Abstract
In Drosophila melanogaster, female-specific expression of Sex-lethal (SXL) and Transformer (TRA) proteins controls sex-specific alternative splicing and/or translation of a handful of regulatory genes responsible for sexual differentiation and behavior. Recent findings in 2009 by Telonis-Scott et al. document widespread sex-biased alternative splicing in fruitflies, including instances of tissue-restricted sex-specific splicing. Here we report results arguing that some of these novel sex-specific splicing events are regulated by mechanisms distinct from those established by female-specific expression of SXL and TRA. Bioinformatic analysis of SXL/TRA binding sites, experimental analysis of sex-specific splicing in S2 and Kc cells lines and of the effects of SXL knockdown in Kc cells indicate that SXL-dependent and SXL-independent regulatory mechanisms coexist within the same cell. Additional determinants of sex-specific splicing can be provided by sex-specific differences in the expression of RNA binding proteins, including Hrp40/Squid. We report that sex-specific alternative splicing of the gene hrp40/squid leads to sex-specific differences in the levels of this hnRNP protein. The significant overlap between sex-regulated alternative splicing changes and those induced by knockdown of hrp40/squid and the presence of related sequence motifs enriched near subsets of Hrp40/Squid-regulated and sex-regulated splice sites indicate that this protein contributes to sex-specific splicing regulation. A significant fraction of sex-specific splicing differences are absent in germline-less tudor mutant flies. Intriguingly, these include alternative splicing events that are differentially spliced in tissues distant from the germline. Collectively, our results reveal that distinct genetic programs control widespread sex-specific splicing in Drosophila melanogaster.
Keywords: alternative splicing, sex, Drosophila, Hrp40
INTRODUCTION
Post-transcriptional regulation of gene expression plays a key role in sex determination in Drosophila melanogaster (Baker et al. 1989; McKeown and Madigan 1992; Cline 1993; Schutt and Nothiger 2000; Forch and Valcarcel 2003; Penalva and Sanchez 2003). In female flies, expression of the RNA-binding protein Sex-lethal (SXL) regulates alternative splicing of Sxl, transformer (tra), and male-specific-lethal 2 (msl-2) transcripts, which allows the synthesis of TRA and SXL proteins and inhibits the synthesis of MSL-2 protein (Nagoshi et al. 1988; Inoue et al. 1990; Bell et al. 1991; Bashaw and Baker 1995; Kelley et al. 1997; Blencowe et al. 2009). Subsequently, TRA regulates alternative splicing of doublesex (dsx) and fruitless (fru) pre-mRNAs, resulting in synthesis of sex-specific protein isoforms of DSX and FRU transcription factors (Baker 1989; Burtis and Baker 1989; Ryner and Baker 1991; Ito et al. 1996; Ryner et al. 1996). Thus, female-specific expression of SXL during the blastoderm stage triggers a cascade of post-transcriptional regulatory events that maintains sexual identity through Sxl autoregulation, establishes somatic sex differentiation and sex courtship behavior through expression of female-specific isoforms DSX and FRU, and represses dosage compensation through inhibition of splicing and translation of msl-2 transcripts (Schutt and Nothiger 2000; Forch and Varcarcel 2003; Penalva and Sanchez 2003). Such orchestration of sex determination by a single RNA-binding protein has become a paradigm for the developmental control of gene expression through post-transcriptional mechanisms.
A classical view of this pathway has been that post-transcriptional regulation acts as an upstream switch that helps to establish comprehensive sex-specific programs of transcriptional control and chromatin remodeling, and that these transcriptional programs are largely responsible for sex determination in the fly. Recent work, however, has documented sex-specific differences in alternatively spliced transcripts of multiple genes (McIntyre et al. 2006; Telonis-Scott et al. 2009) suggesting a more extensive post-transcriptional regulation program that may act together with transcriptional changes to shape sex determination in Drosophila melanogaster. One important question is whether all this extensive diversity of alternative splicing is under the control of the SXL/TRA pathway.
In this article, we confirm extensive differences in alternative splicing between male and female Drosophila transcriptomes, including substantial changes in expression and splicing of RNA binding proteins, using splicing-sensitive microarrays monitoring over 2600 alternatively spliced genes (Blanchette et al. 2005). We further show that regulation by the SXL/TRA pathway can only explain a fraction of those changes. We provide evidence that differential expression of Hrp40/Squid can contribute to sex-specific splicing and document alterations in somatic sex-specific splicing induced by genetic depletion of the germline. Bioinformatic and experimental analyses implicate distinct regulatory programs in the control of sex-specific splicing and, in particular, tissue-restricted sex-specific splicing.
RESULTS
Extensive sex-specific alternative splicing in adult flies
To describe sex-specific differences in alternative pre-mRNA splicing in Drosophila melanogaster, total RNA was purified from triplicate pools of male or female adult flies (1–12 d old), labeled with fluorescent Cy5 or Cy3 fluorescent dyes and hybridized to splicing-sensitive microarrays designed to assess all annotated alternative splicing events in Drosophila using exon-specific and splice-junction-specific probes (Blanchette et al. 2005). The design contains over 19,000 splice-junction probes, which cover more than 9300 alternative splice junctions in 2662 genes. In addition to the analyses described by Blanchette et al. (2005), a sensitive algorithm was employed to maximize the call rate of significant changes in net-expression (changes in splice-junction probes after normalization by gene expression) with limited biological replication, using a simple linear model and a moderated t-statistic (see Materials and Methods).
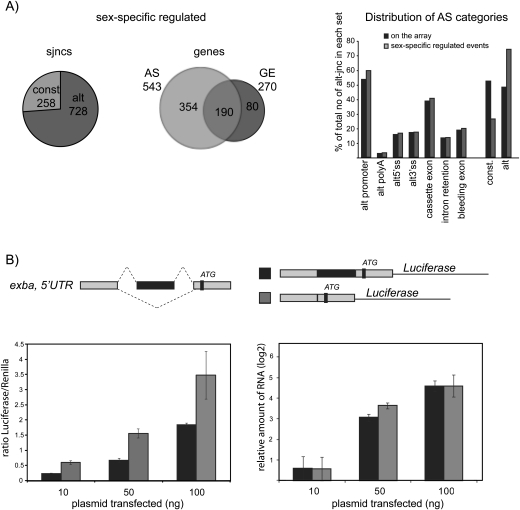
Over 950 splice junctions (sjncs, out of a total of 19,589 monitored by the array and passing nonspecific filters) showed sex-specific differences (determined by a net-fold change cutoff of 2). From the total number of 2662 genes analyzed, these changes correspond to 543 genes (20.4%) (Fig. 1A, middle panel) of which 189 were also differentially expressed at the transcriptional level and 354 genes were differentially regulated exclusively by alternative splicing. These figures suggest extensive involvement of alternative splicing in sex-specific gene expression. In contrast, 269 genes (10.1%) showed differential transcription.
FIGURE 1.
Extensive sex-specific alternative splicing in Drosophila melanogaster. (A) Distribution of sex-specific differences in alternative splicing. (Left panel) Distribution of the 986 sex-specific regulated splice junctions (sjncs), between sjncs annotated as alternative (alt) or constitutive (const). (Middle panel) Distribution of gene expression (GE) and alternative splicing (AS) changes between male and female flies. (Right panel) Distribution of classes of alternative splicing events for all the sjncs represented on the array (black bars) and those sex-differentially regulated (gray bars). (B) Alternative splicing of a cassette exon in the 5′ UTR of extra bases (exba) affects translational efficiency in cell culture. The 5′ UTRs of exba, including the endogenous translational start (ATG), were cloned in frame with the firefly luciferase gene in a Cu2+ inducible Drosophila vector (top panel). Luciferase activity (normalized to the activity of cotransfected Renilla) for three independent experiments is represented for different amounts of transfected plasmids (left panel). Black bars correspond to the longer and gray bars to the shorter isoforms of exba 5′ UTRs, respectively. The levels of 5′ UTR-exba-luc mRNA from the same experiments were quantified by real-time PCR and presented as relative amounts (right panel).
As expected, Sxl, tra, msl-2, and dsx showed clear sex-specific isoform expression in adult flies measured by quantitative RT-PCR (Fig. 5A, below). Analysis of the microarray predictions for 30 additional genes by quantitative RT-PCR showed 93% (28/30) validation of transcriptional and splicing changes (a change was considered validated when the net expression value of the corresponding probe was determined as twofold (log2 ratio sjnc – log2 ratio of GE) or higher by qRT-PCR for three biological replicas). These results imply that a significant fraction (∼18%) of alternative splicing events in Drosophila show sex-specific differences (for examples of these differences, see Figs. 2, 5B; Supplemental Table 1). The average magnitude of the 28 validated changes was four- to eightfold, with 12 changes reaching 100-fold, i.e., a significant fraction of these alternative splicing events show radically different patterns of splicing between male and female flies. Analysis of the distribution of splicing changes among different categories of splicing events and alternative start and polyadenylation sites showed, as expected, that changes were more prominent in alternative rather than constitutive splice junctions (74% of alternative vs. 26% of junctions annotated as constitutive) (Fig. 1A, left panel). Considering the alternative junctions, sex-specific changes affected similarly different classes of alternative splicing events (Fig. 1A, right panel).
FIGURE 5.
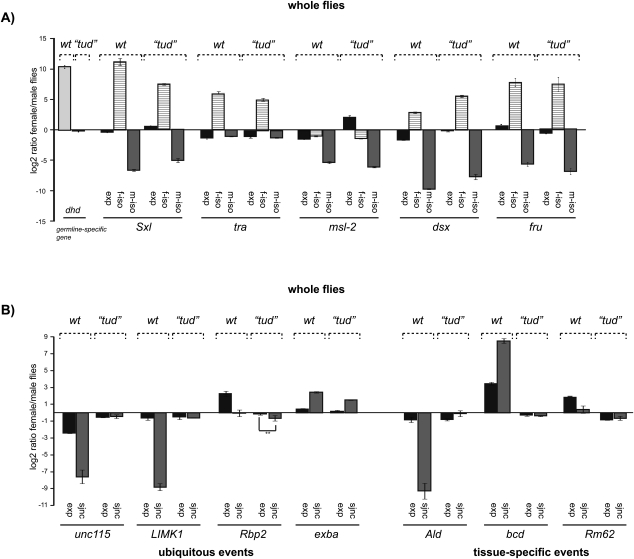
Analysis of sex-specific splicing in germline-less tudor mutant flies. (A) Transcription and sex-specific isoform expression of classical sex-specific events are not affected in germline-less mutant flies (labeled as “tud”). Quantitative RT-PCR in female and male wild type (wt) and germline-less “tud” flies were carried out as in Figure 3. A control gene (deadhead, dhd) (Parisi et al. 2004) served as a marker for the absence of germline in females flies. (B) Patterns of sex-specific splicing in microarray-predicted sex-specific splicing events in germline-less tud flies. Splicing patterns were analyzed as in Figure 3 for four events showing ubiquitous splicing changes (unc115, LIMK1, Rbp2, exba) and three tissue-specific events (Ald, bcd, Rm62) comparing female and male adult wild-type (wt) or “tudor” flies. (**) Statistically significant difference reproducibly observed in more than three biological replicas.
FIGURE 2.
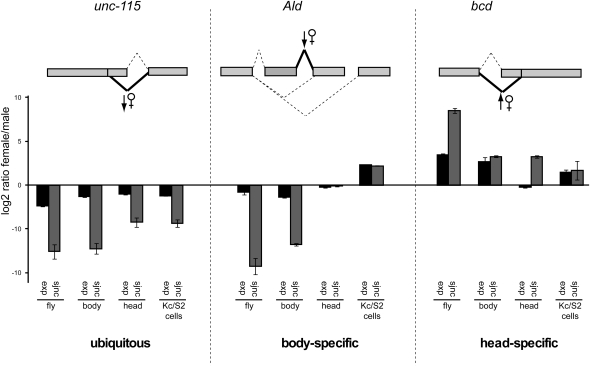
Ubiquitous and tissue-restricted regulation of sex-specific alternative splicing. (Top) Schematic representation of alternative splicing patterns corresponding to the genes indicated and of splice junctions differentially regulated in female flies, as predicted by the microarray data. (Bottom) Quantitative RT-PCR analysis of gene expression (exp) and alternative junctions (sjnc) in female vs. male flies, bodies and heads, as well as Kc vs. S2 cell lines. The alternative junctions analyzed correspond to those indicated in bold in the top panel. The three events exemplify three categories of sex-specific changes observed in more than 30 other genes (Supplemental Table 1): unc-115 is an example of ubiquitous sex-specific differences observed throughout the fly and also in cell lines; Ald is an example of sex-specific differences observed only in the body but not in heads or cell lines; bcd represents an event displaying sex-specific differences only in the head.
Significantly, gene ontology analyses revealed a significant enrichment of sex-specific splicing changes in genes with annotated functions linked to sexual differentiation, further supporting the concept that alternative splicing plays diverse roles in sexual dimorphism. Interestingly, another category of enriched genes involves various functions related to post-transcriptional regulation of gene expression, including pre-mRNA splicing, consistent with the relevance of these processes in Drosophila sex determination (Table 1; see below).
TABLE 1.
GO analysis of enriched categories in biological processes
Our results offer multiple examples in which sex-specific splicing changes are predicted to have a significant impact on protein domain composition and structure. In addition, changes in the sequences of 5′ untranslated regions (UTRs) are also prominent among the sex-specific differences in transcript structure detected in our experiments. These can be either associated with the use of alternative promoters or be due to alternative splicing events in transcripts generated from a single promoter. Alternative 5′ UTRs can play important roles in the regulation of mRNA stability, translation efficiency, and localization (Hughes 2006). To test the possibility that some of the observed splicing changes in the 5′ UTR have functional consequences, we focused on one of the sex-specific alternative 5′ UTRs revealed by our microarray analyses and validated by qRT-PCR. The gene encoding the translation factor extra bases (also known as eIF-5c or krasavietz) (Lee et al. 2007) contains a cassette exon in its 5′ UTR, which is sex-specifically spliced (Fig. 1B). The alternative 5′ UTRs were cloned upstream of the Luciferase open-reading frame in an expression vector and Luciferase activity was measured in extracts from transfected S2 cells. RNA levels were analyzed in parallel to assess differences in reporter mRNA accumulation. The results of Figure 1B indicate that constructs expressing the shorter 5′ UTR display higher luciferase activity. Because the overall levels of mRNAs containing the longer and shorter 5′ UTRs were comparable, we conclude that the higher luciferase activity is likely due to the higher translational efficiency of transcripts harboring the shorter 5′ UTR.
In summary, our genome-wide screen for sex-specific alternative splicing in the adult fly documents extensive differences in alternative splicing between male and female flies, which can contribute to changes in protein structure or abundance, affecting, preferentially, genes with functions in sex determination and RNA processing. Our results confirm and expand recent reports by McIntyre and colleagues (McIntyre et al. 2006; Telonis-Scott et al. 2009).
Sex- and tissue-specific alternative splicing
Classical examples of sex-specific alternative splicing, like those affecting the genes Sxl, tra, and dsx, occur throughout the fly (Baker and Ridge 1980; Cline 1984) and can be recapitulated in male (S2) and female (Kc) cell lines (see below). On the other hand, gonads are the most sexually dimorphic tissue and can be a key source of sex-biased transcription (Ellegren and Parsch 2007) and splicing (Telonis-Scott et al. 2009). To test whether other examples of sex-specific splicing revealed by our microarray results occur in diverse tissues or show a more restricted distribution, RNA was separately isolated from heads and bodies of male and females flies and alternative splicing events in 28 genes with diverse functions were analyzed by quantitative RT-PCR (Supplemental Table 1). All except one gene (bicoid [Fig. 2, right panel], which showed differences only in the head), showed sex-specific differences in the body, perhaps reflecting the larger contribution of body parts to the tissue mass of the fly. Ten genes displayed the same differential pattern of alternative splicing in heads and bodies, as exemplified by the relative use of an alternative donor site in the unc-115 gene (Fig. 2, left panel). The other 17 genes showed sex-specific differences in the body but not in the head RNA preparations, as shown for inclusion of two alternatively spliced exons in the Aldolase (Ald) gene (Fig. 2, middle panel).
We therefore conclude that while some genes display uniform sex-specific patterns of alternative splicing in different parts of the animal, other sex-specific splicing differences are restricted to specific organs or tissues. This implies that, in addition to the genetic program that ubiquitously controls alternative splicing of key sex determination genes (Baker and Ridge 1980; Cline 1984), additional mechanisms must exist to establish tissue-restricted, sex-specific splicing events. These results also suggest that a significant fraction (perhaps 1/3, given that 10/28 of the analyzed alternative splicing events display differences in both bodies and heads) of the sex-specific alternative splicing differences detected by our microarray cannot be simply explained by the RNA contribution of distinct gonads and germline in the two sexes.
To further substantiate these observations, the splicing pattern of the 28 genes was compared in RNAs purified from two embryonic cell lines (Kc and S2 cells). These cell lines display sex-specific splicing of the genes in the classical pathway (Fig. 3A) and expression of 21 of the 28 genes was detectable in these cells. Interestingly, 13 out of 15 events displaying tissue-restricted splicing patterns (e.g., Ald and bcd) did not display splicing differences between these cell lines, while eight out of eight events found to be ubiquitously regulated in flies (e.g., unc-115) were also differentially regulated in Kc and S2 cells (Fig. 2; Supplemental Table 1). These results indicate that Kc and S2 cells largely recapitulate programs of alternative splicing regulation that occur ubiquitously (including the classical pathway of sex-specific splicing decisions affecting sex regulatory factors), but do not recapitulate most of the regulatory events that show tissue-restricted sex-specific splicing. These observations again imply that distinct regulatory mechanisms operate to establish the extensive differences in splicing patterns observed between male and female flies.
FIGURE 3.
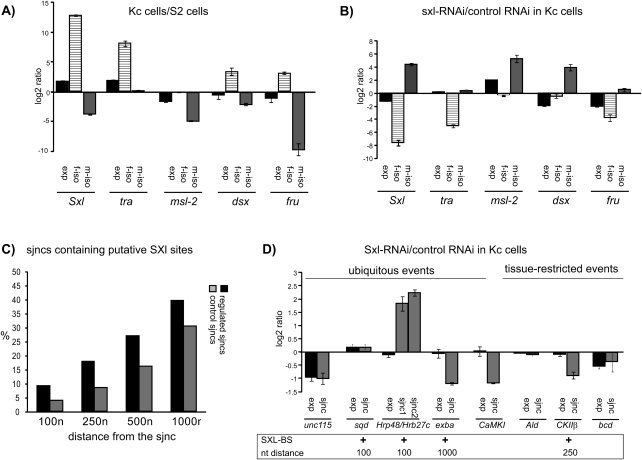
Regulation of sex-specific splicing by SXL. (A) Alternative splicing pattern of the classical sex regulators (Sex-lethal [Sxl], transformer [tra], male specific lethal-2 [msl-2], doublesex [dsx] and fruitless [fru]) in female Kc and male S2 cells quantified by RT-PCR and represented as a ratio between the levels of male (m-iso) and female (f-iso) isoforms in the two cell lines. (exp) Gene expression values. (B) Reversal of sex-specific splicing patterns of sex determination genes in Kc cells upon Sex-lethal knockdown. Analysis of gene expression as well as of female- or male-specific isoforms was carried out as in A and represented as a ratio between RNAs isolated from cells in which Sex-lethal RNA was knocked down by RNAi and control RNAi. (C) Bioinformatic analysis of potential SXL-binding sites in 700 sex-regulated (black bars) and 700 non-sex-regulated sjncs (gray bars) in a window of 100, 250, 500, and 1000 nt adjacent to the splice site (see Materials and Methods for search criteria). (D) Quantitative-RT-PCR analysis of the effect of Sxl knockdown in Kc cells on alternative splicing of genes predicted by the microarray analysis. Analyses were carried out as in B and results of sex-specific differences are represented as in previous figures for eight genes: Unc-115, hrp40/sqd, Hrp48/Hrb27c, exba, and CaMKI from the group of genes showing ubiquitous sex-specific regulation and Ald, CKIIβ, and bcd from the tissue-restricted category. The presence of computationally predicted SXL binding sites is indicated below the graph, including the distance window in nucleotides (nt) from the splice site within which the SXL binding site is located.
SXL- and Tra-independent sex-specific alternative splicing
Extensive previous work has demonstrated that binding of SXL or TRA/TRA-2 complexes to their cognate binding sites regulates female-specific splicing of genes like Sxl, tra, msl-2, dsx, and fru (for review, see Schutt and Nothiger 2000; Forch and Valcarcel 2003). Identification of SXL and TRA/TRA-2 binding sites can indeed predict targets of these proteins (Robida et al. 2007). We have used these sequence motifs to search for SXL and TRA/TRA2 binding sites in the vicinity of the regulated splice junctions revealed by our microarray experiments. For this, we retrieved sequences in a 100-nucleotide (nt) window upstream of and downstream from each regulated splice site (a 5′ and a 3′ splice site for each sjnc) and searched for SXL and TRA/TRA-2 binding sites using string matching (see Materials and Methods). An equivalent number of splice junctions not sex-specifically regulated were used as a control set. TRA/TRA2 binding sites (defined by the motif [T/A]C[T/A][T/A]C[A/G]ATCAACA [Heinrichs et al. 1998]) were only found in dsx, a known target, even when low stringent criteria were used. We searched for SXL sites (using a 17-nt uridine stretch with up to two guanosines, based upon previous SELEX results and the natural high-affinity sequence located adjacent to the non-sex-specific 3′ splice site of tra (Singh et al. 1995), and found that 9% of the sjncs contain potential SXL-binding sites using stringent selection criteria (Fig. 3C). A significantly lower number of sites were found in the control set (4%). Next we considered the possibility that regulatory sites could also be located further away from the splice sites, and extended the search to lengths of up to 1000 nt (Fig. 3C) and used less stringent binding-site criteria (not shown). In a window of 1000 nucleotides, about one-third of the sjncs contain potential SXL binding sites (39% vs. 30% in control sjncs). While these figures suggest that additional SXL targets can be found among these regulated events, no SXL binding site could be identified for the majority of regulated splice sites, even under low stringency criteria, implying that these sex-specifically regulated splicing events are unlikely to be under direct control of SXL.
To further explore this concept and test whether those events bearing potential SXL sites are under the control of this protein, experiments were carried out to knock down Sxl expression by RNAi in female Kc cells. RNAi of Sxl in Kc cells resulted in reduced levels of female isoforms and/or increased levels of male isoforms of the known SXL targets Sxl, tra, and msl-2 (Fig. 3B). Six of the eight ubiquitously regulated events analyzed contained SXL binding sites within a 1000-nt window. Three of the eight events (in the genes Hrp48/Hrb27c, exba, and CaMKI) showed consistent (albeit somewhat limited) changes upon Sxl knockdown (Fig. 3D; Supplemental Table 1). In contrast, only five of the 18 genes showing tissue-restricted sex-specific splicing contained putative SXL binding sites within a 1000-nt window from the regulated junction and only one of them (CKIIβ) was affected, also mildly, by Sxl knockdown (Fig. 3D; Supplemental Table 1).
These results indicate that while new potential SXL targets can be identified, no evidence for SXL-mediated regulation is found for a large fraction of the sex-specific splicing events. Consistent with this, microarray experiments comparing RNA from mock- and Sxl- RNAi Kc cells showed only very limited changes in splicing patterns (data not shown), again suggesting that only a small number of events are regulated in a similar manner to tra, msl2, or Sxl.
Taken together, our results argue that a large fraction of sex-specific alternative splicing events are regulated by factors distinct from the classical cascade of post-transcriptional regulators at the top of the sex determination pathway.
Ubiquitous RNA binding proteins as regulators of sex-specific splicing
One possibility to explain sex-specific changes in splicing not involving the classical sex-specific regulators is that the overall expression or the expression of particular alternatively spliced isoforms of ubiquitous RNA binding proteins differs between the sexes. Indeed, recent work by Telonis-Scott et al. (2009) documented instances of sex-specific splicing of genes encoding RNA binding proteins. To evaluate the generality of this phenomenon, two approaches were taken.
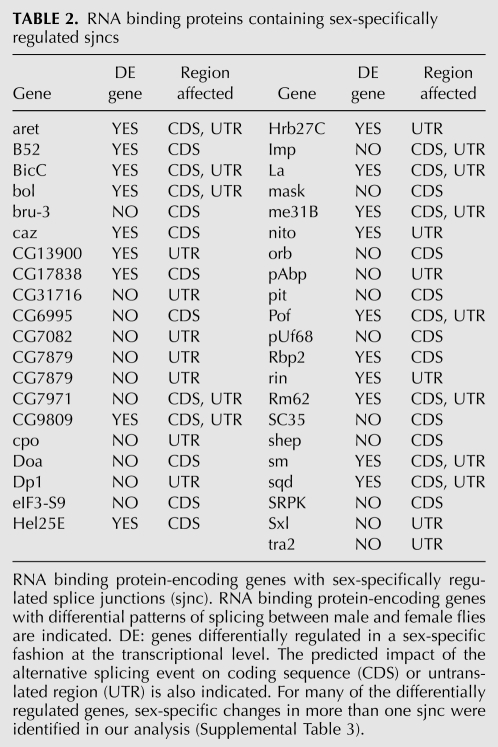
First, we carried out several gene ontology analyses of our microarray data. RNA processing, including splicing regulation, featured prominently among the top enriched categories, together with expected categories like sex determination or developmental regulation of cell division (Table 1; Supplemental Table 2). This was not the case when genes regulated only at the transcriptional level were considered, which showed enrichment in gene functional groups such as spindle organization and nuclear migration (Supplemental Table 2), nor was this the case for genes enriched in alternative splicing in other biological comparisons using the same microarray platform (Blanchette et al. 2005, 2009; Hartmann et al. 2009). Second, a list of 217 Drosophila genes with Entrez Gene identifiers related to RNA-binding proteins, including splicing regulators, was compiled (Lasko 2000; Mount and Salz 2000), of which 83 genes produce multiple transcripts and were analyzed by our arrays. Sex-specific splicing differences were detected in 97 sjncs corresponding to nearly 50% (39) of them (Table 2; Supplemental Table 3). In contrast, differential gene expression was detected for 20 of the 83 genes, where 19 of them in fact also showed changes in alternative splicing. Thus, in total, 40 (48%) of the RNA-binding protein-coding genes considered in this analysis show sex-specific differences either at the level of transcription (1), splicing (20), or both (19). This was not the case for a set of 170 transcription factors compiled by Pfreundt et al. (2010), which included 77 genes represented in our arrays, of which only six showed differential expression and 11 differential splicing (minimum Fisher's Exact test P-value > 0.7, compared to <0.001 for expression or <0.0001 for alternative splicing changes in RNA binding factors).
TABLE 2.
RNA binding proteins containing sex-specifically regulated sjncs
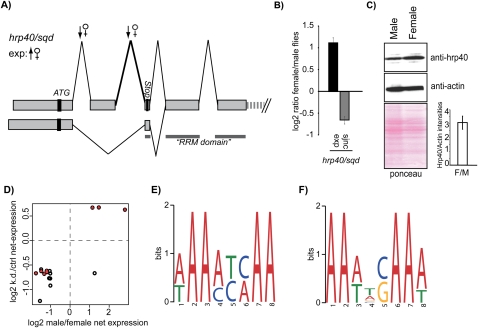
To further explore this concept, we focused on the Drosophila hnRNP hrp40/squid gene, which was found to be sex-specifically regulated transcriptionally and by alternative splicing (Fig. 4A,B) such that expression of the protein is predicted to be higher in female flies. Indeed, Western blot analysis using female and male fly extracts confirmed that Hrp40 levels are higher in female flies (Fig. 4C). Consistent with the possibility that differential expression of hrp40/squid contributes to sex-specific splicing, a statistically significant overlap was found between microarray-detected sex-specific splicing differences and changes in alternative splicing caused by depletion of hrp40/squid in S2 cells (Blanchette et al. 2009). Specifically, 2% of the sex-specific changes were also found upon hrp40/squid knockdown, representing 9% of the alternative splicing changes induced by depletion of this protein. While limited, the overlap is statistically highly significant (Fisher's Exact test P-value < 0.001, <0.0001 when the comparison is restricted to annotated alternative junctions, corresponding to odds-ratio values of 4.0–6.8). Furthermore, when the direction of the splicing changes between the male vs. female and hrp40/sqd knockdown vs. ctrl were compared, a significant correlation was found (Pearson coefficient 0.82, 0.95 when restricted to annotated alternative junctions) (Fig. 4D).
FIGURE 4.
A function for hrp40/squid in sex-specific splicing. (A) Schematic representation of splicing patterns of the hrp40/squid gene and splice junctions differentially regulated in female flies. ATG indicates the translational start site. Inclusion of exon 2 leads to an mRNA containing a premature stop codon (the pattern that is down-regulated in female flies), while the skipping of exon 2 produces the mRNA encoding full-length hrp40/squid protein. The gray line indicates exonic regions encoding the RNA binding domain (RRM). Only the sizes of exons are at scale. (B) Validation by quantitative RT-PCR of sex-specific differences in expression and alternative splicing of the hrp40/squid gene predicted by the microarray. Expression (exp) was quantified by amplification of a constitutive exon; sjnc indicates real-time PCR quantification using as one of the primers a splice-junction oligonucleotide corresponding to the junction indicated by the thicker line in A. (C) Western blot comparing levels of Hrp40/Squid protein in male and female flies (top) and loading controls (β-actin) (middle) and ponceau staining (bottom). Quantification of the relative levels of Hrp40/Squid vs. actin in female vs. male flies (F/M) for three independent experiments is shown in the lower-right panel. (D) Comparison of splice junction changes between hrp40/sqd knockdown vs. ctrl and male vs. female. Circles represent the intersection between the fold changes observed for a particular splice junction in the two experimental setups. Red triangles indicate splice junctions annotated as the alternative. Clustering at the lower-left and upper-right quadrants indicates correlation in the direction of splicing changes. (E) Top-enriched motif identified in exonic regions proximal to sex-specifically regulated sjncs. (F) Top-enriched motif identified in all regions proximal to sjncs differentially regulated upon hrp40/squid knockdown (Blanchette et al. 2009).
Also consistent with a contribution of Hrp40/Squid to sex-specific splicing, related sequences were extracted as the top enriched motifs near sex-specific and Hrp40/Squid-regulated splice sites using MEME (Fig. 4E,F; Bailey and Elkan 1994; see Materials and Methods).
Collectively, these results argue that differential expression and alternative splicing of hnRNP proteins like hrp40/squid contribute to the establishment of sex-specific splicing patterns in Drosophila (see also Discussion).
Germline and the regulation of sex-specific splicing
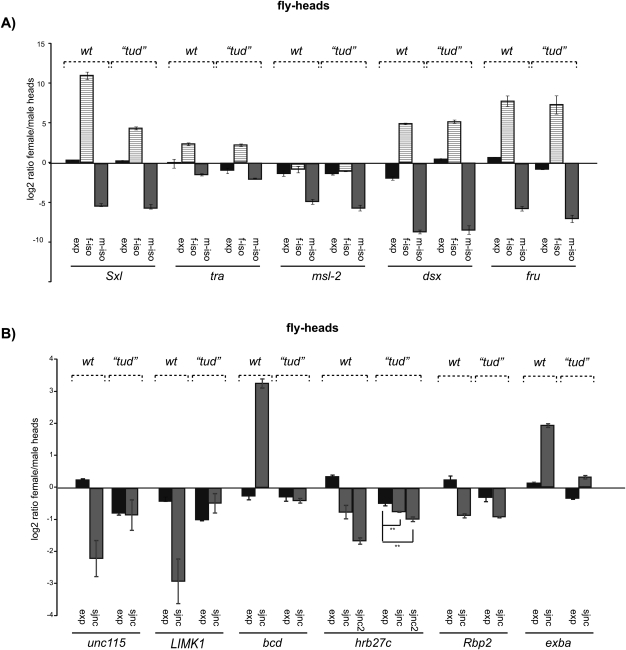
A significant fraction of the sex-specific differences in gene expression can be attributed to the presence of dimorphic gonads and germline (Ellegren and Parsch 2007; Telonis-Scott et al. 2009). To assess the impact of germline in sex-specific splicing we generated flies lacking a germline using the maternal effect mutant tudor (Schupbach and Wieschaus 1986). RNA was isolated from male and female flies and patterns of alternative splicing of Sxl, msl-2, tra, dsx, and the 28 genes showing sex-specific differences in splicing patterns described above were analyzed by quantitative RT-PCR. Sex-specific patterns of alternative splicing of the master regulatory genes Sxl, tra, msl-2, and dsx were largely maintained in these germline-less flies (Fig. 5A). In contrast, differences in sex-specific alternative splicing were no longer detectable in germline-less flies for 24 of the remaining 28 genes analyzed (Fig. 5B; Supplemental Table 1), confirming the important contribution of the germline to sex-specific splicing. Strikingly, however, these included not only genes sex-specifically spliced in the body, but also genes that were found to be differentially regulated both in the body and the head, and even bicoid, which is sex-specifically spliced only in the head (Fig. 5B). To assess whether splicing is specifically affected in the head of germline-less flies or whether the result was due to the larger contribution of the body to whole fly preparations, we isolated RNA from female and male heads of germline-less flies and tested by RT-PCR the 11 genes that were found to be sex-specifically spliced in wild-type heads. Heads of germline-less flies displayed normal sex-specific splicing of Sxl, msl-2, tra, dsx, and fru (Fig. 6A). Three (exba, Hrp48/Hrb27c, and rbp2) of the four genes that maintained sex-specific splicing pattern in germline-less flies kept their sex-dependent splicing pattern in the heads of these flies. One gene (jigR1) for which sex-specific splicing was apparently lost in germline-less flies, preserved however its sex-specific splicing in the head of these mutant flies. The remarkable result, however, was that seven of the 11 genes that showed sex-specific splicing in the head lost their sex-specific differences in the germline-less mutant flies (Fig. 6B; Supplemental Table 1).
FIGURE 6.
Analysis of sex-specific splicing in germline-less tudor mutant heads. (A) Alternative splicing of classical sex determination regulatory genes is not affected in germline-less (tud) fly heads. Patterns of expression and sex-specific isoforms were analyzed as in Figure 3 for the indicated genes in RNA preparations from wild-type (wt) and tud mutant female and male fly heads. (B) Alternative splicing of additional sex-specific splicing differences between female and male in adult fly heads of wild-type and germline-less (tud) flies. Results from six genes are represented as fold changes between female and male flies. (**) Statistically significant differences, reproducibly observed in more than three biological replicas.
Taken together, these results suggest either a novel effect of a reduced dose of tudor gene products on splicing regulation, or a relevant contribution of germline determination to sex-specific splicing. This contribution could directly result from the absence of germline for those genes sex-specifically spliced in these cells or, intriguingly, be the indirect consequence of the absence of germline on sex-specific splicing events occurring in other tissues.
DISCUSSION
In this manuscript we focus on the molecular mechanisms responsible for the extensive sex-specific differences in alternative splicing observed in Drosophila (McIntyre et al. 2006; Telonis-Scott et al. 2009). Our data indicate that in addition to the regulatory cascade triggered by female-specific expression of Sex-lethal, other mechanisms operate to sculpt sex-specific transcriptomes, including qualitative/quantitative differences in expression of RNA binding proteins like Hrp40/Squid and possibly long-distance effects of the germline on somatic cells. Analysis of whole flies, heads, bodies, and cell lines support the notion that distinct regulatory programs for sex-specific splicing coexist, even within one cell type.
Extensive sex-specific splicing
Classical genetic and molecular work on Drosophila sex determination has provided a model in which early expression of the Sex-lethal protein in female embryos triggers a cascade of alternative splicing events culminating in the production of sex-specific isoforms of the transcription factors Doublesex and Fruitless (for review, see Baker et al. 1989; McKeown and Madigan 1992; Cline 1993; Schutt and Nithinger 2000; Forch and Valcarcel 2003; Penalva and Sanchez 2003). This, in turn, establishes differential transcription of a multitude of genes that drive somatic sex differentiation and sexual behavior. This model pictures RNA processing regulation as an initial switch affecting a few key master genes, which is necessary to establish transcriptional programs that will be largely responsible for sex determination. Recent work by McIntyre and colleagues (McIntyre et al. 2006; Telonis-Scott et al. 2009) and our own genome-wide analysis estimate that 11%–20% of all multitranscript genes in Drosophila display sex-biased expression of alternative transcripts. These results suggest that regulation of RNA processing not only provides an initial switch, but also contributes to an extensive remodeling of the sex-specific gene expression program. Similar conclusions were recently reached analyzing alternative splicing in male and female liver samples of different mouse strains using Exon arrays (Su et al. 2008) and of three primates (humans, chimpanzees, and rhesus macaques) using RNASeq (Blekhman et al. 2010). Therefore, extensive sex-specific splicing may be a general phenomenon in sex-dimorphic animals. This view is consistent with the emerging realization that alternative pre-mRNA splicing is a widespread phenomenon in multicellular organisms (Stolc et al. 2004; Kim et al. 2007; Xing and Lee 2007; Pan et al. 2008; Wang et al. 2008) that can contribute programs of gene expression regulation of significant biological value, parallel and complementary to those targeting the transcription process (Pan et al. 2004; Blanchette et al. 2005; Calarco et al. 2007; Ben-Dov et al. 2008; Nilsen and Graveley 2010). It seems likely that the features of post-transcriptional regulation that provide switches, feedback loops, and robustness in gene control (Louis et al. 2003) will be exploited in biologically meaningful ways by at least a fraction of the hundreds of the newly identified sex-specifically spliced genes.
Our results provide examples of sex-specific differences in alternative splicing leading to expression of polypeptides showing differences in key functional domains. Other alternative splicing events affect functionally relevant noncoding regions of mRNAs, including 5′ UTRs. These can be generated either by alternative splicing within the UTR region or by the use of alternative promoters, the latter representing up to 65% of the sex-specific transcript differences detected in our work. Our experiments using the extra bases (exba, eIF5c) gene argue that the alternative 5′ UTRs can lead to quantitative differences in protein expression (Fig. 1B). In addition to exba, sex-specifically expressed alternative 5′ UTRs affect several translation factors, such as pAbp, BicC, etc., and splicing factors (Table 2). This suggests that sex-specific differences in transcriptome may lead to subsequent quantitative and qualitative differences in mRNA processing and/or translation. We propose that these differences can significantly alter the proteome of male and female flies. Alternative splicing coupled to nonsense-mediated decay (NMD) (McGlincy and Smith 2008) is another mechanism that can contribute to differences in protein expression and we have observed potential examples of AS-NMD among the sex-specific alternative splicing differences detected by our microarrays (e.g., Fig. 4).
Mechanisms of regulation
Previous work has documented a variety of mechanisms by which key sex-specific regulators, SXL and TRA, regulate alternative splicing of their target genes (for review, see Forch and Valcarcel 2003; Penalva and Sanchez 2003). These include direct occlusion of binding sites for general splicing factors (Valcarcel et al. 1993; Merendino et al. 1999; Forch et al. 2001), assembly of inhibitory or enhancer complexes targeting early events in spliceosome assembly (Tian and Maniatis 1994; Nagengast et al. 2003; Penn et al. 2008) and even mechanisms targeting late events in the splicing process (Lallena et al. 2002).
Such mechanisms generally assume that switches in splice-site choice are due to the presence or absence of key sex-specific regulators acting upon otherwise identical basal RNP complexes and splicing machineries. This assumption may not be warranted given the prevalence of sex-specific transcriptional and alternative splicing differences among RNA-binding proteins, including splicing factors (Table 2; Telonis-Scott et al. 2009). Indeed, we identified Hrp40/Sqd as an hnRNP protein whose gene displays sex-specific differences in expression and alternative splicing leading to higher expression of the protein in female flies (Fig. 4C). Furthermore, we find an enrichment of a related sequence motif near sex-specific junctions and Hrp40/Squid targets, and a statistically significant overlap between sex-regulated splice junctions and those affected by knockdown of this hnRNP protein in cell lines, as well as an excellent correlation with the predicted direction of the splicing changes. Taken together, these data suggest that sex-specific differences in hrp40/squid expression contribute to sex-specific splicing in Drosophila. Hrp40/sqd was not found to be regulated in Kc cells upon depletion of SXL, suggesting that sex-specific differences in hrp40/squid expression are established through a pathway distinct from the classical sex determination cascade. To our knowledge these results represent the first evidence of a contribution of sex-specific differential expression of RNA binding proteins outside of the classical pathway of sex-specific splicing regulators.
Variations in the expression or activity of general splicing factors are known to occur physiologically, for example, in different tissues, and have been proposed to contribute to tissue-specific splicing, perhaps explaining the relatively low number of tissue-specific splicing regulatory factors identified, particularly in vertebrates (for review, see Chen and Manley 2009). Evidence also exists for core splicing components acting as regulators of alternative splicing (Park et al. 2004) and as cofactors of tissue- or sex-restricted regulators. For example, elegant genetic and molecular work identified mutants in the gene sans fille (snf) that influence Sex-lethal autoregulation (Salz and Flickinger 1996; Stitzinger et al. 1999). SNF is the Drosophila homolog of the mammalian U1A and U2B″ (Polycarpou-Schwarz et al. 1996), which are integral protein components of U1 and U2 snRNPs that recognizes 5′- and 3′-splice sites, respectively.
The results of Telonis-Scott et al. (2009) demonstrate substantial conservation of sex-specific splicing in 11 strains of Drosophila melanogaster and in 7 Drosophila species, particularly among genes expressed in the reproductive tissue. This is in contrast with differences in expression or function of the upstream key regulators of sex determination in different species (Bopp et al. 1996) and even with genetic variation in their expression levels in D. melanogaster (Tarone et al. 2005), which suggest evolutionary diversity and flexibility in the program of master switches. The results of Figure 3 indicate that only the classical sex determinants (Sxl, tra, msl-2, and dsx) and a small number of other genes are affected in their sex-specific splicing patterns by knockdown of the master regulator SXL in Kc cells. Although these differences could be due to limited levels of Sxl knockdown in these experiments, the results at least indicate that the extent of dependence of upstream regulators is different for different genes in the regulatory cascade.
Our bioinformatic analyses indicate that SXL or TRA binding sites can be identified in only a fraction of sex-specific splicing events (Fig. 3C). This concept is also supported by the observation that certain alternative events display sex-specific splicing only in some tissues and not throughout the fly, as is the case for the classical examples Sxl, tra, msl-2, or dsx (Fig. 5A). Given that the sex-specific differences can be recapitulated in Kc vs. S2 cells mostly for ubiquitous sex-specific splicing events and not for tissue-restricted events, the results support the existence of distinct genetic programs that establish ubiquitous vs. tissue-restricted sex-specific splicing.
In this context, the most intriguing observation is the loss of sex-specific splicing in the heads of germline-less mutants, which affects genes that display ubiquitous sex-specific patterns, or even genes such as bcd that display sex-specific splicing only in the head. Two possibilities can be envisioned to explain these results. First, germline-less flies are heterozygous for tudor and RNA or protein products from the tudor locus, could play a direct role in sex-specific splicing. The TUDOR protein contains 11 Tudor domains, a protein motif that recognizes methyl-arginine modifications in target proteins (for review, see Thomson and Lasko 2005). Symmetrical arginine methylation is characteristic of Sm proteins, which are essential components of spliceosomal snRNPs (for review, see Wahl et al. 2009). Indeed, TUD is known to form a complex with two other proteins, Csu1 and Vls, which are relevant for Sm protein methylation (Gonsalvez et al. 2006; Anne et al. 2007). Although defective methylation has not been correlated with snRNP assembly or transport (Khusial et al. 2005), it might lead to defects in sex-specific alternative splicing in certain tissues. Arginine methylation has also been implicated in the control of subcellular localization and function of the mammalian SR protein SF2/ASF, an important regulator of splicing and translation (Sinha et al. 2010). It is therefore possible that tudor mutants, in addition to causing defects in germline formation, cause alterations in the function of spliceosomal complexes.
Second, it is possible that hormone-like signals produced in the germline trigger the activation of pathways in distant tissues, influencing gene expression and alternative splicing. In this context, many sex-specific transcriptional changes were reported to occur in the soma depending on the presence of germline (Parisi et al. 2004). Related examples of such signals are male-derived accessory gland proteins that are transferred via the seminal fluid from males to females, triggering not only local but also profound behavioral changes in the female fly (Chapman 2001; Liu and Kubli 2003; Datta et al. 2008; Ribeiro and Dickson 2010).
MATERIALS AND METHODS
Microarray analysis
Microarray hybridization and data acquisition was performed as previously described (Blanchette et al. 2005). Processing and analysis of microarray data for both sex-specific and hnRNP knockdown experiments (Blanchette et al. 2009), was carried out using the R and Bioconductor software (Gentleman et al. 2004) versions 2.11 and 2.6 and the Bioconductor package limma (Smyth 2004) for background correction using maximum likelihood estimation (Silver et al. 2009), normalization, and differential expression analysis.
A detailed description of the complete calculations can be found in the Supplemental Material. Briefly, gene-level log2 expression ratios were calculated for 2662 genes by summarizing the expression values of the corresponding exon probes using the same method employed by the summarization step of the RMA algorithm (Irizarry et al. 2003). Gene-level ratios were used to find differentially expressed genes and estimate the log2 ratio of net-expression for alternative junctions by subtracting them from the junction probe log2 ratios of expression. Differentially regulated junctions were identified by differential expression analysis of the values of net-expression. For both gene-level expression and net-expression ratios, the differential expression analysis was carried out using simple linear models and moderated t-statistics calculated by the empirical Bayes shrinkage method (Smyth 2004) implemented in the limma package with a cutoff value of 2 for the minimum fold change and a maximum adjusted P-value cutoff of 1%. The hnRNP knockdown data were analyzed similarly, using a common reference design and a smaller cutoff value of 1.5 for the minimum fold-change, due to the narrower dynamic range of these experiments.
Assigning alternative splicing events
Probe sequences and annotations (Blanchette et al. 2005) were downloaded from the NCBI GEO database through accession GPL7508. Due to the turnover of the FlyBase CG-identifiers, transcript and gene assignments were reannotated for all the 42,034 probes using an up-to-date nonredundant set of 25,965 FlyBase and Refseq transcripts (see Supplemental Material) and software from the UCSC Genome Browser (Kent et al. 2002) source code (http://genome.ucsc.edu/admin/cvs.html), in particular the tools txBedToGraph and txgAnalyze, generating a collection of 15,280 genome-wide alternative splicing (AS) event annotations. Junction probes were annotated as alternative or constitutive, depending on whether their flanking exons overlap or not, an intronic region of a different transcript from the same gene, considering also upstream of or downstream from exons in the case of alternative transcription start and polydenylation sites, respectively.
RNA isolation from flies
Bodies and heads were separated by vortexing flies frozen in liquid nitrogen and subsequent separation with a standard household sieve with pores small enough to retain the bodies but not the heads. RNA was isolated from these samples and kept frozen in liquid nitrogen. Flies, bodies, and heads were homogenized directly in Trizol (Invitrogen) using an eppendorf homogenizer and RNA was purified using the RNeasy Miniprep protocol (Qiagen) followed by DNase treatment. The integrity of the RNA was controlled using a Bioanalyzer and only RNA preparations with undetectable degradation of ribosomal RNA peaks were utilized for further analyses.
Generation of germline-less flies
tud1/Df(2R)PF1 mothers were crossed to wild-type males. Progenies were tested for their ability to fertilize (male flies) and lay eggs (female flies) to control for the lack of germline. Additionally, they were tested for their levels of a marker gene, dhd (Fig. 5A).
Primer design and quantitative RT-PCR
Primer design and quantitative RT-PCR was performed as described in Hartmann et al. (2009). Experiments were performed in triplicate and repeated at least three times per biological replica. The data were analyzed using relative quantification (Pfaffl 2001) and were represented as log2 ratios. Changes were considered validated when the net expression value corresponding to the probe was determined as twofold (log2 ratio sjnc – log2 ratio of GE) or higher by qRT-PCR for three biological replicas.
RNAi experiments in Drosophila cell culture
A large number of cells (1.5 × 106) were treated with 15 μg of Sxl dsRNA for 5 d as described in Clemens et al. (2000). Total RNA was isolated following the RNeasy Miniprep protocol (Qiagen), including DNase treatment.
Translation assays
The 5′ UTRs from exba were amplified by PCR from S2-cell cDNA and cloned in frame into the Cu2+ inducible vector pRmHA3 carrying the firefly-luc gene. Plasmids were verified by sequencing. For the 2 × 106 S2 cells were transfected with a total of 200 ng DNA using the Effectene Transfection Reagent (Qiagen) (10, 50, or 100 ng of 5′ UTR-pRmHA3-luc, 10 ng pRmHA3-renilla luciferase for normalization, and pRmHA3, to bring the total DNA to 200 ng). After 24 h, expression was induced with 500 μM Cu2So4 for 3 h. Cells were harvested, washed, and split into two vials so that half of the cells were used to measure luciferase activities, while RNA was isolated from the other half to quantify RNA levels (see below). For luciferase activity assays, cells were lysed in 40 μL 1X lysis buffer (Promega; Dual-Luciferase Reporter Assay System) on ice for 10 min; 10 μL of lysate was immediately used to measure firefly and renilla luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) in a plate reader (TriStar LB941, Berthold Technologies). The ratio of Firefly vs. Renilla activity was calculated. Experiments were done in triplicates and repeated four independent times.
To quantify transcript levels, cytoplasmic RNA was isolated following the RNAeasy protocol (Qiagen). cDNA was generated using AMV-reverse transcriptase (Promega) and a vector specific primer. Real-time PCR was carried out using a primer pair specific for luciferase as described above. The log2 of the relative ratio of Cp values was subsequently calculated.
Western blot analysis
Western blotting was performed as described in Blanchette et al. (2009). Anti-hrp40 antibody was used in 1:2000 and was a gift from M. Blanchette. Anti-beta-actin (Cell Signaling; #4967) was used as a 1:1000 dilution. Intensities of the bands were quantified using MultiGauge from Fujifilm and are presented as the ratio in female/male flies normalized to actin.
SXL binding site search
We considered the SXL binding site, based on the SELEX experiment and sequence conservation in tra (UUUUUGUUGUUUUUUUU), as a 17-nt uridine sequence with up to two guanosines (Singh et al. 1995). We used the list of all possible combinations of this SXL binding site to search for the sequences near the relevant 5′ or 3′ splice sites by standard string matching implemented in Python. For the Tra-binding sites, we searched for the consensus derived from the sequences relevant for the sex-specific splicing regulation of dsx and fru: [T/A]C[T/A][T/A]C[A/G]ATCAACA (Heinrichs et al. 1998).
GO analysis
All Gene Ontology (GO) enrichment analyses were carried out using the conditional hypergeometric test implemented in the package GOstats and the GO annotations available from the Bioconductor project using version 2.6 (March 2010). The previously filtered set of 2662 genes was considered as the gene universe. Only categories with a P-value < 0.05 were considered as enriched.
Motif enrichment analysis
Motif searches using the MEME software (Bailey and Elkan 1994) were applied to proximal regions of 486 annotated alternative sjncs (alternative 5′ss, alternative 3′ss, cassette exon and retained intron) differentially regulated between male and female flies. Proximal regions included 50 nt either in the intronic or exonic regions 5′ or 3′ adjacent to each splice site. Further categories for the analysis include combinations of these regions (intronic + exonic, all regions adjacent to a junction), thus generating seven classes of proximal regions. The 5′ seven intronic nucleotides at 5′ ss and the 30 3′ intronic nucleotides at 3′ ss were not considered to avoid enrichment in splice-site consensus sequences. A similar number of control sequences were selected using the same criteria, from non-sex-regulated junctions and discarding events from genes with undetectable or large expression changes as well as genes displaying sex-specific changes in other regions of the pre-mRNA.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We are grateful to F. Gebauer and G. Pyrowolakis for support and discussions and members of our laboratories for comments on the manuscript. This work was supported by grants from Fundación Marcelino Botín, Fundación Alicia Koplowicz, EURASNET, AICR, Spanish Ministerio de Ciencia e Innovación, Consolider RNAREG, and Excellence Initiative of the German Federal and State Governments (EXC 294). B.H. was supported by an EMBO long-term fellowship and DFG Stipendium. R.C. is supported by a “Ramon y Cajal” research fellowship (RYC-2006-000932) and a grant (TIN2008-00556/TIN) from the Spanish Ministerio de Ciencia e Innovación (MICINN).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2460411.
REFERENCES
- Anne J, Ollo R, Ephrussi A, Mechler BM 2007. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134: 137–146 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Elkan C 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36 [PubMed] [Google Scholar]
- Baker BS 1989. Sex in flies: the splice of life. Nature 340: 521–524 [DOI] [PubMed] [Google Scholar]
- Baker BS, Ridge KA 1980. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94: 383–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Burtis K, Goralski T, Mattox W, Nagoshi R 1989. Molecular genetic aspects of sex determination in Drosophila melanogaster. Genome 31: 638–645 [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Baker BS 1995. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development 121: 3245–3258 [DOI] [PubMed] [Google Scholar]
- Bell LR, Horabin JI, Schedl P, Cline TW 1991. Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65: 229–239 [DOI] [PubMed] [Google Scholar]
- Ben-Dov C, Hartmann B, Lundgren J, Valcarcel J 2008. Genome-wide analysis of alternative pre-mRNA splicing. J Biol Chem 283: 1229–1233 [DOI] [PubMed] [Google Scholar]
- Blanchette M, Green RE, Brenner SE, Rio DC 2005. Global analysis of positive and negative pre-mRNA splicing regulators in Drosophila. Genes Dev 19: 1306–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette M, Green RE, MacArthur S, Brooks AN, Brenner SE, Eisen MB, Rio DC 2009. Genome-wide analysis of alternative pre-mRNA splicing and RNA-binding specificities of the Drosophila hnRNP A/B family members. Mol Cell 33: 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekhman R, Marioni JC, Zumbo P, Stephens M, Gilad Y 2010. Sex-specific and lineage-specific alternative splicing in primates. Genome Res 20: 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe B, Brenner S, Hughes T, Morris Q 2009. Post-transcriptional gene regulation: RNA–protein interactions, RNA processing, mRNA stability and localization. Pac Symp Biocomput 2009: 545–548 [PubMed] [Google Scholar]
- Bopp D, Calhoun G, Horabin JI, Samuels M, Schedl P 1996. Sex-specific control of Sex-lethal is a conserved mechanism for sex determination in the genus Drosophila. Development 122: 971–982 [DOI] [PubMed] [Google Scholar]
- Burtis KC, Baker BS 1989. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56: 997–1010 [DOI] [PubMed] [Google Scholar]
- Calarco JA, Xing Y, Caceres M, Calarco JP, Xiao X, Pan Q, Lee C, Preuss TM, Blencowe BJ 2007. Global analysis of alternative splicing differences between humans and chimpanzees. Genes Dev 21: 2963–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T 2001. Seminal fluid-mediated fitness traits in Drosophila. Heredity 87: 511–521 [DOI] [PubMed] [Google Scholar]
- Chen M, Manley JL 2009. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 10: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci 97: 6499–6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline TW 1984. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 107: 231–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline TW 1993. The Drosophila sex determination signal: how do flies count to two? Trends Genet 9: 385–390 [DOI] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R 2008. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452: 473–477 [DOI] [PubMed] [Google Scholar]
- Ellegren H, Parsch J 2007. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8: 689–698 [DOI] [PubMed] [Google Scholar]
- Forch P, Valcarcel J 2003. Splicing regulation in Drosophila sex determination. Prog Mol Subcell Biol 2003: 127–151 [DOI] [PubMed] [Google Scholar]
- Forch P, Merendino L, Martinez C, Valcarcel J 2001. Modulation of msl-2 5′ splice site recognition by Sex-lethal. RNA 7: 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80 doi: 10.1184/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez GB, Rajendra TK, Tian L, Matera AG 2006. The Sm-protein methyltransferase, dart5, is essential for germ-cell specification and maintenance. Curr Biol 16: 1077–1089 [DOI] [PubMed] [Google Scholar]
- Hartmann B, Castelo R, Blanchette M, Boue S, Rio DC, Valcarcel J 2009. Global analysis of alternative splicing regulation by insulin and wingless signaling in Drosophila cells. Genome Biol 10: R11 doi: 10.1186/gb-2009-10-1-r11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs V, Ryner LC, Baker BS 1998. Regulation of sex-specific selection of fruitless 5′ splice sites by transformer and transformer-2. Mol Cell Biol 18: 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TA 2006. Regulation of gene expression by alternative untranslated regions. Trends Genet 22: 119–122 [DOI] [PubMed] [Google Scholar]
- Inoue K, Hoshijima K, Sakamoto H, Shimura Y 1990. Binding of the Drosophila Sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature 344: 461–463 [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D 1996. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci 93: 9687–9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RL, Wang J, Bell L, Kuroda MI 1997. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387: 195–199 [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D 2002. The human genome browser at UCSC. Genome Res 12: 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusial PR, Vaidya K, Zieve GW 2005. The symmetrical dimethylarginine post-translational modification of the SmD3 protein is not required for snRNP assembly and nuclear transport. Biochem Biophys Res Commun 337: 1119–1124 [DOI] [PubMed] [Google Scholar]
- Kim E, Magen A, Ast G 2007. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res 35: 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallena MJ, Chalmers KJ, Llamazares S, Lamond AI, Valcarcel J 2002. Splicing regulation at the second catalytic step by Sex-lethal involves 3′ splice site recognition by SPF45. Cell 109: 285–296 [DOI] [PubMed] [Google Scholar]
- Lasko P 2000. The Drosophila melanogaster genome: translation factors and RNA binding proteins. J Cell Biol 150: F51–F56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, Cao H, Kim HJ, Lee ZH, Oh SB, et al. 2007. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development 134: 1767–1777 [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci 100: 9929–9933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Holm L, Sánchez L, Kaufman M 2003. A theoretical model for the regulation of Sex-lethal, a gene that controls sex determination and dosage compensation in Drosophila melanogaster. Genetics 165: 1355–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlincy NJ, Smith CW 2008. Alternative splicing resulting in nonsense-mediated mRNA decay: what is the meaning of nonsense? Trends Biochem Sci 33: 385–393 [DOI] [PubMed] [Google Scholar]
- McIntyre LM, Bono LM, Genissel A, Westerman R, Junk D, Telonis-Scott M, Harshman L, Wayne ML, Kopp A, Nuzhdin SV 2006. Sex-specific expression of alternative transcripts in Drosophila. Genome Biol 7: R79 doi: 10.1186/gb-2006-7-8-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M, Madigan SJ 1992. Sex determination and differentiation in invertebrates: Drosophila and Caenorhabditis elegans. Curr Opin Cell Biol 4: 948–954 [DOI] [PubMed] [Google Scholar]
- Merendino L, Guth S, Bilbao D, Martinez C, Valcarcel J 1999. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 402: 838–841 [DOI] [PubMed] [Google Scholar]
- Mount SM, Salz HK 2000. Pre-messenger RNA processing factors in the Drosophila genome. J Cell Biol 150: F37–F44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagengast AA, Stitzinger SM, Tseng CH, Mount SM, Salz HK 2003. Sex-lethal splicing autoregulation in vivo: interactions between SEX-LETHAL, the U1 snRNP and U2AF underlie male exon skipping. Development 130: 463–471 [DOI] [PubMed] [Google Scholar]
- Nagoshi RN, McKeown M, Burtis KC, Belote JM, Baker BS 1988. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell 53: 229–236 [DOI] [PubMed] [Google Scholar]
- Nilsen TW, Graveley BR 2010. Expansion of the eukaryotic proteome by alternative splicing. Nature 463: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Shai O, Misquitta C, Zhang W, Saltzman AL, Mohammad N, Babak T, Siu H, Hughes TR, Morris QD, et al. 2004. Revealing global regulatory features of mammalian alternative splicing using a quantitative microarray platform. Mol Cell 16: 929–941 [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ 2008. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40: 1413–1415 [DOI] [PubMed] [Google Scholar]
- Parisi M, Nuttall R, Edwards P, Minor J, Naiman D, Lu J, Doctolero M, Vainer M, Chan C, Malley J, et al. 2004. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol 5: R40 doi: 10.1186/gb-2004-5-6-r40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Parisky K, Celotto AM, Reenan RA, Graveley BR 2004. Identification of alternative splicing regulators by RNA interference in Drosophila. Proc Natl Acad Sci 101: 15974–15979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalva LO, Sanchez L 2003. RNA binding protein Sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev 67: 343–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JK, Graham P, Deshpande G, Calhoun G, Chaouki AS, Salz HK, Schedl P 2008. Functioning of the Drosophila Wilms'-tumor-1-associated protein homolog, Fl(2)d, in Sex-lethal-dependent alternative splicing. Genetics 178: 737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45 doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfreundt U, James DP, Tweedie S, Wilson D, Teichmann SA, Adryan B 2010. FlyTF: improved annotation and enhanced functionality of the Drosophila transcription factor database. Nucleic Acids Res 38: D443–D447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polycarpou-Schwarz M, Gunderson SI, Kandels-Lewis S, Seraphin B, Mattaj IW 1996. Drosophila SNF/D25 combines the functions of the two snRNP proteins U1A and U2B′ that are encoded separately in human, potato, and yeast. RNA 2: 11–23 [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ 2010. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol 20: 1000–1005 [DOI] [PubMed] [Google Scholar]
- Robida MD, Rahn A, Singh R 2007. Genome-wide identification of alternatively spliced mRNA targets of specific RNA-binding proteins. PLoS ONE 2: e520 doi: 10.1371/journal.pone.0000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner LC, Baker BS 1991. Regulation of doublesex pre-mRNA processing occurs by 3′-splice site activation. Genes Dev 5: 2071–2085 [DOI] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA 1996. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87: 1079–1089 [DOI] [PubMed] [Google Scholar]
- Salz HK, Flickinger TW 1996. Both loss-of-function and gain-of-function mutations in snf define a role for snRNP proteins in regulating Sex-lethal pre-mRNA splicing in Drosophila development. Genetics 144: 95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus E 1986. Germline autonomy of maternal-effect mutations altering the embryonic body pattern of Drosophila. Dev Biol 113: 443–448 [DOI] [PubMed] [Google Scholar]
- Schutt C, Nothiger R 2000. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127: 667–677 [DOI] [PubMed] [Google Scholar]
- Silver JD, Ritchie ME, Smyth GK 2009. Microarray background correction: maximum likelihood estimation for the normal-exponential convolution. Biostatistics 10: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Valcarcel J, Green MR 1995. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268: 1173–1176 [DOI] [PubMed] [Google Scholar]
- Sinha R, Allemand E, Zhang Z, Karni R, Myers MP, Krainer AR 2010. Arginine methylation controls the subcellular localization and functions of the oncoprotein splicing factor SF2/ASF. Mol Cell Biol 30: 2762–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: 3 doi: 10.2202/1544-6115.1027 [DOI] [PubMed] [Google Scholar]
- Stitzinger SM, Conrad TR, Zachlin AM, Salz HK 1999. Functional analysis of SNF, the Drosophila U1A/U2B″ homolog: identification of dispensable and indispensable motifs for both snRNP assembly and function in vivo. RNA 5: 1440–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolc V, Gauhar Z, Mason C, Halasz G, van Batenburg MF, Rifkin SA, Hua S, Herreman T, Tongprasit W, Barbano PE, et al. 2004. A gene expression map for the euchromatic genome of Drosophila melanogaster. Science 306: 655–660 [DOI] [PubMed] [Google Scholar]
- Su WL, Modrek B, GuhaThakurta D, Edwards S, Shah JK, Kulkarni AV, Russell A, Schadt EE, Johnson JM, Castle JC 2008. Exon and junction microarrays detect widespread mouse strain- and sex-bias expression differences. BMC Genomics 9: 273 doi: 10.1186/1471-2164-9-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone AM, Nasser YM, Nuzhdin SV 2005. Genetic variation for expression of the sex determination pathway genes in Drosophila melanogaster. Genet Res 86: 31–40 [DOI] [PubMed] [Google Scholar]
- Telonis-Scott M, Kopp A, Wayne ML, Nuzhdin SV, McIntyre LM 2009. Sex-specific splicing in Drosophila: widespread occurrence, tissue specificity and evolutionary conservation. Genetics 181: 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T, Lasko P 2005. Tudor and its domains: germ cell formation from a Tudor perspective. Cell Res 15: 281–291 [DOI] [PubMed] [Google Scholar]
- Tian M, Maniatis T 1994. A splicing enhancer exhibits both constitutive and regulated activities. Genes Dev 8: 1703–1712 [DOI] [PubMed] [Google Scholar]
- Valcárcel J, Singh R, Zamore PD, Green MR 1993. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature 362: 171–175 [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R 2009. The spliceosome: design principles of a dynamic RNP machine. Cell 136: 701–718 [DOI] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 456: 470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Lee C 2007. Relating alternative splicing to proteome complexity and genome evolution. Adv Exp Med Biol 2007: 36–49 [DOI] [PubMed] [Google Scholar]