Abstract
Autoantibody production is a hallmark of autoimmune diseases such as lupus and rheumatoid arthritis. Accumulating evidence suggests a role of invariant natural killer T cells (iNKT) in their pathogenesis. Mechanisms underlying iNKT role in these diseases, however, remain unclear. Here, we show that iNKTs suppress IgG anti-DNA Ab and rheumatoid factor production and reduce interleukin-10–secreting B cells in a contact-dependent manner, but increase total IgG production and enhance activation markers on B cells via soluble factors. In vivo reconstitution with iNKTs also reduces autoantibody production in iNKT-deficient mice and in SCID mice implanted with B cells. Using an anti-DNA transgenic model, we found that autoreactive B cells spontaneously produce IL-10 and are activated in vivo. In the presence of activated iNKTs, these autoreactive B cells are selectively reduced, whereas non-autoreactive B cells are markedly activated. Since iNKTs recognize CD1d, we reasoned that CD1d might play a role in the differential regulation of autoreactive vs. non-autoreactive B cells by iNKTs. Indeed, autoreactive B cells express more CD1d than non-autoreactive B cells, and CD1d-deficiency in lupus mice exacerbates autoantibody production and enhances Ab response to a self peptide but not to a foreign peptide. Importantly, iNKTs fail to inhibit autoantibody production by CD1d-deficient B cells. Thus, iNKTs inhibit autoreactive B cells in a contact- and CD1d-dependent manner, but activate non-autoreactive B cells via cytokines. Such ability of iNKTs to suppress autoantibody production, without causing global suppression of B cells, has important implications for the development of iNKT-based therapy for autoimmune diseases.
Keywords: Rodent, B cells, T cells, Autoimmunity, Systemic lupus erythematosus
Introduction
Invariant natural killer T (iNKT) cells are CD1d-restricted, lipid Ag-reactive T cells that express an invariant TCR Vα14Jα18 in mice (1–3). Upon activation by glycolipid Ag such as α-galactosylceramide (αGalCer) (4), these cells trans-activate a variety of other cells, including NK cells, T cells, B cells, and dendritic cells (5–9). Activated iNKT cells have been reported to enhance Ab responses against T-dependent and T-independent Ag and pathogens (10–12). These observations suggest a supportive role of iNKT cells on B cell functions.
B cell depletion is emerging as an effective treatment for inflammatory diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (13). Ablation of autoreactive B cells suppresses disease (14) in the New Zealand Black [NZB] x New Zealand White [NZW] F1 (BWF1) model of SLE (15). These studies as well as reports showing prevention of autoimmune disease in B cell-deficient mice (16) emphasize a pivotal role of B cells in these diseases. B cells also promote Ag presentation and produce cytokines such as IL-10, which contribute to the development of these diseases (17, 18).
We have previously reported that CD1d-deficiency worsens autoantibody production and nephritis in BWF1 (19) and pristane-injected BALB/c mice (20), suggesting a role of iNKT cells in regulating autoantibody production. Hence, we investigated the role of iNKT cells on autoantibody production and explored mechanisms underlying iNKT cell effects on autoreactive B cells in this study. Our results show that iNKT cells inhibit IgG autoantibody production in a contact- and CD1d-dependent manner, but leave normal polyclonal IgG responses intact and increase the expression of activation markers on B cells via cytokines. Thus, iNKT cells selectively suppress autoreactive B cells, without impairing normal B cell responses.
Materials and Methods
Mice
NZB, NZW and SCID BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). BWF1 mice that develop T cell-dependent autoantibody-mediated lupus nephritis (15) were bred locally. In some experiments, lupus-like autoantibodies and nephritis were induced in BALB/c mice by pristane injection (20). Vα14Tg BALB/c (21) and Jα18−/− BALB/c (22) mice were provided by Dr. A. Bendelac and Dr. M. Taniguchi, respectively. R4A-γ2bTg mice that have increased numbers of IgG2b anti-dsDNA Ab B cells (23) were provided by Dr. B. Diamond. CD1d−/− BWF1 mice were generated by introgressing CD1d-null allele onto NZB and NZW backgrounds (19). All mice were handled in accordance with the institutional guidelines.
Reagents
For detection of iNKT cells, recombinant soluble dimeric mouse CD1d:Ig was loaded with αGalCer (Kyowa Hakko Kirin Co. Ltd., Tokyo, Japan), and labeled with PE following the manufacturer’s instructions. Synthetic immunostimulatory phosphorothioate-modified oligodeoxynuleotide (CpG-ODN 1826: 5′-TCCATGACGTTCCTGACGTT-3′) was synthesized at the local core facility. LPS and anti-mouse IgM were purchased from Sigma (St. Louis, MO). Peptides A6.1 VH31-45 (p31) (GYFMNWVKQSHGKSL) and hen egg lysozyme (HEL) 106–116 (p106) (NAWVAWRNRCK) were synthesized at the Chiron Laboratories (Clayton, Australia), using F-moc chemistry. The synthetic peptides were analyzed for purity by HPLC and by mass spectrometry, as described previously (24). Peptide p31, an autoantigenic peptide derived from the VH of a pathogenic anti-dsDNA mAb, stimulates T cells that promote anti-DNA Ab production and accelerate lupus nephritis (24, 25). Both p31 and p106 bind MHC class II (I-Ed) and elicit strong immune response in BWF1 mice (25, 26). Animals were immunized with the peptide in CFA s.c. and challenged i.p. with the respective peptide in IFA.
Purification of T, B and iNKT cells
Splenic T cells and B cells were purified using anti-CD90 (Thy1.2) and anti-CD45R (B220) microbeads, respectively, on an autoMACS magnetic cell separator (Miltenyi Biotec, Auburn, CA). In some experiments, T cells were enriched using mouse T cell enrichment columns (R&D Systems, Inc. Minneapolis, MN). To purify iNKT cells, spleen cells were incubated with CD1d- αGalCer dimer on ice for 20 min. After washing, spleen cells were incubated with anti-PE microbeads (Miltenyi Biotec) and iNKT cells positively selected using autoMACS. Purity of T and B cells was >97%, and purity of iNKT cells was >92%.
Flow cytometry
Cells were incubated with anti-CD16/32 (2.4G2) to block FcRγII/III, followed by staining with conjugated mAbs, as described (19). iNKT cells were detected using CD1d-αGalCer dimer and anti-TCRβ (27). Stained cells were analyzed using FACSCalibur (BD Biosciences, San Diego, CA) and CellQuest (Becton Dickinson, San Jose, CA) or FlowJo softwares (Ashland, OR).
Reconstitution of Jα18−/− mice
Recipient Jα18−/− mice were injected i.p. with 5 μg LPS, followed by i.v. transfer of CFSE-labeled purified T cells (5×106) from donor Vα14Tg or Jα18−/− mice. Donor mice were injected i.v. with 4 μg αGalCer or vehicle 90 min prior to harvesting cells. Reconstitution was assessed by detection of CFSE+ cells in peripheral blood cells after 7d (Supplemental Fig. S1). Animals transferred with T cells from αGalCer-injected Vα14Tg mice had higher numbers (≥2-fold) of CFSE+ cells than recipients of control T cells (from αGalCer-injected Jα18−/− mice or vehicle-injected Vα14Tg mice), suggesting an expansion of activated iNKT cells. Spleen cells harvested from these mice on day 7 were cultured for 6d and supernatants tested for autoantibodies.
Reconstitution of SCID mice
BALB/c SCID mice were injected i.p. with 5 μg LPS and 6 μg αGalCer separately, and then transferred i.v. with CFSE-labeled B cells (1×107) isolated from 10-mo-old Jα18−/− mice. SCID mice were then transferred i.v. with 6×106 T (TCRβ+) or iNKT (TCRβ+CD1d-αGalCer dimer+) cells from donor 10-wk-old Vα14Tg mice. Control animals received the same numbers of B220+ cell-depleted spleen cells from Jα18−/− mice. Four days after the transfer, spleen cells harvested from these mice were analyzed for TCRβ and CD1d-αGalCer dimer positive cells to verify the reconstitution of SCID mice with iNKT cells. As expected, the recipients of Jα18−/− T cells had no iNKT cells; and recipients of purified iNKT cells had higher numbers of iNKT cells in their spleens than recipients of purified T cells from Vα14Tg mice (Supplemental Fig. S2). Spleen cells from these mice were cultured in complete medium without any stimulant or with LPS or CpG for 6d, and supernatants assayed for autoantibodies.
Detection of autoantibodies, Ig isotypes and cytokines
Spleen cells (1.5×106/ml) were incubated in complete medium with LPS (10 μg/ml), CpG-ODN (4μg/ml), anti-mouse IgM (10μg/ml) or αGalCer (50ng/ml). Supernatants were collected after 5-6d. IgG anti-DNA Abs were measured by ELISA, as described (14). RF (anti-IgG2aa) was determined by ELISA, as described previously (20). Total Ig and its isotypes were measured by a standard sandwich ELISA (14) using appropriate Ab pairs (Southern Biotechnology Associates Inc., Birmingham, AL). Cytokines were assayed in culture supernatants by ELISA using appropriate mAb pairs and standards, as described (14).
Transwell experiments
Vα14Tg BALB/c mice (2–4-mo-old, 5 mice per group) were injected i.v. with vehicle or αGalCer 90 min before harvesting their spleens. T cells were purified from these spleens using T cell enrichment columns (R&D). These purified T cells were cultured in RPMI 1640 medium (Sigma) with 10% FCS (Life Technologies) with B cells that were purified from the spleens of BALB/c mice 6–12 months after pristane injection (20) using anti-B220 microbeads and autoMACS (Miltenyi Biotec). In transwell experiments, T cells (1×106) in upper wells and the same numbers of B cells in lower wells were cultured using HTS Transwell-24 Plate (Corning, Acton, MA). For the mixed cultures, the same numbers of T cells and B cells were cultured in regular 24-well plates (Corning). B cell stimulants LPS (10μg/ml) or CpG-ODN (2μg/ml) were added to cultures, as indicated. Culture supernatants collected after 5–7d were tested for Abs. Activation marker (CD86) and IL-10 production were assessed in 18h cultures.
Cytokine secretion assay
Cellular sources of cytokines were identified using MACS cytokine secretion assay kit (Miltenyi Biotec), following the manufacturer’s protocol with some modifications (20). Briefly, spleen cells (1×106) were incubated at 37°C for 45min with the cytokine catch reagent that attaches to all leukocytes via CD45 and binds to the specific cytokine that is secreted by the cell. After washing, cells were stained with PE-conjugated cytokine detection Ab, and counterstained with anti-B220 or anti-CD19 and anti-TCRβ Abs, and analyzed by FACSCalibur. To enrich IL-10-secreting B cells, T cell-depleted spleen cells were incubated at 37°C for 45min with the IL-10 catch reagent. After washing, cells were stained with PE-conjugated anti-IL-10 Ab, followed by incubation with anti-PE microbeads. IL-10-secreting cells were then positively selected using AutoMACS (Miltenyi Biotec). More than 90% of such IL-10-enriched cells were B220 positive.
Statistical analysis
Levels of Abs and cytokines, lymphocyte % and numbers were compared using Student’s t or Mann-Whitney U test.
Results
iNKT cell ligand αGalCer suppresses autoantibody production in vitro
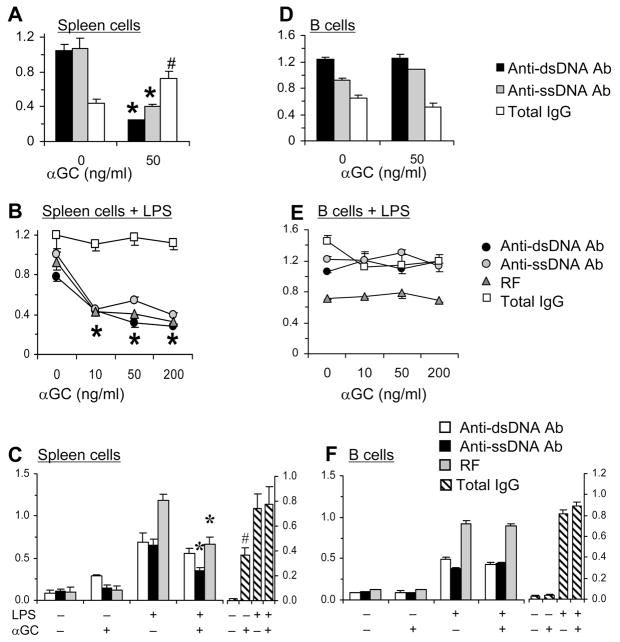
To determine the effects of iNKT cells on autoreactive B cells, we added αGalCer to spleen cell cultures from 6-mo-old BWF1 mice that spontaneously produce IgG autoantibodies (Fig. 1A). Strikingly, while total IgG levels increased, levels of IgG anti-DNA Ab were significantly reduced in the presence of αGalCer. Inhibition of autoantibody production, but not of total IgG, by αGalCer might reflect a selective suppression of in vivo activated autoreactive B cells in diseased BWF1 mice. Hence, we investigated the effect of αGalCer on activated (LPS-stimulated) BWF1 spleen cells. αGalCer still did not reduce total IgG levels, but significantly reduced IgG anti-DNA Ab production in a dose-dependent manner (Fig. 1B). Inhibition of autoantibody production by αGalCer is not limited to anti-DNA, as another autoantibody, RF, is also reduced. These data further confirm the suppressive effect of αGalCer on autoreactive B cells.
FIGURE 1. αGalCer suppresses production of IgG autoantibodies.
Whole spleen cells (A–C) or purified B220+ cells (D–F) were cultured with αGalCer (A, D), LPS plus varying concentrations of αGalCer (B, E), or LPS with or without 50 ng/ml αGalCer (C, F). Supernatants collected on day 5 were assayed for autoantibodies or total IgG, which are expressed as the mean ± SD OD or g/ml, respectively. Results from 6-mo- (A, D) or 3-mo-old μ BWF1 mice (B, E) or 4-mo-old R4A-γ2bTg NZW mice (C, F) are shown. Addition of αGalCer to whole spleen cells, but not to B cells alone, results in reduction in autoantibodies (*p <0.05–0.01, n = 5). Total IgG levels, however, are unaffected or increased in these cultures (#p <0.01 to 0.06). Results are representative of three to five experiments, each using 3–7 animals per group.
αGalCer-mediated suppression of autoantibody production is not limited to BWF1 mice, as addition of αGalCer to spleen cell cultures from IgG2b anti-dsDNA transgenic (R4A- γ2bTg) (23) NZW mice reduced autoantibody levels, but increased total IgG levels (Fig 1C). Thus, the suppressive effects of αGalCer on autoreactive B cells must be quite robust.
αGalCer does not ‘directly’ act on autoantibody producing B cells
αGalCer-induced suppression of autoantibody production in spleen cell cultures (Fig. 1A–C) could be due to αGalCer-mediated direct signaling via CD1d on B cells. However, addition of αGalCer to cultures of purified B cells without (Fig. 1D) or with LPS (Fig. 1E) from BWF1 mice or from R4A-γ2bTg mice (Fig. 1F) had no effect on autoantibody production. Thus, αGalCer confers suppression of autoantibody production through its effects on non-B cells.
αGalCer-mediated regulation of autoantibody production requires the presence of iNKT cells
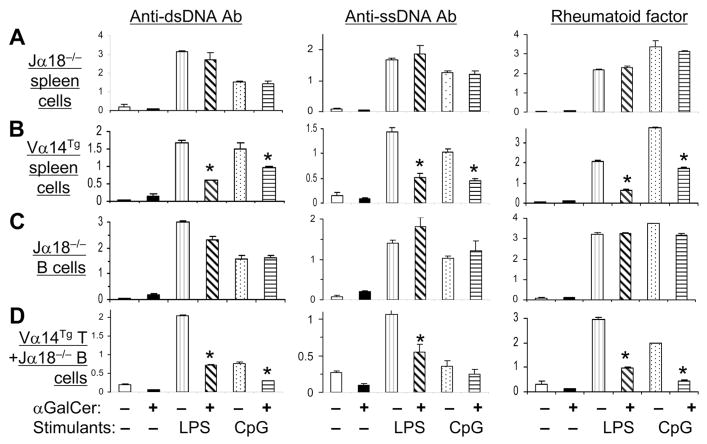
Next, we investigated the role of iNKT cells in αGalCer-mediated suppression of autoantibody production. Spleen cells from iNKT cell-deficient Jα18−/− mice (22) or iNKT cell over-expressing Vα14Tg mice (21) were cultured with B cell stimulants LPS or CpG to induce autoantibody production. Addition of αGalCer to these cultures reduced autoantibody production by spleen cells from Vα14Tg mice (Fig. 2B), but not by spleen cells from Jα18−/− (Fig. 2A) or CD1d−/− mice (data not shown). To determine if Jα18−/− B cells that have never been exposed to iNKT cells are amenable to suppression by αGalCer-activated T cells, we cultured Jα18−/− B cells with αGalCer or αGalCer plus Vα14Tg mouse T cells (Fig. 2C, D). Results show that Vα14Tg T cells efficiently suppress Jα18−/− B cells. Thus, suppression of autoantibody production by αGalCer requires the presence of iNKT cells.
FIGURE 2. iNKT cells regulate IgG autoantibody production.
A and B, Spleen cells from Jα18−/− (A) or Vα14Tg (B) mice (both 3-mo-old) were cultured without or with LPS or CpG in the presence or absence of αGalCer. C and D, Purified B cells from Jα18−/− mice were cultured alone (C) or with T cells from Vα14Tg mice (D) in the presence of LPS, CpG and/or αGalCer. Supernatants collected on day 5 were tested for autoantibodies. Results are shown as the mean ± SD of the mean triplicate OD from 3 mice per group (*p <0.05 – <0.01). Results represent four independent experiments.
In vivo regulation of autoantibody production by iNKT cells in reconstituted Jα18−/− and SCID mice
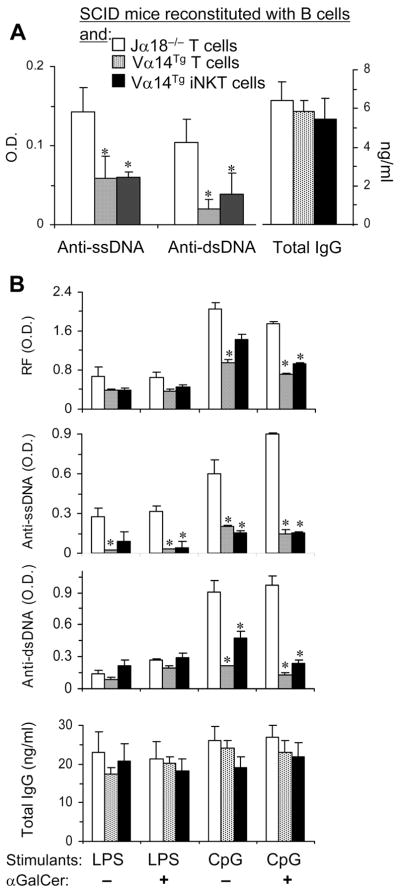
To more directly test the role of iNKT cells on autoantibody production in vivo, Jα18−/− mice were injected with LPS to stimulate B cells and then reconstituted with T cells from vehicle- or αGalCer-treated Vα14Tg mice or with T cells from control (αGalCer-treated Jα18−/−) mice (Supplemental Fig. S1). Anti-DNA Ab and RF production was reduced in Jα18−/− mice that were reconstituted with αGalCer-stimulated Vα14Tg T cells as compared to control mice (Jα18−/− mice that received T cells from αGalCer-treated Jα18−/− mice) (Fig. 3).
FIGURE 3. Reconstitution of Jα18−/− mice with activated iNKT cells suppresses autoantibody production.
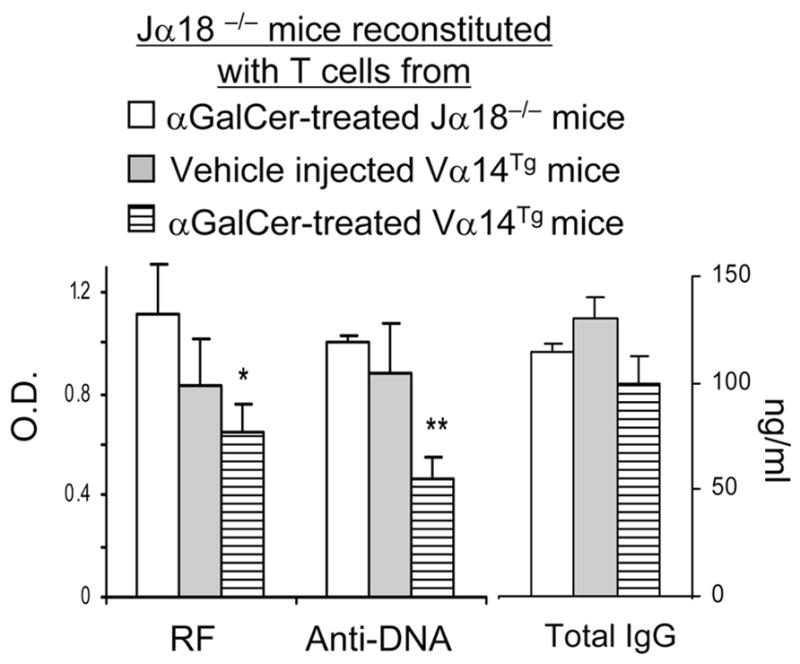
Jα18−/− mice were injected with T cells from αGalCer or vehicle treated Vα14Tg or Jα18−/− mice, as described in Methods. Spleen cells from the reconstituted mice were cultured for 6d and supernatants tested for autoantibodies and total IgG. *p <0.05, **p <0.01; mean ± SE; n = 3 per group. Results represent two similar experiments.
Activation of iNKT cells results in trans-activation of other immune cell types (5, 6, 20). To investigate whether iNKT cell itself or whether trans-activated conventional T cells suppress autoreactive B cells in vivo, we conducted an adoptive transfer experiment in SCID mice that lack B and T cells. SCID mice were injected with B cells from Jα18−/− mice and with purified T (TCRβ+) or iNKT (CD1d-αGalCer dimer+ TCRβ+) cells from Vα14Tg mice (all BALB/c background) (Supplemental Fig. S2). Spleen cells from the reconstituted SCID mice were then tested for autoantibody production. As shown in Fig. 4A, anti-DNA Ab levels are lower in SCID mice reconstituted with Vα14Tg mouse T cells or iNKT cells than in SCID mice reconstituted with Jα18−/− T cells. Spleen cells from the iNKT cell-reconstituted mice continue to produce lower levels of autoantibodies after further in vitro stimulation with LPS or CpG (Fig. 4B). These data further confirm that activated iNKT cells alone can suppress autoantibody production in the absence of conventional T cells.
FIGURE 4. iNKT cells suppress autoantibody production in reconstituted SCID mice.
BALB/c SCID mice were injected i.p. with LPS and αGalCer, and then reconstituted with B cells [Jα18−/− B cells were used to avoid any iNKT cells], and with purified T (Vα14Tg T cells) or CD1d-αGalCer dimer+ T cells (Vα14Tg iNKT cells) from Vα14Tg mice or Jα18−/− spleen cells depleted of B220+ cells (Jα18−/− T cells), as described in Methods. Spleen cells from the reconstituted animals were cultured without any stimulant (A) or with LPS or CPG in the presence or absence of αGalCer (B) for 6d and supernatants assayed for autoantibodies and total IgG. *p <0.05–<0.01; mean ± SE; n = 3–5 mice per group. Results represent two similar experiments.
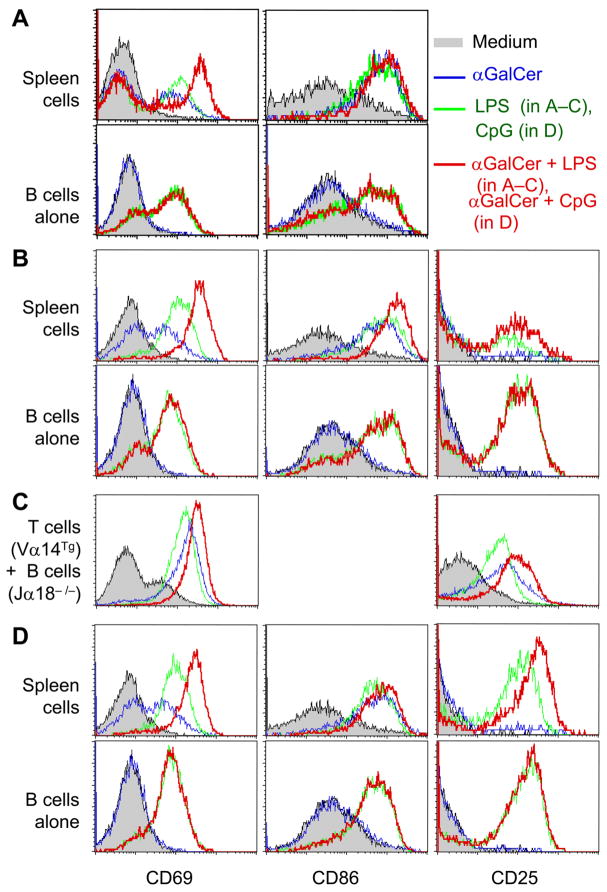
iNKT cells do not cause global suppression of B cells
In contrast to suppressive effects of αGalCer on autoantibody production, addition of αGalCer to whole spleen cell cultures increases the expression of activation markers CD69 and CD86 on B cells in BWF1 or R4A-γ2bTg mice (Fig. 5A, B). αGalCer also enhances these markers on Jα18−/− B cells when co-cultured with Vα14Tg T cells (Fig. 5C). αGalCer further upregulates the expression of these markers on spleen cells stimulated with LPS (Fig. 5A–C) or CpG (Fig. 5D). Again, αGalCer-induced upregulation of activation markers requires the presence of T cells in culture, as there is no effect on activation markers when αGalCer is added to pure B cells alone. Thus, αGalCer-activated T cells generally activate B cells, whereas at the same time they suppress autoantibody production.
FIGURE 5. Effect of activated iNKT cells on B cell activation markers in autoimmune mice.
Whole spleen cells or purified B cells were stimulated overnight with LPS (A–C) or CpG (D) in the presence or absence of αGalCer. Cells from 5-mo-old BWF1 mice (A) or from 4-mo-old R4A anti-DNA transgenic NZW mice (B, D), and mixed Vα14Tg T cells plus Jα18−/− B cells from 2–3-mo-old mice (C) were analyzed. Expression of activation markers is shown on gated B220+ cells. Results are representative of at least five similar experiments.
iNKT cell-mediated suppression of autoantibody production requires cell-cell contact, but increase in B cell activation markers occurs in a contact-independent manner
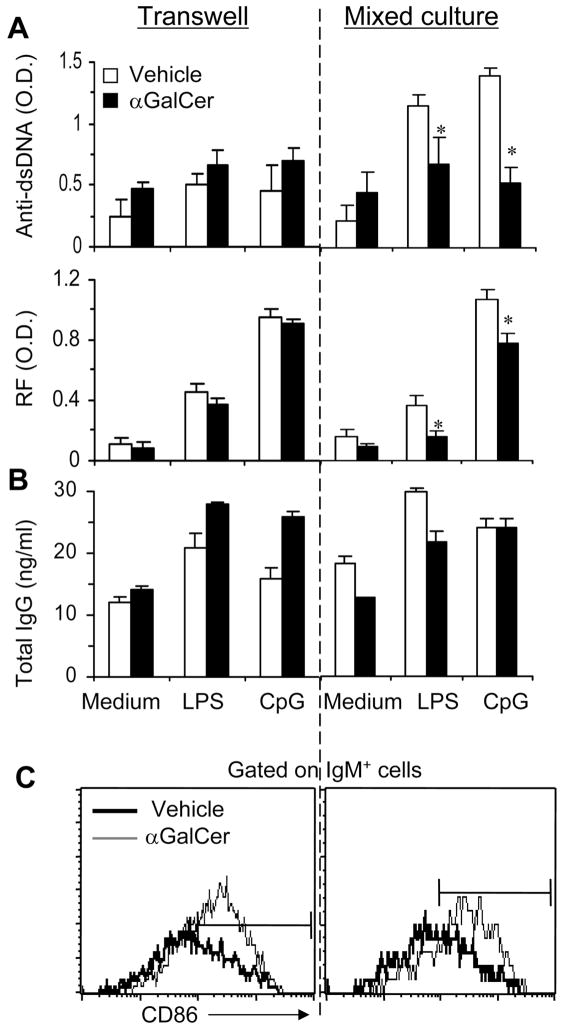
To begin to understand mechanisms underlying iNKT cell-mediated effects on B cells, we performed a transwell culture experiment where T cells from Vα14Tg mice were cultured together or separated in transwells with B cells from pristane-injected mice (Fig. 6). Results show that inhibition of autoantibody production requires direct contact between αGalCer-activated T cells and B cells, whereas increase in the expression of activation markers on B cells occurs both in transwell and mixed cultures. Thus, different mechanisms, i.e., cognate interaction versus cytokine-dependent effects, mediate diverse influences of iNKT cells on B cells.
FIGURE 6. Activated iNKT cells suppress autoantibody production in a contact-dependent manner, but upregulate activation markers on B cells in a contact-independent manner.
Purified T cells from vehicle- or αGalCer-treated Vα14Tg BALB/c mice were cultured with B cells from BALB/c mice (6-mo post-pristane injection; as a source of autoimmune B cells (20)) separately in ‘transwells’ or together in ‘mixed cultures’, as described in Methods. 5th day culture supernatants were tested for autoantibodies (shown as O.D., A) or total IgG (shown as ng/ml, B). The expression of activation marker (CD86) was assessed on B cells after 18 hrs in culture (C). *Anti-DNA and RF levels were reduced in LPS or CpG stimulated ‘mixed’ cultures (p <0.05), but not in transwell cultures. Results from one representative of three experiments are shown as the mean ± SD of mean triplicate values.
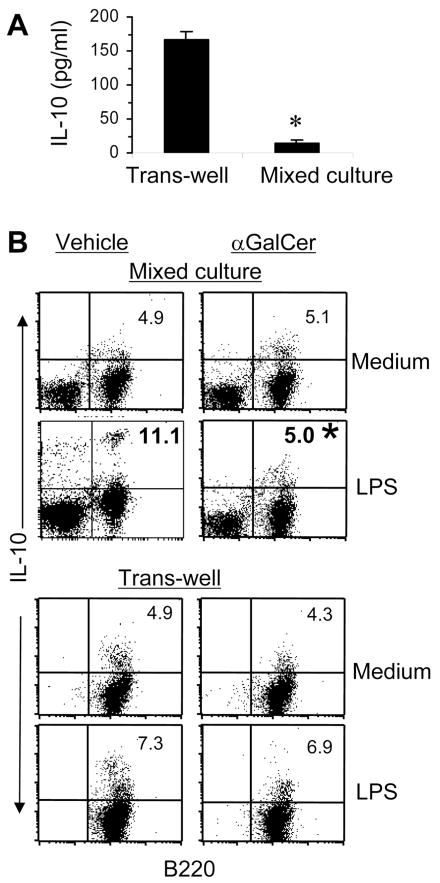
iNKT cells reduce IL-10-producing B cells from autoimmune mice
Next, we measured cytokines, IL-2, IL-4, IL-10, IL-13, TGFβ1 and IFN-γ, in supernatants of Vα14Tg T cells and B cells (from pristane-injected BALB/c mice) cultured together or separately in transwells, as described above in Fig. 6. We found that IL-10 levels were significantly reduced when Vα14Tg T cells and B cells were together in mixed cultures as compared to when the two cell types were separated in transwells (Fig. 7A). Other cytokines were increased or unchanged (data not shown). To determine if iNKT cells inhibit IL-10 production specifically by B cells, we enumerated IL-10 secreting B cells using the cytokine secretion assay (Fig. 7B). Results show that IL-10-secreting B cells are reduced in the mixed cultures, but not in transwells, when LPS-stimulated B cells from autoimmune pristane-injected mice are cultured with αGalCer-primed T cells compared to when they are cultured with T cells from control (vehicle-injected) mice. Taken together, data in Fig. 6 and 7 show that iNKT cells inhibit the production of autoantibodies and IL-10 by B cells in a contact-dependent manner.
FIGURE 7. Activated iNKT cells suppress IL-10 secreting autoimmune B cells.
A, T cells from αGalCer-treated Vα14Tg mice and B cells from pristane-injected mice were cultured separately in ‘transwells’ or together in ‘mixed cultures’. Supernatants collected at 18h were assayed by ELISA for IL-10 (mean ± SD of mean triplicate values). B, B cells from pristane-injected mice and T cells from vehicle- or αGalCer-treated Vα14Tg mice were cultured separately in ‘transwells’ or together in ‘mixed cultures’, as described in Fig. 6. B cells from ‘transwell’ cultures and all cells from ‘mixed cultures’ were collected and analyzed for IL-10 production using the cytokine secretion assay. IL-10-secreting cells are shown as the percent of B cells. Results are representative of three independent experiments, each using cells pooled from 3–4 mice per group (* p <0.05).
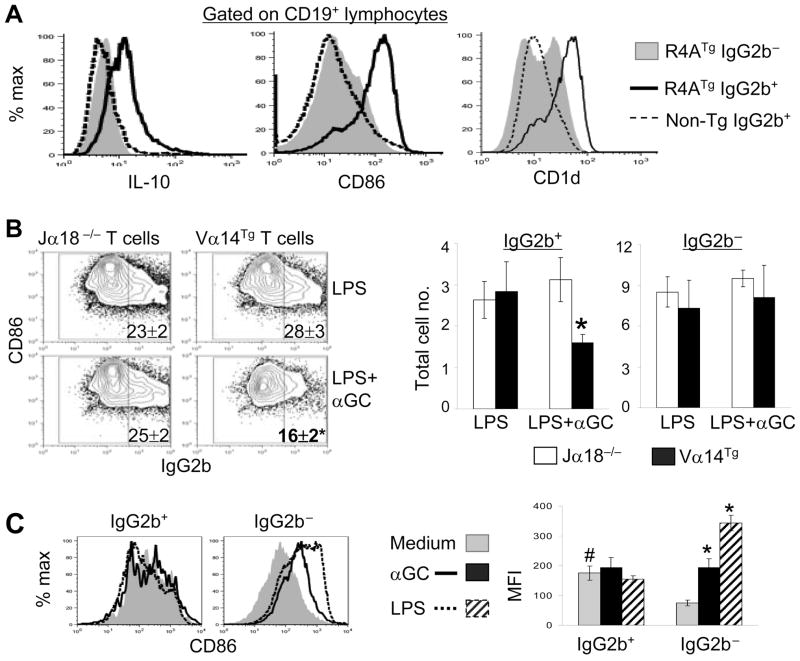
Autoreactive B cells spontaneously produce IL-10 and are selectively inhibited by iNKT cells
The above findings led us to ask whether autoreactive B cells preferentially secrete IL-10. To address this, we used anti-DNA transgenic mice, R4A γ2b, where anti-DNA B cells can be detected using flow cytometry (23, 28). Intracellular staining of freshly isolated spleen cells for IL-10 showed that anti-DNA B cells (IgG2b+ cells from R4A mice) expressed more IL-10 than non-anti-DNA B cells (IgG2b− cells from R4A mice or IgG2b+ cells from non-transgenic mice) (Fig. 8A). Additionally, co-culture of R4A spleen cells with iNKT cells along with LPS and αGalCer significantly reduced the proportion and number of anti-DNA (IgG2b+) B cells, without affecting the number of non-autoreactive B cells – R4A mouse IgG2b− cells (Fig. 8B) and IgG2b+ B cells from non-transgenic mice (not shown in figure). Thus, anti-DNA B cells that spontaneously produce IL-10 are selectively inhibited by iNKT cells, whereas non-autoreactive B cells are not reduced in presence of iNKT cells.
FIGURE 8. Differences between autoreactive and non-autoreactive B cells in cytokine production, surface markers, and response to in vitro stimuli.
A, Analysis of freshly isolated spleen cells from anti-DNATg mice. Freshly isolated spleen cells from R4A γ2bTg or WT control mice were stained for CD19, CD86 and CD1d, and intracellular IgG2b and IL-10. Histograms show IL-10, CD86 and CD1d expression on gated CD19+ IgG2b+ or CD19+ IgG2b− cells. Anti-DNA B cells (R4A IgG2b+) expressed more IL-10, CD86, and CD1d than non-autoreactive B cells (R4A IgG2b− and normal mouse IgG2b+ cells) (MFI, p < 0.05–0.001). B, Effect of iNKT cells on anti-DNA B cells. R4A spleen cells were co-cultured without or with iNKT cells (sorted T cells from Jα18−/− or Vα14Tg mice, respectively) with LPS or LPS+αGalCer for 3d. Cells were analyzed for the expression of CD86 and intracellular IgG2b on gated CD19+ B cells. Numbers on dot plots indicate the percentages of IgG2b+ cells. Total numbers of IgG2b+ and IgG2b− cells are shown in bar diagrams (*p <0.05 to 0.005 compared to various controls). C, Response of anti-DNA B cells to in vitro stimuli. R4A spleen cells were cultured with medium alone, αGalCer, or LPS for 3d. Lymphocytes were analyzed for CD86 expression on gated CD19+ IgG2b+ and CD19+ IgG2b− B cells. CD86 expression was higher on anti-DNA (IgG2b+) than on non-autoreactive (IgG2b−) B cells in unstimulated cultures (# p <0.00002). In vitro stimulation with αGalCer or LPS did not significantly increase CD86 expression on anti-DNA B cells, but significantly increased CD86 expression on non-autoreactive B cells (*p < 0.00001; mean ± SE of mean triplicates; n = 3 mice each group). Results are representative of two independent experiments. αGC, αGalCer.
Autoreactive and non-autoreactive B cells differ in terms of in vivo activation status and response to in vitro stimuli
The above data showing IL-10 production by freshly isolated anti-DNA B cells, but not by non-autoreactive B cells, suggest that autoreactive B cells might be activated in vivo. Indeed, CD86 expression was higher on R4A IgG2b+ cells than on R4A IgG2b− cells or non-transgenic IgG2b+ cells in freshly isolated spleen cells (Fig. 8A). Intriguingly, however, the addition of αGalCer or LPS to R4A spleen cells did not further increase CD86 expression on anti-DNA B cells, whereas both stimuli markedly increased CD86 expression on non-autoreactive B cells (Fig. 8C). Autoreactive B cells, however, are not completely unresponsive, as the addition of LPS+ αGalCer and purified iNKT cells to R4A mouse B cells at 1:2 ratio can increase CD86 expression on both IgG2b+ and IgG2b− cells (data not shown). Thus, iNKT cell stimulation reduces the numbers of autoreactive B cells and does not activate them further, whereas it markedly increases the numbers of activated non-autoreactive B cells.
Autoreactive B cells express more CD1d than non-autoreactive B cells
We have thus far shown that iNKT cells reduce autoantibody production in a contact-dependent manner, and they seem to differentially interact with autoreactive vs. non-autoreactive B cells. Since iNKT cells recognize CD1d (1–3), we reasoned that differences in CD1d expression between autoreactive and non-autoreactive B cells might explain their differential regulation. Indeed, CD1d expression was higher on anti-DNA B cells than on non-autoreactive B cells (Fig. 8A). Addition of αGalCer to R4A spleen cells or co-culture of R4A B cells with iNKT cells isolated from αGalCer-treated mice further increased CD1d expression on anti-DNA B cells, but had no effect on CD1d expression on non-autoreactive B cells (data not shown). LPS, however, had little effect on CD1d expression on both autoreactive and non-autoreactive B cells.
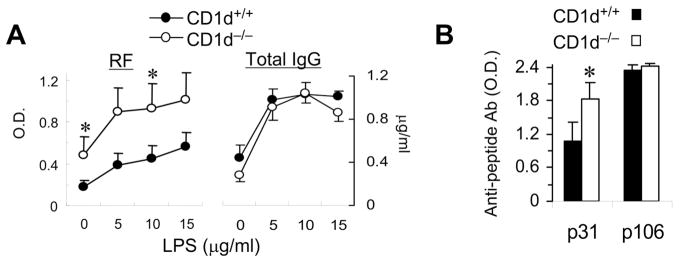
CD1d deficiency increases anti-self Ab responses, but does not affect Ab response to a foreign Ag peptide
If CD1d expression on autoreactive B cells plays a regulatory role in autoantibody production, CD1d-deficiency might increase autoantibody production. In fact, we have reported that CD1d−/− BWF1 mice have increased levels of circulating IgG anti-DNA Abs and experience more severe lupus compared to their wild-type (WT) littermates (19). Here, we show that production of RF by spleen cells was higher in CD1d−/− BWF1 mice than in their WT littermates (Fig. 9A). Total IgG levels in these cultures, however, were not different between CD1d−/− and CD1d+/+ BWF1 mice (Fig. 9A).
FIGURE 9. Effect of CD1d deficiency on self vs. non-self antibody responses in autoimmune-prone mice.
A,Spleen cells from CD1d−/− BWF1 mice or control littermates (10-mo-old) were cultured with or without LPS. Supernatants collected on day 5 were assayed for autoantibodies and total IgG. B, CD1d−/− BWF1 mice and control littermates (10-wk-old) were immunized with a self (p31) or a foreign (p106) peptide, in CFA and challenged with the peptide in IFA, as described in Methods. Sera collected on day 14 post-challenge were assayed for IgG anti-peptide Abs. Results are shown as the mean ± SD of the mean triplicate O.D. from 6 mice per group (*p <0.05). Results are from one representative of two independent experiments.
To further examine if CD1d-deficiency selectively increases autoimmune responses while leaving normal IgG responses intact, young CD1d−/− BWF1 mice and their WT littermates were immunized with self (p31) or foreign (p106) peptides that are known to induce strong immune responses in BWF1 mice (24, 26). Immunized animals were monitored for serum anti-peptide Abs (Fig. 9B). Interestingly, whereas CD1d−/− and CD1d+/+ mice mounted equivalent responses to the foreign peptide, the Ab response against the self-peptide was higher in CD1d−/− mice than in WT littermates. Thus, in vivo autoreactive B cell responses are heightened in the absence of CD1d in autoimmune-prone conditions.
iNKT cells inhibit autoantibody production in CD1d-dependent manner, but total IgG production is unaffected by CD1d expression on B cells
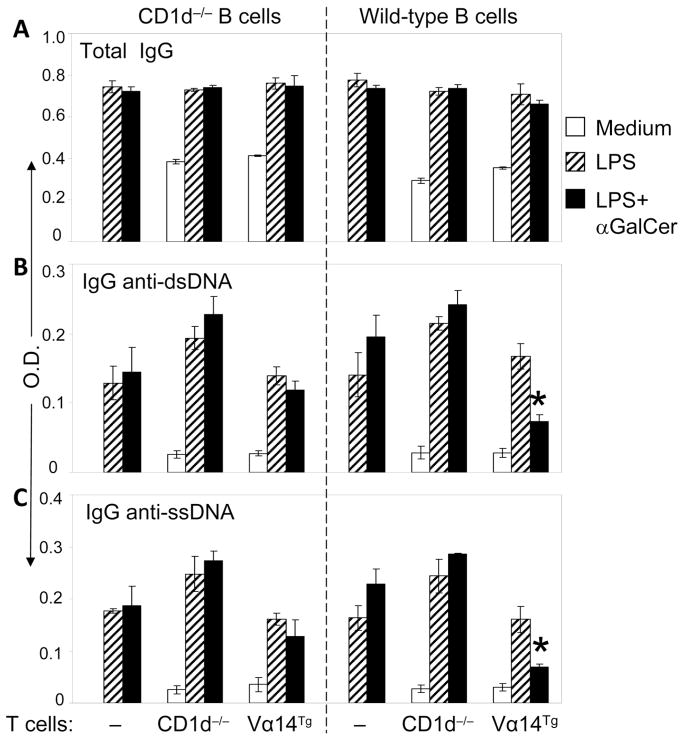
To directly test the role of CD1d on iNKT cell-mediated inhibition of autoantibody production, we co-cultured CD1d−/− or WT B cells without or with iNKT cells (T cells from CD1d−/− or Vα14Tg mice, respectively). As shown in Fig. 10, the addition of activated iNKT cells significantly reduced the production of IgG anti-DNA autoantibodies by WT B cells, but had no effect on anti-DNA Ab production by CD1d-deficient B cells. As shown above, iNKT cells did not reduce total IgG levels in co-cultures with CD1d−/− or WT B cells. Thus, inhibition of autoantibody production by iNKT cells requires CD1d expression on B cells.
FIGURE 10. iNKT cells inhibit autoantibody production in a CD1d-dependent manner.
Purified B cells from 5-mo-old CD1d−/− or WT mice were co-cultured with purified T cells from CD1d−/− or Vα14Tg mice (all BALB/c) in the presence of medium alone, LPS, or LPS+αGalCer for 6 d (B cells alone were not cultured with medium alone). Supernatants were assayed for total IgG (A), and IgG anti-dsDNA and anti-ssDNA autoantibodies (B, C). Results are expressed as the mean ± SE of mean triplicate OD (*p <0.01–0.0001, anti-dsDNA; <0.05–0.001, anti-ssDNA).
Discussion
In this article, we report that iNKT cell ligand αGalCer suppresses IgG autoantibody production in vitro and in vivo. In contrast, αGalCer exposure does not reduce or, in some instances, increases total polyclonal IgG production, and upregulates activation markers on B cells. These αGalCer-induced effects on B cells are mediated by iNKT cells, since: a) addition of αGalCer to purified B cells alone has no effect on IgG production or activation markers; b) addition of αGalCer to spleen cells from Vα14Tg mice, but not those from Jα18−/− mice, suppresses autoantibody production; c) αGalCer-activated T cells suppress autoantibody production by B cells from Jα18−/− mice in vitro as well as in vivo upon adoptive transfer; d) transfer of purified iNKT cells into SCID mice reconstituted with B cells from Jα18−/− mice suppresses autoantibody production. Using anti-DNATg mice, we show that iNKT cells reduce the numbers of autoreactive B cells, but activate non-autoreactive B cells. Consistently, CD1d-deficiency enhances IgG autoantibody and anti-self peptide responses, but leaves total IgG and anti-foreign peptide IgG responses unaffected. Finally, iNKT cells reduce autoantibody production in a contact- and CD1d-dependent manner. These data strongly indicate a regulatory role of CD1d and CD1d-reactive iNKT cells on autoantibody production.
Several studies have shown that iNKT cells can potentiate Ab responses (10–12). For example, human iNKT cells stimulate Ab production by B cells in the presence of αGalCer (11). CD1d/iNKT cell dependence is also reported in contact allergen-induced IgM production by murine B cells (29). However, others have found Ab production to be independent of iNKT cells (30). In this article, we show that whereas CD1d-deficiency does not impair the production of total polyclonal IgG in vitro or IgG Ab against a foreign peptide in vivo, αGalCer-stimulated iNKT cells enhance total IgG levels in cultures. Addition of an anti-CD3 stimulated iNKT cell line to BWF1 B cells also increases total IgG levels (31). Additionally, co-cultures of anti-CD3 stimulated iNKT cells and B cells increases the levels of total IgM and IgM anti-DNA Abs, but not IgG anti-DNA Abs (31, 32). Such increase in IgM Abs is not blocked by an anti-CD1d mAb (32), suggesting that direct CD1d-dependent interactions between iNKT cells and B cells do not mediate such iNKT cell-induced B cell stimulatory effects. Indeed, our data show that activated iNKT cells increase total IgG levels and B cell activation markers even when the two cells are separated in transwell cultures. Collectively, iNKT cells seem to increase total polyclonal IgM and IgG levels and B cell activation markers via soluble factor(s) such as IL-4 (8).
In contrast to the above B cell stimulatory effects, activated iNKT cells suppress IgG anti-DNA Ab and RF production (Fig. 1–4, 6, and 10) when the iNKT cells and B cells are cultured together (Fig. 6). Our data in this article show suppressive effects of iNKT cells on spontaneous autoantibody production in old BWF1 mice as well as on induced autoantibody responses in young BWF1, anti-DNATg NZW, pristane-injected BALB/c, Vα14Tg BALB/c and Jα18−/− BALB/c mice in vitro or in reconstituted Jα18−/− and SCID mice in vivo. In resonance with these findings, CD1d−/− BWF1 mice exhibit increased autoantibody levels (19) and mount a strong Ab response to a self peptide, p31 (Fig. 9) that has been shown to promote anti-DNA Ab production (24). Consistently, in a model where injections of syngeneic apoptotic cells transiently trigger autoantibody production (33), the absence or reduction of iNKT cells as well as absence of CD1d-expression on B cells leads to increased autoreactive B cell activation, but does not affect the activation of B cells reactive to NP-OVA (33). The regulatory effects of CD1d-reactive iNKT cells on autoreactive B cells must be important in the development of lupus, as BWF1 and BALB/c mice rendered deficient in CD1d experience more severe spontaneous or induced lupus, respectively, than their WT littermates (19, 20).
Our results show that addition of αGalCer to purified B cells alone has no direct effects on IgG production (Fig. 1D–F and F; Fig. 2C), activation markers on B cells (Fig. 5), and B cell proliferation (our unpublished data). Thus, αGalCer-mediated B cell effects must be conducted by iNKT cells themselves or by another cell type enlisted by iNKT cells. That addition of Vα14Tg T cells to purified B cells can modulate B cell functions (Fig. 2D and 5C) suggests that iNKT cells or trans-activated conventional T cells can mediate αGalCer effects on B cells and that NK or other cells are not required in this process. Furthermore, ability of iNKT cells alone to confer autoantibody suppression upon transfer into reconstituted SCID mice (Fig. 4) suggests that the presence of conventional T cells is also not required for iNKT cell-mediated suppression of autoantibody production. Thus, iNKT cells can directly suppress autoreactive B cells. These results, however, do not exclude the synergistic or additive roles of trans-activated NK, T or dendritic cells in conferring protection from autoimmune disease in vivo.
We have explored several mechanisms whereby iNKT cells might regulate autoreactive B cells.First, we found a significant reduction in IL-10-producing B cells in presence of iNKT cells (Fig. 7). This finding is important, because autoreactive B cells from patients with SLE produce increased amounts of IL-10 (18), and autoreactive B cells in anti-DNATg mice spontaneously produce more IL-10 than non-autoreactive B cells (Fig. 8A). These anti-DNA B cells are selectively reduced in the presence of iNKT cells (Fig. 8 B). In vivo neutralization of IL-10 has been shown to reduce autoantibody production in reconstituted hu-SCID mice (18) and to suppress lupus nephritis in BWF1 mice (34). A pilot trial of an anti-IL-10 mAb also showed reduced disease activity in SLE patients (35). Thus, ability of iNKT cells to inhibit IL-10-producing B cells may underlie their capacity to suppress autoantibody production.
iNKT cells recognize CD1d-bound lipid antigens (2, 4). Increased expression of CD1d on autoreactive B cells compared to non-autoreactive B cells (Fig. 8A) suggests a mechanism whereby iNKT cells might discriminate between autoreactive vs. non-autoreactive B cells. In fact, iNKT cell-mediated inhibition of autoantibody production requires CD1d expression on B cells (Fig. 10). Suppression of autoantibodies by iNKT cells also requires contact between iNKT and B cells (Fig. 6). These data suggest that iNKT cells directly interact with autoreactive B cells via high levels of CD1d on their surface. In previous studies, CD1d on B cells has been suggested to promote iNKT cell-dependent tolerance (36) and protection from inflammation (37) by facilitating iNKT–B cell interactions. Ongoing studies will determine whether such interaction might further activate and cause activation-induced cell death of autoreactive B cells that are already activated in vivo (Fig. 8A). In contrast, iNKT cells induce a marked activation of naïve non-autoreactive B cells (Fig. 8C) and elicit a prompt polyclonal response (10, 31, 38).
In summary, we report that iNKT cells inhibit IL-10–secreting, CD1d+ autoreactive B cells in a contact- and CD1d-dependent manner, whereas they activate non-autoreactive B cells via cytokines. Further elucidation of mechanisms underlying discriminate regulation of autoreactive vs. normal B cells by iNKT cells will help pave the way for iNKT cell-based therapies for human autoantibody-associated diseases, such as SLE where reduced numbers of iNKT cells is considered to be a primary defect (39). The iNKT cell-based therapy is particularly appealing, given the limited polymorphism in CD1 genes (2), which obviates one of the major hurdles of therapies aimed at MHC class I and II system. Enhancing this appeal is our finding that iNKT cells suppress harmful autoreactivity without causing global suppression of B cells.
Supplementary Material
Acknowledgments
This work is supported in part by National Institutes of Health grants AR50797, AR56465 and AI80778. XW is recipient of the Meyer Investigator Award from the Arthritis Foundation Southern California Chapter. PJK is recipient of the Beginning-Grant-in-Aid grant from the American Heart Association.
We thank Drs. A. Bendelac (Chicago, IL), B. Diamond (Long Island, NY), and M. Taniguchi (Yokohama, Japan) for Vα14Tg, R4ATg, and Jα18−/− mice, respectively; Kyowa Hakko Kirin Co. Ltd. (Tokyo, Japan) for providing αGalCer; and M. Shlomchik for providing rheumatoid factor detection reagents. We appreciate helpful suggestions from Drs. J. Braun, K. Dorshkind and L. Van Kaer.
Abbreviations used in this paper
- αGalCer
α-galactosylceramide
- HEL
hen egg lysozyme
- iNKT cells
invariant natural killer T cells
- RF
rheumatoid factor
- SLE
systemic lupus erythematosus
- Tg
transgenic
- WT
wild-type
Footnotes
Disclosure
The authors have no financial conflict of interest.
References
- 1.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626– 1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 5.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 6.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 7.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M, Yahata T, Meng H, Koda T, Nishimura S, Kawano T, Taniguchi M, Nishimura T. alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 10.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha- galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killerT lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI- anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:20–27. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 14.Fan GC, Singh RR. Vaccination with minigenes encoding V(H)-derived major histocompatibility complex class I-binding epitopes activates cytotoxic T cells that ablate autoantibody-producing B cells and inhibit lupus. J Exp Med. 2002;196:731–741. doi: 10.1084/jem.20020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn BH, Singh RR. Animal models of systemic lupus erythematosus. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus. 7. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 299–355. [Google Scholar]
- 16.Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- 17.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 18.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol. 2003;171:2142–2153. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- 21.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 23.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 24.Singh RR, Hahn BH, Tsao BP, Ebling FM. Evidence for multiple mechanisms of polyclonal T cell activation in murine lupus. J Clin Invest. 1998;102:1841–1849. doi: 10.1172/JCI3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RR, Kumar V, Ebling FM, Southwood S, Sette A, Sercarz EE, Hahn BH. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh RR, Hahn BH, Sercarz EE. Neonatal peptide exposure can prime T cells and, upon subsequent immunization, induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–1621. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR. Repeated α-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J Immunol. 2003;171:4439–4446. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- 28.Kawabata D, Venkatesh J, Ramanujam M, Davidson A, Grimaldi CM, Diamond B. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One. 5:e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molano A, Park SH, Chiu YH, Nosseir S, Bendelac A, Tsuji M. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J Immunol. 2000;164:5005–5009. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 31.Forestier C, Molano A, Im JS, Dutronc Y, Diamond B, Davidson A, Illarionov PA, Besra GS, Porcelli SA. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175:763–770. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 32.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–1222. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 207:943–952. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcon-Segovia D, Wijdenes J, Galanaud P, Emilie D. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur J Immunol. 2002;32:848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 39.Wither J, Cai YC, Lim S, McKenzie T, Roslin N, Claudio JO, Cooper GS, Hudson TJ, Paterson AD, Greenwood CM, Gladman D, Pope J, Pineau CA, Smith CD, Hanly JG, Peschken C, Boire G, Fortin PR. Reduced proportions of natural killer T cells are present in the relatives of lupus patients and are associated with autoimmunity. Arthritis Res Ther. 2008;10:R108. doi: 10.1186/ar2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.