Abstract
Focal adhesion kinase (FAK) has been implicated in the development of cancers including those of the breast. Nevertheless, the molecular and cellular mechanisms by which FAK promotes mammary tumorigenesis in vivo are not well understood. Here, we show that targeted deletion of FAK in mouse mammary epithelium significantly suppresses mammary tumorigenesis in a well characterized breast cancer model. Ablation of FAK leads to the depletion of a subset of bipotent cells in the tumor that express both luminal marker keratin 8/18 and basal marker keratin 5. Using mammary stem/progenitor markers including aldehyde dehydrogenase, CD24, CD29 and CD61, we further revealed that ablation of FAK reduced the pool of cancer stem/progenitor cells in primary tumors of FAK targeted mice, and impaired their self-renewal and migration in vitro. Finally, through transplantation in NOD-SCID mice, we found that cancer stem/progenitor cells isolated from FAK targeted mice have compromised tumorigenicity and impaired maintenance in vivo. Together, these results demonstrate a novel function of FAK in maintaining the mammary cancer stem/progenitor cell population, and provide a novel mechanism by which FAK may promote breast cancer development and progression.
Introduction
The mammary gland is a dynamic organ undergoing significant developmental changes during puberty, pregnancy, lactation, and involution. Numerous studies have provided strong evidence for the existence of mammary stem cells (MaSCs) capable of self-renewal and differentiation into the basal and luminal lineages comprising the functional mammary epithelium (1, 2). Recently, populations enriched in MaSCs have been isolated from adult virgin mice using cell surface markers CD24 and CD29 or CD49f (3, 4). Further analysis revealed that these populations are basal epithelial cells and are negative for steroid hormone receptor ERα (5). These studies suggest that MaSCs reside in the basal compartment of the mammary epithelium, and that integrins may play essential roles in MaSCs. Indeed, a recent study has shown that deletion of β1 integrin in basal mammary epithelial cells significantly impaired the regeneration potential of MaSCs (6).
Breast cancer is a genetically heterogeneous disease that may arise from the malignant transformation of normal MaSCs and/or progenitor cells (7, 8). These stem-cell-like cancer initiating cells, or mammary cancer stem cells (MaCSCs), share several characteristics of normal MaSCs, including self-renewal and differentiation to produce the cell type heterogeneity in breast cancers. Experimental support for the cancer stem cell hypothesis was first provided by studies in human leukemia by showing that a small population of leukemic stem cells could transfer the disease to the recipient mice in transplantation (9). This concept is extended to the solid tumors by identifying a subpopulation of highly tumorigenic cells with stem cell properties from human breast cancers (10), and subsequently in other human cancers (7, 8). Given the critical role of MaCSCs in breast cancer development and progression, the characterization of key signaling proteins and pathways that regulate MaCSC self-renewal and maintenance will be crucial for understanding mammary carcinogenesis as well as the development of novel treatment strategies targeting the MaCSC pool.
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that plays a major role in mediating signal transduction by integrins as well as growth factor receptors in the regulation of cell adhesion, migration, survival, proliferation and differentiation in a variety of cells (11, 12). FAK has been implicated in the development of breast cancer and other malignancies (13, 14). In normal human breast tissue, FAK is expressed at low levels, whereas noninvasive ductal carcinoma in situ (15, 16) and invasive breast cancer (16-18) overexpress FAK. In a large population-based study of breast tumor samples, high FAK expression was shown to be associated with an aggressive phenotype exemplified by high mitotic index, estrogen and progesterone receptor negativity, and HER-2/neu overexpression (18). FAK expression is required for the early phase of lung metastasis of mammary adenocarcinoma in a rat syngeneic xenograft model (19). Furthermore, intrinsic FAK activity controls orthotopic breast carcinoma metastasis through the regulation of urokinase plasminogen activator expression (20), and promotes a MAPK-associated angiogenic switch during breast tumor progression (21). Despite the accumulating evidence in strong support of a role of FAK in breast cancer, the molecular and cellular mechanisms by which FAK promotes mammary tumorigenesis in vivo remain to be characterized.
Genetically engineered mouse (GEM) models offer powerful tools to analyze the molecular and cellular mechanisms of breast cancer induction and progression. Numerous GEM models of human breast cancer have been developed (22). Of these, the polyomavirus middle T (PyVT) transgenic model (in which PyVT oncoprotein is driven by MMTV-LTR promoter) has been well characterized (23, 24), and shown many morphological, histological and molecular biomarker similarities to those of human breast cancers that are associated with poor prognosis, including the loss of estrogen and progesterone receptors and the persistent expression of HER-2/neu and Cyclin D1 (24). Several recent studies using different GEM models of breast cancer have strongly suggested that mammary tumorigenesis may originate from the bipotent mammary stem/progenitor cells. For example, in the MMTV-PyVT induced mammary tumors, high content of stem-like cells as labeled by markers of MaSC/progenitor cells including CD24, CD29 and CD61 has been documented (25), suggesting that MMTV-driven oncogene expression may hit MaSCs and/or progenitor cells and lead to their malignant transformation. In a spontaneous mammary tumor model developed in the conditional Brac1/p53 knockout mice, expansion of a subpopulation of tumor cells expressing normal MaSC markers (CD29hiCD24med) was found to correlate with Cisplatin resistance (26). In another study, Zhang et al has shown that a subset of tumor-initiating cells, isolated from a syngeneic p53-null mouse mammary tumor model, resides in the Lin−CD29hiCD24hi population that closely resembles the population of mammary stem/progenitor cells (27).
To explore the potential role of FAK in MaCSCs, we introduced the MMTV-PyVT breast cancer model into the mice with FAK conditional knockout in the mammary epithelial cells (MaECs) that were developed previously in our lab (28). We found that ablation of FAK leads to a reduced pool of MaCSCs. Furthermore, deletion of FAK significantly affected these cells in both self-renewal and migration in vitro. Finally, through transplantation in immunodeficient NOD-SCID mice, we showed that FAK-null MaCSCs exhibited compromised tumorigenicity and impaired maintenance in vivo. These observations provide support for the cancer stem cell hypothesis and suggest that targeting critical signaling molecules, such as FAK, in the MaCSCs could potentially be an effective therapy for breast cancer.
Materials and Methods
Mice
Floxed FAK and MFCKO mice have been described previously (28, 29). MMTV-PyVT transgenic mice (23) were obtained from the Mouse Repository of MMHCC at NCI (Frederick, MD). Mice genotyping for FAK, Cre and PyVT alleles were performed as described previously (23, 29). Mice were palpated every 7 days after weaning, and the size of tumors was measured with a caliper and recorded. Mice were housed and handled according to local, state, and federal regulations and all experimental procedures were carried out according to the guidelines of IACUC at the University of Michigan.
Mammary glands whole-mounts, histology, immunohistochemistry and immunofluorescent labeling
Mammary glands were excised and whole-mounts stained with carmine alum were analyzed as described previously (28). Mammary tumors or lungs were harvested from mice and subjected to analysis by histology, immunohistochemistry or immunofluorescent labeling, as described previously (28, 30). The following antibodies were used: FAK (1:200, Santa Cruz Biotechnology, Catalog #: SC-558), PyVT (1:500, Santa Cruz Biotechnology, Catalog #: SC-53481), keratin 8/18 (1:400, American Research Products, Catalog #: 03-GP11) and keratin 5 (1:1000, Covance, Catalog #: PRB-160P). Texas-red and FITC labeled secondary antibodies (Jackson Labs) were used at a dilution of 1:250. Nuclei were counterstained with DAPI/antifade (Invitrogen).
Protein extraction, SDS-PAGE and Western blotting
Mammary glands or primary tumor samples were harvested during necropsy and snap frozen in liquid nitrogen, grinded with a mortar and pestle, and proteins were extracted using a triple detergent buffer as described previously (28). They were then subjected to SDS-PAGE and Western blotting analysis as described previously (28, 30).
Preparation of mammary tumor cells
After 4-5 weeks of tumor appearance, primary tumors or tumor transplants were removed and dissociated mechanically and enzymatically to obtain single-cell suspension. Briefly, tumor tissues were minced and dissociated in Ham’s F12/Dulbecco’s modified Eagle’s medium (F12:DMEM; 1:1; Invitrogen) supplemented with 10 mM Hepes, 2% bovine serum albumin (BSA; Fraction V; Invitrogen), 5 mg/ml insulin, 0.5 mg/ml hydrocortisone, 10 ng/ml cholera toxin, 300 U/ml collagenase and 100 U/ml hyaluronidase (all from Sigma, St Louis, MO, USA) at 37°C for 2-4h. Tumor cells were collected by centrifuging the cell suspension at 100 g for 10 min followed by one wash with F12/DMEM. The resulted tumor cell pellet was further digested for 5 min in 0.05% trypsin/0.025% EDTA (Gibco) solution to generate a single-cell suspension. An equal volume of F12/DME/H supplemented with 5% FBS was added to stop the digestion. The cell suspension was filtered twice through a 40-um nylon mesh (BioDesign Inc., New York, N.Y., USA). Following centrifugation at 100 g, the pellet was resuspended in F12/DMEM with a reduced calcium concentration (0.06 mM, StemCell technologies) supplemented with 5 U/ml dispase (Collaborative Biomedical Products,,USA). To remove red blood cells, the pellets were treated with ammonium chloride solution. Tumor cells were prepared from tumors developed in multiple mice and pooled for the following analyses.
Flow cytometry analysis
Freshly isolated tumor cells were subjected to flow cytometry analyses as described previously (25, 31, 32). Lin− cells were obtained by removing CD45-, CD31- and Ter119- positive cells using the EasySep biotin selection kit (StemCell Technologies, BC, Canada). PE-conjugated anti-mouse CD24, FITC-conjugated anti-mouse CD29, and biotin-conjugated anti-mouse CD61 were from BD Biosciences, Biolegend and eBiosciences, respectively. FACS analysis was performed using a FACStarPLUS (Becton Dickinson, Palo Alto, CA, USA) flow cytometer. ALDEFLUOR assay was performed using ALDEFLUOR kit from StemCell Technologies, Durham, NC, USA according to manufacture’s protocols.
Mammosphere culture
Mammosphere culture of sorted ALDH+ and ALDH− tumor cells was performed as previously described with minor modifications (33). Single cells were plated in 6-well ultra-low attachment plates (Corning, Acton, MA, USA) at a density of 5000 cells/ml in primary culture and 2000 cells/ml in subsequent passages. Cells were grown in a serum-free mammary epithelial basal medium (MEBM) (Cambrex, Walkerville, MD, USA) supplemented with B27 (Invitrogen), 20 ng/mL EGF (BD Biosciences), antibiotic-antimycotics (1x, Invitrogen), 20ug/ml Gentamycin, 1 ng/ml Hydrocortisone, 5 μg/ml insulin and 100 μM beta-mercaptoethanol (Invitrogen) for 7-10 days. For counting mammospheres, the content of all wells was collected, pooled, and transferred to a regular 96 well-plate (flat bottom) in 100 ul of completed MEBM. Mammospheres settled in these conditions were counted within 30 min under a microscope at low magnification.
Boyden chamber assay
Cell migration assays using modified Boyden chamber (Neuro Probe, MD) were performed as described previously (29).
Tumor cell transplantation
ALDH+ and ALDH− tumor cells were suspended in PBS with different concentrations. They were then mixed with Matrigel (BD biosciences) (1:1), and implanted into the No.4 inguinal mammary fatpads of NOD-SCID female mice (8-week-old, from Jackson Laboratory). To ensure that tumors cells are implanted, the No.4 inguinal mammary fatpad was first exposed using aseptic surgery procedure, tumor cells are then directly injected into the mammary fatpad. Mice with tumor cell injection were examined by palpation every week for two months.
Statistical analysis
Statistical significance was evaluated by paired T-test, using p < 0.05 as indicative of statistical significance. Kaplan-Meier tumor-free survival data were compared using the log-rank test.
Results
MaEC-specific deletion of FAK suppresses mammary tumor formation and progression
We recently generated MaEC-specific FAK conditional KO mice (designated as MFCKO with FAKf/f;MMTV-Cre genotype) by crossing the FAK floxed mice (designated as CNT with FAKf/f genotype) with MMTV-Cre transgenic mice (28). To test whether deletion of FAK in MaECs could affect breast cancer development in the MFCKO mice, we crossed the MFCKO and CNT mice with the MMTV-PyVT transgenic mice which is a widely used mouse model that develops metastatic breast cancer induced by the PyVT oncoprotein (23). Three cohorts of female mice with the genotypes FAKf/f;MMTV-Cre;MMTV-PyVT (designated Target mice), FAKf/f;MMTV-PyVT (designated PosCNT mice) and FAKf/+;MMTV-Cre;MMTV-PyVT (designated CreCNT mice) were established (see Supplementary Fig. S1) and mammary tumor development in these mice was monitored by physical palpation. Approximately half of PosCNT and CreCNT mice developed palpable mammary tumors by the age of 11 weeks (T1/2 = 11 weeks) and there was no statistical difference between these two groups (Fig. 1A). In contrast, Target mice showed a significantly increased tumor-free interval (T1/2= 17 weeks) compared to the PosCNT and CreCNT mice. The average number of tumors per mouse was also decreased for the Target mice compared to PosCNT mice (Fig. 1B). Consistent with these results, whole mount staining of mammary glands from Target and control mice at 5, 6 and 8 weeks of age showed significantly reduced hyperplastic nodules in the Target mice (Fig. 1C). By 8 weeks of age, approximately 50% of the epithelial surfaces were occupied by the hyperplastic nodules in the PosCNT mice, but this ratio was only about 10% for the Target mice.
Figure 1. Targeted disruption of FAK in the mammary epithelium suppresses mammary tumor formation.

(A) Kaplan-Meier analysis of mammary tumor development in the PosCNT (n=30), CreCNT (n=20) and Target (n=30) mice. Target vs PosCNT or CreCNT: P< 0.01 by the log-rank test.
(B) Average number (±SD) of palpable mammary tumors per mouse per genotype at the indicated ages. Target versus PosCNT: P< 0.05 by the two-way ANOVA.
(C) Representative mammary gland whole-mounts from 5-, 6- and 8-week-old PosCNT (a, c and e) and Target (b, d and f) mice. The arrow marks hyperplastic nodules. Scale bar= 5 mm.
(D) Immunoblotting analysis of the lysates from primary tumors of 4 different (1-4) PosCNT and Target mice as well as mammary glands from the mice using antibodies against FAK, PyVT, and vinculin.
We next analyzed FAK deletion in the tumors developed in the Target mice. Fig. 1D shows a significantly diminished expression of FAK in the tumor samples from the Target mice compared to control mice, as expected. In contrast, comparable levels of PyVT oncoprotein expression were found in the Target and control mice, suggesting that the reduced mammary tumor formation is not due to changes in PyVT expression in the Target mice. These results were further verified by immunohistochemical analysis, as FAK expression was only detected in the tumor cells of the control, but not the Target mice (Fig. 2A). As an internal control, FAK was detected in the blood vessels (arrows) of the same tumor sample of Target mice, confirming that FAK is specifically deleted in MaECs, but not endothelial cells. As expected, PyVT oncoprotein was expressed at comparable levels in both samples.
Figure 2. Analyses of protein expression, tumor growth and metastasis in Target mice.
(A) Sections from the primary tumors of the PosCNT (a, c) and Target (b, d) mice were analyzed by immunohistochemistry using antibodies against FAK (a, b) or PyVT (c, d). The arrows mark blood vessels in panel b. Scale bars= 200 μm.
(B) Lysates of 6 tumors in 3 different PosCNT (P1-P6) and Target (T1-T6) mice were prepared and analyzed by immunoblotting using antibodies against various proteins as indicated.
(C) Mean cumulative mammary tumor volume (±SD) (upper panel) and weight (±SD) (low panel) for each genotype at indicated times after primary tumor appearance were plotted and analyzed. * P<0.05 and ** P<0.01 when Target mice is compared to either PosCNT or CreCNT mice.
(D) Lung sections (4–6 sections per mouse) were prepared at 8 weeks after the primary mammary tumor onset, stained with hematoxylin and eosin (H&E) and the micrometastatic nodules were quantitated under a microscope. Upper panels are representative images with <1, 1–5, or >5 metastases per lung section, and lower panel shows percentages of mice of the indicated genotype with <1, 1–5, or >5 metastases per lung section. Scale bar = 2 mm. ** P<0.01 when Target mice is compared to either PosCNT or CreCNT mice.
The expression of FAK and several downstream signaling molecules in isolated tumor cells were further analyzed (Fig. 2B). Similar to the results in Fig.1D, FAK expression was abolished in the tumor cells from the Target mice whereas PyVT oncoprotein was expressed at comparable levels in all tumor samples. Furthermore, we did not observe increased expression or phosphorylation of the FAK related kinase Pyk2, although other reports suggested that Pyk2 may be up-regulated when FAK is inactivated under some conditions (34). Interestingly, expression of cyclin D1 was reduced in the tumor cells from the Target mice, which is consistent with the previous reports showing regulation of cyclin D1 by FAK in fibroblasts (35) and MaECs (28). Tyrosine phosphorylation of p130cas, one of the major downstream targets of FAK in the regulation of cell migration (36, 37), was reduced in the Target tumor cells. Together, these results demonstrated that FAK is efficiently deleted in the mammary cancer cells of Target mice, and this deletion significantly suppresses mammary tumor formation in MMTV-PyVT mouse model.
Given the observed correlation between FAK expression and/or activation with tumor progression and metastasis in other studies (13, 14), we examined whether deletion of FAK in the Target mice could affect tumor growth and lung metastasis. Measurements of the average size of the mammary tumors at weekly intervals for the three cohorts of the mice showed that tumors in the Target mice grew at a slower rate compared to those in the control mice, as measured by either tumor volume or weight (Fig. 2C). At 5 weeks after the initial detection of primary tumors, lung metastases were detected in about 60% of the PosCNT and CreCNT mice, but not in the Target mice, suggesting that lung metastasis was decreased in the Target mice. By 8 weeks, many Target mice also developed lung metastasis. However, quantitation of the metastatic nodules in lung sections revealed a significant reduction of the number of metastatic nodules in the Target mice compared to the controls (Fig. 2D). It should be noted, however, that the reduced tumor growth may contribute (at least in part) to the decreased number of lung colonies observed in the Target mice, and that future studies will be necessary to show a direct effect of FAK deletion on suppression of metastasis. Together, these results suggest that deletion of FAK reduces tumor growth and possibly also suppresses metastasis in vivo.
Ablation of FAK leads to the depletion of a subset of bipotent cells expressing both luminal and basal epithelial markers
One important feature of PyVT induced mammary tumors is that they have many characteristics similar to those of human breast cancer classified as poorly differentiated invasive ductal carcinoma (23, 24). To determine if ablation of FAK in mammary tumor changes its differentiation status, we stained tumors of CreCNT and Target mice using markers of MaECs including luminal marker keratin 8/18 (CK8/18) and basal epithelial marker keratin 5 (CK5). Both the CreCNT and Target tumors showed strong CK8/18 labeling for the vast majority of tumor cells, with no apparent difference between each genotype observed (Fig. 3A, upper panels). However, significant differences in CK5 labeling were found in mammary tumors of CreCNT and Target mice. In tumor nodules of early carcinoma stage (Fig. 3A, middle panels), we found that CK5 was present mainly at the basal cell lining that surrounds tumor nodules. Interestingly, clusters of CK5-positive cells, probably resulted from cellular proliferation, are frequently found at the periphery of tumor nodules of CreCNT (left panel, arrows), but not of Target mice (right panel). In some tumor samples, basal cells inside the tumor nodules are also found in CreCNT mice (left panel, arrow heads), but not Target mice (right panel). In tumor nodules of CreCNT mice at late carcinoma stage, the CK5 positive cells were widely present and comprised a significant portion of tumor whereas this subset of CK5-positive cells was relatively low in the tumors of Target mice (Fig. 3A, bottom panels). These results suggest that targeted deletion of FAK leads to the depletion of basal cells and a more differentiated pattern of luminal cells in mammary tumors. To further characterize this subset of CK5 positive cells, we performed double immunofluorescent labeling using both CK5 and CK8/18 antibodies. As shown in Fig. 5B, co-expression of both markers was detected for many of the basal cells in the tumors of CreCNT mice (about 5.05±1.00%, arrows in panel g). However, in tumors of Target mice, cells with coexpression of both markers were significantly decreased (about 0.31±0.12%, arrows in panel h), suggesting a role of FAK in maintaining the bipotent progenitor population.
Figure 3. Ablation of FAK depleted a subset of basal cells that co-express luminal and basal markers in the tumors of Target mice.
(A) Sections from the primary tumor of CreCNT (a, c, e) and Target (b, d, f) mice were analyzed by immunohistochemistry using antibodies against CK8/18 (a, b) and CK5 (c-f). The arrows in panel c mark clusters of basal cells in the periphery of tumor nodules of CreCNT mice in early carcinoma stage, and arrowheads mark basal cells present in the tumor nodules. Panels e and f are from sections of tumor of CreCNT and Target mice, respectively, in late carcinoma stage. Scale bars= 200 μm.
(B) Sections from the primary tumor of CreCNT (a,c,e,g) and Target (b,d,f,h) mice were analyzed by double labeling immunofluorescence using antibodies against CK8/18 and CK5. Arrows in panels g and h indicate bipotent cells with co-expression of both markers. Scale bars=200 μm.
Figure 5. MaCSCs isolated from the primary tumors of the Target mice have reduced capacity for self-renewal and migration in vitro.

(A) Freshly isolated tumor cells of a CreCNT mouse were sorted for ALDH+ and ALDH− cells, and analyzed for primary (P1) and secondary (P2) mammosphere formation under suspension culture conditions. Results are generated from eight separated incubations for each sample and are representative of two independent experiments. **P<0.001 in comparison to values for ALDH+ cells.
(B) ALDH+ tumor cells of CreCNT and Target mice were analyzed for primary (P1) and secondary (P2) formation of mammospheres. Results are generated from six separated incubations for each sample and are representative of two independent experiments. Data for a separate experiment as well as images of mammospheres are shown in Supplementary Fig. S3C. **P<0.001 in comparison to values for cells isolated from CreCNT mice. Bar: 200 μm.
(C) Unsorted, ALDH− and ALDH+ tumor cells of CreCNT mice were analyzed using Boyden chamber assay for their migratory capacity. Results are drawn from three independent experiments.
(D) ALDH+ tumor cells of CreCNT and Target mice were analyzed in Boyden chamber assay to compare their migratory capacity. Results are drawn from three independent experiments. **P<0.001 in comparison to values for cells isolated from CreCNT mice. Representative images of migrated cells are shown in Supplementary Fig. S4.
Ablation of FAK reduces the content of MaCSCs
The loss of a bipotent progenitor population in Target mice suggests that deletion of FAK may affect MaCSCs (6). To test this, we first examined the content of MaCSCs in tumors of CreCNT and Target mice, using identified markers of MaSC/progenitor including CD24, CD29 (3) and CD61 (5). In MMTV-PyVT mouse model, the cancer stem-like population has been recently shown as Lin−CD24+CD29+CD61+ (25). Examination of the tumor cells for these markers revealed a significant decrease of the Lin−CD24+CD29+CD61+ subpopulation (gated on viable Lin− CD24+ tumor cell population) in the Target mice (32%, Fig. 6A, right panel) compared to that from the CreCNT mice (67%, Fig. 6A, left panel). We also examined tumor cells isolated from the two types of mice for aldehyde dehydrogenase (ALDH) activity, as the ALDEFLUOR-positive (ALDH+) cell population has been shown to be enriched for MaCSCs (31). As shown in Fig. 6B, under the similar gating criteria, the ALDH+ population in freshly isolated tumor cells was significantly decreased from 15.5% in CreCNT mice to 4.6% in the Target mice. Together, these studies demonstrated that ablation of FAK in mammary cancer cells reduces the content of MaCSCs.
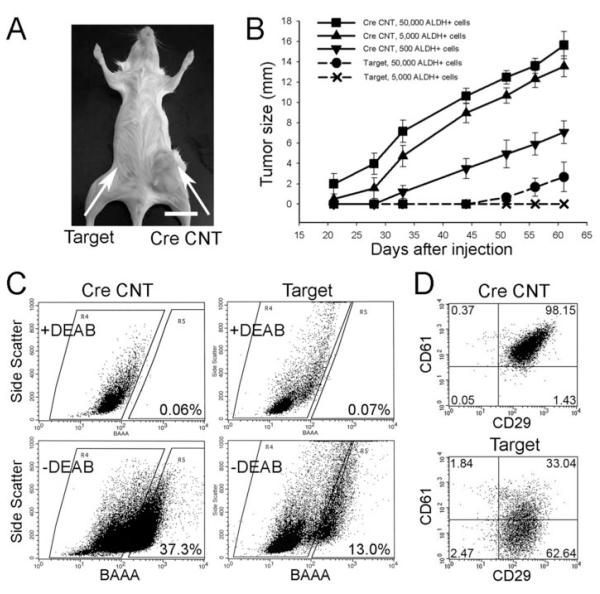
Figure 6. MaCSCs isolated from the Target mice have reduced tumorigenicity and impaired maintenance in NOD-SCID mice following transplantation.
(A) Tumor grown in a NOD-SCID mouse from 500 CreCNT ALDH+ tumor cells after 3 months of observation (arrow on the right side) whereas 500 Target ALDH+ tumor cells injected in the No.4 inguinal mammary fatpad (arrow on the left side) failed to generate tumor at the same time point. Bar=1 cm.
(B) Tumor growth curves resulted from ALDH+ tumor cells of CreCNT and Target mice at different dilutions.
(C) ALDEFLUOR assay of freshly isolated tumor cells from secondary tumors (at 1 month after their appearance) developed from 500 CreCNT (left panels) and 50,000 Target ALDH+ tumor cells (right panels). The percentage of ALDH+ cells for each tumor transplant was determined under similar gating criteria. Results are representative of two separate experiments.
(D) Tumor cells dissociated from secondary tumors derived from 500 CreCNT (upper panel) or 50,000 Target ALDH+ tumor cells (lower panel) were depleted of CD45 and CD31 positive cells and labeled with CD24, CD29 and CD61 antibodies. The MaCSC population in each tumor transplant was gated as Lin−CD24+CD29+CD61+ under the same gating criteria. Results are representative of two separate experiments.
Ablation of FAK leads to impaired self-renewal and migration of MaCSCs in vitro
To investigate the potential role of FAK in the regulation of MaCSCs, we employed mammosphere formation assay as a measure for self-renewal of MaCSCs in vitro (31, 33, 38, 39). We first isolated ALDH+ and ALDH− cells from tumors of CreCNT mice, and cultivated primary (P1) and secondary (P2) mammospheres in suspension culture. As shown in Fig.5A, the ALDH+, but not ALDH− cells, have significantly higher capacity to generate mammospheres at the first and second passages, confirming the nature of ALDH+ cells as the subset of cells possessing stem/progenitor activities. Next, we examined mammosphere formation of ALDH+ cells isolated from tumors of CreCNT and Target mice. As shown in Fig. 5B, the ALDH+ cells in Target mice have significantly lower capacity to generate mammospheres than those isolated from CreCNT mice. In addition, the size of mammospheres produced from Target cells is also smaller than that of CreCNT tumor cells (see Supplementary Figs. S3A and S3B). The reduction in both the number and size of mammospheres derived from Target mice suggests that FAK deletion results in impaired self-renewal of MaCSCs.
In MMTV-PyVT tumor model, MaCSCs isolated based on markers of CD24, CD29 and CD61 have been shown to have higher migratory activity compared to corresponding non-stem-like cells (25). To evaluate if MaCSCs isolated on ALDH activity have similar property, we sorted ALDH+ and ALDH− cell populations from freshly isolated tumor cells of CreCNT mice, and assessed their migration using a transwell migration assay. Fig. 5C shows a significantly higher migration for ALDH+ cells compared to unsorted and ALDH− cells. Such high migratory activity of ALDH+ tumor cells supports ALDH as a marker of MaCSCs in mice. In Fig. 5D, we further explored if FAK deletion affects the migration of MaCSCs. We found that the migration of Target ALDH+ cells is decreased by about 70% relative to CreCNT ALDH+ cells. These results suggest an important role of FAK in the regulation of migration of MaCSCs.
Ablation of FAK impairs tumorigenicity and maintenance of MaCSCs in vivo
To assess if deletion of FAK affects MaCSCs autonomously, we isolated ALDH+ and ALDH− tumor cells from CreCNT and Target mice and transplanted them into NOD-SCID recipient mice. We found that ALDH+ tumor cells of CreCNT displayed tumorigenicity at dilution ranging from 100 to 50,000 cells/injection (Supplementary Table 1). However, for ALDH+ cells of Target tumor sample, only 50,000 cells/injection, but not 5,000 or 500 cells/injection, generated tumors after two months of observation, suggesting that ablation of FAK in MaCSCs significantly compromised their tumorigenicity in vivo. The capacity of ALDH− tumor cells to generate tumors in NOD-SCID mice was also tested. While ALDH− tumor cells isolated from the Target mouse displayed no tumorigenicity for dilution up to 50,000 cells/injection, ALDH− tumor cells from the CreCNT mouse displayed tumorigenicity at 50,000 and 5,000, but not 1,000, cells/injection. This low tumorigenicity of CreCNT ALDH− cells may be caused by contamination of ALDH+ cells during cell sorting. This possibility is confirmed by FACS analysis of tumor cells from recipient mice with 50,000 ALDH− cells injected, as 13% of these cells were found to be ALDH+ (Supplementary Fig. S5). We then measured the growth of tumors in the recipient mice. Fig 6A shows that 500 ALDH+ tumor cells of CreCNT generated large tumors (arrow on the right), whereas 500 ALDH+ cells of Target tumor samples failed to generated palpable tumors, at 3 months after injections (arrow on the left). Fig. 8B shows that ALDH+ tumor cells from CreCNT mice at 50,000, 5,000, and 500 cells/injection all developed palpable tumors earlier than that of 50,000 ALDH+ cells from Target mice. Together, these results confirm that ablation of FAK in MaCSCs leads to increased tumor latency and decreased capacity to recapitulate tumors.
Lastly, in order to determine if deletion of FAK impairs the maintenance of MaCSCs, we isolated cells from the secondary tumors derived from 500 CreCNT and 50,000 Target ALDH+ tumor cells, respectively, and determined the corresponding MaCSC content. Fig. 6C shows that tumor developed in recipient mouse from injection of 500 CreCNT ALDH+ tumor cells contained 37.3 % ALDH+ population, while that from injection of 50,000 Target ALDH+ cells contained only 13.0 % ALDH+ cells, suggesting that deletion of FAK in mammary tumor impaired the maintenance of MaCSCs. In a complementary experiment, we also examined the tumors in the recipient mice using CD24, CD29, and CD61 as markers. Fig. 6D shows that the Lin−CD24+CD29+CD61+ subpopulation gated on viable Lin−CD24+ tumor cells in tumor derived from 50,000 Target ALDH+ cells dropped to about 33% comparing to 98% in tumor derived from 500 CreCNT ALDH+ cells. All together, these results support the idea that deletion of FAK impairs the maintenance of MaCSCs, resulting in a reduced MaCSC pool that contributes to the decreased growth and metastasis of mammary tumors in the Target mice.
Discussion
Although previous studies has implicated a role for FAK in breast cancer and other malignancies (11-14), the molecular and cellular mechanisms by which FAK stimulate mammary tumorigenesis in vivo are not well understood. Our studies presented here identify a novel function for FAK in the regulation of MaCSCs, providing significant and new insights into the mechanisms of FAK promotion of breast cancer initiation and progression. Our finding that FAK plays a role in the maintenance of MaCSCs is supported by a number of previous observations. Dontu et al. have shown that human MaSCs and progenitor cells can form mammospheres in suspension culture and be propagated using this in vitro system (33). Because the majority of primary MaECs undergoes apoptosis in suspension (a process termed anoikis), the ability of MaSCs to propagate through mammospheres suggests that they possess the ability to survive and proliferate in an anchorage-independent manner. Interestingly, expression of constitutively active FAK in MDCK cells rendered them resistance to anoikis (40), suggesting that activation of FAK in MaCSCs may be important for their self-renewal and maintenance in vitro and possibly in vivo. Our findings are also consistent with the studies that identified CD49f (α6-integrin) (4) and CD29 (β1-integrin) (3) as specific markers of MaSCs in mice, and a more recent study demonstrating that ablation of β1-integrin in the basal compartment affects MaSCs (6).
The most important prediction of cancer stem cell hypothesis is that malignant tissue stem/progenitor cells, such as MaCSCs, are the main culprit of cancers that drive tumorigenicity, recurrence and metastasis (7, 8). Our results showing the reduced content and impaired maintenance of MaCSCs in the Target mouse, which has significant suppression of mammary tumorigenesis, lend support for this hypothesis. McLean et al have shown that inactivation of FAK in the epidermis significantly suppressed both tumor formation and malignant progression in the skin (41). It would be interesting to determine whether deletion of FAK in the epidermis also affects epidermal stem cells as a mechanism of suppression of tumor formation and progression. While this possibility has not been directly tested, it is worthwhile to note that disruption of FAK in keratinocytes did not affect their survival and proliferation in vitro (41). This is in contrast to the findings from us and others that FAK deletion in MaECs significantly decreased proliferation of MaECs and mammary tumor cells both in vitro and in vivo (28, 42). Thus, it remains possible that integrin signaling through FAK may play a preferential role in MaSCs and MaCSCs in breast cancer while affecting the formation and/or progression of cancers through other mechanisms in the skin and other tissues.
Lahlou et al reported very recently that deletion of FAK in MaECs blocked breast cancer formation and progression (42). The conclusion drawn from this paper is in agreement with our studies. However, it is important to note that the conclusion was reached from different, and perhaps complementary, set of data utilizing independent mouse models. Lahlou et al observed only a slight decrease in the tumor development and no difference in the metastasis in the FAK conditional KO mice. However, they showed that all malignant primary tumors and metastatic nodules in the FAK conditional KO mice expressed FAK, which were derived from those cells in which FAK was not deleted (termed escapees) due to the estimated 65% deletion efficiency in their model. These observations led the authors to conclude that both initial formation and metastasis of breast tumor require FAK. In contrast, we obtained almost 100% deletion of FAK in both luminal and basal epithelial cells in our mouse models (Supplementary Fig. S6). These models allowed us to demonstrate more directly a role for FAK in mammary tumorigenesis in the Target mice. Furthermore, the absence of FAK in the primary tumors in the Target mice allowed us to further investigate the molecular and cellular mechanisms involved, which were not possible in the model of Lahlou et al because all tumors were derived from the “escapees” that expressed FAK (42).
Our results showing a role of FAK in maintaining MaCSCs raise the possibility that integrins or growth factor receptor tyrosine kinases, the upstream activators of FAK, may also be involved in breast cancer through their regulation of MaCSCs. Indeed, previous studies have shown that blocking antibodies to β1 integrin prevented the malignant phenotype of human breast carcinoma cells in vitro (43), and ablation of integrin β1 abolished the development and progression of breast cancer in the MMTV-PyVT mouse model (44). A recent study has revealed that ablation of β1 integrin in the basal compartment of mammary epithelium impaired the regenerative potential of MaSCs (6), suggesting that a role of β1 integrin in breast cancer could be explained by its function in maintaining MaCSCs. In addition to β1 integrin, β4 integrin and its downstream signaling pathways have been shown to promote mammary tumorigenesis through transactivation of HER2/neu signaling (45). Interestingly, HER2 has recently been shown to regulates the MaCSC population to drive tumorigenesis and invasion (46). FAK has not been found as a major mediator of integrin β4 signaling, and additional studies will be necessary to clarify whether integrin β4 might also influence breast cancer development through its effects on MaSCs either directly or indirectly via the HER2/neu receptor tyrosine kinase. In this regard, it is interesting to note that expansion of MaSCs was observed in the MMTV-Wnt1, but not the MMTV-HER2/neu mice, in a recent report (3). While a number of markers have been identified for MaSCs and MaCSCs, it is not known whether these markers function to promote self-renewal of the stem cells (i.e. as a “functional” marker). It is possible (and even likely) that integrin β1 (or other integrins that also activate FAK) would serve as such a functional marker. The integrin-FAK signaling pathway may play an essential role in mediating regulation of MaSCs and MaCSCs by the mammary stroma and the tumor microenvironments, respectively, which may provide the niches crucial for the self renewal of the stem cells.
We noted that MaCSCs isolated from primary tumors of Target mice have severely impaired tumorigenicity in NOD-SCID mice comparing to those isolated from primary tumors of CreCNT mice. However, deletion of FAK, although substantially inhibited, did not completely eliminate tumor regeneration potential of MaCSCs. It is likely that while it is important, integrin-FAK signaling is only one of the contributing pathways in the regulation of MaSCs and MaCSCs in breast cancer. Several developmental signaling pathways including Notch, Wnt and hedgehog signaling have been demonstrated to play critical roles in the regulation of various stem cells (47-50). Abnormal functions and regulations of components of these signaling pathways are often associated with different cancers, implicating potential roles of these signaling pathways in the cancer stem cells derived from different tissue origin (7, 8). It would be interesting to determine the relative contributions and potential cross-talks of integrin-FAK signaling with other signaling pathways. These studies also suggest that the use of a combination of inhibitors for multiple signaling pathways might be more effective than blockade of single pathway regulating MaCSCs.
Supplementary Material
Supplementary Table 1. Tumorigenicity of Aldefluor-positive and –negative cells from Cre CNT and Target tumors following transplantation in NOD-SCID mice
Fig. S1. Genotyping of three different mouse strains Tail DNA were prepared and analyzed by PCR using primers specific for FAK (Flox and WT alleles), Cre, and PyVT.
Fig. S2. Decreased content of MaCSCs in Target mice compared to CreCNT mice analyzed in another independent experiment Tumor cells pooled from Cre CNT and Target mice after 40 days of first tumor appearance were used to assess the MaCSC content using Aldefluor assay. The gate of ALDH+ population (p5) for each type of tumor cells was determined using DEAB control in which the ratio of p5 of gated living cells is 0.1%.
Fig. S3. Mammosphere formation of ALDH+ tumor cells of Target and Cre CNT mice (A, B) Representative images (expt shown in Fig. 5B) of mammospheres derived from ALDH+ cells of pooled tumor cells of Cre CNT (A) and Target (B) mice. (C) Results from a separate independent experiment are shown. The data are drawn from 6 independent incubations for each passage. **, P<0.001
Fig. S4. Migration of ALDH+ tumor cells isolated from Cre CNT and Target mice is severely decreased Tumor cells pooled from Cre CNT and Target mice after 30-40 days of first tumor appearance were used to enrich MaCSCs using Aldefluor assay. Freshly sorted ALDH+ cells of Cre CNT (a) and Target (b) were analyzed using Boyden chamber assay and migrated cells were counted after Giemsa staining compared to CreCNT.
Fig. S5. The tumorigenicity of ALDH1− tumor cells of Cre CNT mice may be resulted from contaminated ALDH1+ cells Secondary tumors derived from 50,000 ALDH1− tumor cells of Cre CNT contain a relatively low (around 13%) population of ALDH1+ tumor cells, which may be resulted from contaminated ALDH1+ cells during cell sorting.
Fig. S6. Examination of Cre activity in the mammary epithelium of Rosa26 mice Transgenic mice expressing MMTV-Cre were crossed with Rosa26 mice. F1 virgin female offspring (4-week-old) were euthanized. The number 4 inguinal mammary fatpads were fixed and subjected to X-gal staining. Cre activity was shown in almost 100% of mammary epithelial cells including basal myoepithelial (arrows) and luminal epithelial (arrowheads) cells.
Figure 4. Decreased MaCSC content in the primary tumors of Target mice.
(A) Freshly isolated tumor cells from CreCNT (left) and Target (right) mice were depleted of CD45 and CD31 positive cells and labeled with CD24, CD29 and CD61 antibodies. The MaCSC population in each strain of mice was gated as Lin−CD24+CD29+CD61+ (upper right quad) under the same gating criteria. Results are representative of two separate experiments. In the second experiment, 58.32% and 28.08% Lin−CD24+ cells are CD29+CD61+ for CreCNT and Target tumor cells, respectively.
(B) ALDEFLUOR assay of freshly isolated tumor cells of CreCNT (left panels) and Target (right panels) mice. The percentage of ALDH+ cells was determined under similar gating criteria (resided in gate R3). Results are representative of two separate experiments. In the second experiment, 23.37 % and 8.28 % tumor cells are ALDH+ for CreCNT and Target tumor cells, respectively (see Supplementary Fig. S2).
Acknowledgments
We are grateful to the University of Michigan Cancer Center Flow Cytometry core and C. Bian for technical assistance. We thank Drs. Steve Weiss, Yang Liu, Christophe Ginestier, and members of Guan laboratory for critical reading of the manuscript and helpful comments. This research was supported by NIH grant GM48050 and in part by a grant from the Weatherwax Foundation of the University of Michigan Comprehensive Cancer Center to J.-L. Guan.
References
- 1.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–30. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Mammary Stem Cells and Mammopoiesis. Cancer Res. 2006;66:9798–801. doi: 10.1158/0008-5472.CAN-06-2254. [DOI] [PubMed] [Google Scholar]
- 3.Shackleton M, Vaillant Fo, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 4.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 5.Asselin-Labat ML, Shackleton M, Stingl J, et al. Steroid hormone receptor status of mouse mammary stem cells. Journal of the National Cancer Institute. 2006;98:1011–4. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 6.Taddei I, Deugnier MA, Faraldo MM, et al. beta1 Integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–22. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–90. doi: 10.1158/0008-5472.CAN-05-3153. discussion 95-6. [DOI] [PubMed] [Google Scholar]
- 8.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annual Review of Medicine. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 12.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 13.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–53. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 14.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 15.Lightfoot HM, Jr., Lark A, Livasy CA, et al. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88:109–16. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 16.Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–23. [PubMed] [Google Scholar]
- 17.Owens LV, Xu L, Craven RJ, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–5. [PubMed] [Google Scholar]
- 18.Lark AL, Livasy CA, Dressler L, et al. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18:1289–94. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 19.van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for Focal Adhesion Kinase in the Early Phase of Mammary Adenocarcinoma Lung Metastasis Formation. Cancer Res. 2005;65:4698–706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- 20.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–40. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 21.Mitra SK, Mikolon D, Molina JE, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–84. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 22.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–72. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 23.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouros-Mehr H, Bechis SK, Slorach EM, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer cell. 2008;13:141–52. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Behbod F, Atkinson RL, et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68:4674–82. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy T, Wei H, Shen TL, et al. Mammary Epithelial-specific Deletion of the Focal Adhesion Kinase Gene Leads to Severe Lobulo-Alveolar Hypoplasia and Secretory Immaturity of the Murine Mammary Gland. J Biol Chem. 2007;282:31766–76. doi: 10.1074/jbc.M705403200. [DOI] [PubMed] [Google Scholar]
- 29.Shen TL, Park AY, Alcaraz A, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng X, Wu X, Druso JE, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc Natl Acad Sci U S A. 2008;105:6638–43. doi: 10.1073/pnas.0802319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 33.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. Embo J. 1998;17:5933–47. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–77. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci U S A. 1995;92:10678–82. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase- promoted cell migration. J Cell Biol. 1998;140:211–21. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 39.Zucchi I, Sanzone S, Astigiano S, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci U S A. 2007;104:10476–81. doi: 10.1073/pnas.0703071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean GW, Komiyama NH, Serrels B, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahlou H, Sanguin-Gendreau V, Zuo D, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–7. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White DE, Kurpios NA, Zuo D, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Guo W, Pylayeva Y, Pepe A, et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 46.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008 doi: 10.1038/onc.2008.207. doi:10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166–75. doi: 10.1016/j.ceb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 49.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–9. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 50.Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–7. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Tumorigenicity of Aldefluor-positive and –negative cells from Cre CNT and Target tumors following transplantation in NOD-SCID mice
Fig. S1. Genotyping of three different mouse strains Tail DNA were prepared and analyzed by PCR using primers specific for FAK (Flox and WT alleles), Cre, and PyVT.
Fig. S2. Decreased content of MaCSCs in Target mice compared to CreCNT mice analyzed in another independent experiment Tumor cells pooled from Cre CNT and Target mice after 40 days of first tumor appearance were used to assess the MaCSC content using Aldefluor assay. The gate of ALDH+ population (p5) for each type of tumor cells was determined using DEAB control in which the ratio of p5 of gated living cells is 0.1%.
Fig. S3. Mammosphere formation of ALDH+ tumor cells of Target and Cre CNT mice (A, B) Representative images (expt shown in Fig. 5B) of mammospheres derived from ALDH+ cells of pooled tumor cells of Cre CNT (A) and Target (B) mice. (C) Results from a separate independent experiment are shown. The data are drawn from 6 independent incubations for each passage. **, P<0.001
Fig. S4. Migration of ALDH+ tumor cells isolated from Cre CNT and Target mice is severely decreased Tumor cells pooled from Cre CNT and Target mice after 30-40 days of first tumor appearance were used to enrich MaCSCs using Aldefluor assay. Freshly sorted ALDH+ cells of Cre CNT (a) and Target (b) were analyzed using Boyden chamber assay and migrated cells were counted after Giemsa staining compared to CreCNT.
Fig. S5. The tumorigenicity of ALDH1− tumor cells of Cre CNT mice may be resulted from contaminated ALDH1+ cells Secondary tumors derived from 50,000 ALDH1− tumor cells of Cre CNT contain a relatively low (around 13%) population of ALDH1+ tumor cells, which may be resulted from contaminated ALDH1+ cells during cell sorting.
Fig. S6. Examination of Cre activity in the mammary epithelium of Rosa26 mice Transgenic mice expressing MMTV-Cre were crossed with Rosa26 mice. F1 virgin female offspring (4-week-old) were euthanized. The number 4 inguinal mammary fatpads were fixed and subjected to X-gal staining. Cre activity was shown in almost 100% of mammary epithelial cells including basal myoepithelial (arrows) and luminal epithelial (arrowheads) cells.