were studied; in each case, the duration between target jumps (2.9–3.1 s) and the location of the target were unpredictable: (1) Horizontal saccades between equidistant targets of 5°, 10°, and 15°, away from and back to the midline on the tangent screen (Fig. 1b). (2) Target jumps aligned on one eye to elicit horizontal disjunctive saccades of ~5°, 10°, and 15° (Müller paradigm, Fig. 1d); in the case of INO patients, we aligned the targets to test adduction of the affected eye. (3) As a control experiment, horizontal saccades between equidistant targets at near, lying on an arc at 35 cm, of 5° and 10° away from and back to the midline (Fig. 1c); this required a sustained convergence angle of about 10°. Saccades to equidistant target jumps were in the direction of the aligned eye; e.g., if the right eye was aligned on near and far targets during Müller testing, then saccades between equidistant targets to the right of midline were tested (Fig. 1).
Data analysis
Search coil signals (voltages) were filtered (bandwidth 0–150 Hz) before digitization at 500 Hz or 1.0 kHz. Eye velocity and acceleration were calculated from eye position as previously described (Liao et al. 2008). Saccades were defined as target-directed eye movements faster than 10°/s; their onset and offset were determined by when the velocity rose above or fell below a 10°/s threshold, respectively. We checked each subject’s record to make sure that this approach was appropriate and discarded data contaminated by blinks or non-saccadic eye movement. For Müller responses, some subjects showed sustained, high-frequency conjugate eye oscillations during the vergence movement that followed the initial saccade; this behavior has been previously described in detail (Ramat et al. 1999b). Accordingly, we applied the following analytic procedure: (1) If there were multiple local maxima in the peak velocity profile of the response to a target jump, we analyzed only the first saccadic peak, even if the velocity did not fall below our 10°/s threshold. (2) We defined the end times of the abducting and adducting eyes’ saccades as the same; generally, the abducting eye velocity fell below our threshold first. We then calculated the amplitudes, peak velocities, and peak accelerations for both eyes. Note that although some variance was inevitable in estimates of saccade amplitude for combined saccade-vergence movements, measurements of peak velocity and acceleration could be reliably measured. Since each saccade varied in size, even to the same-sized target jump, we calculated the ratios of the movements of the adducting eye to the movements of the abducting eye for each gaze shift. In patients, our analysis focused on adducting movements of the eye with INO and abduction of the fellow eye (Fig. 1b–d). Finally, before embarking on this method of analysis, which was aimed at measuring the initial saccadic pulse of each response, we checked its reliability on the range of data obtained from all subjects. Although subjects did vary as to whether they showed high-frequency oscillations following the initial saccade during the Müller paradigm, each subject’s behavior tended to be stereotyped. Furthermore, our approach of measuring the ratio of peak velocity or peak acceleration for the corresponding adducting and abducting movements of each response minimized any small analytic differences that may have arisen on a response-to-response basis. We used descriptive statistics and analysis of variance with a significance value of P < 0.05 (unless otherwise stated) in order to determine whether there were differences in adducting/abducting ratios of peak velocities or peak accelerations for saccades made under the three test conditions in each individual and between the control group and INO group.
Results
Representative individual responses made during each of the three test paradigms are shown in Fig. 2. A control subject’s responses to equidistant far target jumps, equidistant near target jumps, and the Müller paradigm are shown in Fig. 2a, b, and c, respectively; corresponding responses from a patient with right INO are shown in Fig. 2d, e, and f.
Figure 3 summarizes the adducting eye/abducting eye ratios of peak velocity (top panel) or peak acceleration (bottom panel) for all subjects as box plots of percentiles, as indicated in the top panel; several findings are evident. First, control subjects showed adduction/adduction ratios close to 1.0 in response to far equidistant (FE, blue) target jumps; specifically, group mean ratio (±SD) of adducting/abducting peak velocity was 0.96 ± 0.07 and of adducting/abducting peak acceleration was 0.94 ± 0.09. Second, ratio values for responses to near equidistant (NE, red) target jumps were similar to those for far equidistant jumps; specifically, group mean ratio of adducting/abducting peak velocity was 0.95 ± 0.09 and of adducting/abducting peak acceleration was 0.93 ± 0.12. Third, control subjects showed a significantly increased adducting/abducting ratio (P < 0.001) during the Müller test paradigm (M, green); specifically, the group mean ratio during the Müller test paradigm was 1.25 ± 0.14 for peak velocity and 1.03 ± 0.12 for peak acceleration. Thus, there was a group percentage increase of adducting/abducting ratio by 30% for peak velocity and 10% for peak acceleration during the Müller test paradigm.
Fig. 3.
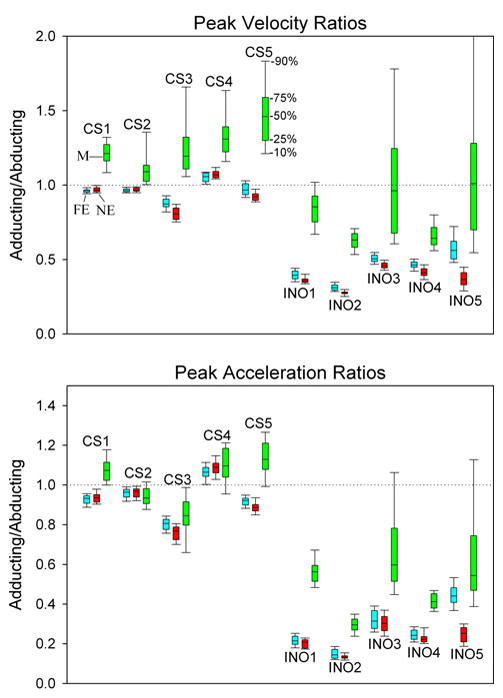
Box plot summary of ratios of peak velocity (top) and peak acceleration (bottom) of adducting/abducting components of each saccade; percentiles are indicated (50% corresponds to the median value). For each subject, the ratio is presented in the order of far equidistant targets (FE, blue), near equidistant targets (NE, red) or during the Müller paradigm (M, green). For each control subject (CS), FE and NE are similar, but M is generally greater. All INO patients show much smaller ratios that control subjects, reflecting their slowed adduction due to MLF demyelination. INO patients also show a large increase of adducting/abducting ratios during the Müller paradigm
Fourth, INO patients showed adducting/abducting ratios that were significantly smaller than control subjects (P < 0.001); specifically, the group mean ratio was 0.45 ± 0.10 for peak velocity and 0.27 ± 0.11 for peak acceleration; this was expected since their adducting saccades were slowed. Fifth, INO patients showed small differences between peak velocity ratios and peak acceleration ratios for far versus near equidistant target jumps, with the exception of INO5, for whom the difference was greater (Fig. 3); specifically, group mean ratio of adducting/abducting peak velocity was 0.37 ± 0.07 and of adducting/abducting peak acceleration was 0.22 ± 0.06. Sixth, an unexpected finding was that during the Müller test paradigm, the relative increase of adducting;/abducting ratios was much larger for INO patients than for control subjects, group mean increase being 82% for peak velocity and 78% for peak acceleration. Thus, group median ratio was 0.82 ± 0.18 for peak velocity and 0.48 ± 0.12 for peak acceleration.
Next, we asked whether ability of INO patients to increase their adducting/abducting ratios for peak velocity and acceleration during the Müller test paradigm versus during saccades to equidistant targets was due to increased adduction or decreased abduction. We used phase-plane plots of velocity versus displacement (change in position) to make this distinction (Serra et al. 2008); an example is shown in Fig. 4. The control subject (CS) makes a smaller, slower movement of both her adducting (Fig. 4a) and abducting (Fig. 4b) eyes during the Müller paradigm than during saccades between far equidistant targets. A patient with INO makes a large, fast abducting movement between equidistant targets and a much smaller movement during the Müller paradigm (Fig. 4d). In contrast, his adducting eye shows little difference in size or speed for movements between equidistant targets of the Müller paradigm (Fig. 4c). Thus, the increase in the ratio of adducting/abducting peak eye velocity during the Müller paradigm (also evident in Fig. 2) by this INO patient can be attributed to changes of his abducting, not adducting, saccades. Of the five cases of INO, adduction velocity during the Müller paradigm showed a small (mean < 3.5°/s) increase in two, a small decrease in two, and no change in one, compared with similar-sized movements between equidistant targets. Thus, the large change in adducting/abducting ratios of peak velocity during the Müller test paradigm versus during saccades to equidistant targets in INO patients (Fig. 3) were due to changes in movements of the abducting eye. Therefore, evidence to support a contribution of vergence burst neurons (Hypothesis 1) during the Müller paradigm was lacking.
Fig. 4.
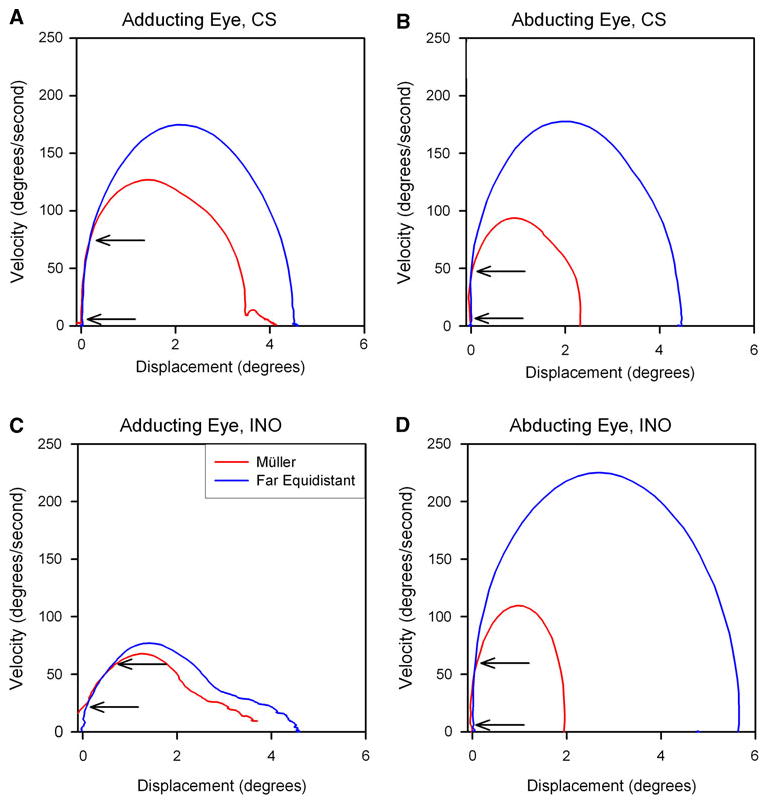
Comparison of initial part of responses to jumps of a target on the tangent screen (far equidistant target, blue) or of a target aligned on the left eye (Müller paradigm–red) that required similar-sized movements. Responses are displayed as phase-plane plots and correspond to the time plots shown in Fig. 2. a The control subject (CS) makes a smaller, slower movement of both her adducting (a) and abducting (b) eyes during the Müller paradigm. A patient with INO makes a large, fast abducting movement between equidistant targets and a much smaller movement during the Müller paradigm (d). In contrast, his adducting eye shows little difference in size or speed for movements between equidistant targets of the Müller paradigm (c). Thus, the increase in the ratio of adducting/abducting peak eye velocity during the Müller paradigm (evident in Fig. 3) in this INO patient can be attributed to changes of his abducting, not adducting, saccades. Note that in each case, the initial portion of the phase plane is similar for eye movements under both test conditions (arrows); the possible significance of this is discussed in the text
Another interesting finding is evident in Fig. 4. In each case, the initial portion of the phase plane is similar for movements of that eye under both test conditions (arrows). The significance of this finding is addressed in the Discussion.
Discussion
We set out to test two hypotheses to account for the coordination of horizontal gaze shifts made between visual targets either lying at similar distances (requiring conjugate eye movements) or shifts between targets lying at different distances aligned on the visual axis of one eye—the Müller paradigm. Hypothesis 1, which incorporates the concept of a conjugate saccade command and a superimposed vergence velocity signal from VBN (Mays et al. 1986), predicts that similar-sized adduction movements of the eye affected by INO will be faster during the Müller paradigm than during saccades made between equidistant targets. Our cases of INO did not show consistent behavior to support this prediction (for example, Figs. 2c–f and 4c). Thus, our findings weigh against a vergence velocity pulse contributing substantially to adduction during the disjunctive gaze shifts that occur with the Müller paradigm. Much more impressive were changes in abducting movements, which were smaller and slower during the Müller paradigm (for example, Figs. 2c–f and 4d). Although some caution is required in extrapolating from this relatively small number of cases of INO to normal saccade-vergence interactions in general, our findings are more consistent with disjunctive control of horizontal saccades (Hypothesis 2). A further test of the hypothesis would be to perform the same experiments in patients with complete internuclear ophthalmoplegia, in which a destructive lesion such as infarction (rather than demyelination in MS) abolishes any saccadic pulse reaching medial rectus motoneurons via the MLF. In this case, only vergence drives could lead to adduction.
In a prior study of rapid horizontal gaze shifts between equidistant targets or during the Müller paradigm (Ramat et al. 1999a), we presented a case in support of Hering’s law (Hypothesis 1), but noted that there were some discrepancies, especially for near-to-far movements during the Müller paradigm. In the present study, we chose to measure horizontal movements in INO, reasoning that any contribution of vergence burst neurons to adducting movements during the Müller paradigm would be more easily detected, since the saccadic pulse is low-pass filtered due to demyelination of the MLF; however, our results did not support Hypothesis 1. What could be the explanation for a discrepancy between the conclusions of these two studies?
Patients with INO are known to show substantial adaptation of their eye movements to compensate for their loss of ability to generate conjugate movements (Zee et al. 1987). Thus, when the unaffected eye is patched for several days, forcing the eye with adduction paresis to view, increased saccadic pulses are evident by overshoots of abduction in the unaffected eye. Such behavior is often the case during normal binocular viewing in patients with INO, as it was in our patients (Fig. 2d and e, arrows). Only after the paretic eye is patched for several days may the abducting eye make accurate saccades. On a shorter time scale, it has been shown that patients with INO who are subjected to a “fatigue test” by making horizontal saccades at 2 Hz for 10 min develop either increased INO (i.e., increased difference of the peak velocity of abducting and adducting eyes) or make adaptive changes to reduce their disconjugacy (Matta et al. 2009). The latter adaptations appear to have at least two components: increased vergence responses and decreased speed of their abducting eye (disjunctive adaptation of saccades). Thus, several mechanisms probably contribute to the behavior of saccades in INO. Our current study suggests that patients with chronic INO may show overshooting of abducting saccades between equidistant targets (to maximize movements of the adducting eye, Fig. 2d and e) but be able to generate smaller saccadic pulses during fixation shifts between targets lying at different distances (based on the abduction movements in Fig. 4c and d). In other words, they may have developed an increased ability to modify the size of abducting saccadic pulses to compensate for their decreased ability to control the size of adducting saccades.
One other interesting finding from our phase-plane analysis was that the initial movement during saccades—of either the adducting or the abducting eye—was similar for saccades between equidistant targets and during the Müller paradigm (arrows on Fig. 4). We suggest that this initial stereotyped movement of all horizontal saccades may correspond to the hypothetical mechanism of post-inhibitory rebound (PIR) discharge of burst neurons that occurs when inhibition from omnipause neurons is suddenly removed (Ramat et al. 2004). This mechanism could be an explanation for the inability to suppress the production of a saccade even in the aligned eye. This phenomenon might also correspond to the bias term of Cullen and Guitton (1997) and explain the initial similarities of saccades of different sizes evident in Fig. 1 by Robinson (1964). In normal subjects, conjugacy of the initial portion of all saccades may account for our finding peak acceleration values close to 1.0 even with responses during the Müller paradigm. Only after this initial, high-acceleration component of the burst neuron discharge does it appear that different discharge rates of burst neurons govern the size and speed of the ensuing saccade made by each eye.
Taken together, the findings of this study of combined horizontal saccade-vergence movements suggest that normally there is: (1) an initial, high-acceleration transient that is conjugate, due to the initial burst of activity by all neurons in the PPRF reflecting PIR; (2) a subsequent fast component due to disjunctive saccades, driven by burst neurons with monocular preferences; and (3) a final disjunctive component that is slower and due to the vergence system. Comparison of responses from normal subjects and individuals with INO indicate that the first two components are dependent on projections through the MLF, whereas the third component is generated in the midbrain. More behavioral and electrophysiological studies are required to test this hypothetical scheme.
Acknowledgments
Supported by the Office of Research and Development, Medical Research Service, and MS Centers of Excellence, Department of Veterans Affairs; NIH grant R01 EY06717, and the Evenor Armington Fund. We are grateful to Dr. W. M. King for his critical comments, to Drs. K. E. Cullen, and P. D. R. Gamlin for helpful advice, and to Dr. Ke Liao for technical assistance.
Contributor Information
Athena L. Chen, Department of Neurology, Veterans Affairs Medical Center and Case Western Reserve University, Cleveland, OH 44106-5040, USA
Stefano Ramat, Systems and Computer Science, University of Pavia, Pavia, Italy.
Alessandro Serra, Department of Neurology, Veterans Affairs Medical Center and Case Western Reserve University, Cleveland, OH 44106-5040, USA.
Susan A. King, Department of Neurology, Veterans Affairs Medical Center and Case Western Reserve University, Cleveland, OH 44106-5040, USA
R. John Leigh, Email: rjl4@case.edu, Department of Neurology, Veterans Affairs Medical Center and Case Western Reserve University, Cleveland, OH 44106-5040, USA. Veterans Affairs Medical Center and Case Western Reserve University, Cleveland, OH 44106-5040, USA.
References
- Collewijn H, Erkelens CJ, Steinman RM. Voluntary binocular gaze-shifts in the plane of regard: dynamics of version and vergence. Vision Res. 1995;35:3335–3358. doi: 10.1016/0042-6989(95)00082-p. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. I. Relationship to eye movement dynamics during head-fixed saccades. J Neurophysiol. 1997;78:3259–3282. doi: 10.1152/jn.1997.78.6.3259. [DOI] [PubMed] [Google Scholar]
- Davis SL, Frohman TC, Crandall CG, Brown MJ, Mills DA, Kramer PD, Stuve O, Frohman EM. Modeling Uhthoff’s phenomenon in MS patients with internuclear ophthalmoparesis. Neurology. 2008;70:1098–1106. doi: 10.1212/01.wnl.0000291009.69226.4d. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Gnadt JW, Mays LE. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. J Neurophysiol. 1989a;62:70–81. doi: 10.1152/jn.1989.62.1.70. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Gnadt JW, Mays LE. Lidocaine-induced unilateral internuclear ophthalmoplegia: effects on convergence and conjugate eye movements. J Neurophysiol. 1989b;62:82–95. doi: 10.1152/jn.1989.62.1.82. [DOI] [PubMed] [Google Scholar]
- Horn AKE, Büttner-Ennever JA, Suzuki Y, Henn V. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol. 1997;359:350–363. doi: 10.1002/cne.903590212. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. Initiation of disjunctive smooth pursuit in monkeys: evidence that Hering’s law of equal innervation is not obeyed by the smooth pursuit system. Vision Res. 1995;35:3389–3400. doi: 10.1016/0042-6989(95)00134-z. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. New ideas about binocular coordination of eye movements: is there a chameleon in the primate family tree? Anat Rec. 2000;261:153–161. doi: 10.1002/1097-0185(20000815)261:4<153::AID-AR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. Neural basis of disjunctive eye movements. Ann N Y Acad Sci. 2002;956:273–283. doi: 10.1111/j.1749-6632.2002.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Kumar AN, Han Y, Dell’osso LF, Durand DM, Leigh RJ. Directional asymmetry during combined saccade-vergence movements. J Neurophysiol. 2004 doi: 10.1152/jn.00858.2004. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Serra A. Taking the temperature of MS with INO. Neurology. 2008;70:1063–1064. doi: 10.1212/01.wnl.0000304347.56133.b4. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements (Book/DVD) 4. Oxford University Press; New York: 2006. [Google Scholar]
- Liao K, Walker MF, Joshi A, Reschke M, Leigh RJ. Vestibulo-ocular responses to vertical translation in normal human subjects. Exp Brain Res. 2008;185:553–562. doi: 10.1007/s00221-007-1181-z. [DOI] [PubMed] [Google Scholar]
- Matta M, Leigh RJ, Pugliatti M, Aiello I, Serra A. Using fast eye movements to study fatigue in multiple sclerosis. Neurology. 2009;73:798–804. doi: 10.1212/WNL.0b013e3181b6bbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE. Has Hering been hooked? Nat Med. 1998;4:889–890. doi: 10.1038/nm0898-889. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- McConville K, Tomlinson RD, King WM, Paige G, EQNA Eye position signals in the vestibular nuclei: consequences for models of integrator function. J Vestib Res. 1994;4:391–400. [PubMed] [Google Scholar]
- Ramat S, Zee DS. Binocular coordination in fore/aft motion. Ann N Y Acad Sci. 2005;1039:36–53. doi: 10.1196/annals.1325.005. [DOI] [PubMed] [Google Scholar]
- Ramat S, Das VE, Somers JT, Leigh RJ. Tests of two hypotheses to account for different-sized saccades during disjunctive gaze shifts. Exp Brain Res. 1999a;129:500–510. doi: 10.1007/s002210050920. [DOI] [PubMed] [Google Scholar]
- Ramat S, Somers JT, Das VE, Leigh RJ. Conjugate ocular oscillations during shifts of the direction and depth of visual fixation. Invest Ophthalmol Vis Sci. 1999b;40:1681–1686. [PubMed] [Google Scholar]
- Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res. 2004;160:89–106. doi: 10.1007/s00221-004-1989-8. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human saccadic eye movement. J Physiol (Lond) 1964;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Liao K, Matta M, Leigh RJ. Diagnosing disconjugate eye movements: phase-plane analysis of horizontal saccades. Neurology. 2008;71:1167–1175. doi: 10.1212/01.wnl.0000327525.72168.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Dynamics of abducens nucleus neuron discharges during disjunctive saccades. J Neurophysiol. 2002;88:3452–3468. doi: 10.1152/jn.00331.2002. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Choi JT, Cullen KE. Discharge dynamics of oculomotor neural integrator neurons during conjugate and disjunctive saccades and fixation. J Neurophysiol. 2003;90:739–754. doi: 10.1152/jn.00123.2003. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen JAM, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol. 1981;45:417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- Van Horn MR, Cullen KE. Dynamic characterization of agonist and antagonist oculomotoneurons during conjugate and disconjugate eye movements. J Neurophysiol. 2009;102:28–40. doi: 10.1152/jn.00169.2009. [DOI] [PubMed] [Google Scholar]
- Zee DS. Internuclear ophthalmoplegia: pathophysiology and diagnosis. Baillieres Clin Neurol. 1992;1:455–470. [PubMed] [Google Scholar]
- Zee DS, Hain TC, Carl JR. Abduction nystagmus in internuclear ophthalmoplegia. Ann Neurol. 1987;21:383–388. doi: 10.1002/ana.410210411. [DOI] [PubMed] [Google Scholar]
- Zee DS, FitzGibbon EJ, Optican LM. Saccade-vergence interactions in humans. J Neurophysiol. 1992;68:1624–1641. doi: 10.1152/jn.1992.68.5.1624. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gamlin PD, Mays LE. Antidromic identification of midbrain near response cells projecting to the oculomotor nucleus. Exp Brain Res. 1991;84:525–528. doi: 10.1007/BF00230964. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Premotor commands encode monocular eye movements. Nature. 1998;393:692–695. doi: 10.1038/31489. [DOI] [PubMed] [Google Scholar]


