Abstract
Background
Recent studies have examined the associations between perfluorooctanoic acid (PFOA) levels in cord blood and maternal plasma with lowered birth weight and gestational age in humans; however, no study has examined these effects in a population of known high PFOA exposure. Residents drinking PFOA-contaminated water from the Little Hocking Water Association (LHWA) in Washington County, Ohio have serum PFOA levels approximately 80 times those in the general U.S. population.
Objectives
To compare birth weights and gestational ages of neonates born to mothers residing in zip codes with water service provided completely, partially or not at all by the LHWA.
Methods
Multiple logistic and linear regression analyses were performed on singleton neonatal birth weight data supplied by the Ohio Department of Health to examine the associations between LHWA water service category (used as a surrogate for PFOA exposure) with mean birth weight, mean gestational age, the likelihood of low birth weight (<2500 grams), and the likelihood of preterm birth (<37 completed weeks of gestation). All models were adjusted for maternal age, gestational age, sex, race and population-level socioeconomic status.
Results
The incidence of low birth weight, preterm birth, mean birth weight and mean gestational age of neonates did not significantly differ among water service categories.
Conclusion
Markedly elevated PFOA exposure, as categorized by water service category, is not associated with increased risk of lowered birth weight or gestational age. This study does not confirm earlier findings of an association between PFOA and lowered birth weight observed at normal population levels.
Keywords: Perflurooctanoic Acid, PFOA, C8, Birth Weight, Low Birth Weight, Gestational Age, Preterm Gestation, Fetal Development, Little Hocking Water Association
INTRODUCTION
Perfluorooctanoic acid (PFOA) and its salts are fully fluorinated, eight carbon (C8) organic compounds that have been utilized for decades in a variety of commercial and manufacturing processes. Ammonium perfluorooctanoate (APFO), in particular, is used in the production of fluoropolymers and fluoroelastomers such as tetrafluoroethylene and has surfactant uses as plasticizers, lubricants, wetting agents and emulsifiers [1]. PFOA is highly water soluble, not easily biodegradable and persistent in the environment [2]. Global bio-monitoring studies have revealed that PFOA is ubiquitously found in ground and surface water as well as wildlife [3, 4]. PFOA is also extremely persistent in the human body where it has a serum half-life of approximately 4 years [5, 6]. In the general U.S. population, median serum PFOA values have been measured at 4 to 5 ng/mL [7].
Rodent studies have shown that chronic PFOA exposure is associated with developmental toxicity [8–10]. Studies conducted by Butenhoff across two generations of rats revealed decreased weight gain in the offspring of dams who were orally dosed 30 mg/kg of PFOA per day during gestation [11]. A statistically significant increase in mortality was observed in both male and female pups in addition to reduced body weight after weaning and throughout the remainder of the study. Both sexes experienced delays in reaching sexual maturity. Similarly, Lau confirmed dose-dependent fetal toxicity of PFOA in mice and observed early pregnancy loss, delayed fetal growth and development, compromised postnatal survival, and sex-specific alterations in pubertal maturation [12]. Complete resorption of litters was observed in dams receiving 40 mg/kg of PFOA per day during gestation. Weight gain in dams that carried pregnancy to term was significantly lower at 20 mg/kg while post-natal survival was compromised and growth deficits were observed at dosages greater than or equal to 5 mg/kg. Fetal weight was also significantly reduced at oral dosages as low as 20 mg/kg.
PFOA is known to cross the murine placenta and is present in murine milk [13]. Despite some understanding of the physiological dynamics of PFOA, the precise mechanism by which it and other perfluorinated compounds disrupt fetal growth is largely uncertain. Some researchers have suggested mechanisms that include altered lipid metabolism, inhibited glycogen mobilization, disrupted thyroid hormone levels, diminished fetal nutrient uptake as well as direct fetal toxicity [5, 9, 12, 14–19]. White also suggested that abnormal lactational development of mice may play an additional role in early growth retardation seen in developmentally exposed offspring [20].
In humans the gestational effects of PFOA exposure have recently been considered [21–23]. Apelberg reported a cross sectional analysis of human neonates born to mothers in an urban hospital in which cord blood PFOA was found to have a negative but not statistically significant association with birth weight [24]. A significant negative association was also observed between PFOA, head circumference and ponderal index while no association was observed between PFOA and newborn length or gestational age. Similarly, Fei reported on a study of randomly selected Danish mothers who gave birth between 1996 and 2001 and found an analogous association between maternal plasma PFOA levels and reduced birth weight [22]. Despite these outcomes, both authors posited whether confounding limited their observations. Apelberg noted the potential confounding of reduced plasma volume, diet (via the usage of food products with PFOA-containing packaging), under-nutrition and a study population that was prone to more risk factors for adverse birth outcomes than the general population (e.g. asthma, substance abuse and infection). Additionally, both investigations were limited by the use of study populations with serum PFOA levels similar to those reported in the general population and neither included subjects with significantly elevated and wide-ranging PFOA concentrations.
Studies that relate biomarkers of exposure to birth metrics displaying normal biological variation (e.g. birth weight) may also be prone to confounding by maternal physiology [25]. In communities lacking distinctive sources of environmental contamination, such as those studied by Apelberg and Fei, variation in individual PFOA levels may result from differing uptake and excretion mechanisms of random background exposure. Conceivably, the same metabolic differences that account for interindividual blood levels may also produce concomitant variation in birth weight. To isolate the causal effects of PFOA, Savitz has suggested that it may be more appropriate to examine populations with significantly increased PFOA exposure (to ensure that variations in PFOA levels among individuals are not solely attributable to maternal physiology) and to focus on outcomes such as low birth weight that fall outside the range of normal variation and have direct consequences on neonatal morbidity and mortality [25].
We have previously demonstrated that residents serviced by the Little Hocking Water Association (LHWA) in Washington County, Ohio are exposed to significant amounts of PFOA through residential drinking water contaminated by local industry. Mean serum PFOA levels in these residents are approximately 80 times higher than the general population [26, 27]. LHWA residents were found to have the highest recorded serum levels of PFOA in a general population with levels approaching or exceeding those of workers engaged in the fluoropolymer production process. Due to their exposure, this population presents a unique opportunity for continued research regarding the associations between PFOA exposure and developmental characteristics such as birth weight and gestational age. A thorough understanding of these associations is of particular importance given that both low birth weight and premature birth are risk factors for the development of chronic diseases later in life including coronary heart disease, hypertension, type-2 diabetes and epilepsy [28–31].
In this study we examined whether mean birth weights, incidence of low birth weight (<2500 grams), incidence of preterm birth (<37 completed weeks of gestation) and mean gestational ages of singleton neonates born to mothers residing within zip codes fully or partially serviced by the LHWA differed significantly from singleton neonates born to mothers residing in zip codes within Washington County not serviced by the LHWA.
METHODS
Study Design
This cross-sectional study received approval from the University of Pennsylvania Institutional Review Board as well as the Institutional Review Board of the Ohio Department of Health. This study was determined to be exempt from the Health Insurance Portability and Accountability Act (HIPAA). There was no requirement for informed consent due to the nature of the de-identified archival data that were analyzed. This study was also endorsed by the Decatur Community Advisory Committee, a joint partnership between residents of the Little Hocking and surrounding communities, local healthcare providers, members of the Ohio Environmental Protection Agency (Ohio EPA) and the University of Pennsylvania School of Medicine. De-identified, record-level, archival data were obtained from the Ohio Department of Health (ODH) Center for Vital Health Statistics for all births occurring in Washington County, Ohio from January 1, 2003 until September 1, 2005. This period was selected as it was prior to the implementation of several interventions designed to reduce PFOA exposure in the LHWA. January 1, 2003 was chosen as the study start date since it marked both the beginning of the first full year in which PFOA levels were measured by the Ohio EPA in the LHWA and the establishment of the relationship between the University of Pennsylvania and the Little Hocking community through a grant from the Environmental Justice Program of the NIEHS. For each record, birth weight, gestational age (based on last menstrual period), plurality, neonatal sex, race, mother's age and zip code of the mother's residence were provided.
Public Water Service Categories
Residents of Washington County are serviced by several local public water facilities: the LHWA, Belpre Water, Marietta Water and Warren Water. On the basis of public water supply and PFOA sampling of water distributed by these facilities, the zip codes in Washington County were divided into three categories. The first category obtained public water service exclusively from the LHWA. This category (“LHWA Only”) comprised the zip codes of 45724, 45742 and 45784. The second category (“Partial LHWA”) included zip codes with water service in part from the LHWA, the Belpre Water System, and others. This category comprised zip codes 45712, 45713, 45714, 45729 and 45787. The third category comprised the zip codes in Washington County entirely outside the service area of the LHWA or Belpre Water System (“No LHWA”). Zip codes in this category were: 43787, 45711, 45715, 45717, 45721, 4523, 45734, 45744, 45745, 45746, 45750, 45767, 45768, 45773, 45786, 45788, and 45789. The major suppliers of public drinking water to zip codes in this category were Marietta Water and Warren Water.
Results of PFOA sampling conducted by the Ohio EPA were available for several of the public water facilities in Washington County (Table 1). Water sampling results indicated substantial PFOA contamination in the LHWA and, to a lesser extent, Belpre Water. The Ohio EPA did not sample public water systems where, in its opinion, there was no prospect of PFOA contamination. In the cases of zip codes completely and partially serviced by the LHWA, data reflect mean levels of multiple samples taken during 2002–2005. For zip codes not serviced by the LHWA in which PFOA contamination did not occur, sampling took place in 2007. A small number of residents in Washington County also use private wells for their residential water. Surveys conducted by the Ohio EPA indicate that PFOA levels in water from private wells ranged from not detectable to a mean value (±SD) of 12.4 ppb ± 6.9. Detectable levels in private wells followed the general pattern of the distribution observed in public water supplies with the highest levels of PFOA found in the zip codes comprising the Little Hocking water service area.
TABLE 1.
Mean PFOA Levels (μg/L) in Public Water Supplies for Washington County, Ohio
| Public Water Facility |
Mean† (±SD), Median (Range) PFOA (μg/L) |
Dates of Sampling |
|---|---|---|
| LHWA | 6.78 (4.2) 5.7 (1.7 – 17.1) | 2002 – 2005 |
| Belpre | 0.21 (0.027) 0.22 (0.17 – 0.24) | 2002 – 2005 |
| Marietta | 0.0065 (0.0074) 0.0049 (0 – 0.017) | 2007 |
| Warren | 0.007 (0.012) 0.0 (0.0 – 0.021) | 2007 |
This value is the average and median of PFOA levels in each of the production wells of the indicated public water facility, as measured by the Ohio State Department of Environmental Protection
Stratification by public water service was also motivated by our previous investigation of a stratfied random sample of residents from the LHWA service area. Our findings demonstrated that residential drinking water service was the major determinant of serum PFOA levels with a smaller contribution arising from the consumption of locally-grown fruits and vegetables. Air exposure played no discernable role. The median serum PFOA level within this random sample was 354 ppb. Home use of a carbon-based water filter reduced PFOA levels by about one quarter, but 70% of residents had serum levels in excess of 200 ppb. The only significant occupational contribution was from work in production areas at a fluoropolymer manufacturing facility, which employed mostly males [26].
Index of Socioeconomic Status
Since indicators of socioeconomic status (SES) were not included in the ODH database, seven commonly used population-level metrics of SES were obtained from the U.S. Census Bureau and the 2000 Decennial Census for each zip code within Washington County [32, 33]. These measures included: the percentage of persons aged 16 years and older who are in the labor force but are unemployed, the percentage of persons living below the federal poverty level, median household income, median value of owner-occupied housing units, the percentage of persons aged 25 years and older with less than a high school education, the percentage of persons aged 25 years and older who completed at least four years of college, and the percentage of households that average one or more persons per room. Based on these measures, an index of SES was calculated. Metric ranges were divided equally into five intervals and each zip code received a score of 1 to 5 based on its value within the range. For metrics with positive associations with SES (median income, median home value and percentage of persons completing college), a zip code received a score of 1 if its metric value fell within the lowest interval of the range and a 5 if its metric fell within the highest. Conversely, for metrics with negative associations with SES (percentage unemployment, percentage of persons below the federal poverty level, percentage of households with one or more occupant per room and percentage of persons without a high school education), a zip code received a score of 5 if its metric value fell within the lowest interval of the range and a 1 if its metric fell within the highest. Scores for all seven metrics were summed to create an overall index of SES for each zip code. Each unit increase in SES index represented either an average increase of $5,485 in income, a 7.2% increase in the number of individuals with a high school education, a 5.2% increase in the number of individuals with a four year college degree, a 6.5% reduction in individuals living below the federal poverty level, a $17,500 increase in home value, an 11.6% reduction in unemployment or a 5.4% reduction in crowding. Individuals residing within the same zip code were assigned the same index.
Study Population
The ODH provided a complete dataset for 1619 live born neonates born to residents of Washington County, Ohio between January 1, 2003 and September 1, 2005. Of the 1619 neonates in the dataset, 56 were not singleton births and omitted from further analysis. An additional 203 neonates were born in the county during this time interval, but were also eliminated from the analysis because they lacked complete records for birth weight, gestational age or one of the requested covariates. Of the 203 censored records, 100, 62 and 41 were removed from the “No LHWA”, “Partial LHWA” and “LHWA Only” water service categories, respectively. Among censored records for which birth weight and gestational age data were available, the mean (± SD) birth weight was 3226 grams ± 629 and the mean gestational age was 38.5 weeks ± 1.8. Neither mean was significantly different from the means calculated for neonates included in the study (p=0.35 and p=0.68, respectively). With the exception of zip code 45750, the average number of subjects included in this study from each zip code was 40.
Neonates with birth weights less than 1000 grams (N=8) and gestational ages less than 27 weeks (N=7) were evaluated for the potential of reporting error. After a review of population-based studies which characterized live born, extremely low birth weight neonates, outliers were considered valid if birth weights were plausible for corresponding gestational ages and plurality [34, 35]. No record expressed overt evidence of error and none were eliminated from subsequent analysis. Three infants born at <500 grams after 19, 20 and 21 weeks of gestation remained in the analysis despite their unlikely long term viability since the effect of PFOA on gestational age was a study endpoint. Repeat analysis with their exclusion (and the exclusion of 8 neonates with gestational ages >45 weeks) did not alter the conclusions of this study.
Statistical Analysis
Data are presented as means ± standard deviation for continuous data (e.g. birth weight, gestational age) and frequencies and percents for categorical data (e.g. water service categories). To compare birth weights and gestational ages across the exposure groups a one-way analysis of variance (ANOVA) was used. A chi-squared analysis was utilized to assess differences in neonatal sex, race, and frequency of low birth weight across water service categories while a one sample t-test for a proportion was performed to estimate whether the incidence of low birth weight and preterm birth differed significantly from the national average. A Kruskal-Wallis test assessed differences in SES index. Logistic regression was performed to examine univariate relationships between the covariates of interest and estimate the odds ratio for low birth weight (<2500 grams), preterm birth (<37 completed weeks of gestation) and 95% confidence intervals across the levels of each covariate. Multiple logistic regression models were used to compare the odds ratios of low birth weight and preterm birth between each water service category after adjusting for neonatal sex, race, gestational age, gestational age squared, gestational age cubed, maternal age and SES index. Maternal age was stratified into categories that have known associations with gestational outcome and which are commonly used in the literature [36–40]. Univariate and multiple linear regression models were constructed to examine the changes in mean birth weight, mean gestational age and 95% confidence intervals attributable to each covariate. With a sample size of 1555 and alpha set at 0.05, this investigation had an 80% power of detecting a true difference of approximately 126 grams in birth weight and 0.6 weeks in gestational age among water service categories. All statistical analyses were performed using STATA 9.1 (StataCorp., College Station, TX).
RESULTS
The 1555 singleton neonates included in this study comprised 777 (50%) males and 778 (50%) females. The demographics according to race were as follows: White, 1519 (97.7%); African-American 19 (1.2%); Other, 17 (1.1%). Mean age of mothers was 26.1 years ± 5.6 (range 14 – 44 years). Mean gestational age of neonates was 38.3 weeks ± 2.4 (range 19 – 47 weeks). Mean birth weight for the entire cohort, regardless of water service category, was 3264g ± 547 (range: 313 – 6262 grams). Seventy-six percent of newborns (N=1175) were born to mothers residing in zip codes without service from the LHWA; 13% of newborns (N=212) were born to mothers residing in zip codes partially serviced by the LHWA; and 11% of newborns (N=168) were born to mothers residing within zip codes exclusively serviced the LHWA. Descriptive data for each water service category are provided (Table 2). While no statistically significant differences were observed for maternal and neonatal characteristics across water service categories, small but statistically significant differences were noted for SES (p<0.05).
TABLE 2.
Maternal and Neonatal Demographic and Socioeconomic Characteristics by Water Service Category
| Water Service Category |
N |
% Male |
%White/%Black/%Other |
Mean ± SD Median (Range) Maternal Age (Years) |
Mean ± SD Median (Range) Gestation (Weeks) |
Mean ± SD Median (Range) Birth Weight (Grams) |
Mean ± SD Median (Range) SES Index |
|---|---|---|---|---|---|---|---|
| LHWA Only | 168 | 43 | 99.4 / 0.0 / 0.6 | 26.9 ± 4.9 27 (16 – 40) | 38.3 ± 2.0 38 (29 – 45) | 3276 ± 422 3263 (2103 – 4447) | 31.8 ± 1.6 32 (29 – 33) |
| Partial LHWA | 212 | 52 | 96.7 / 2.8 / 0.5 | 26.7 ± 6.3 25.5 (17 – 43) | 38.1 ± 2.1 38 (28 – 47) | 3284 ± 463 3289 (993 – 4528) | 29.3 ± 2.6 29 (15 - 33) |
| No LHWA | 1175 | 51 | 97.6 / 1.1 / 1.3 | 26.0 ± 5.5 25 (14 – 44) | 38.4 ± 2.6 39 (19 – 47) | 3260 ± 576 3304 (313 – 6262) | 30.2 ± 2.6 31 (16 – 33) |
Differences in SES Index across water services categories were statistically signficant (p>0.05). All SES data were extracted from the 2000 Federal Census [33].
The incidence estimates of low birth weight (<2500 grams) for zip codes comprising the LHWA Only and Partial LHWA water service categories were significantly lower than the national low birth weight incidence of 8.2% (p<0.01; Table 3) [41]. Though not shown, no zip code in Washington County had an incidence of low birth weight that was significantly greater than the national average. For Washington County as a whole, the total incidence of low birth weight was 7.0% (95% CI: 5.73% – 8.28%).
TABLE 3.
Frequency of Normal and Low (<2500g) Birth Weight and Incidence (per 100 Live Births) of Low Birth Weight by Water Service Category (January 2003 – August 2005)
| Water Service Category |
Normal Birth Weight (≥2500 g) |
Low Birth Weight (<2500 g) |
Incidence of Low Birth Weight (95% CI) |
|---|---|---|---|
| LHWA Only | 162 | 6 | 3.6 (1.3 – 7.6)* |
| Partial LHWA | 204 | 8 | 3.8 (1.6 – 7.3)* |
|
No LHWA
|
1080 |
95 |
8.1 (6.6 – 9.8) |
| TOTAL | 1446 | 109 | 7.0 (5.80 – 8.4) |
Indicates estimates that are significantly different (p<0.05) from 8.2%, the national incidence of low birth weight [41]. Although not shown, no zip code in Washington County had an incidence of low birth weight that was significantly greater than the national average.
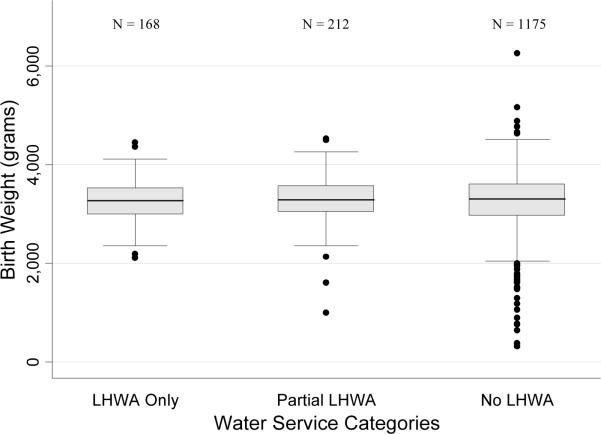
There were no statistically significant differences in unadjusted birth weights among water service categories (LHWA Only: 3276g ± 422; Partial LHWA: 3284g ± 463; No LHWA: 3260g ± 576; p=0.80) (Table 2; Figure 1). After adjustment, the odds ratio of low birth weight for neonates born to mothers in the Partial LHWA service category was decreased (0.37, p=0.021) while the odds of low birth weight for neonates in the LHWA Only service category did not significantly differ from the odds of low birth weight for neonates in the No LHWA service category (p>0.05; Table 4). The multiple logistic model did, however, predict that increasing gestational age significantly reduced the odds of low birth weight while a maternal age of 20–24 years increased the likelihood of low birth weight relative to mothers aged 25–29 years (p<0.001, p<0.05, respectively). In the multiple linear regression model, decreasing gestational age, maternal age <20 years, maternal age 20–24 years and female sex significantly reduced the expected mean birth weight (p<0.001; Table 4). LHWA Only and Partial LHWA water service categories, as well as SES index, were not statistically significant covariates in this model and did not alter the expected mean neonatal birth weight (p>0.05).
FIGURE 1. Birth Weight Distributions Among Water Service Categories (January 2003 – August 2005).
Boxplots in Figure 1 depict median birth weight and interquartile range (IQR) for each LHWA water service category. Whiskers extend to the highest data point located ≤ 1.5 IQR. Outliers greater than 1.5 times the IQR are indicated with a black dot.
TABLE 4.
Logistic and Linear Regression Coefficients Modeling the Effect of Each Covariate on the Odds Ratio of Low (<2500g) Birth Weight and Mean Birth Weight
| Logistic Regression Modeling the Odds Ratio of Low (<2500g) Birth Weight |
Linear Regression Modeling Of Mean Birth Weight (Grams) |
||||
|---|---|---|---|---|---|
| Covariate |
N |
Odds Ratio (95% CI) |
Adjusted† Odds Ratio (95% CI) |
Coefficient (95% CI) |
Adjusted† Coefficient (95% CI) |
| Maternal Age | |||||
| < 20 years | 173 | 1.40 (0.67 – 2.96) | 0.93 (0.38 – 2.24) | −115 (−210 – −20.8)* | −113 (−195 – −30.3)* |
| 20 – 24 years | 488 | 2.31 (1.37 – 3.88)** | 1.99 (1.12 – 3.54)* | −121 (−190 – −52.6)** | −101 (−160 – −42.0)** |
| 25 – 29 years | 477 | 1.0 | 1.0 | - | - |
| 30 – 34 years | 285 | 1.65 (0.89 – 3.05) | 1.32 (0.67 – 2.64) | −36.4 (−116 – 43.4) | −7.87 (−76.5 – 60.8) |
| 35 – 39 years | 101 | 0.85 (0.29 – 2.53) | 0.93 (0.29 – 3.04) | 86.3 (−30.5 – 203) | 68.4 (−32.0 – 169) |
| > 39 years | 31 | 1.43 (0.32 – 6.36) | 0.84 (0.12 – 5.69) | 0.25 (−197 – 198) | 107 (−64.2 – 277) |
| Maternal Race | |||||
| White | 1519 | 1.0 | 1.0 | - | - |
| Black | 19 | 0.74 (0.10 – 5.60) | 0.99 (0.12 – 7.93) | −32.6 (−280 – 215) | −20.0 (−232 −192) |
| Other | 17 | 1.78 (0.40 – 7.87) | 2.45 (0.53 – 11.4) | −138 (−400 – 124) | −180 (−404 – 43.2) |
| Neonate Sex | |||||
| Male | 777 | 1.0 | 1.0 | - | - |
| Female | 778 | 1.06 (0.72 – 1.56) | 1.34 (0.86 – 2.11) | −59.6 (−114 – −5.32)* | −81.5 (−128 – −34.9)** |
| Gestational Age †† | |||||
| Linear | 1555 | 0.67 (0.53 – 0.85)** | 0.67 (0.53 – 0.84)** | 68.6 (55.3 – 82.0)** | 72.3 (58.9 – 85.7)** |
| Quadratic | 1555 | 1.02 (0.99 – 1.06) | 1.03 (0.99 – 1.06) | −7.96 (−10.7 – −5.25)** | −7.34 (−10.0 – −4.64)** |
| Cubic | 1555 | 1.00 (1.00 – 1.00)* | 1.00 (1.00 – 1.00)* | −0.19 (−0.34 – −0.04)** | −0.17 (−0.32 – −0.02)* |
| Water Service Category | |||||
| LHWA Only | 168 | 0.45 (0.18 – 0.98)* | 0.43 (0.17 – 1.12) | 16.3 (−72.3 – 104) | −8.81 (−86.1 – 68.5) |
| Partial LHWA | 212 | 0.42 (0.21 – 0.93)* | 0.37 (0.16 – 0.86)* | 24.2 (−55.9 – 104) | 31.3 (−38.1 – 101) |
| No LHWA | 1175 | 1.0 | 1.0 | - | - |
| SES Index | 1555 | 0.97 (0.90 – 1.04) | 0.96 (0.88 – 1.04) | 5.41 (−5.23 – 16.04) | 6.90 (−2.48 – 16.3) |
Adjusted for all six covariates in the model.
(p<0.001)
(p<0.05). All covariates except Gestational Age and SES Index were categorical variables with levels indicated.
Gestational Age was modeled in weeks centered about 40 with linear, quadratic and cubic terms. Coefficients of the linear regression models are given in grams. Logistic regression coefficients for Gestational Age and SES represent the change in odds ratio per unit increase of the covariate.
In the analysis of gestational age, the incidence estimates of preterm birth (<37 completed weeks of gestation) for all water service categories were not statistically significantly different from the national preterm birth incidence of 12.7% (p>0.05; Table 5) [41]. Though not shown, none of the individual zip codes in Washington County had statistically significant differences (elevations) in the incidence of preterm birth as compared to the national average (p>0.05). The incidence rate of preterm birth in Washington County as a whole was 12.9% (95% CI: 11.2% – 14.5%).
TABLE 5.
Frequency of Normal and Preterm (<37 Weeks) Birth and Incidence (per 100 Live Births) of Preterm Birth by Water Service Category (January 2003 – August 2005)
| Water Service Category |
Normal Term Birth (≥37Weeks) |
Preterm Birth (<37Weeks) |
Incidence of Preterm Birth (95% CI) |
|---|---|---|---|
| LHWA Only | 150 | 18 | 10.7 (6.47 – 16.4) |
| Partial LHWA | 190 | 24 | 11.3 (7.4 – 16.4) |
|
No LHWA
|
1017 |
158 |
13.4 (11.5 – 15.5) |
| TOTAL | 1355 | 200 | 12.9 (11.2 – 14.6) |
None of the incidence estimates of preterm birth for each Water Service Category are significantly different (p>0.05) from 12.7%, the national incidence of preterm birth [41]. Although not shown, no zip code in Washington County had an incidence of premature birth that was significantly greater than the national average.
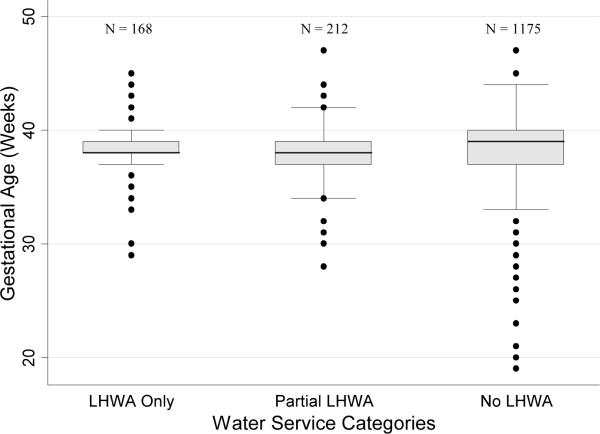
No statistically significant differences were observed among unadjusted gestational ages between water service categories (LHWA Only: 38.3 weeks ± 2.0; Partial LHWA: 38.1 weeks ± 2.1; No LHWA: 38.4 weeks ± 2.6, p = 0.47) (Figure 2; Table 2a). After adjustment, none of the covariates, including LHWA water service category, significantly decreased the likelihood of preterm birth (p>0.05; Table 6). Similarly, in the multiple linear regression model, none of the covariates significantly decreased the expected mean neonatal gestational age (p>0.05; Table 6).
FIGURE 2. Gestational Age Distributions Among Water Service Categories (January 2003 – August 2005).
Boxplots in Figure 2 depict median gestational age and interquartile range (IQR) for each LHWA water service category. Whiskers extend to the highest data point located ≤ 1.5 IQR. Outliers greater than 1.5 times the IQR are indicated with a black dot.
TABLE 6.
Multiple Logistic and Linear Regression Coefficients Modeling the Effect of Each Covariate on the Odds Ratio of Premature Gestational Age (<37 Weeks) and Mean Gestational Age
| Logistic Regression Modeling the Odds Ratio of Premature (<37 Weeks) Birth |
Linear Regression Modeling Of Mean Gestational Age (Weeks) |
||||
|---|---|---|---|---|---|
| Covariate |
N |
Odds Ratio (95% CI) |
Adjusted† Odds Ratio (95% CI) |
Coefficient (95% CI) |
Adjusted† Coefficient (95% CI) |
| Maternal Age | |||||
| < 20 years | 173 | 1.32 (0.78 – 2.21) | 1.32 (0.78 – 2.21) | 0.32 (−0.10 – 0.74) | 0.33 (−0.10 – 0.76) |
| 20 – 24 years | 488 | 1.39 (0.95 – 2.04) | 1.39 (0.95 – 2.04) | −0.06 (−0.37 – 0.25) | −0.06 (−0.37 – 0.24) |
| 25 – 29 years | 477 | 1.0 | 1.0 | - | - |
| 30 – 34 years | 285 | 1.33 (0.86 – 2.07) | 1.36 (0.87 – 2.12) | −0.27 (−0.62 – 0.09) | −0.27 (−0.62 – 0.09) |
| 35 – 39 years | 101 | 0.61 (0.27 – 1.38) | 0.62 (0.27 – 1.41) | 0.13 (−0.40 – 0.65) | 0.14 (−0.39 – 0.66) |
| > 39 years | 31 | 1.96 (0.77 – 5.00) | 2.02 (0.79 – 5.22) | −0.67 (−1.56 – 0.22) | −0.62 (−1.51 – 0.27) |
| Maternal Race | |||||
| White | 1519 | 1.0 | 1.0 | - | - |
| Black | 19 | 1.81 (0.59 – 5.51) | 1.80 (0.58 – 5.55) | −0.13 (−1.24 – 0.98) | −0.11 (−1.22 – 1.00) |
| Other | 17 | 0.42 (0.06 – 3.22) | 0.39 (0.05 – 2.97) | 0.19 (−0.98 – 1.36) | 0.27 (−0.90 – 1.45) |
| Neonate Sex | |||||
| Male | 777 | 1.0 | 1.0 | - | - |
| Female | 778 | 0.87 (0.65 – 1.17) | 0.87 (0.65 – 1.18) | 0.17 (−0.08 – 0.41) | 0.17 (−0.07 – 0.41) |
| Water Service Category | |||||
| LHWA Only | 168 | 0.78 (0.46 – 1.30) | 0.76 (0.44 – 1.28) | −0.04 (−0.44 – 0.35) | −0.01 (−0.40 – 0.41) |
| Partial LHWA | 212 | 0.82 (0.52 – 1.30) | 0.82 (0.51 – 1.31) | −0.24 (−0.59 – 0.12) | −0.24 (−0.60 – 0.12) |
| No LHWA | 1175 | 1.0 | 1.0 | - | - |
| SES Index | 1555 | 1.02 (0.96 – 1.09) | 1.03 (0.97 – 1.10) | −0.02 (−0.06 – 0.03) | −0.02 (−0.07 – 0.03) |
Adjusted for all five covariates in the model. All covariates except SES Index were categorical variables with levels indicated. p>0.05 for all values. Coefficients of the linear regression models are given in weeks. Logistic regression coefficients for SES Index represent the change in odds ratio per unit increase of the covariate.
DISCUSSION
The results of our analysis indicate that zip codes comprising the Partial LHWA and LHWA Only water service categories were not associated with low birth weight, lowered mean birth weight, preterm birth or reduced mean gestational age as compared to No LHWA zip codes and national birth metrics. In contrast, we found that decreasing gestational age and maternal age 20–24 years were associated with an increased likelihood of low birth weight, while maternal age <24 years, female sex and decreasing gestational age were associated with lowered mean birth weight. These findings were expected on the basis of published reports and support the robustness of our study design to detect known associations of birth outcome [42–45]. Since residents of the LHWA Only service category are known to have high levels of PFOA exposure, this study does not appear to confirm earlier reports which suggested an inverse association between PFOA exposure and birth weight [22, 24].
Although the Partial LHWA service category was associated with decreased odds of low birth weight, we considered this finding to be either the result of chance (p=0.021) or the evidence of other factors besides PFOA exposure which were not considered in our model. The strength of the association was moderate (adjusted odds ratio 95% CI: 0.16 – 0.86), but the association itself was opposite of the suspected direction and not consistent with any expected dose response relationship among the water service categories. Residual confounding may also explain why an increased likelihood in low birth weight and lowered mean birth weight were not observed at both extremes of maternal age.
To correlate birth weight and gestational age data with PFOA, we utilized residential public water service category as a surrogate marker for maternal PFOA exposure and, indirectly, for fetal exposure since PFOA crosses the placenta [13, 46]. We have previously shown that the median serum PFOA level in a stratified random sample of residents serviced by the LHWA was 354 ppb, approximately 80 times the national median value of 4 to 5 ppb [7, 26]. Given this observation, we considered population PFOA exposure to be highest among zip codes exclusively serviced by the LHWA, intermediate in zip codes partially serviced by the LHWA and lowest in zip codes not serviced by the LHWA. This conclusion is supported by our previous assessment of PFOA exposure in Washington County as well as sampling data from the Ohio EPA which indicated that the average concentration of PFOA in the LHWA water supply was more than twenty times greater than that of the Belpre Public Water System and a hundred times greater than that of the Marietta and Warren public water facilities.
Based on this gradient of exposure, we conclude that our results are not consistent with the published reports of Apelberg and Fei regarding the association between PFOA and lowered birth weight. Apelberg reported a 104 gram decrease in birth weight for every 2.7-fold ppb increase in cord blood PFOA while Fei reported a 10.6 gram decrease in birth weight for every ppb unit increase in maternal serum PFOA concentration [22, 24]. Since the average difference in PFOA levels between the LHWA Only and No LHWA water service categories was almost two orders of magnitude greater than the levels studied by Apelberg and Fei, we expected to find an association at least as large as those previously reported. Since no association was observed, our results suggests that if any relationship between PFOA and birth weight exists, the magnitude of the association is likely less than previously estimated or greater at lower levels of PFOA. In contrast, our findings agreed with Apelberg and Fei in suggesting that no significant association exists between PFOA exposure and reduced gestational age.
The negative association between PFOA and birth weight found by Apelberg and Fei at low, general population exposure levels may reflect the influence of confounding. Apelberg noted the potential for confounding by factors such as reduced maternal plasma volume expansion, fast-food diet and the potentially higher incidence of substance abuse and infectious disease in an urban cohort. Unlike the mostly suburban residents studied in this analysis, urban residents may also be at risk for other chemical exposures that negatively impact birth weight and remain unstudied. Studies of low-level exposure may also be confounded by maternal physiology [25].
Our failure to detect an association between PFOA and reduced human birth weight is consistent with experimental animal studies. Growth and developmental delays observed in mice began to occur at serum PFOA levels (10 ppm) substantially greater than the levels observed in LHWA residents without occupational exposure [11, 12]. Though highly exposed in comparison to the general U.S population, the levels of serum PFOA among residents serviced by the LHWA may not be sufficiently high to affect birth outcomes. Nonetheless, caution is warranted when extrapolating the results of murine toxicological studies to human populations particularly since the serum half-life of PFOA differs dramatically between species.
This study has potential limitations. In particular, the lack of individual exposure levels may introduce exposure misclassification (resulting from the consumption of drinking water outside LHWA supply areas, the use of bottled water or a home filter and the mobility of pregnant women) into our study. Mothers residing outside the LHWA service area may have consumed contaminated water at work or during critical windows of fetal development when the impact of that exposure would be most significant [47, 48]. Misclassification of these mothers into the No LHWA water service category would potentially bias our associations towards the null. Although mobility data are not available, the results of our prior investigations regarding PFOA exposure in the LHWA service area suggest that misclassification resulting from filtered or bottled water use may be minimized since the vast majority of LHWA residents consumed some public water (82% consumed public water exclusively) and had serum PFOA levels well above background levels for the general US population [26, 27]. Our previous study also revealed that the exclusive use of bottled water was small (3%) and that individuals who utilized a carbon water filter had only mild decreases in their serum PFOA levels (25%).
SES misclassification, resulting either from a methodological fault in the SES index calculation or the application of population-level metrics to individuals, must also be considered a potential limitation. To explore the former possibility, however, we considered several alternative calculations of the SES index. The first utilized income as a marker of SES; the second used income, education and poverty level; the third utilized all seven metrics, but applied greater weight to metric scores of income, education and poverty level; and the fourth controlled for smaller differences in metrics by censoring zip codes with extreme values. None of the recalculated indexes were significant when introduced into the regression models. Although these findings do not avoid the limitation of assigning population-level characteristics to individuals, we believe that if a dramatic effect of PFOA on birth outcome were occurring, some indication of this association would have been apparent despite this shortcoming.
Residual confounding is another potential limitation of our study. Although both the linear and logistic regression models adjusted for the potential confounding effect of several covariates, the database supplied by the ODH lacked information on additional known determinants of birth weight and gestational age including: parity, smoking status of the mother, asthma, prevalence of diabetes, maternal body mass index, hypertension, infection and the mother's nutritional status prior to pregnancy [49–53]. However, we have no reason to believe that these determinants would significantly vary across water service categories in a county with a relatively homogenous population.
We also must consider the possibility that the use of a data set with live born neonates may skew our results. If exposed neonates were more likely to be stillborn, or if exposure adversely affected their viability in early gestation, their exclusion from the data set could potentially bias our associations towards the null. Nevertheless, there is no indication that stillborn rates vary within Washington County and in murine models pregnancy loss was only observed at the highest levels of exposures (40ppm) which were greater than the concentrations of PFOA observed in the LHWA population [11].
Lastly, the ODH database may be subject to reporting error; however, almost all births occurred in four local community hospitals that service residents from all zip codes in Washington County. In a recent review of its vital statistics database, the ODH observed a 90% congruence between the reporting of birth weight on birth certificates and medical records and a 60% congruence for the reporting of gestational age [54]. If reporting errors do exist in the data set, their randomness across water service categories would likely not bias the results in favor of a particular association. Moreover, the ability of the study to correctly identify decreasing gestational age, young maternal age and female sex as known predictors of reduced birth weight suggests that any such errors are minimal.
In summary, our findings suggest that previously reported negative associations between birth weight and PFOA may be non-causal. We confirm the lack of an association between PFOA exposure and gestational age. The population of the LHWA, with high levels of PFOA exposure among community residents, may be appropriate for further research regarding potentially adverse effects of PFOA on fetal and childhood development including the use of case-control studies that utilize individual serum PFOA levels.
ACKNOWLEDGEMENTS
This study was supported in part by grant ES12591 from the Environmental Justice Program of the U.S.National Institute for Environmental Health Sciences (NIEHS), National Institutes of Health, and by P30 Core Center Grant ES013508 from the NIEHS.
The authors would like to thank the following individuals whose contributions were essential to the completion of this manuscript: John Paulson, Ohio Department of Health; Tom Light, West Virginia Department for Public Health; Kathleen Meckstroth, Ohio Department of Health - Washington County; Ellen Mumma, PFOA Community Study Coordinator; Sarah Wallace, Ohio EPA - Southeastern District; Steve Williams, Ohio EPA; Robin Griffin, Little Hocking Water Association; and Sandra Hickey, Marietta City Department of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT The authors declare they have no competing financial interests.
REFERENCES
- 1.Giesy JP, Kannan K. Perfluorochemical surfactants in the environment. Environ Sci Technol. 2002;36(7):146A–152A. doi: 10.1021/es022253t. [DOI] [PubMed] [Google Scholar]
- 2.Hori H, et al. Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches. Environ Sci Technol. 2004;38(22):6118–24. doi: 10.1021/es049719n. [DOI] [PubMed] [Google Scholar]
- 3.Kannan K, et al. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35(8):1593–8. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita N, et al. Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere. 2008;70(7):1247–55. doi: 10.1016/j.chemosphere.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 5.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci. 2003;28(2):49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 6.Olsen GW, et al. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med. 2003;45(3):260–70. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 7.Calafat AM, et al. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115(11):1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29(1):160–9. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy GL, Jr., et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–84. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 10.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198(2):231–41. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Butenhoff JL, et al. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196(1–2):95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Lau C, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2):510–8. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 13.Hinderliter PM, et al. Perfluorooctanoate: Placental and lactational transport pharmacokinetics in rats. Toxicology. 2005;211(1–2):139–48. doi: 10.1016/j.tox.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Abbott BD, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci. 2007;98(2):571–81. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- 15.Chang SC, et al. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) Toxicology. 2008;243(3):330–9. doi: 10.1016/j.tox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Luebker DJ, et al. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology. 2005;215(1–2):149–69. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Martin MT, et al. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci. 2007;97(2):595–613. doi: 10.1093/toxsci/kfm065. [DOI] [PubMed] [Google Scholar]
- 18.Thibodeaux JR, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 2003;74(2):369–81. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- 19.Wolf CJ, et al. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95(2):462–73. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 20.White SS, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 21.Apelberg BJ, et al. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol. 2007;41(11):3891–7. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- 22.Fei C, et al. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–82. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei C, et al. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am J Epidemiol. 2008;168(1):66–72. doi: 10.1093/aje/kwn095. [DOI] [PubMed] [Google Scholar]
- 24.Apelberg BJ, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–6. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savitz DA. Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect. 2007;115(11):A528–9. doi: 10.1289/ehp.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emmett EA, et al. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med. 2006;48(8):759–70. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emmett EA, et al. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med. 2006;48(8):771–9. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Hofman PL, et al. Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab. 1997;82(2):402–6. doi: 10.1210/jcem.82.2.3752. [DOI] [PubMed] [Google Scholar]
- 30.Kramer MS, et al. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics. 1990;86(5):707–13. [PubMed] [Google Scholar]
- 31.Sun Y, et al. Gestational age, birth weight, intrauterine growth, and the risk of epilepsy. Am J Epidemiol. 2008;167(3):262–70. doi: 10.1093/aje/kwm316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, et al. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57(3):186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Bureau of Census [Accessed: September 2008];Census 2000: Summary File 3. Available from: http://factfinder.census.gov. [Google Scholar]
- 34.Malloy MH. Impact of cesarean section on neonatal mortality rates among very preterm infants in the United States, 2000–2003. Pediatrics. 2008;122(2):285–92. doi: 10.1542/peds.2007-2620. [DOI] [PubMed] [Google Scholar]
- 35.Morse SB, et al. Racial and gender differences in the viability of extremely low birth weight infants: a population-based study. Pediatrics. 2006;117(1):e106–12. doi: 10.1542/peds.2005-1286. [DOI] [PubMed] [Google Scholar]
- 36.Branum AM, Schoendorf KC. The influence of maternal age on very preterm birth of twins: differential effects by parity. Paediatr Perinat Epidemiol. 2005;19(5):399–404. doi: 10.1111/j.1365-3016.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 37.Cleary-Goldman J, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 Pt 1):983–90. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 38.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332(17):1113–7. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 39.MacLeod S, Kiely JL. The effects of maternal age and parity on birthweight: a population-based study in New York City. Int J Gynaecol Obstet. 1988;26(1):11–9. doi: 10.1016/0020-7292(88)90191-9. [DOI] [PubMed] [Google Scholar]
- 40.Scholl TO, et al. Young maternal age and parity. Influences on pregnancy outcome. Ann Epidemiol. 1992;2(5):565–75. doi: 10.1016/1047-2797(92)90001-7. [DOI] [PubMed] [Google Scholar]
- 41.Martin JA, et al. Births: Final Data for 2005. National Vital Statistics Reports. 2007;56(6):1–104. [PubMed] [Google Scholar]
- 42.Vital Statistics of the United States. Volume I: Natality. National Center for Health Statistics; 2003. [Google Scholar]
- 43.Alexander GR, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 44.Alexander GR, et al. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynecol. 1998;41(1):114–25. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Martin JA, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56(6):1–103. [PubMed] [Google Scholar]
- 46.Midasch O, et al. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health. 2007;80(7):643–8. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 47.Pryor JL, et al. Critical windows of exposure for children's health: the reproductive system in animals and humans. Environ Health Perspect. 2000;108(Suppl 3):491–503. doi: 10.1289/ehp.00108s3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108(Suppl 3):451–5. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blondel B, et al. The impact of the increasing number of multiple births on the rates of preterm birth and low birthweight: an international study. Am J Public Health. 2002;92(8):1323–30. doi: 10.2105/ajph.92.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Angelo D, et al. Preconception and Interconception Health Status of Women Who Recently Gave Birth to a Live-Born Infant --- Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 Reporting Areas, 2004. MMWR Surveillance Summaries. 2004;56(SS10):1–35. [PubMed] [Google Scholar]
- 51.Stevens-Simon C, McAnarney ER. Adolescent maternal weight gain and low birth weight: a multifactorial model. Am J Clin Nutr. 1988;47(6):948–53. doi: 10.1093/ajcn/47.6.948. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki K, et al. Is maternal smoking during early pregnancy a risk factor for all low birth weight infants? J Epidemiol. 2008;18(3):89–96. doi: 10.2188/jea.JE2007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan KS, Thomson NC. Asthma in pregnancy. Am J Med. 2000;109(9):727–33. doi: 10.1016/s0002-9343(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 54.Paulson J, Solano-McGuire S, Edinger E. Assessing birth certificate data quality in Ohio. American Public Health Association, 133rd Annual Meeting & Exposition; Philadelphia, PA. December 12, 2005. [Google Scholar]