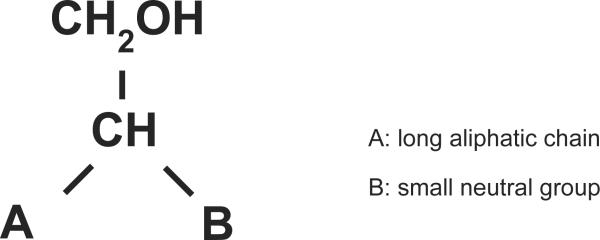Abstract
A phospholipase A2 was identified from MDCK cell homogenates with broad specificity toward glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylglycerol. The phospholipase has the unique ability to transacylate short chain ceramides. This phospholipase is calcium-independent, localized to lysosomes, and has an acidic pH optimum. The enzyme was purified from bovine brain and found to be a water-soluble glycoprotein consisting of a single peptide chain with a molecular weight of 45 kDa. The primary structure deduced from the DNA sequences is highly conserved between chordates. The enzyme was named lysosomal phospholipase A2 (LPLA2) and subsequently designated group XV phospholipase A2. LPLA2 has 49 percent of amino acid sequence identity to lecithin cholesterol acyltransferase and is a member of the αβ-hydrolase superfamily. LPLA2 is highly expressed in alveolar macrophages. A marked accumulation of glycerophospholipids and extensive lamellar inclusion bodies, a hallmark of cellular phospholipidosis, is observed in alveolar macrophages in LPLA2−/− mice. This defect can also be reproduced in macrophages that are exposed to cationic amphiphilic drugs such as amiodarone. In addition, older LPLA2−/− mice develop a phenotype similar to human autoimmune disease. These observations indicate that LPLA2 may play a primary role in phospholipid homeostasis, drug toxicity, and host defense.
Keywords: Lysosomal phospholipase A2 (LPLA2), 1-O-acylceramide synthase, phospholipase A2, transacylase, amiodarone, cationic amphiphilic drugs, lecithin-cholesterol acyltransferase (LCAT), LCAT like lysophospholipase (LLPL), alveolar macrophages (AMs), pulmonary surfactant, phospholipidosis
1. Introduction
The lysosome is an intracellular acidic compartment containing more than 50 digestive enzymes including proteases, nucleases, glycosidases, phosphatases, sulfatases, lipases, phospholipases and esterases. These enzymes, collectively known as hydrolases, are present within the lumen of the lysosome. They are characterized by an acidic pH optimum and are critically important for the degradation of intracellular and extracellular macromolecules that are trafficked to the lysosome by endocytic, phagocytic, and autophagic pathways [1, 2].
The best characterized lysosomal phospholipase is acidic sphingomyelinase [3]. This phospholipase hydrolyzes sphingomyelin to form ceramide and phosphorylcholine. Inherited deficiencies in acidic sphingomyelinase cause Niemann-Pick type A and B disease. Additional glycerophospholipid specific phospholipase activities that are localized to lysosomes have long been recognized based on their measured activities. In general, these phospholipases may be classified into four types, phospholipase A1, A2, C and D, according to the specific cleavage site of the substrate (Fig. 1). Phospholipase A1 and A2 cleave the acyl ester bonds of sn-1 and sn-2 position, respectively, of glycerophospholipids and produce free fatty acid and the corresponding lyso-glycerophospholipid. Phospholipase C and D cleave the phosphodiester bonds linked to the diacylglycero group and alkoxy group (head group), respectively. Phospholipase Cs produce diacylglycerol and a phosphorylated alcohol; phospholipase Ds produce phosphatidic acid and an alcohol. In distinction to the acid sphingomyelinase, no clinically significant human disease has been ascribed to deficiencies in lysosomal phospholipase A, C, or D to date.
Fig. 1. Cleavage sites of glycerophospholipids by phospholipases.
R1, R2 and X refer to the fatty acids and the polar group, respectively, of the stereospecifically numbered phospholipids.
In 1967, Mellors and Tappel reported the existence of a phospholipase A activity in the soluble fraction obtained from rat liver lysosomes [4]. The enzyme released free fatty acid from both sn-1 and sn-2 positions of phosphatidylcholine and phosphatidylethanolamine, and was characterized by Ca2+-independent activity and an acid pH optimum. Similar phospholipase A activities in rat liver lysosomes were reported by other groups [5, 6]. Later, Franson et al. identified a phospholipase A1 (PLA1) and A2 (PLA2) in the soluble fraction of rat liver lysosomes [7]. Lysosomal phospholipase A activities were subsequently reported in a number of additional tissues and cell types including rat myocardial preparations [8], rabbit alveolar macrophages [9], arterial smooth muscle cells [10], rat testes [11], kidney cortex [12], and mouse peritoneal macrophages [13].
The PLA1 and PLA2 of rat liver lysosomes reported by Franson were separated by gel filtration, suggesting that lysosomal PLA1 is a protein distinct from lysosomal PLA2 [7]. Both enzymes have similar properties such as water solubility, Ca2+-independent activity, an acid pH optimum and lysosomal localization. The lysosomal PLA1 was characterized by enhanced enzyme activity when the substrate was dispersed into inert detergent [14]. By contrast, the non-ionic detergent, Triton X-100, inhibited lysosomal PLA2 activity [13], suggesting that the enzyme requires a lipid bilayer structure to catalyze the substrate degradation.
In 1980, an acidic phospholipase C activity in rat tissue homogenates obtained from adipose tissue, brain, diaphragm, duodenum, heart, small intestine, kidney, liver, lung, skeletal muscle and spleen was reported [15]. In addition, lysosomal phospholipase C was identified from the soluble fractions of rat liver [16–18] and kidney [12] lysosomes, and canine cardiac sarcoplasmic reticulum [19]. The lysosomal phospholipase C has an acid pH optimum and no requirement of divalent metal ions. A phospholipase D activity may also be present in lysosomes [20].
Lysosomal PLA1 was subsequently purified from rat liver and kidney. Hostetler et al. purified five lysosomal PLA1s with molecular weights between 20,000 and 90,000 from rat liver [21]. The differences in molecular weights between the isolated enzymes were due to distinct isoelectric points and carbohydrate moieties. Each enzyme demonstrated an acid optimum pH, the absence of a requirement of divalent metal ions, and a higher specificity toward sn-1 position compared to the sn-2 position of glycerophospholipids. The lysosomal PLA1 purified from rat kidney cortex was a glycoprotein with an isoelectric point of pH 5.4 and an apparent molecular weight of 30 kDa [22]. However, the lysosomal PLA1 gene and encoded amino acid sequence have not been reported.
Unlike well-characterized PLA2s such as cytosolic and secretory PLA2s [23], there have only been limited reports addressing the purification and cloning of lysosomal PLA2. A lysosomal-type Ca2+-independent PLA2 was isolated by the Fisher group from rat lungs and cloned from a human myeloblast cell line [24]. The characterized enzyme was identical to peroxiredoxin 6 and displayed bi-functional enzyme activities that included glutathione peroxidase as well as phospholipase A2 activities. The investigators proposed that the enzyme has the unique property of acting as a glutathione peroxidase when localized to the cytoplasm and acting as a phospholipase A2 when localized to the lysosome [25]. More recently, the localization of the enzyme in acidic vesicles was reported to depend on a novel 10 amino acid peptide located at positions 31–40 of the protein [26], suggesting that there is a binding equilibration between peroxiredoxin 6 localized to the cytoplasm and the lysosomal membrane. Presently, it still remains unsettled whether peroxiredoxin 6 functions as a lysosomal enzyme.
In 1996 a novel enzyme activity was reported and identified as a 1-O-acylceramide synthase. The activity was characterized by the transacylation of N-acetylsphingosine on the 1-hydroxyl site by the sn-2 fatty acid of phosphatidylcholine. The product of this reaction was a 1-O-acylceramide (1-O-acyl- N-acetylsphingosine). However, the further purification of this enzyme from the soluble and lysosomal factions of MDCK cells revealed the presence of dual transacylase and phospholipase A2 activities under acidic conditions [27]. The enzyme was subsequently purified, sequenced, cloned, and expressed [28, 29]. Upon further characterization, the enzyme was observed to demonstrate predominately function as a phospholipase A2. Thus the enzyme is now known as lysosomal phospholipase A2 (LPLA2) or group XV phospholipase A2.
The study of lysosomal proteins has been informative not only in the elucidation of important cellular functions but also for the identification of the mechanistic basis for several diseases, collectively termed lysosomal storage disorders. Additionally, each group of phospholipase A2s have been associated with novel biological functions [23]. Thus there is substantial motivation to study the biochemical and biological functions of LPLA2, the focus of this review.
2. Discovery of a ceramide transacylase/acidic phospholipase A2 in lysosomes
For the last two decades, sphingolipids, most notably ceramide, have been the object of considerable study. Ceramide has been proposed to be a lipid mediator of a range of cellular functions including growth, proliferation, differentiation, and apoptosis [30]. One set of reagents that have been useful to interrogate these roles have been specific inhibitors of sphingolipid metabolism. The development and use of small molecule inhibitors of glucosylceramide synthase was first proposed by Norman Radin for the treatment of glycosphingolipidoses [31]. D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) was identified as the first reversible inhibitor of the cerebroside synthase [32]. Subsequently a series of PDMP homologs [33–35], which block glucosylceramide synthase activity at lower IC50s, were identified and more recently have been tested in clinical trials for the treatment of type 1 Gaucher disease [36, 37]. During the development of these inhibitors, it was appreciated that when cultured cells were treated with PDMP and related homologues, ceramide content increased in the presence of some inhibitors and resulted in growth inhibition, cytotoxicity, and apoptosis [38]. These observations were originally interpreted to reflect the accumulation of ceramide as substrate for glucosylceramide synthase. However, with the development of novel homologues with sphingosyl backbones and of PDMP homologues with nanomolar sensitivity, the dissociation of ceramide accumulation from glucosylceramide depletion led us to question whether glucosylceramide synthase inhibition results in substrate accumulation. Specifically, the glucosylceramide synthase inhibitor 1-pyrrolidino-1-deoxyceramide was found to inhibit cerebroside formation without elevating cell ceramide content [39]. Additionally, D-threo-4'-hydroxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol and D-threo-1',4'ethylenedioxyphenyl-2-palmitoylamino-3-pyrrolidino-propanol were observed to inhibit glucosylceramide synthase at circa 10 nM but only increase cell ceramide levels at 100 fold greater concentrations [35].
These data suggested that the PDMP related compounds increase ceramide levels secondary to the inhibition of a metabolic pathway distinct from glucosylceramide synthase. (By contrast, N-butyl-deoxynojirimycin, an imino sugar developed as an α-glucosidase inhibitor, represents a second class of glucosylceramide synthase inhibitors, does not raise ceramide levels [40].) A study was initiated to determine which metabolic pathway involved in the synthesis or catabolism of ceramide was targeted by the glucosylceramide synthase inhibitor. Well established ceramide associated enzyme activities were assayed in the presence and absence of PDMP. These included ceramide synthase, neutral, acidic, and alkaline ceramidases, sphingomyelin synthase, sphingomyelinase, and ceramide kinase. No inhibitory or stimulatory effects of PDMP were detected against these anabolic or catabolic pathways [41].
An alternative strategy to identify this secondary site of action was therefore employed. The tritiated ceramide analog, N-acetylsphingosine (NAS), was prepared from acetic anhydride and [3H]sphingosine in order to follow the ceramide metabolism in MDCK cells. Surprisingly, N-acetyl-[3H]sphingosine was esterified when incubated with MDCK cell homogenate under acidic conditions. When MDCK cells were labeled with radioactive N-acetyl-[3H]sphingosine, a fraction of the radioactive NAS was metabolized and converted to not only expected sphingolipids such as sphingosine, C2-sphingomyelin, C2-glucosylceramide, long chain ceramide, long chain sphingomyelin and long chain glucosylceramide but also an unknown compound which was identified as 1-O-acyl-NAS [27]. These radioactive products accumulated in the cells in a time dependent manner. These findings suggested the existence of a previously uncharacterized metabolic pathway involving phospholipids and ceramide catalyzed by a novel enzyme. However, in 1979, Okabe and Kishimoto reported formation of 1-O-acylceramide in rat brain [42], suggesting the existence of a metabolic pathway for the acylation of the hydroxyl group at the C1 position of ceramide in the cell.
The acylated ceramide was labile in the presence of base, consistent with the presence of an acyl and not ether linkage. To establish whether the C-1 or C-3 hydroxyl was the site of the acylation, the reaction product was treated with 3-dichloro-5, 6-dicyanobenzoquinone, an agent that converts the hydroxyl group vicinal to the 4,5 double bond on the sphingosine base to a ketone. A ketone at C-3 was formed, consistent with the transfer of the fatty acyl group to the C-1 and not C-3 hydroxyl in the transacylase reaction [27]. The substrate for the acylation was next identified. Neither free fatty acids nor acyl-CoAs could serve as substrates for the formation of the 1-O-acylceramide. Rather, the glycerophospholipids, particularly phosphatidylcholine and phosphatidylethanolamine were acyl group donors. Greater than 80 percent of the enzyme activity that catalyzed the acylation was recovered in the soluble fraction of the cell homogenate [27]. Subcellular fractionation subsequently demonstrated that the same enzyme activity was localized to the lysosomal fraction in MDCK cells [29].
The enzyme was named 1-O-acylceramide synthase (ACS). When liposomes consisting of phosphatidylethanolamine (PE) labeled at the sn-2 position with radioactive acyl group and NAS were incubated with the soluble fraction from MDCK cells, the formation of radioactive 1-O-acyl-NAS and release of radioactive fatty acid was observed to occur concurrently. However, the release of radioactive fatty acid was also observed in the absence of NAS [27]. This observation indicated that ACS had both phospholipase A2 as well as transacylase activities. In other words, the acyl group at the sn-2 position of phospholipid could be transferred to the hydroxyl group at the C1 position of a short chain ceramide in the transacylase reaction as well as to the hydroxyl group of water in phospholipase A2 reaction (Fig. 2). The enzyme activity was independent of calcium ion. Therefore, it was concluded that ACS is a lysosomal calcium-independent phospholipase A2, and the transacylase activity of ACS was attributed to this phospholipase A2 reaction mechanism.
Fig. 2. Enzymatic reactions of LPLA2 with its dual transacylase and phospholipase A2 activities.
LPLA2 recognized phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol and phosphatidylserine as substrates. The respective lyso-phosphoglycerides are produced regardless of whether N-acetylsphingosine is present. The acyl-LPLA2 intermediate is shown.
3. Biochemical properties of lysosomal phospholipase A2 (LPLA2)
3.1 Purification of ACS
ACS activity was detected in the soluble fractions prepared from mouse brain, liver and kidney [27]. Because the soluble fraction from mouse brain showed the highest enzyme activity compared to other organs, bovine brain was chosen as a starting material for the purification of ACS. A post-mitochondrial supernatant obtained from bovine brain homogenate was prepared with a hypotonic neutral buffer and followed through a series of steps including ammonium sulfate fractionation, DEAE-Sephacel, phenyl-Sepharose, S-Sepharose, Sephdex G-75, concanavalin A-agarose, and heparin-Sepharose chromatography. As a result of this purification, 4.5 μg of highly purified ACS was obtained from 3 kg of bovine brain [28]. The isolated protein separated as a single band at a molecular weight of 45 kDa on SDS polyacrylamide gel electrophoresis. The gel filtration column chromatography by G-75 indicated that ACS activity could be recovered at the fractions corresponding to a molecular weight of 40 kDa. In addition, ACS was strongly bound to concanavalin A agarose gel column and released by a buffer containing 500 mM α-methyl-mannoside [28]. These results indicated that intact ACS is a water-soluble high mannose rich glycoprotein consisting of a single polypeptide chain with a molecular weight of 45 kDa and exists as a monomer. As predicted from the original characterization [27], the purified ACS had a pH optimum at 4.5, both phospholipase A2 and transacylase activities, and the absence of a calcium ion requirement for enzyme activity. The purified ACS was insensitive to dithiothreitol, N-ethylmaleimide and phospholipase A2 inhibitors such as (E)-6-(bromomethylene) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one and nonadecyltetraenyl trifluoromethyl ketone [28]. Characteristic of other described lysosomal phospholipase A2s obtained from mammalian tissues, liposomes were a preferred substrate when compared to micelles in the ACS reaction. It was concluded that ACS represented a unique phospholipase A2 compared to previously isolated phospholipase A2 family members.
In 1999, Shinozaki and Waite reported the partial purification of a phosphatidylglycerol-selective lysosomal phospholipase A2 (PG-LPLA2) from RAW 264.7, macrophage like cells [43]. They suggested that the physiological role of PG-LPLA2 was to catalyzed the first step in the biosynthesis of bis(monoacylglycero)phosphates. Interestingly, the physicochemical properties and substrate and inhibitor specificities of PG-LPLA2 are similar to those of LPLA2 [28, 44], consistent with the possibility that PG-LPLA2 is identical to LPLA2.
3.2 Cloning of LPLA2
The purified enzyme was subjected to tryptic digestion and the partial amino acid sequences were characterized by mass spectrometry. The full length protein was expressed and a BLAST search revealed that the protein was highly homologous to a protein named lecithin-cholesterol acyltransferase like lysophospholipase (LLPL) as reported by Taniyama et al. [45]. LLPL was originally identified as a gene product induced in THP-1 cells (a macrophage cell line) following stimulation with phorbol ester and β-VLDL. However, LLPL was characterized as a lyso-phospholipid lipase. In addition, there were other discrepancies in the biochemical properties attributed to LLPL when compared to LPLA2. These differences included the subcellular location, molecular weight, pH optimum of enzyme activity, and substrate specificity. Thus a determination was sought as to whether LPLA2 was the product of the LLPL gene.
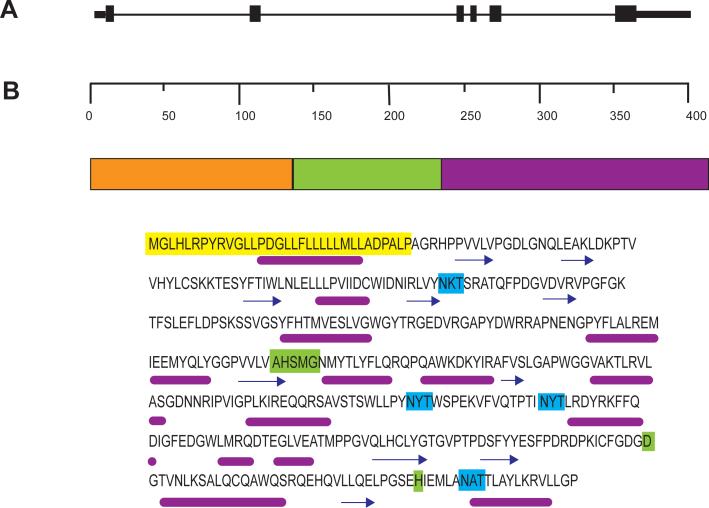
The LPLA2 gene was successfully cloned from human, mouse and bovine sources. The primary structure of LPLA2 deduced from DNA sequences encoding LPLA2 is highly preserved between the three species. The human gene maps to 16q22.1 and consists of 6 exons. The catalytic domain is encoded in exon 5. The primary structure is characterized by consensus sequences that include a signal peptide cleavage site and the lipase motif AXSXG found in serine hydrolases (where A, S, G, and X denote alanine, serine, glycine, and any amino acid respectively). In addition, there are several postulated N-glycosylation sites, 4 in the human and mouse LPLA2 and 3 in bovine LPLA2 (Fig. 3A) [29].
Fig. 3.
A. Genomic structure of LPLA2. The gene consists of 6 exons localized to chromosome 16q22.1. B. The protein structure and amino acid sequence of LPLA2. The deduced domains were derived from the Ginzu program starting with PSI-Blast. Domain 2 (residues 133 – 234) is highly homologous with the catalytic domain of lecithin coholesterol acyltransferase and is underlined. Predicted functions for this domain include palmitoyl-protein hydrolase, phospholipase, lipase, sterol esterase, and carboxylesterase activities. No predicted activites can be assigned to the first (residues 1 – 132) and third (residues 235 – 412) domains. The signal peptide is highlighted in yellow. The lipase motif and additional catalytic amino acids are highlighted in green. The glycosylation sites are highlighted in turquoise. Below the amino acid sequent is the PSIPRED predicted structure [105]. Helices are denoted by a magenta line and coil regions are denoted by arrows. C. Minimum-evolution tree of LPLA2 and LCAT proteins. The numbers in parentheses represent NCBI protein GIs for corresponding LPLA2 or LCAT proteins. The protein sequences were first aligned using the Clustal program within the MEGA 4.0 package [106]. Evolutionary distances were computed by using the Tamura-Nei method [107]. Bootstrap analysis using 1,000 repetitions provided support for individual nodes [108]. The numbers next to the branches are the percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates). The tree is drawn to scale; branch lengths are in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Scale bar indicates nucleotide substitutions per site.
LPLA2 has 49% amino acid sequence identity to lecithin cholesterol acyltransferase (LCAT). Both enzymes belong to the αβ-hydrolase superfamily [46, 47] and have the amino acid residues forming the catalytic triad structure (serine, aspartic acid and histidine) (Fig. 3B) [29, 48, 49]. LCAT is a serum protein secreted from liver. LCAT cleaves the sn-2 acyl-ester of phosphatidylcholine and like LPLA2 acts as both a phospholipase A2 and an acyl-transferase in the absence and the presence, respectively, of cholesterol in the reaction. LCAT also has the GXSXG lipase motif containing the catalytic serine. The phylogenetic tree constructed for plant, animal, fungal and bacterial LCAT-like proteins shows a high degree of relatedness between phospholipid: diacylglycerol acyltransferase (PDAT), LCAT and LPLA2 [50]. Each enzyme family possesses phospholipase A activity and catalyzes transacylation in the presence of acceptor molecules. In addition, the deduced amino acid sequences of LCAT-like proteins show highly conservative regions including a catalytic site between the proteins. A minimum evolutionary tree comparing mouse, rat, and human forms of LCAT and PLA2 is shown in figure 3C.
In order to establish that the LPLA2 gene product was in fact the source of the 1-O-acylceramide synthase activity, COS-7 cells were transfected with the mouse LPLA2 (mLPLA2, mLLPL) gene conjugated with Flag, HA or c-myc at the carboxy-terminal end. The soluble fraction prepared from the transfected cells demonstrated both transacylase and PLA2 activities under acidic conditions [29]. When the expressed protein was immunoprecipitated using an anti-c-myc antibody the same activities were detected, demonstrating that LPLA2 protein itself contains the acyltransferase and phospholipase A2 activities. Similar to the report of Taniyama et al., LPLA2 did not show any LCAT activity [29, 45]. However, unlike their report, LPLA2 did not exhibit significant lysophospholipase activity under either acidic or neutral conditions. Both soluble fractions obtained from the transfectants of human LPLA2 and bovine LPLA2 also showed acyl-ceramide synthase activity. The conclusion was that ACS is encoded by the same gene as LLPL.
Molecular weights of mouse, human and bovine LPLA2s conjugated with c-myc were 51 kDa, 50 kDa and 47 kDa, respectively, according to Western blot analysis using anti-c-myc antibody. Each molecular weight of LPLA2 was converted to 42 kDa after N-glycanase treatment, which is equivalent to the molecular weight of the core protein of LPLA2 without the signal peptide. These results indicated that LPLA2 undergoes post-translational modifications including a signal peptide cleavage and N-glycosylation. The de-glycosylation of LPLA2 resulted in no loss of the enzyme activity [29]. The glycosylation of LPLA2 potentially may mediate the secretion and translocation of LPLA2, and perhaps the protection of LPLA2 from lysosomal proteases.
As detailed above, LPLA2 has a consensus amino acid sequence of lipase motif consisting of AXSXG (Fig. 3B). The serine residue in the motif is a catalytic site with a nucleophilic hydroxyl group and essential in the hydrolytic reaction. The site directed mutation of the serine to alanine of LPLA2 resulted in the complete elimination of the enzyme activity [29]. Another feature of LPLA2 is a catalytic triad of amino acids containing the same serine residue that may play in a critical role for formation of an acyl-enzyme intermediate. In a proposed reaction mechanism of LPLA2 the enzyme cleaves the acyl-ester bond of glycerophospholipids and forms an acyl-LPLA2 intermediate via the catalytic serine residue. When either water or a short chain ceramide such as NAS are presented to the intermediate as acceptors in the reaction, free fatty acid or a 1-O-acyl-short chain ceramide are produced (Fig. 2). In this manner, LPLA2 may function like classic serine proteases such as trypsin, chymotrypsin, and elastase [51]. As demonstrated for these enzymes, during catalysis there is a nucleophilic attack of the hydroxyl oxygen of the serine residue on the carbonyl bond to be cleaved. An acyl-enzyme intermediate is transiently formed as part of this reaction. The active site in each serine protease includes a serine residue, a histidine residue, and an aspartate residue. The nucleophilic serine is created by the transfer of a proton from the serine hydroxyl to the imidazole ring of the histidine, as the adjacent carboxyl is H-bonded to the histidine.
Thus the cloning and expression studies revealed that LLPL is identical to ACS. Based on the properties of ACS, the ACS may primarily act as a phospholipase degrading glycerophospholipids within the lysosome rather than an anabolic enzyme responsible for the formation of 1-O-acylceramides. Thus, ACS is more appropriately termed lysosomal phospholipase A2 (LPLA2).
3.3 Structure and function of LPLA2
A signal sequence and three domains can be identified within the LPLA2 protein (Fig. 3A). LPLA2 has 49% amino acid sequence identity to LCAT, primarily within the catalytic domain. A catalytic triad and four cysteine residues are conserved between LPLA2 orthologues and between LPLA2 and LCAT [29, 48]. These conserved amino acids are thought to play a crucial role in determining the substrate specificity as well as maintaining the structure of LCAT. Despite the similarities in structural homology between LPLA2 and LCAT, there are significant differences between the two enzymes, most notably the pH optimum of enzyme activity and the acceptor specificity in transacylase reaction [29]. These conserved amino acid residues in LPLA2 were further investigated by site directed mutagenesis in order to more fully discern these differences [52].
Each amino acid residue in the catalytic triad of mouse LPLA2 was individually mutated to alanine. In each case, single alanine substitutions of serine-198, aspartic acid-360, and histidine-392 resulted in the complete loss of enzyme activity. Thus each amino residue in the catalytic triad of LPLA2 is essential for the enzyme activity. There is a minor difference in the lipase motif between LCAT (GXSXG) and LPLA2 (AXSXG). The substitution of alanine to glycine of the lipase motif of LPLA2 also resulted in a reduction of both the transacylase and esterase activities, consistent with the interpretation that the substitution affects the enzyme activity but not specificity to the substrate [52]. A helical wheel alignment creates a hydrophobic domain and is found in proximity to the catalytic serine residue in both LCAT and LPLA2 [53]. This helical wheel may play a critical role in the binding to the phospholipid substrate and may account for the difference in the lipase motif. A homologous structure is shared by triacylglycerol lipase. The crystal structure of the triacylglycerol lipase from Pseudomonas glumae supports this mechanism [54].
Two of the four conserved cysteine residues C65 and C89 (C1 and C2) are located within the N-terminal region. The other two cysteines C330 and C371 (C3 and C4) are located in the C-terminal region. Each cysteine residue was also substituted with an alanine. Four single (C1, C2, C3 or C4), two double (C1/C2 and C3/C4), and one quadruple (C1, C2, C3 and C4) LPLA2 substitutions were created. The quadruple mutation, the double mutation at C1 and C2, and the single mutation at C1 or C2 resulted in the elimination of LPLA2 activity [52]. By contrast, when a single substitution at C3 or C4 or a double substitution at C3 and C4 was created, 5, 35, and 30 percent, respectively, of the original activity of LPLA2 remained [52]. These data suggest that the two cysteine residues in the C-terminal region of LPLA2 are not required to form a disulfide bond for the preservation of LPLA2 activity.
An amphipathic helix is predicted to be present within the most hydrophobic region in the N-terminal region of LPLA2. This helix is likely involved in the interaction between LPLA2 and the lipid membrane. LCAT contains a very similar region that is essential for the binding to lipoprotein surface and is thought to be crucial for interfacial activation of the enzyme [55, 56]. The two conserved cysteines in both enzymes are present within this region. Thus, the mutation of C1 or C2 of LPLA2 may disrupt the enzyme/membrane interaction and eliminate the enzyme activity. Intact LCAT contains two disulfide bonds formed between the conserved cysteine residues in N-terminal and C-terminal regions [57], indicating that the disulfide bonds are essential for the enzyme activity of LCAT. These findings led to the consideration of whether or not LPLA2 possesses free cysteine residues or disulfide bonds.
To confirm the presence of free cysteine residues, the purified bovine brain LPLA2 was studied. The bovine enzyme only contains the four conserved cysteine residues but no additional cysteines. The enzyme was applied to an organomercury column. The LPLA2 activity was completely absorbed to the column and eluted with the buffer containing 200 mM 2-mercaptoethanol (2-ME), suggesting that LPLA2 has at least two free cysteine residues. In addition, the migration of LPLA2 without 2-ME treatment was faster than LPLA2 treated with 2-ME treated in the presence of SDS on SDS-polyacrylamide gel electrophoresis [52]. In SDS-PAGE, a denatured protein with one or more intramolecular disulfide bonds behaves as a smaller size molecule compared to the same denatured protein with intramolecular disulfide bonds that have been reduced with thiol reagents [58]. Thus, LPLA2 may contain at least one intra-molecular disulfide bond.
In aggregate, these observations suggest that unlike LCAT, two of the conserved cysteine residues are reduced in LPLA2. One disulfide bond between C1 and C2 and free cysteine residues at C3 and C4 as well as the triad in LPLA2 appear to be required for the full expression of LPLA2 activity. In addition, C1/C2 and C3/C4 seem to be involved in the enzyme-lipid interaction and the catalytic reaction, respectively. The structural similarities and proposed roles for the catalytic triads are summarized for LPLA2 and LCAT (Fig. 4). An important caveat for this model is that these studies were based on the purified bovine enzyme. Whether or not this model can be generalized to the murine or human protein will require additional study.
Fig. 4. Comparison of the secondary structures of mouse LPLA2 and LCAT.
Shown are the sites of the homologous cysteines and the catalytic triads.
3.4. Positional specificity of LPLA2
Phospholipase A2s predominantly cleave acyl groups at the sn-2 position of glycerophospholipids. However, this is not always observed. For example, the positional specificity of LCAT toward various phospholipid substrates is dependent on the length of acyl chain at the sn-2 position in the PC molecule [59]. Recently, it has been suggested that the positional specificity of PLA2 toward acyl groups of glycerophospholipids is determined by the physicochemical characteristics of acyl groups at the sn-1 and sn-2 positions of glycerophospholipids, as well as the physicochemical state of the membrane integrating the substrate [43, 60].
The observed transacylation of NAS by LPLA2 provided a novel opportunity to explore the relative PLA1 versus PLA2 activities of this enzyme since various 1-O-acyl-ceramides are readily separated by thin layer chromatography. Argentation high performance thin layer chromatography plate was employed for this purpose [61]. First, liposomes consisting of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), dicetyl phosphate and N-acetylsphingosine (NAS) were incubated with the soluble fraction obtained from MDCK cells that had been stably transfected with or without the mouse LPLA2 (mLPLA2) gene. Virtually, no O-acyl-NAS formation was detected in the extracts of cells not transfected with the mLPLA2 gene [61]. By contrast, two 1-O-acyl-NASs, 1-O-palmitoyl-NAS and 1-O-oleoyl-NAS, were found when using the cell extracts prepared from the mLPLA2 transfectants. The formation of 1-O-oleoyl-NAS was three-fold faster that of 1-O-palmitoyl-NAS. When liposomes containing 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (OPPC) were incubated with the mLPLA2 soluble fraction, the formation of 1-O-oleoyl-NAS was five-fold faster than that of 1-O-palmitoyl-NAS [61]. Additionally, the lysophospholipase A1 activity in the soluble fraction was found to be significantly lower than the phospholipase A activities in the same fraction. These results demonstrated that mLPLA2 could act on acyl groups at both sn-1 and sn-2 positions of POPC and OPPC. Various 1-palmitoyl-2-unsaturated acyl-sn-glycero-3-phosphocholines were used as acyl donors. The transacylation of the unsaturated acyl group from the sn-2 position of the donor to NAS was favored compared to that of the palmitoyl group from the sn-1 position. A notable exception was observed for 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC). Although the formation of 1-O-palmitoyl-NAS from PAPC was four-fold faster than that of 1-O-arachidonoyl-NAS, it was highly dependent on the lipid composition of the liposomes. A similar positional specificity of the soluble cell fraction was also observed with phosphatidylethanolamine. Purified recombinant mLPLA2 obtained from HEK 293 cells showed the same positional specificity as the cell extract obtained from mLPLA2 gene-transfectants. In aggregate, these observations indicate that mLPLA2 has broad positional specificity for both the sn-1 and sn-2 acyl groups in glycerophospholipids such as phosphatidylcholine and phosphatidylethanolamine [61].
The human peroxisomal calcium-independent PLA2γ (iPLA2γ) is characterized by a positional specificity that is similar to LPLA2 [60]. Incubation of LPLA2 or iPLA2γ with PAPC results in a rapid release of palmitic acid and accumulation of 1-hydroxy-2-arachidonoyl-sn-glycero-3-phosphocholine. This lysophospholipid is a precursor of 2-arachidonoyl monoglycerol, a natural product in mammalian tissues [62–64], an endogenous ligand of the cannabinoid receptor [65, 66], and a COX2 substrate [67].
3.5. Acceptor specificity of LPLA2
In general, LPLA2 catalyzes the transfer of the acyl group of the sn-2 position of phospholipid to the hydroxyl group at the C1 position of a short chain ceramide such as NAS under acidic conditions. However, the efficiency of the acylation on long chain ceramides is much lower than that of NAS [27]. In addition, LPLA2 does not esterify cholesterol under either acidic or neutral conditions [29]. To examine the common features of acceptors in the transacylase reaction by LPLA2, purified recombinant mouse LPLA2 was incubated with liposomes containing different acceptor molecules [68]. When 1-O-hexadecyl-2-acetyl-sn-glycerol (HAG) was used as an acceptor, the purified enzyme efficiently esterified HAG under acidic conditions. HAG is an alkylacylglycerol with a primary alcohol group at the C3 position and structurally very similar to NAS. The product produced from HAG was 1-O-hexadecyl-2-acetyl-3-acylglycerol. HAG competed with NAS and inhibited the esterification of NAS in a dose dependent manner with an IC50 of 25 μM.
To better understand the structural requirements of the alcohol compound as an acceptor of the acyl group, the transacylation of simpler alcohols such as 1-O-hexadecyl-glycerol (HG) and monoacylglycerides was investigated. HG, 1- or 3-palmitoyl-sn-glycerol and 2-palmitoylglycerol were converted to 1,3-alkylacylglycerol, 1,3-diacylglycerol and 1,2-diacylglycerol, respectively, by the enzyme. HG and monoacylglycerol inhibited the esterification of NAS by the enzyme with IC50 at 35 μM and 45 μM, respectively. In addition, the enzyme esterified glycerol at a high concentration (30%, w/v) to produce 1- or 3-acyl-sn-glycerol and 1,3-diacylglycerol, but not 2-acylglycerol. Taken together, these findings indicate that the desirable acceptor molecules for LPLA2 are primary alcohols with one long carbon chain and one small non-polar residue linked to the C2 position of ethanol (Fig. 5) [68].
Fig. 5. Common features of acceptor lipophilic alcohols recognized by LPLA2.
A denotes a long aliphatic carbon chain. B denotes a small neutral group such as -H, -OH, -OCH3, -OCOCH3 and –NHCOCH3.
As expected from this structural requirement, the enzyme esterified other natural bioactive lipophilic alcohols such as anandamide (ANA) and 2-oleoylethanolamide (OEA) [68]. ANA inhibited formation of 1-O-acyl-NAS by the enzyme in a dose dependent manner with an IC50 of 45 μM. Thus, some bioactive lipophilic primary alcohols, including HAG [69–71], HG [72], ANA and OEA [73], in the cell may be esterified and inactivated via LPLA2 within acidic compartments.
3.6. The role of anionic lipids in the LPLA2 reaction
In the initial characterization of the LPLA2 assay, it was recognized that the phospholipase exhibits an increased activity towards zwitterionic phosphatidylcholine liposomes containing negatively charged lipids under acidic conditions [27]. To understand the effect of negatively charged lipids on LPLA2 activity, purified recombinant mLPLA2 was used as the source of LPLA2 and sulfatide, a lipid that is resistant to LPLA2, was chosen as a negatively charged lipid. LPLA2 activity was monitored by NAS transacylation [44].
Sulfatide incorporated into DOPC/NAS liposomes enhanced LPLA2 activity under acidic conditions. The enhancement by sulfatide was linear at molar ratios of sulfatide to DOPC up to 10 % and weakened by the concentration dependent addition of NaCl [44]. Amiodarone (AMD), a cationic amphiphilic drug that interacts with negatively charged lipids and zwitterionic phospholipids [74], reduced the LPLA2 activity against DOPC/sulfatide/NAS liposomes in a concentration dependent manner. These data suggest that LPLA2 interacts with the anionic lipid membrane containing substrate through an electrostatic interaction. LPLA2 is characterized by a pH optimum of 4.5 on DOPC/sulfatide/NAS liposomes. However, the activity of LPLA2 was found to be decreased with at increased pH. The transacylase and phospholipase activities were almost non-existent at a pH greater than 6 in this assay system. The isoelectric point of mouse LPLA2 is estimated to be 5.9 from its primary structure suggesting that LPLA2 may be an anionic molecule at pH greater than 5.9. Thus the electrostatic interaction of LPLA2 with anionic lipid membrane surfaces is probably weakened at pH 5.9 and greater. In support of this mechanism, we observed that galactosylceramide, a neutral glycosphingolipid and precursor of sulfatide, had no effect on LPLA2 activity when compared to sulfatide. Thus the negatively charged sulfate group in the sulfatide molecule enhances the interaction of LPLA2 with the liposomes under acidic conditions [44].
The sedimentation of LPLA2 with liposomes was evaluated by very high-speed centrifugation and was significantly enhanced by sulfatide but not galactosylceramide. The enhancement was markedly reduced by raising NaCl, AMD or the proton concentration in the reaction mixture [44]. In general, lipolytic enzymes need to non-specifically access membranes for their activation before interacting with the substrate [75–77]. These experiments indicate that LPLA2 adsorbs to the negatively charged lipid membrane surface through an electrostatic attraction and enhances enzyme activity against otherwise insoluble substrates. Stated differently, the negatively charged lipids present within lipid membranes play a crucial role in enhancing the rate of phospholipid hydrolysis by LPLA2 at lipid-water interfaces.
By contrast, recombinant LPLA2 exhibited esterase activity when p-nitro-phenylbutyrate (p-NPB) was dispersed and used as a monomeric substrate. Unlike the phospholipase A2 activity, the considerable esterase activity was detected over wide range pH, including neutral pH. The esterase activity was not inhibited by the addition of NaCl or AMD [44]. In addition, the study using p-NPB showed initial burst by LPLA2 in pre-steady state, indicating that LPLA2 could form an acyl-enzyme intermediate. The stoichiometry of LPLA2 to the substrate was estimated to be 1 to 1 from the burst size. Interestingly, 1-O-butanoyl-NAS was produced when p-NPB was incubated with LPLA2 in the presence of NAS even under neutral conditions [44, 78]. Therefore the catalytic reaction of LPLA2 is independent of the binding reaction of LPLA2 to the membrane. These findings further support the proposed reaction mechanism (Fig. 3). We believe that the dual enzyme activity of LPLA2 is due to formation of an acyl-enzyme intermediate via the catalytic serine residue in the reaction.
Several reports on the interfacial interaction and the catalytic reaction of secretory phospholipase A2s (sPLA2s) have shown that these enzymes adsorb to the lipid-water interface via electrostatic as well as hydrophobic interactions (the interfacial activation of the enzymes) and bind a phospholipid monomer into the catalytic site, resulting in the activated enzyme-substrate formation and release product [79]. Subsequently, the enzymes may diffuse on the membrane surface in a “scooting” or “hopping” mode to bind the substrate. LPLA2 may share the common principle in the lipid-water facial reaction as sPLA2. Studies of lysosomal PLA1 prepared from purified lysosomes to anionic lipid membranes show a similarity of the reaction mechanism of lysosomal PLA1 to that of LPLA2 [80, 81].
4. Biological functions of LPLA2
What roles this novel lysosomal hydrolase might play in normal physiological states and disease were next considered. Proteomic analyses have identified over 85 unique gene products that localize to the lysosome [82]. Approximately 76 of these proteins are well characterized. A partial or complete loss of function of approximately 50 of these proteins result in clinically significant diseases, commonly associated with the accumulation of substrate within the lysosome. In considering the large family of phospholipase A2s, a number of interesting and important phenotypes are associated with a loss of function [23]. Thus having identified and biochemically characterized LPLA2, we were motivated to explore potential functions for this enzyme.
4.1. LPLA2 expression in rat and mouse alveolar macrophages
Measurements of the tissue specific expression of LPLA2 mRNA and activity demonstrated that the enzyme was ubiquitously expressed in every tissue and cell type analyzed, including brain, kidney, heart, liver, spleen, and lung. The observation that pulmonary surfactant might be catabolized by alveolar macrophages (AMs) led us to evaluate this cell type in greater detail. Rat AMs were isolated by bronchoalveolar lavage and assayed for LPLA2 activity. Surprisingly, the specific activity of LPLA2 in the soluble fraction obtained from AMs was more than 50 fold higher than that from whole lung. The increased LPLA2 activity in the AMs was also 50 fold greater compared to peritoneal macrophages or peripheral blood monocytes [83].
Real time PCR demonstrated that tissue distribution of LPLA2 messenger closely correlated with LPLA2 activity, suggesting that LPLA2 expression is regulated by transcriptional level. In addition, when real time PCR study was compared to either macrophage associated cytosolic Ca2+-independent phospholipase A2 or peroxiredoxin 6 the AM specific changes were only associated with LPLA2. Significantly, Kim et al. reported that peroxiredoxin 6 is a lysosomal-type Ca2+-independent phospholipase A2 important in pulmonary function [24]. Western blot analysis using anti-LPLA2 antibody confirmed that LPLA2 is highly expressed in rat AMs [83]. The LPLA2 expression and activity in mouse shows a similar tissue distribution of LPLA2 to rat (unpublished data).
Pulmonary surfactant is a surface-active agent that stabilizes the alveolar volume by reducing surface tension of the lung. Pulmonary surfactant consists of 90% lipid and 10% protein, including surfactant proteins A, B, C, and D. Greater than 90 percent of the lipid consists of phosphatidylcholine and phosphatidylethanolamine. The largest fraction of PC is comprised of dipalmitoyl-PC. The surfactant is synthesized at alveolar type II epithelial cells and degraded at the type II cells and alveolar macrophages (AMs) [84]. GM-CSF deficient (GM-CSF−/−) mice develop the progressive accumulation of surfactant lipids and proteins [85]. GM-CSF knockout mice display a phenotype similar to a human disorder of surfactant catabolism known as pulmonary alveolar proteinosis. Bi-transgenic mice generated from GM-CSF−/− mice by transgenic expression of GM-CSF gene under the surfactant protein C promoter are able to correct alveolar proteinosis [86]. These mice were utilized to explore a possible role for LPLA2 in surfactant metabolism. AMs from GM-CSF−/− mice exhibited significantly lower LPLA2 activity and expression than wild type mice. By contrast, the transgenic mice over expressing GM-CSF exhibited greater LPLA2 activity and protein levels than did wild type mice [83]. These findings are consistent with a role for LPLA2 in AM dependent surfactant phospholipid catabolism.
4.2. Transcriptional regulation of LPLA2
The strong association between murine GM-CSF expression and LPLA2 activity in alveolar macrophages raised the possibility that LPLA2 gene transcription is related to the presence of this growth factor. This possibility was explored with the macrophage cell line THP-1. mRNA levels and LPLA2 activity was measured following exposure of this cell line to several agents. 1,25 Dihydroxy-vitamin D3, interferon-γ, and GM-CSF all failed to increased mRNA and activity. Phorbol ester, on the other hand, increased LPLA2 mRNA and activity [87]. THP1 cells are of monocytic origin and are known to acquire increased phagocytic ability upon treatment with phorbol myristate acetate. This was consistent with the findings of Taniyama et al. who used the THP-1 line in their initial subtraction cloning work to identify LPLA2 as a novel gene that was responsive to VLDL and phorbol ester stimulation [45].
Also observed, however, was that all-trans-retinoic acid enhanced mRNA expression and enzyme activity of LPLA2, indicating the possible induction of LPLA2 through the RXR. These changes in mRNA and LPLA2 activity occurred in a concentration and time dependent fashion. The induction was not, however, the result of changes in mRNA stability. Both 9-cis- and 13-cis-retinoic acids were as effective as all-trans-retinoic acid in inducing these changes. To delineate whether the retinoic acid signaling was secondary to RAR or RXR nuclear receptor activation, TTNPB and methoprene acid were used as RAR and RXR agonists, respectively. Methoprene acid was as effective as all-trans-retinoic acid in inducing LPLA2mRNA and activity. TTNPB increased mRNA and activity levels, albeit to a lesser extent. In aggregate, these data suggest that retinoic acid stimulates the LPLA2 gene activation of LPLA2 via an RXR-dependent pathway [87].
4.3. LPLA2 knockout mice
LPLA2 knockout mice were created by double conditional gene targeting via Cre/loxP and Flp/FRT systems in an effort to better understand the biological function of LPLA2 [88]. LPLA2−/− mice were generated by systemic deletion of exon 5 of LPLA2 gene, which encodes the lipase motif. A systemic loss of LPLA2 activity was observed. However, the mice were viable, displaying normal fertility and fecundity. The homozygous LPLA2−/− mice display normal life span.
Biochemical phenotyping of the mice demonstrated a global defect in the ability of tissues to degrade glycerophospholipids under acidic conditions as measured by the 1-O-acylceramide synthase assay. The phospholipid accumulation appeared earliest and was most evident in the AMs, occurring at 3 months of age. The lung phospholipid accumulation was evident in the bronchoalveolar lavage fluid with significant elevations of PE and PC by one year of age. Dipalmitoyl-PC was significantly elevated, suggesting that this surfactant specific phospholipid is a substrate for LPLA2. Consistent with this accumulation was the presence of markedly enlarged alveolar macrophages with a foam cell appearance in mice as young as 3 months of age.
To confirm the presence of the defect in phospholipid catabolism, AMs of 3 month old wild type and knockout mice were further studied. The total phospholipid content of the LPLA2−/− mouse AMs was twice that of the LPLA2+/+ mouse AMs. Thin layer chromatography of the lipid extract of AMs showed a marked increase of glycerophospholipids but not sphingomyelin in the LPLA2−/− mouse. Phosphatidylethanolamine and phosphatidylcholine levels in the LPLA2−/− mouse AMs were 4 times and twice, respectively, higher than those of the LPLA2+/+ mouse AMs [88]. When isolated wild type and LPLA2−/− AMs were treated with liposomes containing 1-palmitoyl-2-[14C]-oleoyl-sn-3-glycero-phosphorylcholine, [14C]-labeled oleic acid was detected in the wild-type mouse AM, but not the knockout macrophages.
Peritoneal macrophages and spleen also exhibited an increase in phospholipid content in young LPLA2−/− mice. The total phospholipid content in the LPLA2−/− mouse PMs was 40% higher than that of the LPLA2+/+ mouse PMs. The total phospholipid content in the LPLA2−/− mouse spleen was 30% higher than LPLA2+/+ mouse spleen. Phosphatidylethanolamine and phosphatidylcholine in the LPLA2−/− mouse spleen were 100% and 30%, respectively, higher than those of the LPLA2+/+ mouse spleen. The increase of glycerophospholipids in LPLA2-knockout mouse spleen is likely due to the presence of splenic macrophages that also lack the catabolic pathway for glycerophospholipids because of a deficiency of LPLA2. By contrast, the total phospholipid content in other tissues, including heart, liver, kidney, brain and thymus, was not much significantly different between the LPLA2+/+ and LPLA2−/− mice, although in each tissue the phospholipid content was trended higher in the LPLA2−/− mouse than LPLA2+/+ mouse.
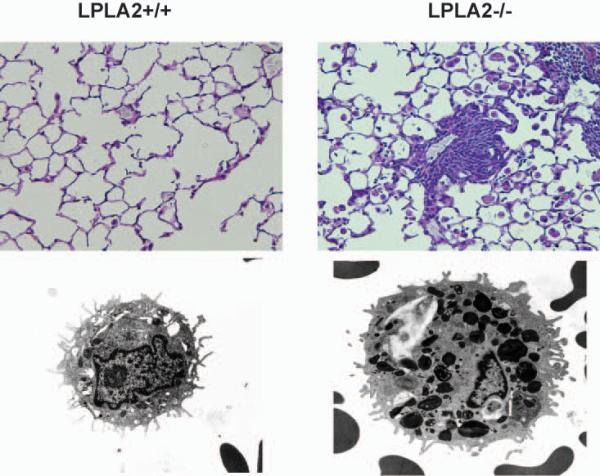
By light microscopy the lungs of the LPLA2−/− mice were characterized by a marked increase in the numbers of AMs. The macrophages were significantly larger than the normal resident alveolar macrophages (Fig. 6). Electron micrography revealed that AMs in LPLA2 knockout mice contain numerous lamellar inclusion bodies, indicative of cellular phospholipidosis (Fig. 6). Such lamellar inclusion bodies were only rarely present in the LPLA2+/+ mouse AMs. Similar inclusion bodies also appeared in the peritoneal macrophages although the frequency of lamellar inclusion appearance in the LPLA2+/+ mouse peritoneal macrophages was lower than in the LPLA2−/− mouse AMs. These results indicate that phospholipid accumulation resulting from a defect in LPLA2 is linked to inordinate lamellar body accumulation, a form of phospholipidosis [88].
Fig. 6. Light microscopy of lung and transmission electron micrographs of AMs obtained from LPLA2+/+ and LPLA2−/− mice.
The PAS stain compares the lungs of 18 month old LPLA2+/+ and LPLA2−/− mice. Shown are increased numbers and size of alveolar macrophages in the knockout mice. The transmission electron micrographs were taken at the same magnification.
Recently, Taniyama et al. created the LPLA2 deficient mice and crossed them on an apo E null background. The double knockout mice were characterized by the presence of more atherosclerosis under high fat feeding [89]. When our group performed similar studies, crossing the LPLA2−/− mice on either an apo E or LDL receptor null background, we failed to see a similar phenotype (unpublished results).
More recently we have observed that LPLA2 null mice older than one year develop lymphoid hypertrophy, glomerulonephritis, and abnormal serologies including anti-dsDNA antibodies, positive anti-nuclear antibodies, and high circulating immunoglobulin levels. This autoimmune phenotype resembles systemic lupus erythematosus. The splenic macrophages are characterized by the inability to digest apoptotic bodies that have been endocytosed. Thus, LPLA2 deficiency may potentially be linked to macrophage associated phospholipidosis and underlie some forms of autoimmunity.
4.4. Secretion and uptake of LPLA2
In many types of hematopoietic cells and melanocytes, the lysosomal compartment has a bifunctional role. These may include lytic and secretory functions [90, 91]. Macrophages have secretory lysosomes that store acid hydrolases and that are selectively released in response to specific stimuli [92]. Resident mouse peritoneal macrophages were reported to secrete an acidic phospholipase A2 from lysosomes into the culture medium following exposure to phagocytic stimuli such as zymosan [93]. This acidic phospholipase activity is distinct from the small secreted PLA2s (sPLA2s) characterized by their low molecular weights (14–18 kDa) and high number of disulfide bonds [23]. Unlike the well studied sPLA2s, the PLA2 released from peritoneal macrophage lysosomes had not been characterized on a molecular basis.
A monoclonal antibody was raised against LPLA2. Using this reagent against AMs, the localization of LPLA2 to late endosomes and lysosomes was confirmed. LPLA2 was released following exposure to zymosan in a dose and time dependent manner. When AMs from LPLA2 null mice were exposed to recombinant LPLA2, the lipase was rapidly incorporated into the macrophages. The uptake of LPLA2 into the cells was significantly inhibited in the presence of 10 mM α-methyl-mannoside but not mannose-6-phosphate. Interestingly, the levels of phospholipids in the knockout mice AMs were markedly reduced by treatment with the recombinant LPLA2 and almost normalized to wild type levels [94]. These facts indicate that LPLA2 is a secreted protein as well as a lysosomal enzyme and that extracellular LPLA2 is taken up by macrophages via a mannose receptor and trafficked to acidic compartments such as late endosomes and lysosomes, where LPLA2 functions as a hydrolase. Although the release of lysosomal acid enzymes from macrophages and other secretory cells by phagocytic stimuli has been well documented, the biological and physiological purposes of lysosomal enzyme release have yet to be delineated.
4.5. LPLA2 and cationic drug induced phospholipidosis
Cellular phospholipidosis is a significant cause of toxicity resulting from prolonged exposure to many commonly used drugs. Examples of such agents include fluoxetine, azithromycin, and amiodarone. In addition, the development of several otherwise effective drug candidates has been terminated once this form of toxicity is observed. Drugs that are associated with phospholipidosis are chemically characterized by the presence of an aromatic group and an amine that becomes protonated under acidic conditions and are therefore termed cationic amphiphilic drugs [95, 96]. PDMP, the glucosylceramide synthase inhibitor that led to the original identification of the 1-O-acylceramide synthase, conforms to the structure of a CAD.
The pathological consequences of CAD-induced phospholipidosis at the cell or organ level are not well understood. One CAD, amiodarone (AMD), is an anti-arrhythmic agent used in the treatment of a wide range of cardiac tachyarrhthmias. These include ventricular and supraventricular arrhythmias. Although highly effective, AMD is often limited in its use by numerous side effects [97, 98]. The most serious complication is pulmonary toxicity associated with the accumulation of phospholipid in the lung, most commonly in the alveolar macrophages.
Four hypotheses have been proposed for the induction of phospholipidosis [99]. Under the first mechanism CADs bind to phospholipids and render them resistant to phospholipase catabolism. The second mechanism proposes that CADs bind to lysosomal phospholipases and limit the enzyme's ability to catalyze phospholipid hydrolysis. The third mechanism suggests that CADs stimulate phospholipid synthesis in the cell. The fourth mechanism proposes that if a CAD induces the dissociation of a lysosomal hydrolase from the lysosomal membrane, then the hydrolase is subject to the degradation by other lysosomal catabolic enzymes [100]. Presently, none of the proposed mechanisms fully account for the tissue specific differences seen following exposure to individual CADs or for differences in the phospholipid profiles observed for specific drugs. Thus while there may be evidence supporting each of these mechanisms, the molecular basis for CAD-induced phospholipidosis remains to be established.
Both AMD and PDMP induce the accumulation of glycerophospholipids and formation of numerous multi-lamellar inclusion bodies in MDCK cells [101]. Compared to amiodarone, a higher concentration of PDMP was required to induce the lysosomal lipid accumulation. Although both compounds inhibit LPLA2 activity in a concentration dependent manner, the IC50 of PDMP for LPLA2 activity was three times higher than that of AMD. By contrast, the amphiphilic amine compound tetracycline had no effect on phospholipid levels in MDCK cells or on LPLA2 activity. In addition and as discussed below, the incorporation of AMD into anionic lipid membrane reduces the binding affinity of LPLA2 to the membrane by a decrease in the electrostatic interaction and results in a decrease of LPLA2 activity (Fig. 7). These data suggest that the inhibition of LPLA2 by CADs may be the basis for this form of drug toxicity. In support of this hypothesis, the phenotype of LPLA2-deficient mouse AMs is indistinguishable from AMD induced phospholipidosis [88]. However, a more extensive study on LPLA2 activity in the presence of a larger panel of CADs has yet to be performed.
Fig. 7. Effect of increasing cationic amphiphilic drug (CAD) concentration on the interaction of LPLA2 with lipid membrane.
PL denotes phospholipid.
To better discern the potential basis for LPLA2 activity in phospholipidosis, the role of negatively charged membrane lipids in regulating LPLA2 activity was explored. Negatively charged glycerophospholipids, including phosphatidylglycerol, cardiolipin, phosphatidylserine, phosphatidic acid, phosphatidylinositol and bis(monoacylglycero)phosphate (BMP), significantly enhance LPLA2 activity [44]. In particular, BMP is the most potent enhancer. BMP is highly resistant to LPLA2 and localizes in acidic compartments in the cell [102, 103]. BMP is concentrated in intra-endosomal and intra-lysosomal membranes and not limiting membranes in late endosomes and lysosomes, respectively, and the degradation of glycosphingolipids in lysosomes requires glycosidase, activator protein and anionic lipids such as BMP [104]. The surfaces of intra-endosomal and intra-lysosomal membranes in acidic compartments are thought to be a platform for the lipid–water interfacial reaction of lysosomal lipolytic enzymes. Thus, BMP may work as an endogenous stimulator of LPLA2 reaction in the lysosome and late endosome. The unique conformation of BMP confers the ability of this lipid to persist in its anionic form under lysosomal conditions.
In drug induced phospholipidosis, CADs are translocated into acidic compartments within the cell and incorporated to intra-endosomal and intra-lysosomal membranes that are negatively charged. As a result, the membranes in the compartments are neutralized and become insensitive to LPLA2 (Fig. 7). Therefore in some cases of CAD-induced phospholipidosis the impairment of phospholipid catabolism may result from inhibition of the binding of LPLA2 to the surface of intra-endosomal and intra-lysosomal membranes into which the CAD has been incorporated.
6. Conclusions and future directions
In this article we have reviewed the current understanding of LPLA2, one of the most recently identified lysosomal hydrolases. Although we originally named this enzyme lysosomal phospholipase A2 (LPLA2), it is now officially classified as group XV PLA2. When compared to other well characterized PLA2s, LPLA2 is unique in its low pH optimum and ability to transacylate a variety of lipophilic alcohols. In this regard, LPLA2 more closely resembles LCAT. LPLA2 recognizes a wide range of phospholipid substrates and the hydrolysis of which requires electrostatic interactions with anionic lipids in the lysosome or late endosome.
LPLA2 activity is required for the catabolism of endogenous cellular lipids as demonstrated by a robust phenotype in the LPLA2 null mouse. The expression of LPLA2 and activities in all cell types tested to date are consistent with a ubiquitous role for LPLA2 in lysosomal phospholipid degradation. But the early formation of alveolar macrophage foam cells and increased surfactant phospholipid levels in the knockout mice suggest that LPLA2 is not only a marker of the terminally differentiated alveolar macrophage but is important for normal surfactant catabolism. Our preliminary and currently unpublished finding of an autoimmune phenotype in older LPLA2 knockout mice also suggests a role for this enzyme in the digestion of apoptotic bodies. Lysosomes also are involved in the catabolism of endogenously presented lipids. An important and as yet unexplored question is whether LPLA2 is important for the defense against microbial infection through degradation of the cell walls of bacteria and other pathogens.
Perhaps the most important question is whether an inherited or acquired deficiency in LPLA2 is the basis for clinically significant disease. The complete or partial loss of activity of any particular lysosomal enzyme has a high likelihood of resulting in a significant clinical syndrome. To date no defined disorder has yet to be linked to LPLA2. However, LPLA2 itself, an assay for the measurement of plasma LPLA2 activity, and the LPLA2 null mouse phenotype have only recently been described.
Acknowledgements
We are grateful to Norman Radin for his careful review of this article. This work was supported by NIH 1RO1 AR056991-01 and a Merit Award from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–92. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- [2].Turk B, Turk V. Lysosomes as “suicide bags” in cell death: myth or reality? J Biol Chem. 2009;284:21783–7. doi: 10.1074/jbc.R109.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schuchman EH. Acid sphingomyelinase, cell membranes and human disease: lessons from Niemann-Pick disease. FEBS Lett. 2010;584:1895–900. doi: 10.1016/j.febslet.2009.11.083. [DOI] [PubMed] [Google Scholar]
- [4].Mellors A, Tappel AL. Hydrolysis of phospholipids by a lysosomal enzyme. J Lipid Res. 1967;8:479–85. [PubMed] [Google Scholar]
- [5].Fowler S, De Duve C. Digestive activity of lysosomes. 3. The digestion of lipids by extracts of rat liver lysosomes. J Biol Chem. 1969;244:471–81. [PubMed] [Google Scholar]
- [6].Stoffel W, Trabert U. Studies on the occurrence and properties of lysosomal phospholipases A1 and A2 and the degradation of phosphatidic acid in rat liver lysosomes. Hoppe Seylers Z Physiol Chem. 1969;350:836–44. doi: 10.1515/bchm2.1969.350.2.836. [DOI] [PubMed] [Google Scholar]
- [7].Franson R, Waite M, LaVia M. Identification of phospholipase A 1 and A 2 in the soluble fraction of rat liver lysosomes. Biochemistry. 1971;10:1942–6. doi: 10.1021/bi00786a031. [DOI] [PubMed] [Google Scholar]
- [8].Franson R, Waite M, Weglicki W. Phospholipase A activity of lysosomes of rat myocardial tissue. Biochemistry. 1972;11:472–6. doi: 10.1021/bi00753a028. [DOI] [PubMed] [Google Scholar]
- [9].Franson RC, Waite M. Lysosomal phospholipase A 1 and A 2 of normal and bacillus calmette guerin-induced alveolar macrophages. J Cell Biol. 1973;56:621–7. doi: 10.1083/jcb.56.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ishikawa Y, Nishide T, Sasaki N, Shirai K, Saito Y, Yoshida S. Hydrolysis of low-density lipoprotein phospholipids in arterial smooth muscle cells. Biochim Biophys Acta. 1988;961:170–6. [PubMed] [Google Scholar]
- [11].Ellis LC, Boccio JR, Cunningham MJ, Groesbeck MD, Cosentino MJ. Rat testicular phospholipase A2 activity: pH optima, its cellular and subcellular distribution in the gonad, and some factors that may modulate its activity. Journal of Andrology. 1981;2:94–102. [Google Scholar]
- [12].Hostetler KY, Hall LB. Inhibition of kidney lysosomal phospholipases A and C by aminoglycoside antibiotics: possible mechanism of aminoglycoside toxicity. Proc Natl Acad Sci U S A. 1982;79:1663–7. doi: 10.1073/pnas.79.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wightman PD, Dahlgren ME, Hall JC, Davies P, Bonney RJ. Identification and characterization of a phospholipase C activity in resident mouse peritoneal macrophages. Inhibition of the enzyme by phenothiazines. Biochem J. 1981;197:523–6. doi: 10.1042/bj1970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robinson M, Waite M. Physical-chemical requirements for the catalysis of substrates by lysosomal phospholipase A1. J Biol Chem. 1983;258:14371–8. [PubMed] [Google Scholar]
- [15].Hostetler KY, Hall LB. Phospholipase C activity of rat tissues. Biochem Biophys Res Commun. 1980;96:388–93. doi: 10.1016/0006-291x(80)91227-9. [DOI] [PubMed] [Google Scholar]
- [16].Matsuzawa Y, Hostetler KY. Properties of phospholipase C isolated from rat liver lysosomes. J Biol Chem. 1980;255:646–52. [PubMed] [Google Scholar]
- [17].Kunze H. Intralysosomal glycerophospholipid catabolism in liver: hydrolysis of amino alcohol-containing phospholipids and their metabolites. Biochim Biophys Acta. 1993;1169:273–9. doi: 10.1016/0005-2760(93)90251-4. [DOI] [PubMed] [Google Scholar]
- [18].Richards DE, Irvine RF, Dawson RM. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J. 1979;182:599–606. doi: 10.1042/bj1820599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gamache DA, Fawzy AA, Franson RC. Preferential hydrolysis of peroxidized phospholipid by lysosomal phospholipase C. Biochim Biophys Acta. 1988;958:116–24. doi: 10.1016/0005-2760(88)90252-4. [DOI] [PubMed] [Google Scholar]
- [20].Brown FD, Thompson N, Saqib KM, Clark JM, Powner D, Thompson NT, et al. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr Biol. 1998;8:835–8. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- [21].Hostetler KY, Yazaki PJ, van den Bosch H. Purification of lysosomal phospholipase A. Evidence for multiple isoenzymes in rat liver. J Biol Chem. 1982;257:13367–73. [PubMed] [Google Scholar]
- [22].Hostetler KY, Gardner MF, Giordano JR. Purification of lysosomal phospholipase A and demonstration of proteins that inhibit phospholipase A in a lysosomal fraction from rat kidney cortex. Biochemistry. 1986;25:6456–61. doi: 10.1021/bi00369a017. [DOI] [PubMed] [Google Scholar]
- [23].Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- [24].Kim TS, Sundaresh CS, Feinstein SI, Dodia C, Skach WR, Jain MK, et al. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J Biol Chem. 1997;272:2542–50. doi: 10.1074/jbc.272.4.2542. [DOI] [PubMed] [Google Scholar]
- [25].Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem. 2000;275:28421–7. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- [26].Sorokina EM, Feinstein SI, Milovanova TN, Fisher AB. Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L871–80. doi: 10.1152/ajplung.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abe A, Shayman JA, Radin NS. A novel enzyme that catalyzes the esterification of N-acetylsphingosine. Metabolism of C2-ceramides. J Biol Chem. 1996;271:14383–9. doi: 10.1074/jbc.271.24.14383. [DOI] [PubMed] [Google Scholar]
- [28].Abe A, Shayman JA. Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J Biol Chem. 1998;273:8467–74. doi: 10.1074/jbc.273.14.8467. [DOI] [PubMed] [Google Scholar]
- [29].Hiraoka M, Abe A, Shayman JA. Cloning and characterization of a lysosomal phospholipase A2, 1-O-acylceramide synthase. J Biol Chem. 2002;277:10090–9. doi: 10.1074/jbc.M111977200. [DOI] [PubMed] [Google Scholar]
- [30].Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–6. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Radin NS, Arora RC, Ullman MD, Brenkert AL, Austin J. A possible therapeutic approach to Krabbe's globoid leukodystrophy and the status of cerebroside synthesis in the disorder. Res Commun Chem Pathol Pharmacol. 1972;3:637–44. [PubMed] [Google Scholar]
- [32].Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980;26:265–78. doi: 10.1016/0009-3084(80)90057-2. [DOI] [PubMed] [Google Scholar]
- [33].Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman JA, et al. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111:191–6. doi: 10.1093/oxfordjournals.jbchem.a123736. [DOI] [PubMed] [Google Scholar]
- [34].Abe A, Radin NS, Shayman JA, Wotring LL, Zipkin RE, Sivakumar R, et al. Structural and stereochemical studies of potent inhibitors of glucosylceramide synthase and tumor cell growth. J Lipid Res. 1995;36:611–21. [PubMed] [Google Scholar]
- [35].Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274:14662–9. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- [36].Lukina E, Watman N, Arreguin EA, Banikazemi M, Dragosky M, Iastrebner M, et al. A phase 2 study of eliglustat tartrate (Genz-112638), an oral substrate reduction therapy for Gaucher disease type 1. Blood. 2010;116:893–9. doi: 10.1182/blood-2010-03-273151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lukina E, Watman N, Avila Arreguin E, Dragosky M, Iastrebner M, Rosenbaum H, et al. Improvement in hematological, visceral, and skeletal manifestations of Gaucher disease type 1 with oral eliglustat tartrate (Genz-112638) treatment: two-year results of a Phase 2 study. Blood. 2010 doi: 10.1182/blood-2010-06-293902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rani CS, Abe A, Chang Y, Rosenzweig N, Saltiel AR, Radin NS, et al. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J Biol Chem. 1995;270:2859–67. doi: 10.1074/jbc.270.6.2859. [DOI] [PubMed] [Google Scholar]
- [39].Shayman JA, Lee L, Abe A, Shu L. Inhibitors of glucosylceramide synthase. Methods Enzymol. 2000;311:373–87. doi: 10.1016/s0076-6879(00)11097-3. [DOI] [PubMed] [Google Scholar]
- [40].Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–5. [PubMed] [Google Scholar]
- [41].Shayman JA, Abe A. 1-O-acylceramide synthase. Methods Enzymol. 2000;311:105–17. doi: 10.1016/s0076-6879(00)11071-7. [DOI] [PubMed] [Google Scholar]
- [42].Okabe H, Kishimoto Y. In vivo metabolism of ceramides in rat brain. Fatty acid replacement and esterification of ceramide. J Biol Chem. 1977;252:7068–73. [PubMed] [Google Scholar]
- [43].Shinozaki K, Waite M. A novel phosphatidylglycerol-selective phospholipase A2 from macrophages. Biochemistry. 1999;38:1669–75. doi: 10.1021/bi982123q. [DOI] [PubMed] [Google Scholar]
- [44].Abe A, Shayman JA. The role of negatively charged lipids in lysosomal phospholipase A2 function. J Lipid Res. 2009 doi: 10.1194/jlr.M900008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Taniyama Y, Shibata S, Kita S, Horikoshi K, Fuse H, Shirafuji H, et al. Cloning and expression of a novel lysophospholipase which structurally resembles lecithin cholesterol acyltransferase. Biochem Biophys Res Commun. 1999;257:50–6. doi: 10.1006/bbrc.1999.0411. [DOI] [PubMed] [Google Scholar]
- [46].Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- [47].Nardini M, Dijkstra BW. Alpha/beta hydrolase fold enzymes: the family keeps growing. Curr Opin Struct Biol. 1999;9:732–7. doi: 10.1016/s0959-440x(99)00037-8. [DOI] [PubMed] [Google Scholar]
- [48].Jonas A. Lecithin cholesterol acyltransferase. Biochim Biophys Acta. 2000;1529:245–56. doi: 10.1016/s1388-1981(00)00153-0. [DOI] [PubMed] [Google Scholar]
- [49].Wallace AC, Laskowski RA, Thornton JM. Derivation of 3D coordinate templates for searching structural databases: application to Ser-His-Asp catalytic triads in the serine proteinases and lipases. Protein Sci. 1996;5:1001–13. doi: 10.1002/pro.5560050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Noiriel A, Benveniste P, Banas A, Stymne S, Bouvier-Nave P. Expression in yeast of a novel phospholipase A1 cDNA from Arabidopsis thaliana. Eur J Biochem. 2004;271:3752–64. doi: 10.1111/j.1432-1033.2004.04317.x. [DOI] [PubMed] [Google Scholar]
- [51].Whiting AK, Peticolas WL. Details of the acyl-enzyme intermediate and the oxyanion hole in serine protease catalysis. Biochemistry. 1994;33:552–61. doi: 10.1021/bi00168a021. [DOI] [PubMed] [Google Scholar]
- [52].Hiraoka M, Abe A, Shayman JA. Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J Lipid Res. 2005;46:2441–7. doi: 10.1194/jlr.M500248-JLR200. [DOI] [PubMed] [Google Scholar]
- [53].Pollastri G, McLysaght A. Porter: a new, accurate server for protein secondary structure prediction. Bioinformatics. 2005;21:1719–20. doi: 10.1093/bioinformatics/bti203. [DOI] [PubMed] [Google Scholar]
- [54].Noble ME, Cleasby A, Johnson LN, Egmond MR, Frenken LG. The crystal structure of triacylglycerol lipase from Pseudomonas glumae reveals a partially redundant catalytic aspartate. FEBS Lett. 1993;331:123–8. doi: 10.1016/0014-5793(93)80310-q. [DOI] [PubMed] [Google Scholar]
- [55].Peelman F, Vanloo B, Perez-Mendez O, Decout A, Verschelde JL, Labeur C, et al. Characterization of functional residues in the interfacial recognition domain of lecithin cholesterol acyltransferase (LCAT) Protein Eng. 1999;12:71–8. doi: 10.1093/protein/12.1.71. [DOI] [PubMed] [Google Scholar]
- [56].Adimoolam S, Jonas A. Identification of a domain of lecithin-cholesterol acyltransferase that is involved in interfacial recognition. Biochem Biophys Res Commun. 1997;232:783–7. doi: 10.1006/bbrc.1997.6375. [DOI] [PubMed] [Google Scholar]
- [57].Yang CY, Manoogian D, Pao Q, Lee FS, Knapp RD, Gotto AM, Jr., et al. Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme. J Biol Chem. 1987;262:3086–91. [PubMed] [Google Scholar]
- [58].Abe A, Sasaki T. Sulfhydryl groups in glycolipid transfer protein: formation of an intramolecular disulfide bond and oligomers by Cu2+-catalyzed oxidation. Biochim Biophys Acta. 1989;985:38–44. doi: 10.1016/0005-2736(89)90100-4. [DOI] [PubMed] [Google Scholar]
- [59].Subbaiah PV, Liu M, Paltauf F. Role of sn-2 acyl group of phosphatidylcholine in determining the positional specificity of lecithin-cholesterol acyltransferase. Biochemistry. 1994;33:13259–66. doi: 10.1021/bi00249a012. [DOI] [PubMed] [Google Scholar]
- [60].Yan W, Jenkins CM, Han X, Mancuso DJ, Sims HF, Yang K, et al. The highly selective production of 2-arachidonoyl lysophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2gamma: identification of a novel enzymatic mediator for the generation of a key branch point intermediate in eicosanoid signaling. J Biol Chem. 2005;280:26669–79. doi: 10.1074/jbc.M502358200. [DOI] [PubMed] [Google Scholar]
- [61].Abe A, Hiraoka M, Shayman JA. Positional specificity of lysosomal phospholipase A2. J Lipid Res. 2006;47:2268–79. doi: 10.1194/jlr.M600183-JLR200. [DOI] [PubMed] [Google Scholar]
- [62].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- [63].Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- [64].Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- [65].Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, et al. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+-dependent and -independent mechanisms. FEBS Lett. 1998;429:152–6. doi: 10.1016/s0014-5793(98)00581-x. [DOI] [PubMed] [Google Scholar]
- [66].Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794–801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- [67].Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–9. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- [68].Abe A, Hiraoka M, Shayman JA. The acylation of lipophilic alcohols by lysosomal phospholipase A2. J Lipid Res. 2007;48:2255–63. doi: 10.1194/jlr.M700277-JLR200. [DOI] [PubMed] [Google Scholar]
- [69].McNamara MJ, Schmitt JD, Wykle RL, Daniel LW. 1-0-Hexadecyl-2-acetyl-sn-glycerol stimulates differentiation of HL-60 human promyelocytic leukemia cells to macrophage-like cells. Biochem Biophys Res Commun. 1984;122:824–30. doi: 10.1016/s0006-291x(84)80108-4. [DOI] [PubMed] [Google Scholar]
- [70].Bass DA, McPhail LC, Schmitt JD, Morris-Natschke S, McCall CE, Wykle RL. Selective priming of rate and duration of the respiratory burst of neutrophils by 1,2-diacyl and 1-O-alkyl-2-acyl diglycerides. Possible relation to effects on protein kinase C. J Biol Chem. 1988;263:19610–7. [PubMed] [Google Scholar]
- [71].Daniel LW, Small GW, Schmitt JD, Marasco CJ, Ishaq K, Piantadosi C. Alkyl-linked diglycerides inhibit protein kinase C activation by diacylglycerols. Biochem Biophys Res Commun. 1988;151:291–7. doi: 10.1016/0006-291x(88)90592-x. [DOI] [PubMed] [Google Scholar]
- [72].Warne TR, Buchanan FG, Robinson M. Growth-dependent accumulation of monoalkylglycerol in Madin-Darby canine kidney cells. Evidence for a role in the regulation of protein kinase C. J Biol Chem. 1995;270:11147–54. doi: 10.1074/jbc.270.19.11147. [DOI] [PubMed] [Google Scholar]
- [73].Farrell EK, Merkler DJ. Biosynthesis, degradation and pharmacological importance of the fatty acid amides. Drug Discov Today. 2008;13:558–68. doi: 10.1016/j.drudis.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sautereau AM, Tournaire C, Suares M, Tocanne JF, Paillous N. Interactions of amiodarone with model membranes and amiodarone-photoinduced peroxidation of lipids. Biochem Pharmacol. 1992;43:2559–66. doi: 10.1016/0006-2952(92)90144-8. [DOI] [PubMed] [Google Scholar]
- [75].Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzyme kinetics. John Wiley & Sons; Chichester: 2001. [Google Scholar]
- [76].Aloulou A, Rodriguez JA, Fernandez S, van Oosterhout D, Puccinelli D, Carriere F. Exploring the specific features of interfacial enzymology based on lipase studies. Biochim Biophys Acta. 2006;1761:995–1013. doi: 10.1016/j.bbalip.2006.06.009. [DOI] [PubMed] [Google Scholar]
- [77].Winget JM, Pan YH, Bahnson BJ. The interfacial binding surface of phospholipase A2s. Biochim Biophys Acta. 2006;1761:1260–9. doi: 10.1016/j.bbalip.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [78].Segel IH. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady state enzyme systems. Wiley Classics Library; New York: Wiley: 1993. [Google Scholar]
- [79].Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzymology: the secreted phospholipase A(2)-paradigm. Chem Rev. 2001;101:2613–54. doi: 10.1021/cr990139w. [DOI] [PubMed] [Google Scholar]
- [80].Mingeot-Leclercq MP, Laurent G, Tulkens PM. Biochemical mechanism of aminoglycoside-induced inhibition of phosphatidylcholine hydrolysis by lysosomal phospholipases. Biochem Pharmacol. 1988;37:591–9. doi: 10.1016/0006-2952(88)90130-x. [DOI] [PubMed] [Google Scholar]
- [81].Piret J, Schanck A, Delfosse S, Van Bambeke F, Kishore BK, Tulkens PM, et al. Modulation of the in vitro activity of lysosomal phospholipase A1 by membrane lipids. Chem Phys Lipids. 2005;133:1–15. doi: 10.1016/j.chemphyslip.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [82].Lubke T, Lobel P, Sleat DE. Proteomics of the lysosome. Biochim Biophys Acta. 2009;1793:625–35. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Abe A, Hiraoka M, Wild S, Wilcoxen SE, Paine R, Shayman JA. Lysosomal phospholipase A2 is selectively expressed in alveolar macrophages. J Biol Chem. 2004;279:42605–11. doi: 10.1074/jbc.M407834200. [DOI] [PubMed] [Google Scholar]
- [84].Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]
- [85].Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- [86].Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97:649–55. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Abe A, Poucher HK, Hiraoka M, Shayman JA. Induction of lysosomal phospholipase A2 through the retinoid X receptor in THP-1 cells. J Lipid Res. 2004;45:667–73. doi: 10.1194/jlr.M300342-JLR200. [DOI] [PubMed] [Google Scholar]
- [88].Hiraoka M, Abe A, Lu Y, Yang K, Han X, Gross RW, et al. Lysosomal phospholipase A2 and phospholipidosis. Mol Cell Biol. 2006;26:6139–48. doi: 10.1128/MCB.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Taniyama Y, Fuse H, Satomi T, Tozawa R, Yasuhara Y, Shimakawa K, et al. Loss of lysophospholipase 3 increases atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2005;330:104–10. doi: 10.1016/j.bbrc.2005.02.126. [DOI] [PubMed] [Google Scholar]
- [90].Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–78. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- [91].Clark R, Griffiths GM. Lytic granules, secretory lysosomes and disease. Curr Opin Immunol. 2003;15:516–21. doi: 10.1016/s0952-7915(03)00113-4. [DOI] [PubMed] [Google Scholar]
- [92].Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–69. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- [93].Wightman PD, Dahlgren ME, Davies P, Bonney RJ. The selective release of phospholipase A2 by resident mouse peritoneal macrophages. Biochem J. 1981;200:441–4. doi: 10.1042/bj2000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Abe A, Kelly R, Kollmeyer J, Hiraoka M, Lu Y, Shayman JA. The secretion and uptake of lysosomal phospholipase A2 by alveolar macrophages. J Immunol. 2008;181:7873–81. doi: 10.4049/jimmunol.181.11.7873. [DOI] [PubMed] [Google Scholar]
- [95].Kodavanti UP, Mehendale HM. Cationic amphiphilic drugs and phospholipid storage disorder. Pharmacol Rev. 1990;42:327–54. [PubMed] [Google Scholar]
- [96].Reasor MJ, Kacew S. Drug-induced phospholipidosis: are there functional consequences? Exp Biol Med (Maywood) 2001;226:825–30. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- [97].Costa-Jussa FR, Corrin B, Jacobs JM. Amiodarone lung toxicity: a human and experimental study. J Pathol. 1984;144:73–9. doi: 10.1002/path.1711440202. [DOI] [PubMed] [Google Scholar]
- [98].Mason JW. Amiodarone. N Engl J Med. 1987;316:455–66. doi: 10.1056/NEJM198702193160807. [DOI] [PubMed] [Google Scholar]
- [99].Halliwell WH. Cationic amphiphilic drug-induced phospholipidosis. Toxicol Pathol. 1997;25:53–60. doi: 10.1177/019262339702500111. [DOI] [PubMed] [Google Scholar]
- [100].Kolzer M, Werth N, Sandhoff K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004;559:96–8. doi: 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- [101].Abe A, Hiraoka M, Shayman JA. A role for lysosomal phospholipase A2 in drug induced phospholipidosis. Drug Metab Lett. 2007;1:49–53. doi: 10.2174/187231207779814292. [DOI] [PubMed] [Google Scholar]
- [102].Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–7. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- [103].Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- [104].Schulze H, Kolter T, Sandhoff K. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta. 2009;1793:674–83. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- [105].Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- [106].Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- [107].Nei M, Roychoudhury AK. Evolutionary relationships of human populations on a global scale. Mol Biol Evol. 1993;10:927–43. doi: 10.1093/oxfordjournals.molbev.a040059. [DOI] [PubMed] [Google Scholar]
- [108].Efron B, Halloran E, Holmes S. Bootstrap confidence levels for phylogenetic trees. Proc Natl Acad Sci U S A. 1996;93:13429–34. doi: 10.1073/pnas.93.23.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]