Abstract
Background
To explore contextual effects and to test for interactions, this study examined how breast cancer stage at diagnosis among U.S. women related to individual- and county-level (contextual) variables associated with access to health care and socioeconomic status.
Methods
Individual-level incidence data were obtained from the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology and End-Results (SEER) program. The county of residence of women with diagnosed breast cancer (n = 217,299) was used to link NPCR and SEER data with county-level measures of health care access from the 2004 Area Resource File (ARF). In addition to individual-level covariates such as age, race, and Hispanic ethnicity, we examined county-level covariates (residence in a Health Professional Shortage Area, urban/rural residence; race/ethnicity; and number of health centers/clinics, mammography screening centers, primary care physicians, and obstetrician-gynecologists per 100,000 female population or per 1000 square miles) as predictors of stage of breast cancer at diagnosis.
Results
Both individual-level and contextual variables are associated with later stage of breast cancer at diagnosis. Black women and women of “other race” had higher odds of receiving a diagnosis of regional or distant stage breast cancer (P <0.0001 and P = 0.02). With adjustment for age, Hispanics were more likely to receive a diagnosis of later stage breast cancer than non-Hispanics (P <0.0.001). Women living in areas with a higher proportion of black women had greater odds of receiving a diagnosis of regional or late stage breast cancer compared with women living in areas with the lowest proportion of black women. The same was noted for women living in areas with intermediate proportions of Hispanic women (age-adjusted odds ratio [OR], 0.94; 95% confidence interval [CI], 0.92–0.97]. Other important contextual variables associated with stage at diagnosis included the percentage of persons living below the poverty level and the number of office-based physicians per 100,000 women. Women living in counties with a higher proportion of persons living below the poverty level or fewer office-based physicians were more likely to receive a diagnosis of later stage breast cancer than those living in other counties (P < 0.001). In multivariable analysis, residence in areas with a higher proportion of non-Hispanic black women modified the associations of age and Hispanic ethnicity with later stage breast cancer (P = 0.0159 and P = 0.0002, respectively).
Conclusions
This study found that county-level contextual variables related to the availability and accessibility of health care providers and health services can affect the timeliness of breast cancer diagnosis. This information could help public health officials develop interventions to reduce the burden of breast cancer among U.S. women.
Keywords: Breast cancer, cancer prevention and control, screening, stage
INTRODUCTION
Breast cancer stage at diagnosis is used to assess prognosis, plan treatment, and evaluate outcomes [1]. It also can be a useful marker of screening mammography use at the population level. Previous studies have identified several individual-level and area-based variables associated with late stage at breast cancer diagnosis, including lack of adherence to guidelines for screening mammography, age, less education, black race, Hispanic ethnicity, and factors associated with decreased access to care (e.g., lower income, residence in socioeconomically distressed counties, population density, rural residence, residence in medically underserved urban areas, and lack of health care insurance or underinsurance) [2–12]. Breast cancer stage at diagnosis also has been associated with the number of mammography facilities in a county that are certified by the U.S. Food and Drug Administration (FDA) [13] and with treatment at a teaching hospital [14, 15]. Davidson et al. [5] examined stage at breast cancer diagnosis in relation to physician supply and health maintenance organization penetration in California [5]. With adjustment for individual-level factors, women who resided in a neighborhood with greater percentages of female head of households, persons living below the poverty level, less educated persons, and more recent immigrants, were more likely to have breast cancer diagnosed at a later stage. The supply of primary care physicians and radiologists, as well as residence in a county with higher insurance rates, were associated with earlier stage at diagnosis [5, 16].
Previous studies of stage at breast cancer diagnosis have often been limited to data from specific areas, states, or managed care organizations and have not been representative of the overall U.S. population [2, 3, 5, 10, 12]. Data from the National Program of Cancer Registries (NPCR) are more likely to be generalizable to all women in the United States because recent data include more than 90% of the population. A further issue is that previous studies have rarely included a variety of individual and area-based variables related to access to health care simultaneously [5].
In the current study, we examined data from the Centers for Disease Control and Prevention’s NPCR, the National Cancer Institute’s Surveillance, Epidemiology and End-Results (SEER) program, and the Health Resources and Services Administration’s Area Resource File (ARF) to determine if women with breast cancer who live in counties with ecological markers of decreased access to health care (i.e., rural residence, fewer physicians, fewer health centers/clinics) are more likely to receive a diagnosis of late stage disease. We examined whether associations between breast cancer stage at diagnosis and individual-level covariates such as age, race, and Hispanic ethnicity persist after adjusting for contextual factors associated with health care availability and access. We hypothesized that the effects of these individual-level covariates on stage of cancer diagnosis might be modified by contextual factors associated with health care availability and access, including the numbers of primary care physicians, health centers/clinics, and mammography screening centers, as well as rural/nonrural residence and race/ethnicity.
MATERIALS AND METHODS
Data Sources and Analytic Samples
The study population consisted of breast cancer cases reported to NPCR as of January 31, 2007, and to SEER as of November 2006 and made available through a limited-use data file in April 2007 for all invasive cancer sites combined (not just breast cancer). Only primary female breast cancer cases diagnosed in 2004 were included, and duplicate cases were excluded [17]. Cases from 47 U.S. states and the District of Columbia were selected for inclusion in this study. We excluded cases from Maryland because data that met the study criteria were unavailable. We also excluded data from Minnesota and Illinois because of missing county information. Cases with a zip code associated with a military base also were excluded. Stage at breast cancer diagnosis was categorized as local (stage 1), regional (stages II and III), distant (stage IV), or unstaged. In situ breast cancers (SEER summary stage 0) were examined separately. Other individual level explanatory variables available from the cancer registries included Hispanic ethnicity, race, age and sex. The North American Association of Central Cancer Registries Hispanic Identification Algorithm was used to reduce misclassification of Hispanic women. American Indian/Alaska Native (AI/AN) race was linked to the Indian Health Service (IHS) records to minimize misclassification of AI/AN race.
The patient’s county of residence as determined by the county Federal Information Processing Standards (FIPS) code was used to merge individual-level data from the cancer registries with other county-level data sets to obtain the contextual-level variables. We accessed county-level information from multiple data sources: the 2004 ARF and the 2003 U.S. Department of Agriculture (USDA) for data on urban/rural continuum code, the U.S. Census Bureau for 2004 estimates of county female populations, and the FDA for listing of certified mammography centers as of December 2004. The FDA data on mammography facilities were geocoded; we then computed the number of mammography facilities in each county.
The ARF consists of integrated county-level data from various primary data sources, such as the American Hospital Association, the American Medical Association, and the Bureau of the Census [18]. ARF data elements considered as covariates include Health Professional Shortage Areas (HPSAs), number of federally qualified health centers and rural health clinics, number of mammography screening centers, total number of office-based primary care physicians (nonfederal, general practice, and family practice), and number of office-based obstetrician-gynecologists per county. Physician counts included only full-time equivalents for patient care and excluded administrative and research activities. We also restricted physician counts to office-based physicians because we were unable to discern from the ARF whether hospital-based physicians provided inpatient or outpatient care. We derived covariates for medical facilities and medical professionals per 100,000 female population by using the 2004 female population estimates provided by the Census Bureau [19]. Medical facility counts and provider counts per 1000 square miles from 2000 census geography data came from the 2004 ARF. Defining the medical provider counts and facility counts on the basis of both population and area (square miles) incorporates the spatial dimensions of availability and accessibility into the measurement of health care service access. The number of local health care service points from which a woman can receive screening or diagnostic services for breast cancer is a measure of availability, and either time or distance to health care provider locations measures accessibility [20]. To determine non-Hispanic black female county composition and Hispanic female county composition, we used the respective 2004 population estimates from the Census Bureau [19]. We used the 2004 total female population estimates to calculate the county minority female population composition percentages. We stratified county minority female composition on the basis of the tertiles of the respective county minority female composition percentages. To assess potential contextual interactions between population compositions of black females and Hispanic females, we restricted county composition of black females to non-Hispanics to define nonoverlapping comparative subpopulations.
The analysis of urban, suburban, and rural residence used county-level FIPS codes to assign county-level, rural-urban continuum codes using 2003 USDA data [21]. Codes 0–3 correspond to metropolitan areas (including metropolitan areas with populations of about 250,000 to greater than 1 million), codes 4 and 5 correspond to urban populations of 20,000 or greater (but less than 250,000), and codes 6–9 correspond to rural populations and to nonmetropolitan urban populations of up to 19,999. Depending on their county of residence, persons were categorized as residents of either (a) more populated metropolitan areas, (b) suburban areas and smaller metropolitan areas, or (c) rural areas and small towns.
A list containing addresses of mammography centers (8988), updated as of December 2004, was obtained from the FDA. The Geographic Information Systems (GIS) module of the SAS system was used to identify the county where each of these mammography centers was located. To determine the county for each address, we first assigned latitude and longitude values corresponding to the Census Bureau block-level of the address location (geocoding). To perform geocoding, we downloaded prebuilt data from the SAS Institute, created from the Census Tiger files [22]. Of the 8988 mammography centers, latitude and longitude values could be assigned with street-level data (which produces the highest level of precision) for 7176 of the centers (80%). For 1646 (20%) of the centers, the latitude and longitude values assigned were the zip code centroid. A total of 166 (<1%) centers could not be geocoded using street-level data or zip code match. The latitude and longitude coordinates were then used to determine the county boundary where each center was located. After eliminating duplicates (83), centers located at military facilities (17), and those located in U.S.-associated territories (e.g., American Samoa, Guam, Puerto Rico, U.S. Virgin Islands) (154), the county location could be identified for 8721 centers. The county location was used to count the number of mammography centers in each county. Number of centers per 100,000 female population and number of centers per 1000 square miles served as a proxy for community access to mammography screening.
Variable Definitions
All explanatory variables used in the analyses were categorical, with the exception that continuous versions of the variables were used in explanatory analyses to determine colinearity. Race and Hispanic ethnicity were categorized with two variables: a binary variable for Hispanic ethnicity and a separate variable with five nominal categories for race (white, black, Asian/Pacific Islander, American Indian/Alaska Native, or other race). Age was recoded into five groups: 18–29, 30–39, 40–49, 50–64, and older than 65 years. Other continuous variables related to access to care were categorized with quintiles after merging these county-level data to the NCPR/SEER registry data. These variables included number of office-based obstetrician-gynecologists (per 100,000 females and per 1000 square miles), number of office-based primary care physicians (per 100,000 females and per 1000 square miles), number of mammography centers (per 100,000 females and per 1000 square miles), number of health center clinics (per 100,000 females and per 1,000 square miles), and percentage of people living below the poverty level. Other variables included in our analysis were whether a county was designated as an HPSA (2 levels), rural-urban continuum code (3 levels), education code (2 levels), proportion of non-Hispanic black female county residents, and proportion of Hispanic female county residents. In multivariate models, fewer categories for some variables (e.g., age) were included. In addition, numbers of obstetrician-gynecologists and numbers of primary care physicians were combined into one variable.
Statistical Analysis
Statistical analyses were conducted to assess the association between the odds of being diagnosed with a late stage cancer and the identified explanatory variables. First, exploratory analyses consisting of univariate and bivariate distribution of the analytical variables were conducted. The univariate analysis was intended primarily as a quality assurance measure to ensure that the data exhibited known trends for key variables such as age or race. For continuous variables, bivariate analysis consisted of computing Spearman correlation coefficient; for categorical variables, measures of concordance and discordance were computed (Goodman and Kruskal Gamma). Primarily, the purpose of the bivariate analysis was to determine the presence of colinearity among the independent variables.
For multivariate analyses, binary and polytomous logistic regression models were fitted to the data. The following two types of models were fitted: 1) models for ascertaining the effect of each variable after adjusting for age and 2) a final model to assess the effect of each variable in the presence of other confounders and effect modifiers. For the binary logistic regressions, the variable was regional or distant cancer versus local cancer. For the polytomous logistic regression, three levels were used: local, regional or distant, and unstaged. Because observations from the same county or the same facility are likely to be correlated, we had planned to account for such correlations using generalized estimating equations (GEE). However, preliminary analysis showed that, for our data, there was little difference between a GEE model and one that assumed that the observations were independent. To assess the age-adjusted odds of having late stage cancer diagnosed, we fitted separate binary logistic regression where the dependent variables were age and the variable for which adjusted odds were to be computed. Separate polytomous logistic regressions were fitted with age and each dependent variable to determine if the distribution of stage of cancer (local, regional/distant, or unstaged) differed across levels of that dependent variable when the data were adjusted for age. We used contrasts for this process.
We used results from the exploratory analysis and the models with age-adjustment only and each dependent variable to select variables for the final model. The bivariate analysis showed that number of primary care physicians and number of obstetrician-gynecologists were highly correlated (correlation value, 0.87); as a result, they were combined into one variable. For measuring access to care in terms of availability of medical professionals or facilities, we used the population density variable (per 100,000 females) instead of the variable of the number of professionals or medical facilities per 1000 square miles because it had a higher odds ratio. For the final model, interaction terms created from the individual variables (age, Hispanic ethnicity, and race) combined with the contextual variables that remained were added and removed one at a time to determine if any of the county-level variables modified the effect of the individual variables.
RESULTS
A total of 217,299 women with diagnosed breast cancer in 2004 were included in this analysis. Individual and contextual characteristics of the cases are shown in Table 1. About 77% of the women with diagnosed breast cancer were >50 years; 41% were >65 years. About 86% of the women were white and 10% were black. The remaining women were Asian or Pacific Islander (3%), American Indian or Alaska Native (0.4%), or of other race (0.4%); 6% of the women were Hispanic. A majority (83%) of the women lived in metropolitan areas. About 20% lived in counties where more than 16% of residents lived below the poverty level.
Table 1.
Individual and Contextual Characteristics of Women with Diagnosed Breast Cancer in 2004 from the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology and End-Results (SEER)
| Independent Variable1 | Sample Size | Percentage |
|---|---|---|
| Age (years) | ||
| 18–29 | 917 | 0.42 |
| 30–39 | 9,553 | 4.4 |
| 40–49 | 39,988 | 18.4 |
| 50–64 | 77,809 | 35.81 |
| 65+ | 89,024 | 40.97 |
| Race | ||
| White | 185,417 | 86.36 |
| Black | 21,397 | 9.97 |
| American Indian/Alaska Native | 837 | 0.39 |
| Asian/Pacific Islander | 6,299 | 2.93 |
| Other | 764 | 0.36 |
| Hispanic | ||
| No | 204,014 | 93.89 |
| Yes | 13,285 | 6.11 |
| Education (county level) | ||
| Not Low | 192,516 | 88.85 |
| Low | 24,171 | 11.15 |
| County designated as Health Professional Shortage Area | ||
| None of the county | 41,445 | 19.13 |
| Part or all of the county | 175,242 | 80.87 |
| Number of rural health clinics | ||
| < 1 | 161,045 | 74.11 |
| ≥ 1 to < 2 | 25,014 | 11.51 |
| ≥ 2 to < 5 | 21,840 | 10.05 |
| ≥ 5 | 9,400 | 4.33 |
| Rural/Urban code for county3 | ||
| Metro | 179,692 | 82.93 |
| Urban | 32,956 | 15.21 |
| Rural | 4,039 | 1.86 |
| Percent living below poverty level | ||
| ≤ 8.8 | 44,010 | 20.31 |
| > 8.8 to ≤ 11.1 | 46,614 | 21.51 |
| > 11.1 to ≤ 13.4 | 41,980 | 19.37 |
| > 13.4 to ≤ 16.3 | 40,022 | 18.47 |
| > 16.3 | 44,061 | 20.33 |
| Number of mammography centers per 100,000 females2 | ||
| ≤ 1.93 | 44,388 | 20.48 |
| > 1.93 to ≤ 2.34 | 42,126 | 19.44 |
| > 2.34 to ≤ 2.97 | 42,680 | 19.7 |
| > 2.97 to ≤ 3.81 | 43,666 | 20.15 |
| > 3.81 | 43,827 | 20.23 |
| Number of mammography centers per 1,000 square miles | ||
| ≤ 2.78 | 44,701 | 20.63 |
| > 2.78 to ≤ 8.39 | 44,790 | 20.67 |
| > 8.39 to ≤ 20.23 | 42,816 | 19.76 |
| > 20.23 to ≤ 42.93 | 44,423 | 20.5 |
| > 42.93 | 39,932 | 18.43 |
| Number of office based physicians per 100,000 females2 | ||
| ≤ 107 | 44,100 | 20.35 |
| > 107 to ≤ 161 | 44,008 | 20.31 |
| > 161 to ≤ 200 | 45,338 | 20.92 |
| > 200 to ≤ 247 | 39,437 | 18.2 |
| > 247 | 43,804 | 20.22 |
| Number of office based physicians per 1,000 square miles | ||
| ≤ 124 | 44,715 | 20.64 |
| > 124 to ≤ 531 | 43,916 | 20.27 |
| > 531 to ≤ 1,594 | 44,272 | 20.43 |
| > 1,594 to ≤ 3,961 | 47,560 | 21.95 |
| > 3,961 | 36,199 | 16.71 |
| Number of Ob/Gyn physicians per 100,000 females2 | ||
| ≤ 5.93 | 44,725 | 20.64 |
| > 5.93 to ≤ 8.91 | 43,884 | 20.25 |
| > 8.91 to ≤ 10.95 | 45,562 | 21.03 |
| > 10.95 to ≤ 14.10 | 39,083 | 18.04 |
| > 14.10 | 43,433 | 20.04 |
| Number of OB-Gyn physicians per 1,000 square miles | ||
| ≤ 7.24 | 44,721 | 20.64 |
| > 7.24 to ≤ 29.54 | 44,612 | 20.59 |
| > 29.54 to ≤ 90.38 | 42,760 | 19.74 |
| > 90.38 to ≤ 225.29 | 44,977 | 20.76 |
| > 225.29 | 39,592 | 18.27 |
| Percentage of Hispanic females4 | ||
| ≤ 1.60 | 68,535 | 31.63 |
| > 1.60 to ≤ 6.28 | 76,316 | 35.22 |
| > 6.28 | 71,836 | 33.15 |
| Percentage of Non-Hispanic black females4 | ||
| ≤ 1.87 | 68,512 | 31.62 |
| > 1.87 to ≤ 6.44 | 75,439 | 34.81 |
| > 6.44 | 72,736 | 33.57 |
The individual-level characteristics are: age, race, and Hispanic ethnicity. All other variables are characteristics at the county level (contextual variables). Except for number of mammography centers (FDA) and rural code (USDA), the contextual variables came from the Area Resource File (ARF) of HRSA. Cases from Illinois and Minnesota have been removed.
The population used in the computation is the estimated total number of females in 2003 from the US Census Bureau.
From U.S. Department of Agriculture.
From US Census Bureau.
Table 2 presents age-adjusted ORs for predicting stage at diagnosis in 2004. In our sample, stage at diagnosis varied by age, with women aged >65 years having the highest proportion of localized breast cancer (51.6%). Women aged 18–29 years had the highest proportions of regional or distant stage cancer (39.6% and 6.5% respectively) and the second highest proportion of unstaged cancer. All independent variables significantly predicted stage at diagnosis except county designation as an HPSA (0.98; 95% CI, 0.95, 1.01). Of note, black women and women of other race had higher odds of a diagnosis of regional or distant stage breast cancer (P <0.01 and P < 0.05). Hispanic women were more likely to have a diagnosis of later stage breast cancer than non-Hispanic women.
Table 2.
Age Adjusted Odds Ratios and p-Values for Comparing the Distribution in the Outcome (Breast Cancer Stage) to a Referent Cell for Levels of Various Explanatory Variables
| Independent Variable1 | Distribution of Cancer Stage for Each Dependent Variable |
Age Adjusted OR | Confidence Interval for OR | P-Value Level (Binary Logistic Regression) $ | P-Value from Polytomous Regression# | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | In Situ | Localized | Regional | Distant | Unstaged | |||||
| Age (years) | ||||||||||
| 18–29 | 917 | 7.7% | 40.8% | 39.6% | 6.5% | 5.3% | ||||
| 30–39 | 9,553 | 13.6% | 40.0% | 37.2% | 4.6% | 4.5% | ||||
| 40–49 | 39,988 | 22.8% | 41.7% | 28.3% | 3.0% | 4.1% | ||||
| 50–64 | 77,809 | 21.4% | 45.8% | 24.5% | 3.9% | 4.4% | ||||
| 65+ | 89,024 | 16.6% | 51.6% | 20.5% | 4.4% | 6.9% | ||||
| Race | ||||||||||
| White (+) | 185,417 | 19.1% | 48.1% | 23.6% | 3.7% | 5.4% | 1.0 | |||
| Black | 21,397 | 18.8% | 40.7% | 29.6% | 6.6% | 4.4% | 1.48 | (1.43, 1.53) | *** | <.0001 |
| American Indian/Alaska Native | 837 | 17.6% | 41.9% | 24.4% | 5.0% | 11.1% | 1.16 | (0.98, 1.37) | <.0001 | |
| Asian/Pacific Islander | 6,299 | 22.2% | 46.9% | 25.3% | 3.3% | 2.3% | 0.99 | (0.93, 1.05) | <.0001 | |
| Other | 764 | 23.2% | 40.4% | 25.8% | 4.6% | 6.0% | 1.22 | (1.03, 1.45) | * | 0.0203 |
| Hispanic | ||||||||||
| No | 204,014 | 19.4% | 47.3% | 23.9% | 4.0% | 5.4% | 0.85 | (0.82, 0.89) | *** | <.0001 |
| Yes (+) | 13,285 | 17.9% | 44.4% | 28.8% | 4.4% | 4.6% | 1.0 | |||
| Education (county level) | ||||||||||
| Not Low | 192,516 | 19.6% | 47.2% | 23.9% | 3.9% | 5.5% | 0.88 | (0.86, 0.91) | *** | <.0001 |
| Low (+) | 24,171 | 16.6% | 47.7% | 27.1% | 4.8% | 3.8% | 1.0 | |||
| County Designated as Health Professional Shortage Area | ||||||||||
| None of the county | 41,445 | 19.8% | 47.6% | 24.1% | 3.9% | 4.6% | 0.98 | (0.96, 1.01) | <.0001 | |
| Part or all of the county (+) | 175,242 | 19.1% | 47.2% | 24.3% | 4.0% | 5.4% | 1.0 | |||
| Number of rural health clinics | ||||||||||
| < 1 | 161,045 | 19.9% | 46.8% | 23.8% | 4.0% | 5.5% | 0.95 | (0.90, 1.00) | * | 0.0097 |
| ≥ 1 to < 2 | 25,014 | 17.9% | 48.6% | 24.9% | 4.0% | 4.5% | 0.96 | (0.91, 1.02) | 0.0263 | |
| ≥ 2 to < 5 | 21,840 | 17.5% | 47.3% | 25.5% | 3.9% | 5.9% | 1.01 | (0.95, 1.07) | 0.0445 | |
| ≥ 5 (+) | 9,400 | 16.8% | 48.3% | 25.5% | 4.2% | 5.2% | 1.0 | |||
| Rural/Urban code for county3 | ||||||||||
| Metro | 179,692 | 19.8% | 47.3% | 24.1% | 3.9% | 4.8% | 0.95 | (0.92, 0.98) | *** | <.0001 |
| Urban (+) | 32,956 | 16.7% | 47.1% | 24.6% | 4.2% | 7.4% | 1.0 | |||
| Rural | 4,039 | 16.4% | 46.3% | 24.1% | 4.3% | 8.9% | 1.01 | (0.94, 1.10) | 0.0069 | |
| Percent living below poverty level | ||||||||||
| ≤ 8.8 (+) | 44,010 | 21.3% | 47.0% | 22.4% | 3.5% | 5.8% | 1.0 | |||
| > 8.8 to ≤ 11.1 | 46,614 | 20.3% | 47.4% | 23.2% | 3.6% | 5.6% | 1.04 | (1.00, 1.07) | * | 0.0090 |
| > 11.1 to ≤ 13.4 | 41,980 | 18.3% | 48.0% | 24.1% | 3.9% | 5.7% | 1.08 | (1.04, 1.11) | *** | <.0001 |
| > 13.4 to ≤ 16.3 | 40,022 | 18.6% | 48.4% | 25.0% | 4.2% | 3.8% | 1.11 | (1.08, 1.15) | *** | <.0001 |
| > 16.3 | 44,061 | 17.7% | 45.5% | 26.5% | 4.8% | 5.5% | 1.25 | (1.21, 1.29) | *** | <.0001 |
| Number of mammography centers per 100,000 females2 | ||||||||||
| ≤ 1.93 | 44,388 | 19.1% | 47.9% | 24.9% | 4.0% | 4.2% | 0.98 | (0.95, 1.02) | <.0001 | |
| > 1.93 to ≤ 2.34 | 42,126 | 19.2% | 47.8% | 25.3% | 4.0% | 3.7% | 1.00 | (0.96, 1.03) | <.0001 | |
| > 2.34 to ≤ 2.97 | 42,680 | 19.7% | 48.4% | 23.9% | 3.9% | 4.2% | 0.94 | (0.91, 0.97) | *** | <.0001 |
| > 2.97 to ≤ 3.81 | 43,666 | 20.2% | 46.6% | 23.7% | 4.0% | 5.6% | 0.98 | (0.95, 1.01) | <.0001 | |
| > 3.81 (+) | 43,827 | 18.2% | 45.6% | 23.4% | 4.0% | 8.7% | 1.0 | |||
| Number of mammography centers per 1,000 square miles | ||||||||||
| ≤ 2.78 | 44,701 | 16.9% | 47.5% | 24.6% | 4.1% | 6.9% | 1.02 | (0.99, 1.05) | <.0001 | |
| > 2.78 to ≤ 8.39 | 44,790 | 18.5% | 47.3% | 24.2% | 3.8% | 6.3% | 0.99 | (0.96, 1.02) | <.0001 | |
| > 8.39 to ≤ 20.23 | 42,816 | 20.6% | 47.4% | 23.9% | 3.6% | 4.5% | 0.96 | (0.93, 0.99) | * | <.0001 |
| > 20.23 to ≤ 42.93 | 44,423 | 19.9% | 47.0% | 24.5% | 3.9% | 4.6% | 1.00 | (0.96, 1.03) | <.0001 | |
| > 42.93 (+) | 39,932 | 20.7% | 47.0% | 23.9% | 4.5% | 3.9% | 1.0 | |||
| Number of office based physicians per 100,000 females2 | ||||||||||
| ≤ 107 | 44,100 | 17.2% | 46.6% | 25.2% | 4.2% | 6.8% | 1.12 | (1.09, 1.16) | *** | <.0001 |
| > 107 to ≤ 161 | 44,008 | 18.2% | 47.0% | 24.7% | 4.1% | 5.9% | 1.09 | (1.06, 1.12) | *** | <.0001 |
| > 161 to ≤ 200 | 45,338 | 19.7% | 47.8% | 24.7% | 3.9% | 3.9% | 1.05 | (1.02, 1.09) | ** | <.0001 |
| > 200 to ≤ 247 | 39,437 | 20.1% | 47.4% | 23.4% | 3.8% | 5.4% | 1.02 | (0.98, 1.05) | <.0001 | |
| > 247 (+) | 43,804 | 21.2% | 47.4% | 23.1% | 3.8% | 4.5% | 1.0 | |||
| Number of office based physicians per 1,000 square miles | ||||||||||
| ≤ 124 | 44,715 | 16.6% | 46.9% | 25.0% | 4.2% | 7.3% | 1.07 | (1.03, 1.11) | *** | <.0001 |
| > 124 to ≤ 531 | 43,916 | 18.5% | 47.9% | 23.9% | 3.8% | 6.0% | 0.99 | (0.95, 1.02) | <.0001 | |
| > 531 to ≤ 1,594 | 44,272 | 20.6% | 47.5% | 23.8% | 3.6% | 4.6% | 0.97 | (0.94, 1.01) | <.0001 | |
| > 1,594 to ≤ 3,961 | 47,560 | 19.9% | 46.6% | 24.4% | 4.1% | 4.9% | 1.02 | (0.99, 1.06) | <.0001 | |
| > 3,961 (+) | 36,199 | 21.1% | 47.4% | 24.0% | 4.3% | 3.3% | 1.0 | |||
| Number of Ob/Gyn physicians per 100,000 females2 | ||||||||||
| ≤ 5.93 | 44,725 | 17.5% | 46.7% | 24.2% | 4.1% | 7.3% | 1.08 | (1.05, 1.12) | *** | <.0001 |
| > 5.93 to ≤ 8.91 | 43,884 | 18.6% | 47.3% | 25.2% | 4.1% | 4.8% | 1.09 | (1.06, 1.13) | *** | <.0001 |
| > 8.91 to ≤ 10.95 | 45,562 | 19.3% | 47.8% | 24.1% | 3.9% | 4.9% | 1.03 | (1.00, 1.06) | <.0001 | |
| > 10.95 to ≤ 14.10 | 39,083 | 19.8% | 46.4% | 24.0% | 4.0% | 5.8% | 1.06 | (1.02, 1.09) | ** | <.0001 |
| > 14.10 (+) | 43,433 | 21.3% | 47.8% | 23.5% | 3.8% | 3.6% | 1.0 | |||
| Number of OB-Gyn physicians per 1,000 square miles | ||||||||||
| ≤ 7.24 | 44,721 | 16.8% | 47.0% | 24.8% | 4.1% | 7.2% | 1.04 | (1.01, 1.07) | * | <.0001 |
| > 7.24 to ≤ 29.54 | 44,612 | 18.4% | 47.9% | 24.0% | 3.8% | 5.9% | 0.97 | (0.94, 1.01) | <.0001 | |
| > 29.54 to ≤ 90.38 | 42,760 | 20.6% | 47.3% | 23.9% | 3.6% | 4.6% | 0.97 | (0.94, 1.00) | <.0001 | |
| > 90.38 to ≤ 225.29 | 44,977 | 20.2% | 46.5% | 23.8% | 4.1% | 5.3% | 0.99 | (0.96, 1.02) | <.0001 | |
| > 225.29 (+) | 39,592 | 20.6% | 47.5% | 24.6% | 4.3% | 3.1% | 1.0 | |||
| Percentage of Hispanic females4 | ||||||||||
| ≤ 1.60 | 68,535 | 18.6% | 46.7% | 24.1% | 4.1% | 6.5% | 1.00 | (0.97, 1.02) | <.0001 | |
| > 1.60 to ≤ 6.28 | 76,316 | 20.0% | 47.3% | 23.4% | 3.8% | 5.4% | 0.94 | (0.92, 0.97) | *** | <.0001 |
| > 6.28 (+) | 71,836 | 19.2% | 47.6% | 25.2% | 4.0% | 3.9% | 1.0 | |||
| Percentage of Non-Hispanic black females4 | ||||||||||
| ≤ 1.87 | 68,512 | 18.5% | 47.4% | 23.4% | 3.7% | 6.9% | 0.91 | (0.89, 0.93) | *** | <.0001 |
| > 1.87 to ≤ 6.44 | 75,439 | 19.8% | 47.9% | 24.1% | 3.8% | 4.5% | 0.91 | (0.89, 0.93) | *** | <.0001 |
| > 6.44 (+) | 72,736 | 19.4% | 46.4% | 25.1% | 4.5% | 4.6% | 1.0 | |||
The event is regional or distant stage vs. local stage.
(+) indicates reference level.
- < 0.05.
- < 0.01.
< 0.001.
Testing that the distribution of the outcome is the same for the reported and the reference level.
The individual-level characteristics are: age, race, and Hispanic ethnicity. All other variables are characteristics at the county level (contextual variables). Except for number of mammography centers (FDA) and rural code (USDA), the contextual variables came from the Area Resource File (ARF) of HRSA. Cases from Illinois and Minnesota have been removed.
The population used in the computation is the estimated total number of females in 2003 from the US Census Bureau.
From U.S. Department of Agriculture.
From US Census Bureau.
Other important contextual variables associated with stage at diagnosis included the percentage of persons living below the poverty level and the number of office-based physicians per 100,000 females. As the proportion of residents living in poverty increases, the odds of a diagnosis of later stage breast cancer increases, with women living in areas with >16% poverty being 1.25 times more likely to receive a diagnosis of late stage cancer compared with those living in areas with less poverty. Women living in areas with fewer office-based physicians were more likely to receive a diagnosis of later stage breast cancer than those living in other counties (P <0.0001). This relationship also holds true for geographic density, with women living in areas with <124 physicians per 1000 square miles being 1.07 times more likely to receive a diagnosis of a later stage compared with women living in areas with >3961 physicians per 1000 square miles. The number of mammography facilities per 100,000 women and per 1000 square miles was not as predictive. This relationship does not appear to be linear, with women in the third quintile who receive a diagnosis having the lowest chances of receiving a diagnosis of late stage breast cancer compared with those living in areas with the highest density of mammography facilities. Women living in metropolitan counties were less likely to have later stage breast cancer than women living in suburban counties (P <0.0001); there was no significant difference between women living in suburban counties and those living in rural counties.
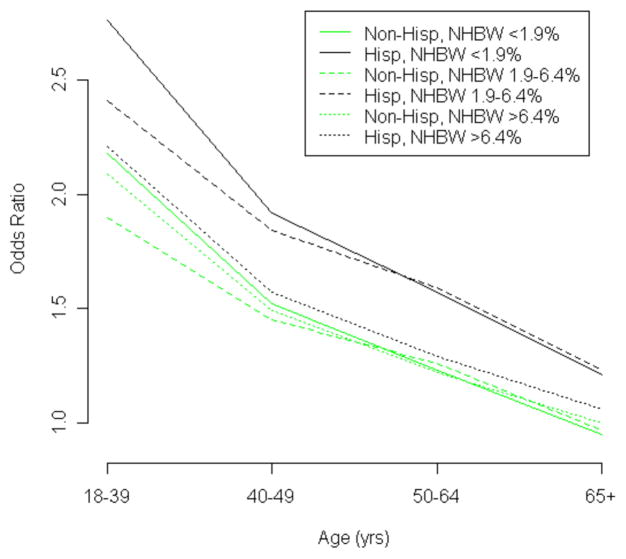
In multivariable analysis (Table 3), after accounting for other covariates in the model, age remained the most significant variable associated with the risk of receiving a diagnosis of late stage cancer. Another important individual risk factor was race: the risk of a late stage cancer diagnosis was 47% higher for blacks compared with whites. Key contextual variables that remained significant included percentage of persons living below the poverty level (risk is 14% higher for those living in areas with the highest poverty level compared with those living in areas with the lowest level) and number of primary care physicians (risk is 13% higher for those living in counties with the lowest number of primary care physicians per 100,000 residents compared with those living in counties with the highest number). Residence in areas with a higher proportion of non-Hispanic black women modified the associations of age (P = 0.0159) and Hispanic ethnicity (P = 0.0002) with later stage breast cancer. Although younger women had higher odds of late stage cancer, the size of the difference varied by the proportion of non-Hispanic black women in the county of residence. Women aged 18–39 years were at increased risk for later stage cancer compared with women aged >65 years, but this effect was larger among women living in areas with a small proportion of non-Hispanic black women (OR = 2.28, 95% CI = 2.10–2.49) compared to those living in areas with a moderate (OR = 1.96, 95% CI = 1.82–2.12) proportion of non-Hispanic black women. Similarly, Hispanics had higher odds of late stage cancer compared with non-Hispanics, but only among women living in areas with a small (OR = 1.27, 95% CI = 1.17–1.38) or moderate (OR = 1.27, 95% CI = 1.19–1.35) proportion of non-Hispanic black women. Hispanic ethnicity was not significantly associated with late stage cancer among those living in areas with a high proportion of non-Hispanic black women (OR = 1.06, 95% CI = 0.98–1.14). Fig. (1) shows the interaction between the proportion of non-Hispanic black women living in a county, age, and Hispanic ethnicity.
Table 3.
Parameter Estimate, Odds Ratios and Confidence Intervals for Odds Ratios from Multivariate Logistic Regression for Regional or Distant vs. Local Stage of Breast Cancer
| Independent Variable1 | Parameter Estimate | P-Value Level | Odds Ratio | Confidence Interval for OR |
|---|---|---|---|---|
| Age (years) | ||||
| 18–39 | 0.7375 | *** | 2.09 | (1.94, 2.26) |
| 40–49 | 0.3960 | *** | 1.49 | (1.42, 1.56) |
| 50–64 | 0.1992 | *** | 1.22 | (1.17, 1.27) |
| 65 (+) | 1.0 | |||
| Race | ||||
| White (+) | 1.0 | |||
| Black | 0.3835 | *** | 1.47 | (1.42, 1.52) |
| American Indian/Alaska Native | 0.1262 | 1.13 | (0.96, 1.34) | |
| Asian/Pacific Islander | 0.0290 | 1.03 | (0.97, 1.09) | |
| Other | 0.1782 | 1.20 | (1.01, 1.42) | |
| Hispanic | ||||
| No (+) | 1.0 | |||
| Yes | 0.0537 | *** | 1.06 | (0.98, 1.14) |
| County Designated as Health Professional Shortage Area | ||||
| None of the County | 0.0278 | 1.03 | (1.00, 1.06) | |
| Part or All of the County (+) | 1.0 | |||
| Rural/Urban code for County3 | ||||
| Metropolitan | −0.0009 | 1.00 | (0.96, 1.03) | |
| Surburban (+) | 1.0 | |||
| Rural | 0.0102 | 1.01 | (0.93, 1.09) | |
| Percent living below poverty level | ||||
| ≤ 8.8 (+) | 1.0 | |||
| > 8.8 to ≤ 11.1 | 0.0283 | 1.03 | (0.99, 1.06) | |
| > 11.1 to ≤ 13.4 | 0.0509 | ** | 1.05 | (1.02, 1.09) |
| > 13.4 to ≤ 16.3 | 0.0660 | *** | 1.07 | (1.03, 1.11) |
| > 16.3 | 0.1269 | *** | 1.14 | (1.09, 1.18) |
| Number of mammography centers per 100,000 females2 | ||||
| ≤ 1.93 | −0.0366 | * | 0.96 | (0.93, 1.00) |
| > 1.93 to ≤ 2.34 | −0.0207 | 0.98 | (0.94, 1.02) | |
| > 2.34 to ≤ 2.97 | −0.0259 | 0.97 | (0.94, 1.01) | |
| > 2.97 to ≤ 3.81 | 0.0140 | 1.01 | (0.98, 1.05) | |
| > 3.81 (+) | 1.0 | |||
| Number of Ob/Gyns and PCPs per 100,000 females2 | ||||
| ≤ 113.21 | 0.1206 | *** | 1.13 | (1.09, 1.17) |
| > 113.21 to ≤ 170.29 | 0.0912 | *** | 1.10 | (1.06, 1.13) |
| > 170.29 to ≤ 210.44 | 0.0516 | ** | 1.05 | (1.02, 1.09) |
| > 210.44 to ≤ 260.42 | 0.0372 | * | 1.04 | (1.00, 1.07) |
| > 260.42 (+) | 1.0 | |||
| Percentage of Hispanic females4 | ||||
| ≤ 1.60 | 0.0048 | 1.00 | (0.97, 1.04) | |
| > 1.60 to ≤6.28 | −0.0286 | * | 0.97 | (0.95, 1.00) |
| > 6.28 (+) | 1.0 | |||
| Percentage of Non-Hispanic black females4 | ||||
| ≤ 1.87 | −0.0478 | 0.95 | (0.91, 1.00) | |
| > 1.87 to ≤6.44 | −0.0315 | 0.97 | (0.93, 1.01) | |
| > 6.44 (+) | 1.0 | |||
| Age x Percent Non-Hispanic black females5 | ||||
| Age 18–39, Pct NonHispanic black ≤ 1.87 | 0.0879 | |||
| Age 18–39, Pct NonHispanic black > 1.87 to ≤ 6.44 | −0.0622 | |||
| Age 40–49, Pct NonHispanic black ≤ 1.87 | 0.0684 | |||
| Age 40–49, Pct NonHispanic black > 1.87 to ≤ 6.44 | 0.0096 | |||
| Age 50–64, Pct NonHispanic black ≤ 1.87 | 0.0590 | |||
| Age 50–64, Pct NonHispanic black > 1.87 to ≤ 6.44 | 0.0596 | |||
| HispanicxPercent Non-Hispanic black females5 | ||||
| Hispanic, Pct NonHispanic black ≤ 1.87 | 0.1840 | |||
| Hispanic, Pct Non-Hispanic black > 1.87 to ≤ 6.44 | 0.1816 | |||
(+) indicates reference level.
- < 0.05.
- < 0.01.
< 0.001.
Testing that the distribution of the outcome is the same for the reported and the reference level.
The individual characteristic variables are: age, race, and Hispanic ethnicity. All other variables are characteristics at the county level (contextual variables). Except for number of mammography centers (FDA) and rural code (USDA), the contextual variables came from the Area Resource File (ARF) of HRSA. Cases from Illinois and Minnesota have been removed.
The population used in the computation is the estimated total number of females in 2003 from the US Census Bureau.
From U.S. Department of Agriculture.
From US Census Bureau.
OR for interactions are not given as they are meaningless without the main effect terms.
Fig. 1.
Odds of receiving a diagnosis of later stage breast cancer by age group for Hispanic versus non-Hispanic women. Reference group is non-Hispanic women aged 65 or older living in countries with more than 6.4% of the population being non-Hispanic black women (NHBW).
DISCUSSION
The current study adds to the sparse but expanding literature about the role of contextual variables in breast cancer stage at diagnosis by evaluating county-level variables related to the availability and accessibility of health care providers and health services. The important contextual variables associated with cancer stage at diagnosis included percentage of persons living below the poverty level and number of office-based physicians per 100,000 females (including primary care providers and obstetrician-gynecologists). Some previous studies have suggested that living in a county with a larger number of physicians is associated with increased use of mammography services [23], and that primary care physician supply is associated with earlier stage at breast cancer diagnosis [5, 9]. Studies of breast and cervical cancer screening in the United States have shown that women with greater access to health care, such as those with health insurance or a higher family income, are more likely to have recent screening tests [24, 25]. Having had a recent physician visit or having a usual source of health care is also predictive of screening adherence [26]. The accessibility of routine health care is also important. For example, persons who live in areas of the United States with more primary care providers might have greater access to cancer screening [27].
In the current study, black women and women of other race had higher odds of receiving a diagnosis of regional or distant stage breast cancer (P <0.0001 and P = 0.02, respectively). After adjustment for age, Hispanic women were more likely to receive a diagnosis of later stage breast cancer than non-Hispanic women. In addition, important two-way interactions were found between an individual-level measure of Hispanic ethnicity (and age) and contextual variables related to racial composition at the county level. Our findings are generally consistent with those reported by Benjamins et al. [23] from their contextual analysis of associations of county-level racial/ethnic composition and use of mammography and other preventive services in the United States. On the basis of data from the Medical Expenditure Panel Survey (MEPS) and ARF (1996–1998), Benjamins et al. [23] found that women living in counties with more blacks were more likely to have regular mammograms. They also found that Hispanic women who lived in counties with high percentages of blacks reported higher levels of use of mammograms and other preventive services compared with Hispanic women living in other counties, suggesting a possible interactive effect between county racial/ethnic composition and individual-level ethnicity. This association may explain why our study found that Hispanic women who live in areas with a high proportion of non-Hispanic black females had a lower risk of receiving a diagnosis of a later stage cancer.
Previous research has indicated that U.S. women who live in rural areas are less likely than women living in urban areas to report mammography screening [23, 28–30]. Rural and nonrural areas differ in health care workforce and provider supply, distance to mammography screening facilities, and use of mammography screening [20, 30, 31]. Urban practices may differ from rural practices in office systems used to promote breast cancer screening [32]. Results from our bivariate analyses suggest that women living in metropolitan areas are less likely to have later stage cancer compared with women living in suburban or rural counties. However, no important associations were observed in the current study with rural/nonrural residence in multivariate analysis.
Our finding that women aged 18–29 years had the highest proportions of regional and distant stage cancer may reflect the fact that screening mammography is not recommended for women aged <40 years, except for those with a pronounced family history of breast or ovarian cancer. Prior studies have shown that younger patients with breast cancer are more likely to have aggressive tumors [33–35]. The two-way interaction observed in multivariate analysis between the individual-level measure of age and contextual variables related to county racial composition suggests that women aged 18–39 years are more likely to receive a diagnosis of later stage breast cancer if they live in a county with a lower proportion of women who are non-Hispanic black. This apparent effect modification by county racial composition may be accounted for by racial differences in the prevalence of breast cancer. Although racial differences in access to health care resources for diagnostic evaluation and treatment among younger women is a further possibility, no significant interactions were observed between age and number of office-based primary care physicians or obstetrician-gynecologists (results not shown).
With respect to limitations, some bias could have occurred because of misclassification of race and Hispanic ethnicity in the cancer registry data. In addition, the ARF data do not capture possible incongruence between county of residence and county of medical service provider or facility, as medical service provider catchment areas do not necessarily correspond to county boundaries [36]. For example, if the nearest medical provider was located in an adjacent county, a woman could receive health services for breast cancer from that provider rather than from one located within her county of residence. A further issue is that our measures represent average supply across populations or geographic areas and therefore do not account for heterogeneity within counties [36]. Contextual analyses that focus on smaller geographic areas (e.g., census tracts) might partially overcome this limitation. We did not include hospital-based outpatient departments providing primary care or gynecology services, which often serve different patient populations than office-based practices [37]. Our measures of provider supply might not reflect the total supply because we did not include nonphysician health care professionals (e.g., nurse practitioners, physicians’ assistants). Similarly, measures of mammography screening centers might not reflect nontraditional screening sites.
In summary, we found that women who were younger, black or Hispanic, or who lived in counties with higher poverty rates or lower primary care physician supply were more likely to have later stage of breast cancer at diagnosis. These findings suggest that disparities in stage at diagnosis occur across communities and populations, with communities with markers of decreased access reporting more advanced stages. The information obtained from this study may be helpful in planning these public health interventions to reduce the burden of breast cancer among U.S. women. Interventions found to be effective in increasing screening mammography among women aged >40 who are members of diverse populations and communities, including client-, provider-, and health care system-based interventions, have been highlighted by the Guide to Community Preventive Services and other evidence reviews [38–41]. Intervention approaches that enhance access to mammography may be especially helpful for low-income women and those who are racial-ethnic minorities [39]. Examples of access-enhancing intervention approaches include the use of vouchers and same-day appointments to reduce out of pocket expenses and overcome structural barriers, help with appointment and scheduling of mammograms, and the use of mobile mammography vans. Interventions that combine different approaches (for example, those aimed at individuals and at health care systems) may be especially helpful among populations with lower mammography rates, given the interplay of individual and health care system or environmental factors [39]. Further research is needed to better understand the contextual effects of race, ethnicity, and number of office-based physicians (including primary care providers and Ob-Gyns) on mammography utilization.
References
- 1.Hunter CP. Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer. 2000;88(Suppl):1193–202. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1193::aid-cncr3>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Menck HR, Mills PK. The influence of urbanization, age, ethnicity, and income on the early diagnosis of breast carcinoma. Cancer. 2001;92:1299–304. doi: 10.1002/1097-0142(20010901)92:5<1299::aid-cncr1451>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518–27. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Weissfeld JL, Weinberg GB, et al. Screening mammography and late-stage breast cancer: a population-based study. Prev Med. 1999;28:572–8. doi: 10.1006/pmed.1999.0483. [DOI] [PubMed] [Google Scholar]
- 5.Davidson PL, Bastani R, Nakazono TT, Carreon DC. Role of community risk factors and resources on breast carcinoma stage at diagnosis. Cancer. 2005;103:922–30. doi: 10.1002/cncr.20852. [DOI] [PubMed] [Google Scholar]
- 6.Barry J, Breen N. The importance of residence in predicting late-stage diagnosis of breast or cervical cancer. Health Place. 2005;11:15–29. doi: 10.1016/j.healthplace.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Amey CH, Miller MK, Albrecht SL. The role of race and residence in determining stage at diagnosis of breast cancer. J Rural Health. 1997;13:99–108. doi: 10.1111/j.1748-0361.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 8.Perkins CI, Wright WE, Allen M, et al. Breast cancer stage at diagnosis in relation to duration of Medicaid enrollment. Med Care. 2001;39:1224–33. doi: 10.1097/00005650-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante JM, Gonzalez EC, Pal N, et al. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13:408–14. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- 10.Gregorio DI, Walsh SJ, Tate JP. Diminished socioeconomic and racial disparity in the detection of early-stage breast cancer, Connecticutt, 1986–1995. Ethnic Dis. 1999;9:396–402. [PubMed] [Google Scholar]
- 11.Lee-Feldstein A, Feldstein PJ, Buchmueller T, Katterhagen G. The relationship of HMOs, health insurance, and delivery systems to breast cancer outcomes. Med Care. 2000;38:705–18. doi: 10.1097/00005650-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy EP, Burns RB, Coughlin SS, et al. Does regular mammography use explain black-white differences in stage at diagnosis among older women? Ann Int Med. 1998;128:729–36. doi: 10.7326/0003-4819-128-9-199805010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Marchick J, Henson DE. Correlations between access to mammography and breast cancer stage at diagnosis. Cancer. 2005;103:1571–80. doi: 10.1002/cncr.20915. [DOI] [PubMed] [Google Scholar]
- 14.Hand R, Sener S, Imperato J, Chmiel JS, Sylvester JA, Fremgen A. Hospital variables associated with quality of care for breast cancer patients. JAMA. 1991;266:3429–32. [PubMed] [Google Scholar]
- 15.Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81:646–9. doi: 10.2105/ajph.81.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decker SL, Hempstead K. HMO penetration and quality of care: the case of breast cancer. J Health Care Finance. 1999;26:18–32. [PubMed] [Google Scholar]
- 17. [accessed on December 9, 2008]; http://www.cdc.gov/cancer/npcr/uscs/data/00_2004_table.htm.
- 18.Bureau of Health Professions. Area Resource File. Rockville, MD: U.S. Department of Health and Human Services, Health Resources and Services Administration; Feb, 2004. [Google Scholar]
- 19.Census Bureau population estimates, as modified by the National Cancer Institute’s Surveillance Epidemiology and End Results Program. [Accessed on 2008 December 9]; Available from: http://www.seer.cancer.gov/popdata.
- 20.Guagliardo M. Spatial accessibility of primary care: concepts, methods and changes. Int J Health Geographics. 2004;3:3. doi: 10.1186/1476-072X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Agriculture. [accessed on December 9, 2008];Economic Research Service, 2003 rural-urban continuum codes. Available from: http://www.ers.usda.gov/data/RuralUrbanContinuumCodes/
- 22. [accessed on December 9, 2008]; http://support.sas.com/rnd/datavisualization/mapsonline/html/geocode.html.
- 23.Benjamins MR, Kirby JB, Bond Huie SA. County characteristics and racial and ethnic disparities in the use of preventive services. Prev Med. 2004;39:704–12. doi: 10.1016/j.ypmed.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 24.O’Malley MS, Earp JAL, Hawley ST, et al. The association of race/ethnicity, socioeconomic status, and physician recommendation for mammography: who gets the message about breast cancer screening? Am J Public Health. 2001;91:49–54. doi: 10.2105/ajph.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 27.Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health. 2004;20:118–24. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin SS, Thompson TD, Hall HI, et al. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer. 2002;94:2801–12. doi: 10.1002/cncr.10577. [DOI] [PubMed] [Google Scholar]
- 29.Duelberg SI. Preventive health behavior among black and white women in urban and rural areas. Soc Sci Med. 1992;34:191–8. doi: 10.1016/0277-9536(92)90096-9. [DOI] [PubMed] [Google Scholar]
- 30.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contexual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008;66:260–75. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Yabroff KR, Lawrence WF, King JC, et al. Geographic disparities in cervical cancer mortality: what are the roles of risk factor prevalence, screening, and use of recommended treatment? J Rural Health. 2005;21:149–57. doi: 10.1111/j.1748-0361.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 32.Engelman KK, Ellerbeck EF, Mayo MS, et al. Mammography facility characteristics and repeat mammography use among Medicare beneficiaries. Prev Med. 2004;39:491–7. doi: 10.1016/j.ypmed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Thomas GA, Leonard RC. How age affects the biology of breast cancer. Clin Oncol (R Coll Radiol) 2009;21:81–5. doi: 10.1016/j.clon.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29–34. doi: 10.1016/j.cdp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Shannon C, Smith IE. Breast cancer in adolescent and young women. Eur J Cancer. 2003;39:2632–42. doi: 10.1016/s0959-8049(03)00669-5. [DOI] [PubMed] [Google Scholar]
- 36.Goodman DC, Mick SS, Bott D, et al. Primary care service areas: a new tool for the evaluation of primary care services. Health Services Res. 2003;38(1 Pt 1):287–309. doi: 10.1111/1475-6773.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regan J. Cancer screening among community health center women: eliminating the gaps. J Ambul Care Manage. 1999;22:45–52. doi: 10.1097/00004479-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Task Force on Community Preventive Services. [Accessed on December 9, 2008];The guide to community preventive services. Available from: http://www.thecommunityguide.org.
- 39.Legler J, Meissner HI, Coyne C, et al. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev. 2002;11:59–71. [PubMed] [Google Scholar]
- 40.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med. 2008;35(Suppl):S34–S55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Baron RC, Rimer BK, Coates RJ, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: a systematic review. 2008;35(Suppl):S56–S66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]