Abstract
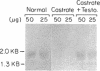
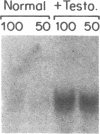
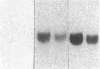
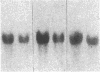
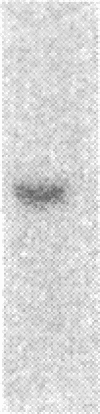
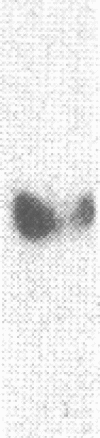
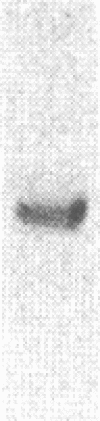
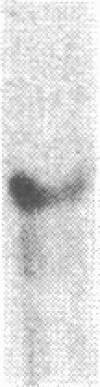
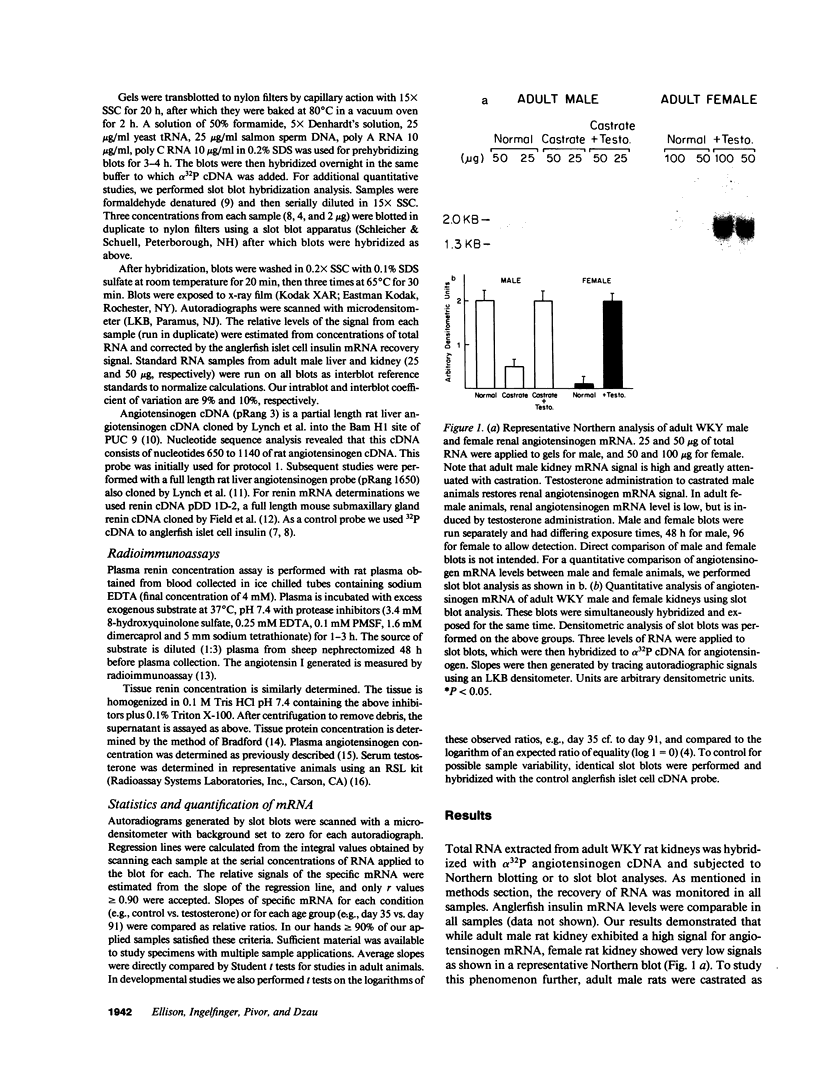
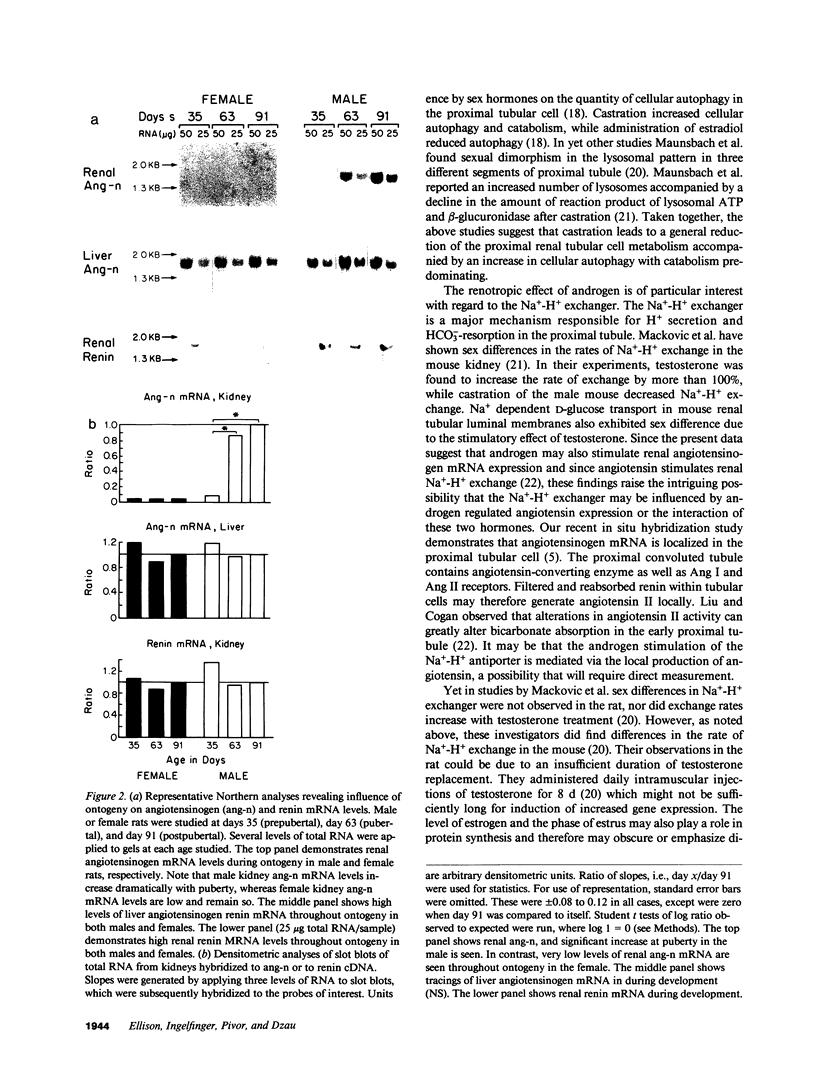
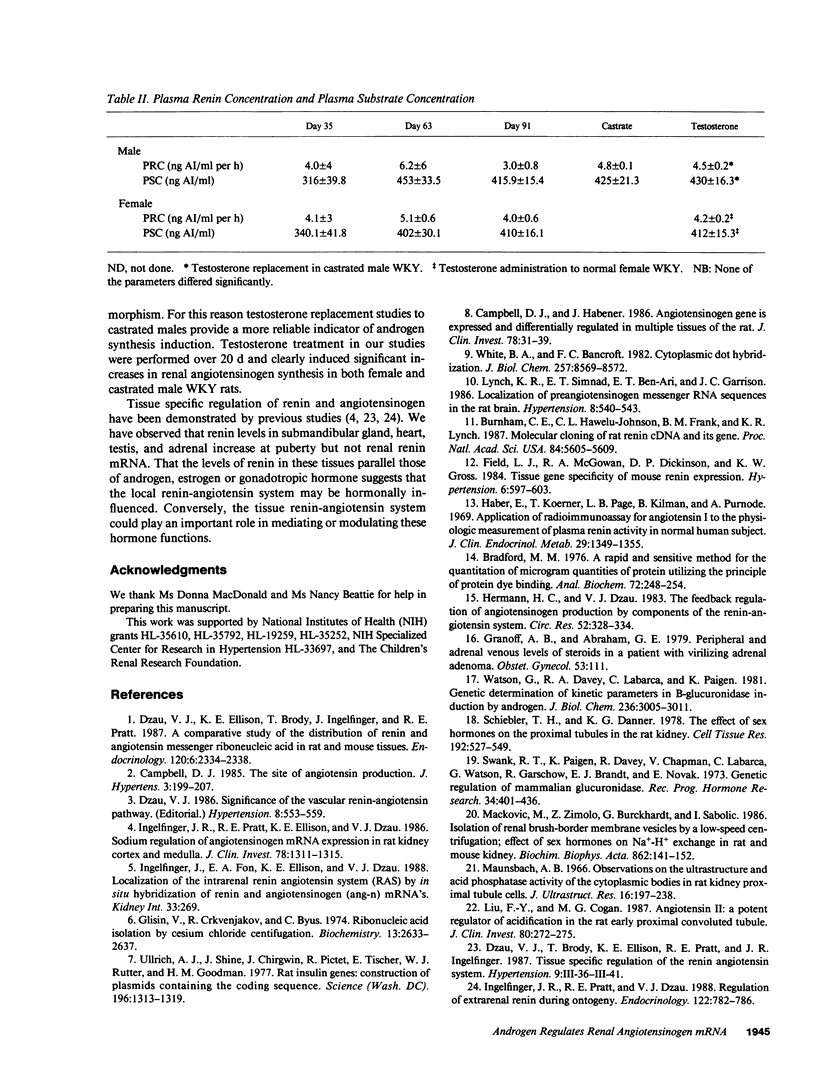
Renal angiotensinogen (ang-n) mRNA concentration in the male WKY rat increases significantly during puberty. Furthermore, renal angiotensinogen mRNA level in the adult female WKY rat is considerably lower than in the male. The present study investigates the role of androgen in differential renal ang-n mRNA expression. Northern and slot blot analyses with alpha-32P labeled ang-n cDNA (pRang 3) demonstrated that castration lowered ang-n mRNA levels in the male kidney by greater than or equal to 60% compared with control, suggesting that androgen may be involved with renal ang-n gene regulation. Moreover, male WKY rats castrated as weanlings and normal adult female WKY rats each implanted with testosterone displayed significant (P less than 0.05) increases in renal ang-n mRNA levels. Our observations, taken together with previous reports that androgen influences proximal tubule morphology and the tubular expression of transport proteins (e.g., Na+/H+ antiporter), may have important physiological implications for understanding the relationship between androgen and angiotensin in the regulation of tubular function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnham C. E., Hawelu-Johnson C. L., Frank B. M., Lynch K. R. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5605–5609. doi: 10.1073/pnas.84.16.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. J., Habener J. F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986 Jul;78(1):31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. J. The site of angiotensin production. J Hypertens. 1985 Jun;3(3):199–207. doi: 10.1097/00004872-198506000-00002. [DOI] [PubMed] [Google Scholar]
- Dzau V. J., Ellison K. E., Brody T., Ingelfinger J., Pratt R. E. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987 Jun;120(6):2334–2338. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- Dzau V. J. Significance of the vascular renin-angiotensin pathway. Hypertension. 1986 Jul;8(7):553–559. doi: 10.1161/01.hyp.8.7.553. [DOI] [PubMed] [Google Scholar]
- Field L. J., McGowan R. A., Dickinson D. P., Gross K. W. Tissue and gene specificity of mouse renin expression. Hypertension. 1984 Jul-Aug;6(4):597–603. doi: 10.1161/01.hyp.6.4.597. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Granoff A. B., Abraham G. E. Peripheral and adrenal venous levels of steroids in a patient with virilizing adrenal adenoma. Obstet Gynecol. 1979 Jan;53(1):111–115. [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Herrmann H. C., Dzau V. J. The feedback regulation of angiotensinogen production by components of the renin-angiotensin system. Circ Res. 1983 Mar;52(3):328–334. doi: 10.1161/01.res.52.3.328. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J. R., Pratt R. E., Dzau V. J. Regulation of extra-renal renin during ontogeny. Endocrinology. 1988 Mar;122(3):782–786. doi: 10.1210/endo-122-3-782. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J. R., Pratt R. E., Ellison K., Dzau V. J. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986 Nov;78(5):1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Angiotensin II: a potent regulator of acidification in the rat early proximal convoluted tubule. J Clin Invest. 1987 Jul;80(1):272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. R., Simnad V. I., Ben-Ari E. T., Garrison J. C. Localization of preangiotensinogen messenger RNA sequences in the rat brain. Hypertension. 1986 Jun;8(6):540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- Macković M., Zimolo Z., Burckhardt G., Sabolić I. Isolation of renal brush-border membrane vesicles by a low-speed centrifugation; effect of sex hormones on Na+-H+ exchange in rat and mouse kidney. Biochim Biophys Acta. 1986 Nov 6;862(1):141–152. doi: 10.1016/0005-2736(86)90478-5. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the ultrastructure and acid phosphatase activity of the cytoplasmic bodies in rat kidney proximal tubule cells. With a comment on their classification. J Ultrastruct Res. 1966 Oct;16(3):197–238. doi: 10.1016/s0022-5320(66)80059-x. [DOI] [PubMed] [Google Scholar]
- Schiebler T. H., Danner K. G. The effect of sex hormones on the proximal tubules in the rat kidney. Cell Tissue Res. 1978 Sep 26;192(3):527–549. doi: 10.1007/BF00212331. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Paigen K., Davey R., Chapman V., Labarca C., Watson G., Ganschow R., Brandt E. J., Novak E. Genetic regulation of mammalian glucuronidase. Recent Prog Horm Res. 1978;34:401–436. doi: 10.1016/b978-0-12-571134-0.50015-6. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Watson G., Davey R. A., Labarca C., Paigen K. Genetic determination of kinetic parameters in beta-glucuronidase induction by androgen. J Biol Chem. 1981 Mar 25;256(6):3005–3011. [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]