Abstract
Post-weaning social isolation (SI) is a model of prolonged mild stress characterized by behavioral and neurochemical alterations. We used SI in C57BL/6J mice to investigate the effects of ethanol (EtOH) in the free-choice drinking paradigm on gene expression and function of γ-aminobutyric acid type A receptors (GABAARs) and the role of neuroactive steroids in the actions of EtOH in the hippocampus. SI stress induced a marked reduction in hippocampal 3α-hydroxy-5α-pregnan-20-one (3α,5α-TH PROG) and was associated with molecular and functional changes of the GABAAR. The gene expression of the α4 and δ subunits was increased in the hippocampus of SI C57BL/6J mice; the expression of the γ2 subunit was decreased whereas that of the α1 did not change. Patch-clamp recordings in dentate gyrus (DG) granule cells obtained from SI C57BL/6J mice revealed a greater enhancement of tonic currents induced by α-(4,5,6,7-tetrahydroisoxazolo[5,4-c] pyridin-3-ol (THIP) compared to that in control C57BL/6J mice. These neurochemical, molecular and functional changes observed in SI C57BL/6J mice were associated with an increased EtOH intake and EtOH preference. Nevertheless, the increase in EtOH consumption did not restore the reduction in hippocampal 3α,5α-TH PROG induced by SI. EtOH self-administration blocked the changes in gene expression of the α4 subunit but not those of the δ and γ2 subunits induced by SI. In addition, EtOH self-administration did not block the SI-induced changes in GABAAR-mediated tonic inhibition in hippocampal granule cells but increased the frequency of basal GABAergic sIPSCs in DG granule cells. We conclude that self-administration of EtOH selectively abolishes the increase of α4 subunit but not other neurochemical, molecular, and functional modifications induced by SI prolonged mild stress.
Keywords: social isolation, stress, ethanol, allopregnanolone, GABAA receptor, gene expression, patch-clamp, hippocampus
Introduction
Social isolation (SI) has been extensively shown to be associated with marked behavioral alterations, such as changes in locomotor activity, anxiety, depression, and aggressiveness in laboratory animals, including mice and rats, suggesting that SI is a stressful condition for these normally gregarious animals and that the abnormal behavioral response of rodents so reared is the product of prolonged stress. We here used SI in C57BL/6J mice as a model of prolonged mild stress by which to investigate the role of neuroactive steroids in the actions of ethanol (EtOH) on the function of γ-aminobutyric acid type A receptors (GABAARs) in the hippocampus. Neurochemical, electrophysiological, and behavioral evidence suggests that the GABAAR might be an important and sensitive site in mediating some of the acute and chronic actions of EtOH (Faingold et al., 1998; Harris, 1999; Ueno et al., 2001). EtOH has been shown to selectively enhance the function of recombinant α4/α6- and δ-containing GABAARs expressed in Xenopus oocytes at concentrations as low as 3–30 mM that resulted ineffective in receptors where the δ subunit was replaced with the γ2 subunit (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). In contrast to γ2-containing receptors, those formed by α4/α6 and δ subunits are located exclusively at extrasynaptic sites and are thought to mediate the tonic inhibitory activity (Mody et al., 1994; Semyanov et al., 2004). Furthermore, extrasynaptic GABAARs, expressed preferentially in the dentate gyrus (DG) and thalamus (α4βδ), and cerebellar granule cells (α6βδ; Semyanov et al., 2004), are characterized by a higher affinity for GABA (about 0.5 μM), display a slower desensitization rate, and are preferentially sensitive to neuroactive steroids (Semyanov et al., 2004). Increase of GABAAR-mediated tonic activity by a low (30 mM) concentration of EtOH has been demonstrated in DG granule cells (Wei et al., 2004). However, it should be noted that other studies are not in agreement (Borghese et al., 2006; Yamashita et al., 2006) and it appears at this stage that the idea that extrasynaptic receptors may represent important targets for the action of EtOH at pharmacologically relevant concentrations needs further experimental evaluation.
Previous work in our and other laboratories has demonstrated that SI in rats is associated with a decrease in plasma and brain levels of neuroactive steroids such as the progesterone metabolites 3α-hydroxy-5α-pregnan-20-one (3α,5α-TH PROG) and 3α,5α-tetrahydrodeoxycorticosterone (3α,5α-TH DOC; Serra et al., 2000). These changes are paralleled by an increase in the expression of the GABAAR α4 and δ subunits throughout the hippocampus (Serra et al., 2006).
The molecular mechanisms that underlie the persistent decrease in the abundance of neuroactive steroids induced by prolonged SI of rats remain unclear. However, consistent with the notion that a “facilitatory trace,” characterized by hyper-responsiveness of the hypothalamic-pituitary-adrenal axis (HPA axis) to new stimuli, may develop during chronic stress (Akana et al., 1992), we have previously demonstrated that the functional response of the HPA axis to an acute stress stimulus is enhanced in SI rats (Serra et al., 2000). More recently, our group showed that the increases in both the activity of the HPA axis and the plasma and brain concentrations of 3α,5α-TH PROG and 3α,5α-TH DOC induced by a systemic injection of EtOH are potentiated by SI in rats (Serra et al., 2003), suggesting that chronic stress may induce a plastic adaptation of neuronal systems that contributes to an increased vulnerability to alcohol abuse. It has been proposed that certain acute actions of EtOH at GABAARs might be mediated by the peripheral secretion of neuroactive steroids (Morrow et al., 1999, 2001; Kumar et al., 2009). Acute EtOH administration indeed increases the concentrations of 3α,5α-TH PROG in the plasma, cerebral cortex, and hippocampus of rats (Barbaccia et al., 1999; VanDoren et al., 2000; Morrow et al., 2001). Furthermore, pretreatment of animals with the 5α-reductase inhibitor finasteride, which inhibits the biosynthesis of 3α,5α-TH PROG, reduced the extent of the EtOH-induced increase in the cerebrocortical levels of 3α,5α-TH PROG and prevented certain neurochemical, electrophysiological, and behavioral effects of EtOH (VanDoren et al., 2000; Khisti et al., 2002). Thus, it has been postulated that certain effects of EtOH on GABAARs may be mediated by neuroactive steroids produced by peripheral organs in response to the activation of the HPA axis. Furthermore, in our previous study (Sanna et al., 2004), we found that EtOH can increase the concentrations of 3α,5α-TH PROG in rat isolated hippocampal slices, with an action independent of the HPA axis activity. This effect resulted in the positive modulation of GABAAR-mediated IPSCs recorded in voltage-clamped CA1 pyramidal neurons in hippocampal slices. This action of EtOH could be prevented by the 5α-reductase inhibitor finasteride, suggesting that the local synthesis 3α,5α-TH PROG from progesterone is required for the modulation of GABAAR function by this drug. Since the GABAAR represents an important target in certain pharmacological actions of EtOH, the functional interaction between neuroactive steroids and GABAARs may also be important in the response to neurobiological effects of EtOH that may depend on subunit composition in specific brain circuits.
The main objective of the present work was to evaluate the role of neuroactive steroids as possible mediators of the actions exerted by EtOH on GABAAR gene expression and function and the interplay with stress. Given that SI rats (Schenk et al., 1990; Wolffgramm, 1990; Hall et al., 1998) and C57BL/6J mice (Lopez et al., 2010) consume increased amounts of EtOH, one of the aims of the present study was to evaluate the effects of voluntary EtOH consumption on different molecular and functional parameters. In particular we evaluated the effect of SI stress on hippocampal neuroactive steroid levels and the gene expression of different GABAAR subunits and related function in the hippocampus of C57BL/6J mice given free-choice EtOH drinking during the SI period.
Materials and Methods
Social isolation stress paradigm
Male C57BL/6J mice were bred in our animal facility, maintained under an artificial 12-h-light (on at 2300), 12-h-dark (off at 1100) cycle at a constant temperature of 23° and 65% humidity. Food and water were freely available at all times. Animal care and handling throughout the experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). After birth in our animal facility, mouse pups were left undisturbed with their mothers until weaning (21 days after birth). After weaning, male mice were selected and randomly divided into two experimental groups as follow: six per cage (group-housed or GH) or one per cage (socially isolated or SI) for the following 6 weeks. Mice were therefore 9 weeks old at the time the experiments were performed. These two groups of animals were used to perform all the procedures listed below.
Spontaneous locomotor activity testing
Independent groups of GH and SI C57BL/6J mice were tested at the end of the 6-week isolation period. To determine general activity levels, gross locomotor activity, and exploration habits, the motility meter was used. Animals were left in the same room in which the apparatus was placed for at least 2 h prior the beginning of experiments to allow for habituation to the environment. Assessment took 60 min during which parameters were collected every 5 min (12 acquisitions total). The open field consisted of different square arenas (20.3 cm × 20.3 cm) assembled with specially designed sound-attenuating shells made of polypropylene, and expanded PVC sheet. The recorder and printer needed to acquire all data were placed in a different room in order to isolate the animals from any noise. The experimental protocol was as follows: individual C57BL/6J mice were placed in the center of the arena and allowed to freely move for 60 min while being tracked by an automated tracking system. At the conclusion of each trial the surface of the arena was cleaned with 70% EtOH. We tested nine GH and nine IS mice. The parameters observed were: horizontal activity, total distance, locomotion time, and rest time.
EtOH drinking behavior: two-bottle choice home cage limited access paradigm
Using a modified sucrose-fading technique (Samson, 1986), C57BL/6J mice were given daily access to EtOH for 2 h in the home cage, beginning at 0.5 h prior to the start of the dark cycle (1100 h). During the 2-h limited access period mice were given a two-bottle choice (EtOH vs. water), and the position of the bottles was alternated on a daily basis. Every day during the 2-h procedure the mice of GH experimental group were individually housed and at the end of the 2-h were again housed in group. For both experimental GH and SI groups the single standard water bottle was removed from each cage and replaced with two 250 ml bottles, one containing EtOH/sucrose solutions and the second one containing water/sucrose at matching sucrose concentration during the first 10 day period of the sucrose-fading procedure and then just water or EtOH as follow: 10% EtOH/5% sucrose for 2 days, 12% EtOH/5% sucrose for 2 days, 15% EtOH/5% sucrose for 2 days, 15% EtOH/2% sucrose for 2 days, 15% EtOH/1% sucrose for 2 days, and then 15% EtOH/0% sucrose as a final solution for the rest of the 6-weeks study. At the end of the 2-h access period, EtOH bottles and water bottles were removed and the one standard water bottle was returned to the home cage. The GH experimental group of mice were re-grouped six per cage (red lights in the colony room minimize disturbance of animals during their dark cycle). Although C57BL/6J mice will readily drink EtOH (Fuller, 1964; Yoneyama et al., 2008), the sucrose-fading procedure was employed to further stabilize their daily intake. The different EtOH and sucrose solutions were prepared as v/v and w/v solutions, respectively. The EtOH solutions were presented at room temperature. EtOH (2 h) and water (2 h) intake were measured daily by weighing the bottles. The difference with the initial weight was calculated in order to establish the amount of fluids drank during the 2-h period. Dependent variables recorded and analyzed include EtOH intake (gram and gram per kilogram), water intake (gram), and total fluid intake (EtOH + water intake). EtOH preference ratio was calculated as the volume of EtOH, divided by the total volume of liquid (EtOH + water) consumed. At the end of the 6-weeks period, the last 2 h session of the two-bottle choice, mice were either sacrificed in the dark and brain samples were collected and immediately used (electrophysiology), or frozen in dry ice and stored at −80°C and then used for the different assays as described below. Another group of animals was used for immunohistochemistry and sacrificed as described below.
Extraction and assay of steroids
Mouse hippocampi were dissected and then frozen at −80°C until steroid extraction. 3α,5α-TH PROG was extracted from hippocampal homogenates and purified as previously described (Serra et al., 2000). The extract residue was dissolved in 5 ml of n-hexane and applied to a SepPak silica cartridge (Waters SpA, Milan, Italy), and eluted components were separated and further purified by HPLC on a 5-m Lichrosorb-diol column (250 by 4 mm, Phenomenex, Torrance, CA, USA) with a discontinuous gradient of 2-propanol (0 to 30%) in n-hexane. Recovery (70–80%) of 3α,5α-TH PROG through the extraction and purification procedures was monitored by addition of a trace amount (6000–8000 cpm, 20–80 Ci/mmol) of 3H-labeled standard (Perkin Elmer Italia, Monza, Milan, Italy) to the brain homogenate. 3α,5α-TH PROG was quantified by radioimmunoassay with specific antibodies generated in sheep (Purdy et al., 1990; Serra et al., 2000). Due to the small amount of plasma that could be obtained from each animal it has not been possible to quantify the plasma concentrations of 3α,5α-TH PROG, corticosterone, and ACTH.
RNA extraction and measurement of GABAAR subunit mRNAs by RNase protection assay
Total RNA was extracted from the frozen hippocampi by using standard procedures routinely employed in our laboratory and messenger RNAs (mRNAs) encoding for the indicated GABAAR subunits were measured by RNase protection assay as previously described (Follesa et al., 1998) by using specific mouse probes (Yu et al., 1996). Twenty-five microgram of total RNA were dissolved in 20 μl of hybridization solution containing 150,000 cpm of 32P-labeled cRNA probe for a specific GABAAR subunit (6 × 107 to 7 × 107 cpm/μg) and hybridization reaction mixture was incubated at 50°C overnight and then subjected to polyacrylamide gel electrophoresis and autoradiography. The amounts of GABAAR subunit mRNAs and internal standard (cyclophilin) mRNA were determined by measurement of the optical density of the corresponding bands on the autoradiogram with a densitometer. The amount of mRNA was expressed in arbitrary units.
Immunohistochemistry
Mice were administered a lethal dose (0.3 ml/g) of Equithesin (1 g of sodium pentobarbital, 4.251 g of choral hydrate, 2.125 g of MgSO4, 12 ml of ethanol, and 43.6 ml of propylene glycol, adjusted to a total volume of 100 ml with distilled water) and were then perfused through the ascending aorta with 20 ml of PBS followed by 50 ml of 4% paraformaldehyde in PBS. The brain was removed, exposed to the same fixative for 2 h, and then transferred to 30% (w/v) sucrose in PBS. Coronal sections (thickness, 16 μm) were cut with a criostat HM 560 (Microm) and these were mounted onto SuperFrost Plus glass slides (Menzel-Glaser, Germany) and stored at −20°C. The day of the experiment, glass slides were towed at room temperature, and sections incubated for 30 min at room temperature with 0.1% phenylhydrazine to block endogenous peroxidase activity. Permeabilization was then performed by incubation for 1 h with 0.2% Triton X-100 in PBS (PBS-T) followed by incubation for 1 h with 10% normal donkey serum (Jackson ImmunoResearch Europe Ltd, Suffolk, UK) in PBS-T. They were then incubated at 4°C overnight with rabbit polyclonal antibodies to the α1, α4, γ2, or δ subunits (Phosphosolutions, CO, USA) at dilutions of 1:300, 1:500, 1:250, or 1:300, respectively, in PBS-T containing 10% normal donkey serum. After several washes, the sections were incubated at room temperature first for 2 h with anti-rabbit biotinylated donkey antibodies to IgG (Jackson ImmunoResearch) diluted 1:200 in PBS-T and then for 30 min with avidin–peroxidase solution (Vectastain Elite Kit; Vector Laboratories), and washed three times with PBS-T after each incubation. The reaction product was visualized by exposure of the sections to 0.4 mM 3,3-diaminobenzidine (Sigma) and 0.01% H2O2. After several washes with PBS, the sections were dehydrated in ethanol, and cleared in xylene, and a coverslip was then applied in the presence of Eukitt mounting medium. Negative control sections either incubated with primary antibodies in the presence of the corresponding peptide antigen or not exposed to primary antibodies did not yield positive staining. Sections were examined with a BX-41 microscope (Olympus) and photographed with an F-View chargecoupled device camera. Semi-quantitative analysis of images was performed with AnalySIS 3.2 software (Soft Imaging System). In each image, the DG molecular layer of the hippocampus was selected by drawing a line surrounding the region of interest (ROI), based on plates 50–54 of the Franklin and Paxinos atlas (Franklin and Paxinos, 1996). The intensity of immunostaining in each ROI was determined as an integral from the intensity value for each pixel and the total number of pixels and was corrected for the background obtained in negative control sections. The intensity values, which represent the abundance of the corresponding GABAAR subunit, were represented by a gray scale and expressed as percentage change relative to the control mice.
Brain slice preparation for electrophysiological experiments
After 6 weeks of isolation and exposure to voluntary EtOH consumption, brain slices were prepared from the different groups of mice. Animals were anesthetized with chloroform and then decapitated. Their brains were transferred rapidly, for extracellular recordings, to a standard artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 d-glucose (pH 7.4, set by aeration with 95% O2/5% CO2) or to a modified ACSF (pH 7.4, set by aeration with 95% O2/5% CO2) containing (in mM): 220 sucrose, 2 KCl, 0.2 CaCl2, 6 MgSO4, 26 NaHCO3, 1.3 NaH2PO4, and 10 d-glucose, for patch-clamp recordings. Coronal brain sections (300 or 400 μm thick) were cut in ice-cold standard or modified ACSF using a Leica VT1200S vibratome (Leica, Heidelberg, Germany). Slices were then transferred immediately to a nylon net submerged in normal ACSF, for at least 40 min at 35°C controlled temperature. Then, following at least 1 h of incubation at room temperature, hemi-slices were transferred to a recording chamber, submerged in normal ACSF with a constant flow of ~2 ml/min. For all recordings, the temperature of the bath was maintained at 33°C.
Extracellular recording of fEPSPs
Field excitatory postsynaptic potential (fEPSP) recordings were obtained from the CA1 region of the stratum radiatum following stimulation of the Schaffer/Commissural afferents. Extracellular recording electrodes were made from borosilicate capillaries with a filament and an outer diameter of 1.5 μm (Sutter Instruments, Novato, CA, USA) filled with 4 M NaCl (1–2 MΩ). For stimulation of afferents, a concentric bipolar electrode was used and this was placed about 300 μm apart from the recording site. Responses were triggered digitally every 20 s using an interval generator (Master 8) and a stimulus isolator by applying a constant current pulse of 60-μs duration and 0.1–0.3 mA intensity, which yielded a half-maximal response. For the input/output curves, stimulation current intensity ranged from 0 to 1 mA with steps of 0.1 mA. fEPSPs were amplified by an Axoclamp 2B amplifier (Axon Instruments, Union City, CA, USA) digitized and analyzed off-line using Clampfit 8.02 software (Axon Instruments). Several kinetic parameters of fEPSP were analyzed, but the slope values were considered for quantifying the responses. To elicit LTP, after a baseline recording of fEPSPs evoked every 20 s at the current intensity set to evoke 50% of the maximal fEPSP response, high frequency stimulation (HFS) consisting of a single train of 100 stimuli at 250 Hz was delivered, and then recording continued for 60 min with stimulations of fEPSP every 20 s, to determine the effect of HFS.
Whole-cell patch-clamp experiments
Whole-cell recordings from hippocampal DG principal neurons were performed as previously described. Recording pipettes were pulled from borosilicate glass on a Fleming Brown micropipette puller (Molecular Devices, Novato, CA, USA). Pipette resistance ranged from 2.5 to 4.5 MΩ when filled with an internal solution containing (in mM): 150 CsCl, 10 Hepes, 5 lidocaine N-ethyl bromide, 2 MgCl2, 3 Mg-ATP, 0.3 Na-GTP, and 10 BAPTA-4K, pH adjusted to 7.2 with CsOH, and osmolarity set to 298 mOsm with sucrose. To record GABA-evoked Cl− currents kynurenic acid (3 mM) was added to the extracellular ACSF recording solution to block NMDA, kainate, and AMPA receptor-mediated currents. Cells were voltage-clamped at −65 mV. We analyzed only recordings with access resistance of <25 MΩ (usually ranging from 9 to 20 MΩ). The series resistance was not compensated, and if access resistance changed by more that 20% during the course of the recording, the cell was discarded. Synaptic currents were recorded with an Axopatch 200-B amplifier (Axon Instruments, Foster City, CA, USA), filtered at 2 kHz, and digitized at 5 kHz. Spontaneous inhibitory postsynaptic current (sIPSC) amplitude, decay time, rise time, and frequency were recorded using peak and event detection software in pClamp9.2 (Union City, CA, USA) and analysis was performed with Minianalysis 60. After initiation of the whole-cell recording, we waited 2–3 min for response to stabilize, and then, recorded baseline activity for approximately 3 min. For the experiments evaluating changes in tonic current in hippocampal DG from different animal groups, 6 min of GABAAR partial agonist α-(4,5,6,7-tetrahydroisoxazolo[5,4-c] pyridin-3-ol; THIP; 3 μM) was perfused to increase tonic GABAergic currents. After THIP perfusion, the GABAA receptor antagonist bicuculline (20 μM) was added to block both phasic and tonic currents. To evaluate the tonic component of GABAergic inhibition, noise variance, and shift in holding current were evaluated.
Statistical analysis
Data are presented as mean ± SEM and compared by one-way or two-way analysis of variance (ANOVA) and Scheffe's test with the use of Statistica software (StatSoft, Tulsa, OK, USA) or Student's t test with the use of Prism software (version 5, Graphpad). A p value of <0.05 was considered statistically significant.
Results
Effects of SI on spontaneous locomotor activity in C57BL/6J mice
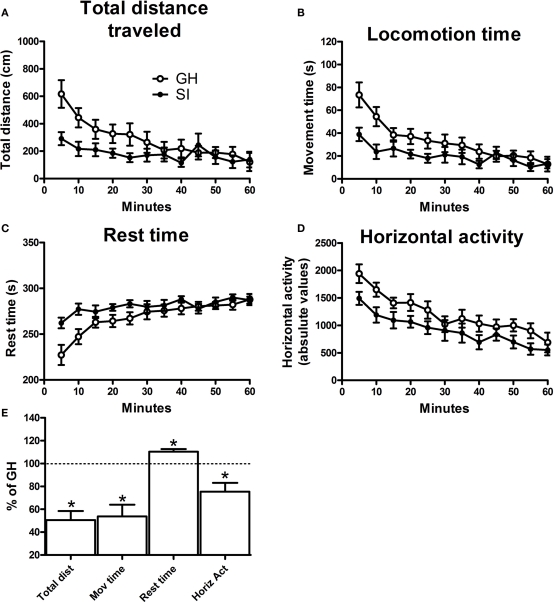
At the end of the isolation period, both GH and SI mice were tested for their spontaneous locomotor activity in a motility meter for 60 min. The data indicate that SI was associated with a reduced locomotor activity compared to animals reared in group. In fact, in SI mice there was a significant decrease in total distance traveled, locomotion time, horizontal activity, and a parallel increase in rest time, compared to GH animals (Figure 1, p < 0.05).
Figure 1.
Spontaneous locomotor activity in isolated (SI) and group-housed (GH) C57BL/6J mice. Locomotor activity was assessed in a motility meter after the 6-week isolation period. The different parameters of motor activity (A) total distance traveled, (B) locomotion time, (C) rest time, and (D) horizontal activity, were averaged in bins of 5 min, for 60 continuous min. Data in graphs (A) through (D) are mean ± SEM (n = 9 per group) of the absolute values of the different measures. Graph (E) summarizes the effects of SI on locomotor activity as measured during the initial 15 min. Data are expressed as mean ± SEM of the percentage vs. GH mice. *p < 0.05 vs. GH animals.
Effects of SI on voluntary EtOH consumption in C57BL/6J mice
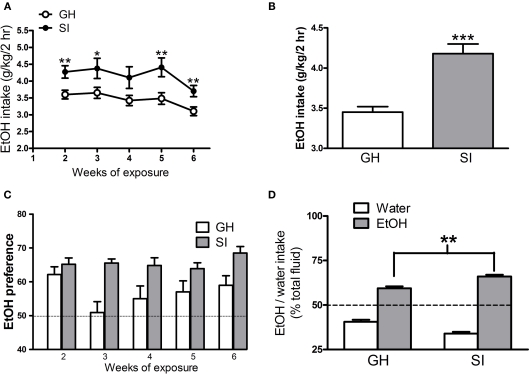
In order to determine whether SI could alter EtOH consumption, we exposed mice to the 2-h free access to EtOH drinking during the 6-week isolation period. Figure 2A reports the daily average intake of 15% EtOH solution, during each week, from week 2 to 6, in both groups of mice. SI mice consumed a significantly higher amount of EtOH compared to GH animals; this difference is already evident at week 2, when animals are first exposed to the 15% EtOH solution, and is maintained through the end of the isolation period. The overall average daily intake of EtOH was 3.4 ± 0.1g/kg/2 h for GH mice and 4.2 ± 0.1 g/kg/2 h for SI animals (Figure 2B, p < 0.001). SI resulted also in an increased preference to EtOH over water. As expected (Phillips and Crabbe, 1991; Belknap et al., 1993; Yoneyama et al., 2008), GH mice display a higher preference to EtOH (Figure 2C), and this effect was significantly increased in SI mice (Figures 2C,D, p < 0.01).
Figure 2.
Effects of SI on voluntary EtOH consumption and preference. (A) The graph shows the daily average EtOH intake in GH and SI C57BL/6J mice during the 5-weeks of exposure to the 15% solution of EtOH. (B) The bar graph shows the average EtOH intake for the whole 5-week period of exposure to 15% EtOH solution in both groups of mice. (C) EtOH preference in GH and SI mice; the graph shows the percent of EtOH self-administered week by week with respect to the total fluid consumed. (D) The graph shows the percent of water or EtOH self-administered with respect to the total fluid consumed during the whole 5-week period (n = 22 for SI and n = 21 for GH mice). *p < 0.05; **p < 0.01; ***p < 0.001 vs. GH mice.
Measurement of body weight during SI and voluntary EtOH consumption in C57BL/6J mice
Body weight of mice was monitored throughout the isolation period. At the time of weaning (PND21) body weight was homogeneous, and at the beginning of the second week of isolation when exposed to 15% EtOH solution, ranged between 16.83 and 20.05 g, among mice of the different experimental groups. At the end of the treatment, weight gain did not differ between GH (+23 ± 2.4%), GH that consumed EtOH (+29 ± 3.8%), and SI mice (+20 ± 2.4%). However, SI mice that consumed EtOH had a reduced weight gain (+15 ± 2.1%) that was statistically significant (p < 0.05; n = 21–22) compared to GH animals.
Effects of voluntary EtOH consumption on the abundance of 3α,5α-TH prog in SI C57BL/6J mice
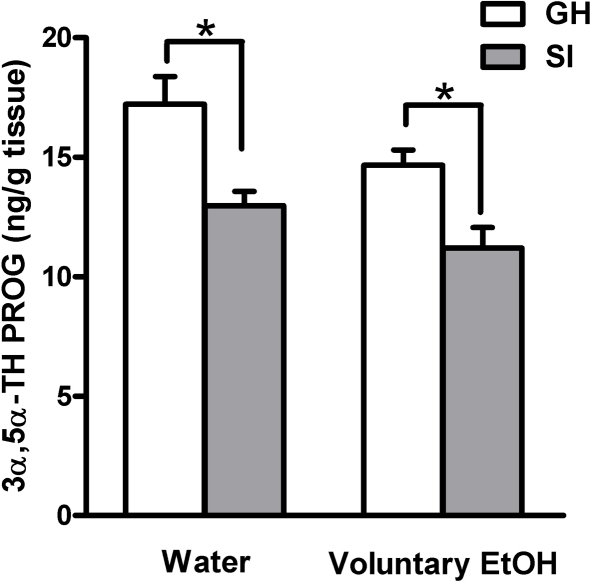
In order to test the hypothesis that increased drinking behavior and EtOH preference induced by SI could restore the concentrations of neuroactive steroids to steady state levels in the hippocampus we measured 3α,5α-TH PROG in both GH and SI mice that had free access to EtOH or water. All measurements were done at the end of the 2-h session the last day of SI. Consistent with our previous data in the rat (Serra et al., 2000), SI of C57BL/6J mice induced a significant decreases in hippocampal concentrations of 3α,5α-TH PROG compared to GH mice (Figure 3; p < 0.05). Voluntary EtOH consumption throughout the SI period for just 2 h at day did not restore the steady state levels of 3α,5α-TH PROG which remained as low as those measured in SI mice that drank water (Figure 3).
Figure 3.
Effect of voluntary EtOH consumption on the concentration of 3α,5α-TH PROG in the hippocampus of group-housed (GH) and socially isolated (SI) C57BL/6J mice. Animals were housed in groups or in isolation for 6 weeks with free access for 2 h a day to EtOH or water as described in the Section “Materials and Methods.” The last day of isolation animals were sacrificed at the end of the 2-h EtOH access session and the hippocampal concentration of 3α,5α-TH PROG was measured. Data are mean ± SEM (n = 9 per group): Two-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test; *p < 0.01 vs. corresponding GH animals as indicated.
Effects of SI on neuronal excitability and LTP induction in the hippocampal CA1 region of C57BL/6J mice
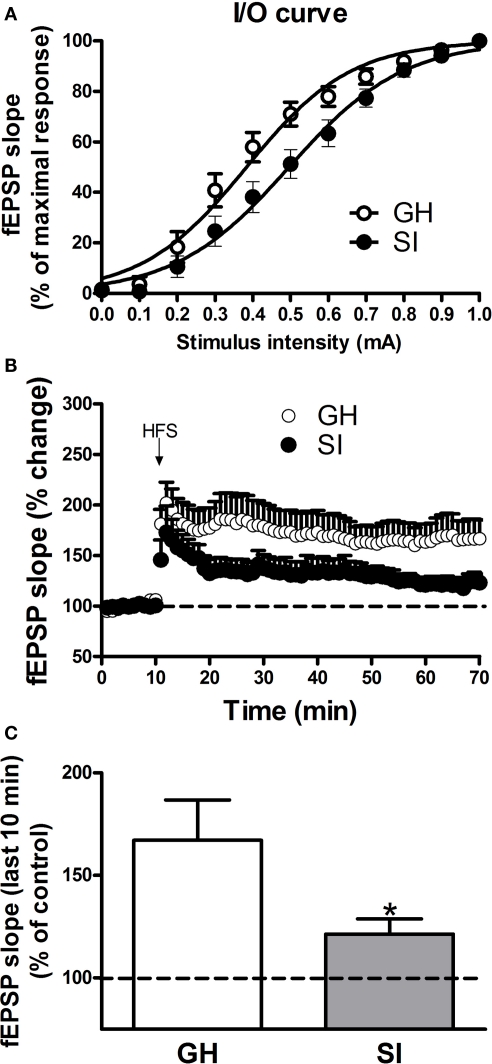
Previous studies reported that SI in rodents altered hippocampal excitability (Bartesaghi, 2004), an effect associated with memory deficits as well as impaired performance in the Morris water maze test (Lu et al., 2003; Bianchi et al., 2006). In order to evaluate the effects of SI on hippocampal excitability in C57BL/6J mice, we recorded fEPSPs in the CA1 region of GH and SI mice. Input/output curves revealed that the intensity value of the stimulation that evoked half-maximal postsynaptic response was significantly enhanced (Figure 4A; p < 0.05) in the CA1 region of SI mice (0.50 ± 0.04 mA) compared to GH animals (0.38 ± 0.03 mA).
Figure 4.
Effects of SI on hippocampal excitability and LTP induction in C57BL/6J mice. Field EPSPs (fEPSPs) were recorded in the dendritic CA1 region of hippocampal slices obtained from group-housed (GH) and socially isolated (SI) animals. (A) Input/output relationship; the curves were constructed by measuring fEPSP slope in response to increasing intensity (from 0 to 1.0 mA) of the stimulation. Data are expressed as the mean ± SEM (n = 6–7) percent of the maximal response. (B) LTP was induced in the CA1 region by HFS of the Schaffer's collateral pathway. Data represent the percent change in slope value induced by HFS with respect to baseline (n = 11–14). (C) The graph illustrate the extent of LTP calculated by averaging the percent of slope change from 50 to 60 min after HFS. *p < 0.01 vs. GH mice.
LTP was induced in the CA1 region by HFS consisting of a single train of 100 stimuli at 250 Hz that was delivered to the afferent Schaffer/Commissural afferents. The degree of LTP, calculated by averaging the slope values of fEPSPs recorded from 50 to 60 min, following HFS, was significantly reduced in SI mice compared to GH mice (Figures 4B,C; p < 0.01; n = 11–14).
Effects of voluntary EtOH consumption on the gene expression of GABAAR subunits in the hippocampus of SI and GH C57BL/6J mice
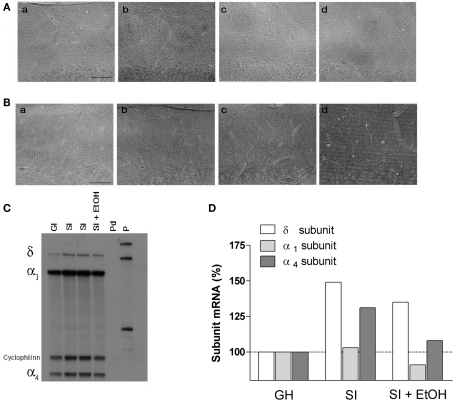
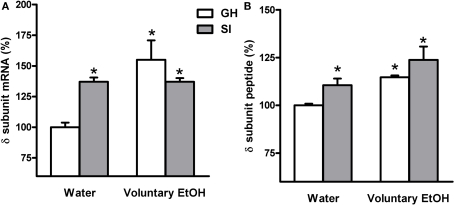
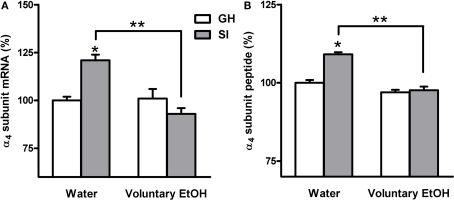
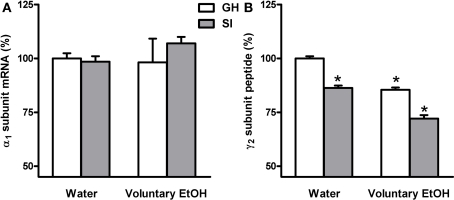
In order to assay whether SI stress is associated with changes in GABAAR gene expression in the hippocampus and whether the increased drinking behavior in the SI mice could reverse these changes we measured both the abundance of mRNA and peptide, in the whole hippocampus or DG respectively, of discrete GABAAR subunits. RNase protection assay and immunohistochemistry measurements (Figure 5) revealed that SI-induced a marked and significant increase in the abundance of both the δ and α4 subunit (Figures 6 and 7A,B; p < 0.05). Moreover, SI did not alter the mRNA levels of the α1 subunit (Figure 8A) but significantly decreased the γ2 subunit peptide levels (Figure 8B; p < 0.05). In hippocampus of mice that had free access to EtOH the gene expression of the GABAAR subunits was also altered. Similarly to our recent finding in the rat (Pisu et al., 2010) EtOH intake induced changes in gene expression in the GH mice namely a marked and statistically significant increase of the δ subunit (Figures 6A,B; p < 0.05) and a significant decrease of the γ2 subunit peptide (Figure 8B; p < 0.05). On the other hand, in mice the intake of EtOH was able to completely block the increase in the abundance of the α4 subunit induced by SI stress in a selective manner (Figures 7A,B; p < 0.05). In fact, EtOH intake did not abolish the effects of SI on the gene expression of neither δ nor γ2 subunit which remained still up-regulated (Figures 6A,B) and down-regulated, respectively (Figure 8B). Moreover, EtOH intake did not have any significant effect on the gene expression of the α1 subunit in the hippocampus of mice from both GH and SI experimental groups (Figure 8A).
Figure 5.
Representative immunohistochemical (A,B) and RNase protection assay (C) analysis images. Distribution of the α4 (A) and δ (B) subunits in molecular layer of DG of the hippocampus slices from GH (a), SI (b), GH with free access for 2 h a day to EtOH (c), and SI mice with free access for 2 h a day to EtOH (d). Scale bar, 50 μm. (C) Autoradiograph on a urea/polyacrylamide electrophoresis gel showing protected fragments of the mRNAs encoding for δ, α1, α4 subunits of GABAAR and cyclophilin (internal standard). On each band, 25 μg of total RNA extracted from the whole hippocampus of individual mice of the indicated experimental groups. Pd = digested probe; P = probe. (D) Semi-quantitative measurement of image (C) of the indicated subunits. Data are expressed as percentage change in optical density of the corresponding bands relative to GH.
Figure 6.
Effects of voluntary EtOH consumption on the gene expression of the δ subunit of the GABAAR in the hippocampus of group-housed (GH) or socially isolated (SI) C57BL/6J mice. Animals were housed in groups (GH) or in isolation (SI) for 6 weeks with free access for 2 h a day to EtOH or water as described in the Section “Materials and Methods.” The last day of SI, mice were sacrificed at the end of the 2-h EtOH access session and the total hippocampal abundance of the mRNA (A) and the DG molecular layer peptide (B) of the δ subunit of the GABAAR was measured by RNase protection assay and immunohistochemistry, respectively. Data are expressed as mean ± SEM of values (n = 19–21 for mRNA and 7–11 for peptide for each experimental group) from three independent experiments and are percentage of the value relative to GH mice drinking water. *p < 0.05; vs. GH mice drinking water (ANOVA and Scheffe's test).
Figure 7.
Effects of voluntary EtOH consumption on the gene expression of the α4 subunit of the GABAAR in the hippocampus of group-housed (GH) or socially isolated (SI) C57BL/6J mice. Animals were housed in groups (GH) or in isolation (SI) for 6 weeks with free access for 2 h a day to EtOH or water as described in the Section “Materials and Methods.” The last day of SI were sacrificed at the end of the 2-h EtOH access session and the abundance of the mRNA (A) and peptide (B), in the whole hippocampus and in the DG molecular layer respectively, of the α4 subunit of the GABAAR was measured by RNase protection assay and immunohistochemistry. Data are expressed as mean ± SEM of values (n = 19–21 for mRNA and 7–11 for peptide for each experimental group) from three independent experiments and are percentage of the value relative to GH mice drinking water. *p < 0.05 vs. GH mice drinking water; **p < 0.05 vs. SI mice drinking water (ANOVA and Scheffe's test).
Figure 8.
Effects of voluntary EtOH consumption on the gene expression of the α1 and γ2 subunits of the GABAAR in the hippocampus of group-housed (GH) or socially isolated (SI) C57BL/6J mice. Animals were housed in groups (GH) or in isolation (SI) for 6 weeks with free access for 2 h a day to EtOH or water as described in the methods. The last day of isolation animals were sacrificed at the end of the 2-h EtOH access session and the abundance of the mRNA encoding for the α1 subunit (A) and of γ2 peptide (B), in the whole hippocampus and in the DG molecular layer respectively, of the of the GABAAR were measured by RNase protection assay and immunohistochemistry. Data are expressed as mean ± SEM of values (n = 19–21 for mRNA and 7–11 for peptide for each experimental group) from three independent experiments and are percentage of the value relative to GH mice drinking water. *p < 0.05 vs. GH mice drinking water (ANOVA and Scheffe's test).
Effects of voluntary EtOH consumption on GABAergic tonic and phasic currents in DG granule cells of GH and SI C57BL/6J mice
Because of the changes in expression of α4, γ2, and δ subunit in response to SI and voluntary EtOH consumption, we next examined GABAergic transmission in granule cells of the DG using hippocampal slices obtained from GH and SI mice that had access, during the 6-week period of SI, to 15% EtOH or water. Granule cells of the DG are characterized by a prominent GABAergic tonic current mediated by extrasynaptic GABAARs containing mainly α4, βn, and δ subunits (Nusser and Mody, 2002) and, in a lower proportion, by extrasynaptic receptors containing the α5 subunit (Glykys et al., 2008). There is also a phasic component to inhibition of these neurons that is attributable to the activation of synaptic GABAARs with an αn, βn, and γ2 subunit composition (Nusser and Mody, 2002). We recorded from granule cells in the voltage-clamp mode (holding membrane potential, −65 mV) with patch pipettes loaded with a high Cl− concentration that gives rise to a Cl− equilibrium potential of ~0 mV. Under these conditions, activation of GABAARs generates inward currents that reflect an outflow of Cl−. GABAergic currents were pharmacologically isolated by the addition of kynurenic acid (3 mM) to ACSF, a broad-spectrum antagonist of ionotropic glutamate receptors.
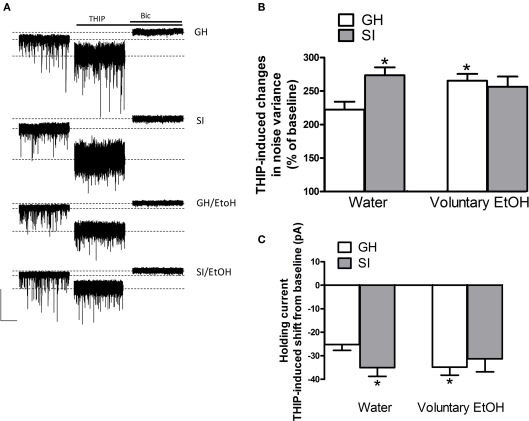
After a baseline period of ~3 min, slice were exposed to THIP (3 μM) for 6 min to activate preferentially high-affinity extrasynaptic GABAARs (Brown et al., 2002; Drasbek and Jensen, 2006). Exposure of granule cells from GH mice to THIP increased current noise variance and shifted the holding current in the negative direction with respect to baseline (Figure 9). The subsequent bath application of bicuculline (20 μM) blocked all GABAergic currents and reduced both noise variance and the holding current compared to baseline (Figure 9). In SI mice, the bath application of THIP resulted in a significant increase in noise variance and a larger holding current negative shift, compared to GH animals (Figure 9; p < 0.05). Tonic current parameters were also significantly increased in GH animals that consumed EtOH. In SI mice that consumed EtOH the effect of THIP on both noise variance and holding current shift, although still increased, was not statistically different compared to GH drinking water.
Figure 9.
Effect of voluntary EtOH consumption on GABAergic tonic current in granule cells of the DG from GH and SI C57BL/6J mice. (A) Representative traces of GABAergic currents recorded in the whole-cell mode (holding potential, −65 mV) from granule cells of the DG are shown (left panel) for hippocampal slices obtained from group-housed (GH) or isolated (SI) mice exposed to water only, and GH and SI animals exposed to EtOH. All recordings were performed in the presence of kynurenic acid (3 mM) to block glutamatergic currents. After a baseline of 3 min, application of THIP (3 μM) induced an increase in noise variance and a negative shift in the holding current. All GABAergic currents were blocked by the application of bicuculline (Bic) at 20 μM. Calibration, 100 pA, 20 s. (B,C), bar graphs summarizing the changes in noise variance (B) and holding current (C) induced by the bath perfusion of THIP in experiments similar to those shown in (A). Data are mean ± SEM (n = 19–25 neurons). *p < 0.05 vs. GH mice exposed to water only (ANOVA followed by Scheffe's test).
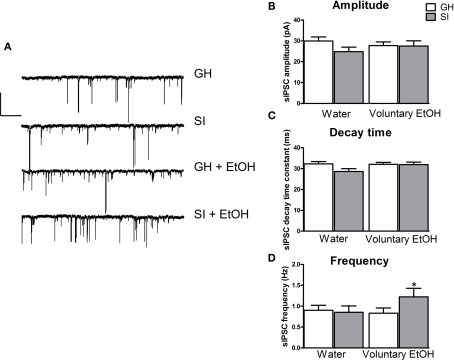
Analysis of the kinetic properties of spontaneous IPSCs recorded in granule cells from the different experimental groups did not reveal statistically significant difference in current amplitude or decay time (Figure 10). In contrast, we found a significant increase (36%) in sIPSC frequency in SI mice that self-administered EtOH during SI period compared to GH mice exposed to water only (Figure 10; p < 0.05).
Figure 10.
Effect of voluntary EtOH consumption on GABAergic phasic currents in granule cells of the DG from GH and SI C57BL/6J mice. (A) Representative traces of GABAergic spontaneous IPSCs (sIPSCs) recorded in the whole-cell mode (holding potential, −65 mV) from granule cells of the DG are shown (left panel) for hippocampal slices obtained from group-housed (GH) or isolated (SI) mice exposed to only water, and GH and SI animals exposed to EtOH. All recordings were performed in the presence of kynurenic acid (3 mM) to block glutamatergic currents. Calibration, 50 pA, 10 s. (B,C), bar graphs summarizing the changes in sIPSC amplitude (B), decay time (C), and frequency (D) in experiments similar to those shown in (A). Data are mean ± SEM (n = 26–31 neurons). *p < 0.05 vs. GH mice exposed to water only (ANOVA followed by Scheffe's test).
Discussion
One of the main goals of the present study was to assess whether the post-weaning SI of C57BL/6J mice could represent a useful model of excessive EtOH self-administration and thus could allow highlighting the molecular and neurochemical mechanisms involved in the interaction between stress and EtOH. In agreement with results of other studies conducted in the rat (Schenk et al., 1990; Hall et al., 1998) and in C57BL/6J mice (Yanai and Ginsburg, 1976; Advani et al., 2007; Lopez et al., 2010) our results show that C57BL/6J mice, exposed to the two-bottle choice paradigm during the 6-week period of post-weaning SI, self-administered higher amounts of EtOH and displayed an enhanced preference to EtOH compared to GH animals. It is interesting that the increase in EtOH self-administration found in our study was apparent already during the second week of isolation, when mice are first exposed to the choice between water and the 15% EtOH solution, suggesting that these young animals might experience a level of stress due to SI from their peers that is sufficient to increase their susceptibility to EtOH early in the SI period. In line with this observation, neonatal stress induced by maternal separation, usually performed during the first 2 weeks of life, also results in an increased EtOH consumption in adult rats (Huot et al., 2001; Ploj et al., 2003; Roman et al., 2005; Gustafsson and Nylander, 2006) and mice (Cruz et al., 2008). On the other hand, Lopez et al. (2010) showed that if mice are reared in isolation during the adult life, they will consume similar amounts of EtOH as those reared in group. Altogether, these results suggest that post-weaning SI of male C57BL/6J mice is a suitable model of excessive EtOH self-administration that is useful to further study the molecular and neurochemical mechanisms involved in the interaction between early life prolonged stress and EtOH abuse in adulthood.
Alterations of a number of behavioral responses, including locomotor activity, and exploratory behavior, caused by SI have been extensively reported in mice and rats (Voikar et al., 2005; Fone and Porkess, 2008; Pietropaolo et al., 2008; Arndt et al., 2009). In our study, SI mice displayed a reduced spontaneous locomotor activity in a novel environment compared to GH animals. Our result differs from those studies where SI caused motor hyperactivity (Pietropaolo et al., 2008). However other authors reported only weak or no changes in such a measure (Voikar et al., 2005; Arndt et al., 2009), while others (Valzelli et al., 1974) reported a reduction of motor activity similar to our study. The reasons of such variability in the changes in locomotor activity in response to SI are not presently known.
The observation that the hippocampal concentration of 3α,5α-TH PROG in SI C57BL/6J mice was decreased is in agreement with previous studies showing similar effects in the cerebral cortex and plasma of both mice (Matsumoto et al., 1999) and rats (Serra et al., 2000). Free access to EtOH tended to slightly reduce the hippocampal concentration of 3α,5α-TH PROG even though this difference was not statistically significant. Thus, our initial hypothesis that increased EtOH intake and EtOH preference could restore the steady state levels of neuroactive steroids in the hippocampus of SI mice was disproved. This result is in agreement with a very recent similar study from our group performed in the rat in which the cerebrocortical levels of 3α,5α-TH PROG remained decreased in SI rats that had free access to EtOH (Pisu et al., 2010). Nevertheless, in the same study it has been shown that voluntary EtOH consumption abolished the hypersensitivity of SI animals to acute novel stress, as demonstrated by the attenuation of the increase in the cerebrocortical concentration of 3α,5α-TH PROG induced by foot shock stress (Pisu et al., 2010).
Analysis of fEPSPs recorded in the hippocampal CA1 region revealed that SI stress caused a right shift of the I/O relationship compared to GH animals, suggesting that SI is associated with a decreased neuronal excitability. Our data are in agreement with other studies showing similar changes in guinea pig CA1 region (Bartesaghi, 2004). A reduced neuronal excitability is also in line with the data showing a decreased degree of LTP induced by HFS in the CA1 region of SI animals found in the present as well as in other studies (Roberts and Greene, 2003). On the other hand, reduced hippocampal excitability and long-term synaptic plasticity may be relevant for the reduced learning and memory deficits that have been reported in SI C57BL/6J mice (Voikar et al., 2005) as well as in rats (Lu et al., 2003; Bianchi et al., 2006).
In agreement with our previous observations in the rat (Serra et al., 2006; Pisu et al., 2010) SI stress induced alterations in the gene expression of the GABAAR subunits in the hippocampus of C57BL/6J mice; namely we observed increased gene expression of both the α4 and δ subunits, decreased expression of the γ2 subunit and no changes in α1. The main finding of our study is that EtOH self-administration in SI mice selectively blocked the changes in gene expression of the α4 subunit but not those of δ and γ2 subunits induced by SI. This specific action of EtOH on the α4 subunit could be related to the anxiolytic action exerted by EtOH. In agreement with this hypothesis the α4 subunit has been associated with increased anxiety (Smith et al., 1998b) and suppression of its expression prevents withdrawal properties of an endogenous steroid (Smith et al., 1998a). Moreover, increased gene expression of the GABAAR α4 subunit induced by EtOH withdrawal can be blocked by treatment with either diazepam or gamma-hydroxybutyric acid during withdrawal (Follesa et al., 2003).
The results of our study also showed that voluntary EtOH intake induced changes in GABAAR gene expression in the GH mice in particular reducing the hippocampal abundance of the γ2 subunit and increasing the expression of the δ subunit. These molecular effects are in agreement with the notion that EtOH exposure can be associated with marked changes in the expression of GABAAR subunits at both the mRNA and protein levels that may vary depending upon the different protocols used to expose the animals to EtOH (forced vs. voluntary), the blood alcohol concentrations reached, and the time of sacrifice after the last EtOH exposure (Grobin et al., 1998; Matthews et al., 1998; Cagetti et al., 2003; Olsen et al., 2005; Liang et al., 2006; Serra et al., 2006; Pisu et al., 2010). These variations may produce differential changes in GABAAR gene expression in discrete brain regions (Grobin et al., 1998, 2000; Matthews et al., 1998; Mehta and Ticku, 1999) and sub-cellular localization (Olsen et al., 2005; Liang et al., 2006). Accordingly, we have previously shown that chronic EtOH treatment can affect GABAAR gene expression in hippocampal neurons (Sanna et al., 2003; Follesa et al., 2005) and cerebellar granule neurons in an opposite manner (Follesa et al., 2005). Thus, our results demonstrated that voluntary EtOH drinking in SI mice had a selective influence on α4 subunit and could not antagonize the up-regulation of δ subunit and down-regulation of γ2, consistent with the observation that chronic EtOH itself up-regulates the δ subunit and down-regulates the γ2 (Sanna et al., 2003; Follesa et al., 2005).
Our results have revealed that, in association with the increased expression of the α4 and δ subunits in the hippocampus of SI mice, there is an enhanced modulatory action of the agonist THIP on GABAergic tonic currents recorded in DG granule cells. Such effect is consistent with previous findings from our lab in SI rats (Serra et al., 2006), further suggesting that SI stress induces an enhancement of surface expression of extrasynaptic GABAARs responsible for mediating the tonic component of the GABAergic inhibition in this neuronal population. Such an effect could be, in turn, critical for counterbalancing the reduced basal levels of neuroactive steroids induced by SI. In addition, we found that voluntary EtOH consumption produced a weak effect on such SI-induced elevation of tonic current. In fact, in SI mice self-administering EtOH the increased effect of THIP was modestly reduced compared to SI self-administering water, and was not statistically significant when compared to control GH animals. Given that EtOH drinking in SI mice selectively counteracts the increased expression of the α4, but not δ, subunit, the attenuation in tonic current may be dependent on such opposite regulation of the α4 subunit by EtOH. On the other hand, the marked increase in THIP-stimulated tonic current observed in DG granule cells obtained from GH mice that self-administered EtOH may be linked to the increased number of the δ subunit containing receptors that could be assembled with α subunits other than the α4 that could dynamically move from extrasynaptic to synaptic sites (Liang et al., 2007), suggesting again that voluntary EtOH consumption may regulate both gene expression and function of α4/δ-containing receptors.
Voluntary EtOH consumption in SI mice was also associated with a selective increase in the frequency of sIPSCs mediated by synaptic GABAARs in DG granule cells, an effect consistent with an enhanced probability of the presynaptic release of GABA from afferent neurons. A number of studies performed in several brain regions have consistently reported that acute application of EtOH affects the presynaptic release of GABA (Carta et al., 2003; Roberto et al., 2003; Ariwodola and Weiner, 2004; Sanna et al., 2004), and more recent observations suggest that its effect may involve an increase in presynaptic calcium release from intracellular stores consequent to the activation of both adenylate cyclase/PKA and PLC/PKC pathways (Kelm et al., 2008, 2010). In our study, hippocampal slices were not perfused acutely with EtOH and the present finding may suggest that EtOH self-administration in SI mice might affect some of the specific molecular mechanisms that are involved in the presynaptic release of GABA.
Analysis of the amplitude and decay time constant of phasic sIPSCs recorded in DG granule cells revealed no significant alterations in response to SI or to EtOH self-administration despite the down-regulation of the γ2 subunit expression in this cell population. This finding is in agreement with other studies where down-regulation of the γ2 subunit expression during the ovarian cycle (Maguire et al., 2005) and during pregnancy (Maguire and Mody, 2008; Sanna et al., 2009) were not accompanied by changes in the kinetic parameters of synaptic currents.
In conclusion, the results of our study suggest that voluntary EtOH consumption might exert an anxiolytic action by selectively blocking the expression of the α4 subunit of GABAAR, which has been associated with increased anxiety (Smith et al., 1998b). Thus, SI might induce an anxiogenic state, generated by an increased expression of the α4 subunit together with a reduced level in neuroactive steroids, that in turn raise EtOH drinking behavior. This later hypothesis could be tested by administering an antisense against the α4 subunits in SI mice and/or exogenous steroids to re-establish the steady state levels. Moreover, the increased expression of α4/δ-containing GABAARs associated with the enhanced tonic current in DG granule cells might be expected to generate a greater sensitivity to the acute effects of low EtOH concentrations of SI mice (Wei et al., 2004). Nevertheless, the relevancy of altered GABAAR electrophysiology and biochemistry here described needs to be further investigated with respect to ethanol and stress related behavior by using appropriate behavioral tests and other pharmacological tools.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Funding for this study was provided by grant 5U01AA016670-03 from the USA National Institute on Alcohol Abuse and Alcoholism.
References
- Advani T., Hensler J. G., Koek W. (2007). Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int. J. Neuropsychopharmacol. 10, 595–607 [DOI] [PubMed] [Google Scholar]
- Akana S. F., Scribner K. A., Bradbury M. J., Strack A. M., Walker C. D., Dallman M. F. (1992). Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology 131, 585–594 10.1210/en.131.2.585 [DOI] [PubMed] [Google Scholar]
- Ariwodola O. J., Weiner J. L. (2004). Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J. Neurosci. 24, 10679–10686 10.1523/JNEUROSCI.1768-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt S. S., Laarakker M. C., van Lith H. A., van der Staay F. J., Gieling E., Salomons A. R., van't Klooster J., Ohl F. (2009). Individual housing of mice – impact on behaviour and stress responses. Physiol. Behav. 97, 385–393 [DOI] [PubMed] [Google Scholar]
- Barbaccia M. L., Affricano D., Trabucchi M., Purdy R. H., Colombo G., Agabio R., Gessa G. L. (1999). Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur. J. Pharmacol. 384, R1–R2 [DOI] [PubMed] [Google Scholar]
- Bartesaghi R. (2004). Effect of early isolation on the synaptic function in the dentate gyrus and field CA1 of the guinea pig. Hippocampus 14, 482–498 10.1002/hipo.10201 [DOI] [PubMed] [Google Scholar]
- Belknap J. K., Crabbe J. C., Young E. R. (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl.) 112, 503–510 [DOI] [PubMed] [Google Scholar]
- Bianchi M., Fone K. F., Azmi N., Heidbreder C. A., Hagan J. J., Marsden C. A. (2006). Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur. J. Neurosci. 24, 2894–2902 [DOI] [PubMed] [Google Scholar]
- Borghese C. M., Storustovu S., Ebert B., Herd M. B., Belelli D., Lambert J. J., Marshall G., Wafford K. A., Harris R. A. (2006). The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J. Pharmacol. Exp. Ther. 316, 1360–1368 [DOI] [PubMed] [Google Scholar]
- Brown N., Kerby J., Bonnert T. P., Whiting P. J., Wafford K. A. (2002). Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol. 136, 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E., Liang J., Spigelman I., Olsen R. W. (2003). Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol. Pharmacol. 63, 53–64 [DOI] [PubMed] [Google Scholar]
- Carta M., Ariwodola O. J., Weiner J. L., Valenzuela C. F. (2003). Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc. Natl. Acad. Sci. U.S.A. 100, 6813–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F. C., Quadros I. M., Planeta Cda S., Miczek K. A. (2008). Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl.) 201, 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek K. R., Jensen K. (2006). THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb. Cortex 16, 1134–1141 [DOI] [PubMed] [Google Scholar]
- Faingold C. L., N'Gouemo P., Riaz A. (1998). Ethanol and neurotransmitter interactions – from molecular to integrative effects. Prog. Neurobiol. 55, 509–535 [DOI] [PubMed] [Google Scholar]
- Follesa P., Floris S., Tuligi G., Mostallino M. C., Concas A., Biggio G. (1998). Molecular and functional adaptation of the GABA(A) receptor complex during pregnancy and after delivery in the rat brain. Eur. J. Neurosci. 10, 2905–2912 [DOI] [PubMed] [Google Scholar]
- Follesa P., Mancuso L., Biggio F., Mostallino M. C., Manca A., Mascia M. P., Busonero F., Talani G., Sanna E., Biggio G. (2003). Gamma-hydroxybutyric acid and diazepam antagonize a rapid increase in GABA(A) receptors alpha(4) subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol. Pharmacol. 63, 896–907 [DOI] [PubMed] [Google Scholar]
- Follesa P., Mostallino M. C., Biggio F., Gorini G., Caria S., Busonero F., Murru L., Mura M. L., Sanna E., Biggio G. (2005). Distinct patterns of expression and regulation of GABA receptors containing the delta subunit in cerebellar granule and hippocampal neurons. J. Neurochem. 94, 659–671 10.1111/j.1471-4159.2005.03303.x [DOI] [PubMed] [Google Scholar]
- Fone K. C., Porkess M. V. (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 32, 1087–1102 [DOI] [PubMed] [Google Scholar]
- Franklin K. B. J., Paxinos G. (1996). The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press [Google Scholar]
- Fuller J. L. (1964). Measurement of alcohol preference in genetic experiments. J. Comp. Physiol. Psychol. 57, 85–88 [DOI] [PubMed] [Google Scholar]
- Glykys J., Mann E. O., Mody I. (2008). Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 28, 1421–1426 10.1523/JNEUROSCI.4751-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin A. C., Matthews D. B., Devaud L. L., Morrow A. L. (1998). The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl.) 139, 2–19 [DOI] [PubMed] [Google Scholar]
- Grobin A. C., Papadeas S. T., Morrow A. L. (2000). Regional variations in the effects of chronic ethanol administration on GABA(A) receptor expression: potential mechanisms. Neurochem. Int. 37, 453–461 [DOI] [PubMed] [Google Scholar]
- Gustafsson L., Nylander I. (2006). Time-dependent alterations in ethanol intake in male wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol. Clin. Exp. Res. 30, 2008–2016 [DOI] [PubMed] [Google Scholar]
- Hall F. S., Huang S., Fong G. W., Pert A., Linnoila M. (1998). Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl.) 139, 210–216 [DOI] [PubMed] [Google Scholar]
- Harris R. A. (1999). Ethanol actions on multiple ion channels: which are important? Alcohol. Clin. Exp. Res. 23, 1563–1570 [PubMed] [Google Scholar]
- Huot R. L., Thrivikraman K. V., Meaney M. J., Plotsky P. M. (2001). Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl.) 158, 366–373 [DOI] [PubMed] [Google Scholar]
- Kelm M. K., Criswell H. E., Breese G. R. (2008). The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J. Neurophysiol. 100, 3417–3428 10.1152/jn.90970.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M. K., Weinberg R. J., Criswell H. E., Breese G. R. (2010). The PLC/IP 3 R/PKC pathway is required for ethanol-enhanced GABA release. Neuropharmacology 58, 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti R. T., Penland S. N., VanDoren M. J., Grobin A. C., Morrow A. L. (2002). GABAergic neurosteroid modulation of ethanol actions. World J. Biol. Psychiatry 3, 87–95 [DOI] [PubMed] [Google Scholar]
- Kumar S., Porcu P., Werner D. F., Matthews D. B., Diaz-Granados J. L., Helfand R. S., Morrow A. L. (2009). The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl.) 205, 529–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Suryanarayanan A., Abriam A., Snyder B., Olsen R. W., Spigelman I. (2007). Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J. Neurosci. 27, 12367–12377 10.1523/JNEUROSCI.2786-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Zhang N., Cagetti E., Houser C. R., Olsen R. W., Spigelman I. (2006). Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 26, 1749–1758 10.1523/JNEUROSCI.4702-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. F., Doremus-Fitzwater T. L., Becker H. C. (2010). Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. [Epub ahead of print]. 10.1016/j.alcohol.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Bao G., Chen H., Xia P., Fan X., Zhang J., Pei G., Ma L. (2003). Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp. Neurol. 183, 600–609 [DOI] [PubMed] [Google Scholar]
- Maguire J., Mody I. (2008). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213 10.1016/j.neuron.2008.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. L., Stell B. M., Rafizadeh M., Mody I. (2005). Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uzunova V., Pinna G., Taki K., Uzunov D. P., Watanabe H., Mienville J. M., Guidotti A., Costa E. (1999). Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology 38, 955–963 10.1016/S0028-3908(99)00018-0 [DOI] [PubMed] [Google Scholar]
- Matthews D. B., Devaud L. L., Fritschy J. M., Sieghart W., Morrow A. L. (1998). Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J. Neurochem. 70, 1160–1166 10.1046/j.1471-4159.1998.70031160.x [DOI] [PubMed] [Google Scholar]
- Mehta A. K., Ticku M. K. (1999). An update on GABAA receptors. Brain Res. Brain Res. Rev. 29, 196–217 [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. (1994). Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 17, 517–525 10.1016/0166-2236(94)90155-4 [DOI] [PubMed] [Google Scholar]
- Morrow A. L., Janis G. C., VanDoren M. J., Matthews D. B., Samson H. H., Janak P. H., Grant K. A. (1999). Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol. Clin. Exp. Res. 23, 1933–1940 [DOI] [PubMed] [Google Scholar]
- Morrow A. L., VanDoren M. J., Penland S. N., Matthews D. B. (2001). The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res. Brain Res. Rev. 37, 98–109 [DOI] [PubMed] [Google Scholar]
- Nusser Z., Mody I. (2002). Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 87, 2624–2628 [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Liang J., Cagetti E., Spigelman I. (2005). Plasticity of GABAA receptors in brains of rats treated with chronic intermittent ethanol. Neurochem. Res. 30, 1579–1588 [DOI] [PubMed] [Google Scholar]
- Phillips T. J., Crabbe J. C. (1991). “Behavioral studies of genetic differences in alcohol action,” in The Genetic Bases of Alcohol and Drug Action, eds Phillips T. J., Crabbe J. C. (New York: Plenum; ), 25–104 [Google Scholar]
- Pietropaolo S., Singer P., Feldon J., Yee B. K. (2008). The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology (Berl.) 197, 613–628 [DOI] [PubMed] [Google Scholar]
- Pisu M. G., Mostallino M. C., Dore R., Maciocco E., Secci P. P., Serra M. (2010). Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats. Eur. Neuropsychopharmacol. [Epub ahead of print]. 10.1016/j.euroneuro.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploj K., Roman E., Nylander I. (2003). Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121, 787–799 10.1016/S0306-4522(03)00499-8 [DOI] [PubMed] [Google Scholar]
- Purdy R. H., Moore P. H., Jr., Rao P. N., Hagino N., Yamaguchi T., Schmidt P., Rubinow D. R., Morrow A. L., Paul S. M. (1990). Radioimmunoassay of 3 alpha-hydroxy-5 alpha-pregnan-20-one in rat and human plasma. Steroids 55, 290–296 10.1016/0039-128X(90)90031-6 [DOI] [PubMed] [Google Scholar]
- Roberto M., Madamba S. G., Moore S. D., Tallent M. K., Siggins G. R. (2003). Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. U.S.A. 100, 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L., Greene J. R. T. (2003). Post-weaning social isolation of rats leads to a diminution of LTP in the CA1 to subiculum pathway. Brain Res. 991, 271–273 10.1016/j.brainres.2003.08.022 [DOI] [PubMed] [Google Scholar]
- Roman E., Gustafsson L., Hyytia P., Nylander I. (2005). Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol. Clin. Exp. Res. 29, 591–601 [DOI] [PubMed] [Google Scholar]
- Samson H. H. (1986). Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol. Clin. Exp. Res. 10, 436–442 [DOI] [PubMed] [Google Scholar]
- Sanna E., Mostallino M. C., Busonero F., Talani G., Tranquilli S., Mameli M., Spiga S., Follesa P., Biggio G. (2003). Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J. Neurosci. 23, 11711–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E., Mostallino M. C., Murru L., Carta M., Talani G., Zucca S., Mura M. L., Maciocco E., Biggio G. (2009). Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J. Neurosci. 29, 1755–1765 10.1523/JNEUROSCI.3684-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E., Talani G., Busonero F., Pisu M. G., Purdy R. H., Serra M., Biggio G. (2004). Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J. Neurosci. 24, 6521–6530 10.1523/JNEUROSCI.0075-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S., Gorman K., Amit Z. (1990). Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol 7, 321–326 10.1016/0741-8329(90)90090-Y [DOI] [PubMed] [Google Scholar]
- Semyanov A., Walker M. C., Kullmann D. M., Silver R. A. (2004). Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 27, 262–269 10.1016/j.tins.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Serra M., Mostallino M. C., Talani G., Pisu M. G., Carta M., Mura M. L., Floris I., Maciocco E., Sanna E., Biggio G. (2006). Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J. Neurochem. 98, 122–133 10.1111/j.1471-4159.2006.03850.x [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M. G., Floris I., Cara V., Purdy R. H., Biggio G. (2003). Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J. Neurochem. 85, 257–263 10.1046/j.1471-4159.2003.01680.x [DOI] [PubMed] [Google Scholar]
- Serra M., Pisu M. G., Littera M., Papi G., Sanna E., Tuveri F., Usala L., Purdy R. H., Biggio G. (2000). Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J. Neurochem. 75, 732–740 10.1046/j.1471-4159.2000.0750732.x [DOI] [PubMed] [Google Scholar]
- Smith S. S., Gong Q. H., Hsu F. C., Markowitz R. S., Ffrench-Mullen J. M., Li X. (1998a). GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392, 926–930 10.1038/31948 [DOI] [PubMed] [Google Scholar]
- Smith S. S., Gong Q. H., Li X., Moran M. H., Bitran D., Frye C. A., Hsu F. C. (1998b). Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J. Neurosci. 18, 5275–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I., Smith D. H., Gong Q. H., Sabado T. N., Li X., Light A., Wiedmann M., Williams K., Smith S. S. (2002). Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat. Neurosci. 5, 721–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Harris R. A., Messing R. O., Sanchez-Perez A. M., Hodge C. W., McMahon T., Wang D., Mehmert K. K., Kelley S. P., Haywood A., Olive M. F., Buck K. J., Hood H. M., Blednov Y., Findlay G., Mascia M. P. (2001). Alcohol actions on GABA(A) receptors: from protein structure to mouse behavior. Alcohol. Clin. Exp. Res. 25, 76S–81S [DOI] [PubMed] [Google Scholar]
- Valzelli L., Bernasconi S., Gomba P. (1974). Effect of isolation on some behavioral characteristics in three strains of mice. Biol. Psychiatry 9, 329–334 [PubMed] [Google Scholar]
- VanDoren M. J., Matthews D. B., Janis G. C., Grobin A. C., Devaud L. L., Morrow A. L. (2000). Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 20, 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V., Polus A., Vasar E., Rauvala H. (2005). Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 4, 240–252 [DOI] [PubMed] [Google Scholar]
- Wallner M., Hanchar H. J., Olsen R. W. (2003). Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Faria L. C., Mody I. (2004). Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 24, 8379–8382 10.1523/JNEUROSCI.2040-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J. (1990). Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl.) 101, 233–239 [DOI] [PubMed] [Google Scholar]
- Yamashita M., Marszalec W., Yeh J. Z., Narahashi T. (2006). Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J. Pharmacol. Exp. Ther. 319, 431–438 [DOI] [PubMed] [Google Scholar]
- Yanai J., Ginsburg B. E. (1976). Increased sensitivity to chronic ethanol in isolated mice. Psychopharmacologia 46, 185–189 10.1007/BF00421390 [DOI] [PubMed] [Google Scholar]
- Yoneyama N., Crabbe J. C., Ford M. M., Murillo A., Finn D. A. (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42, 149–160 10.1016/j.alcohol.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Follesa P., Ticku M. K. (1996). Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res. Mol. Brain Res. 41, 163–168 10.1016/0169-328X(96)00087-3 [DOI] [PubMed] [Google Scholar]