Abstract
Targeted therapy in oncology consists of drugs that specifically interfere with abnormal signaling pathways that are dysregulated in cancer cells. Tyrosine kinase inhibitors (TKIs) take advantage of unique oncogenes that are activated in certain types of cancer, and also target common mechanisms of growth, invasion, metastasis, and angiogenesis. However, many kinase inhibitors for cancer therapy are somewhat nonselective, and most have additional mechanisms of action at the cellular level which are not completely understood. The use of these agents has increased our knowledge of important side effects, of which the practicing clinician must be aware. Recently proposed endocrine-related side effects of these agents include alterations in thyroid function, bone metabolism, linear growth, gonadal function, fetal development, and glucose metabolism, and adrenal function. This review summarizes the most recent data on the endocrine side effects of TKIs.
INTRODUCTION
Dysregulated kinase signaling is invariably involved in the pathogenesis of all types of malignancies. Therefore, enormous efforts are being devoted to the development of small-molecule drugs that regulate the abnormal cancer “kinome”. Protein tyrosine kinases (TKs) catalyze the phosphorylation of specific tyrosine residues on their substrate proteins. TKs are key regulators of signaling pathways involving cellular proliferation, differentiation, and apoptosis (Krause and Van Etten 2005; Schlessinger 2000). Small molecule tyrosine kinase inhibitors (TKIs) are rationally designed compounds that affect TK-dependent oncogenic pathways. They are promising treatments for the therapy of malignant disease and offer excellent targets for selective inhibition (Krause and Van Etten 2005). These agents potentially provide a relatively high therapeutic window with low toxicity in comparison with conventional cytotoxic chemotherapy. However, as we gain experience with the use of TKIs, we are becoming aware of important side effects. This review will outline the endocrine-related side effects associated with TKIs in order to bring the practicing clinician up to date with the current status of the field.
TKIs have become more widespread in use as targeted therapy for a variety of malignancies (Zhang, et al. 2009). One of the first TKIs to demonstrate effectiveness, imatinib, has activity against the BCR-ABL oncoprotein, and has been successful in the treatment of chronic myeloid leukemia (Jabbour, et al. 2007; Ren 2005). Imatinib is also approved for the treatment of recurrent or metastatic gastrointestinal stromal tumors (GIST), in which the c-KIT or platelet-derived growth factor receptor alpha (PDGFRα) TKs may be constitutively activated. (Rubin, et al. 2007). More recently, TKIs have been used in the treatment of neuroendocrine tumors (Kulke, et al. 2008; Raymond 2010). Oncogenic kinases that have been implicated in the development of thyroid cancer, such as RET and BRAF, have emerged as targets for TKI therapy (Lodish and Stratakis 2008; Sherman 2009a). For patients with medullary or differentiated thyroid cancer unresponsive to conventional treatment, TKIs are currently being used in a number of clinical trials (Fox 2009; Gupta-Abramson, et al. 2008; Kloos, et al. 2009; Schlumberger, et al. 2009; Sherman, et al. 2008; Wells, et al. 2010). Further use of these agents for other types of malignancies is outlined in Table 1.
Table 1.
Major Tyrosine Kinase Inhibitors in Clinical Use
| Drug | Tumors treated | Main tyrosine kinases targeted |
Stage of Clinical development |
|---|---|---|---|
| Imatinib | CML, chronic eosinophilic leukemia, Philadelphia positive ALL, GIST |
bcr-abl, c-KIT and PDGFRA |
FDA-approved |
| Dasatinib | CML, Philadelphia chromosome positive ALL |
SRC-family TK, bcr- abl, c-KIT, PDGFRA |
FDA-approved |
| Nilotinib | CML | bcr-abl c-KIT and PDGFRA |
FDA-approved |
| Sunitinib | GIST, RCC | VEGFR2, PDGFRB, c- KIT, FLT3 |
FDA-approved |
| Sorafenib | RCC, hepatocellular carcinoma |
RAF/MEK/ERK VEGFR2/PDGFRs |
FDA-approved |
| Gefitinib | NSCLC | EGFRs | FDA-approved |
| Erlotinib | NSCLC pancreatic cancer |
EGFRs | FDA-approved |
| Lapatinib | HER2 positive breast cancer |
EGFR, ErbB2, Erk-1 and-2, AKT kinases |
FDA-approved |
| Axitinib | Pancreatic, Breast RCC, NSCLC, melanoma, thyroid, renal |
VEGFRs, PDGFRs | Phase II-III |
| Vandetanib | NSCLC, myeloma, thyroid cancer, glioma |
VEGFRs, EGFRs, RET | Phase II-III |
| Vatalanib | Colorectal cancer, pancreatic, neuroendocrine, glioblastoma multiforme |
VEGFRs, PDGFRs, c- KIT, c-Fms |
Phase II-III |
| AEE788 | Glioblastoma multiforme | EGFR, HER2, VEGF2 | Phase I/II |
| Motesanib | GIST, breast cancer, neuroendocrine tumors, thyroid, colorectal, NSCLC |
VEGFRs, PDGFRs, c- KIT, RET |
Phase II/III |
| Dovitinib | Melanoma, RCC, breast, prostate |
FGFR3, VEGFR, PDGFR3, FLT-3,c-KIT |
Phase I/II |
| Tivozanab | RCC, breast, colorectal, NSCLC |
Phase II/III |
All information may be accessed at: http://www.cancer.gov; Abbreviations: CML: Chronic Myeloid Leukemia; ALL: Acute lymphoblastic leukemia, GIST: gastrointestinal stromal tumor; RCC: Renal cell carcinoma; NSCLC: Non-small cell lung carcinoma; EGFR: epidermal growth factor Receptor; PDGFR: platelet derived growth factor receptor, VEGFR: vascular endothelial growth factor receptor; FLT-3: Fms-related tyrosine kinase 3.
There are more that 500 different protein kinases encoded by the human genome; almost all of these kinases phosphorylate substrate proteins via their catalytic ATP binding region (Daub 2010). The TKs include the epidermal growth factor receptor (EGFR), the vascular endothelial growth factor receptors 1 and 2 (VEGFR-1 and VEGFR 2), and the downstream signaling mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK) pathway, among others (Sebolt-Leopold 2008; Tortora, et al. 2008). Platelet derived growth factor (PDGFRA), fibroblast growth factor receptor (FGFR), and serine-threonine kinase mammalian target of rapamycin (mTOR) are additional kinases implicated in oncogenesis (Alvarez, et al. 2006; Meric-Bernstam and Gonzalez-Angulo 2009). In papillary thyroid cancer (PTC), somatic rearrangement of the RET proto-oncogene leads to activated forms of RET, and germline mutations of the RET receptor are pathogenic in medullary thyroid cancer (MTC) (Ciampi and Nikiforov 2007; Wells and Santoro 2009). Activation of the B-Raf proto-oncogene serine/threonine-protein kinase occurs frequently in adult PTC (Xing 2005).
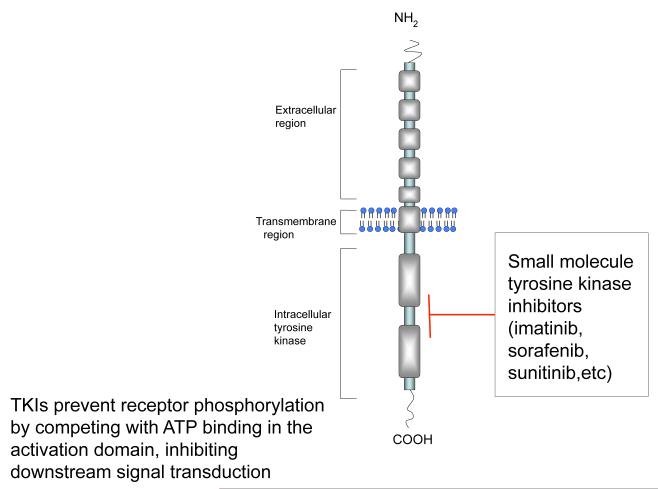
Among the different classes of protein kinases, there is a conservation of the structure of the ATP binding site. TKIs act as small molecules with structural similarity to ATP that serve to disrupt the catalytic activation of TKs (Figure 1). As a result of this homology, many TKIs may have inhibitory activity against a broad range of protein kinases. Many kinase inhibitors are less selective than initially thought and often affect multiple signaling pathways (Daub 2010; Fabian, et al. 2005; Karaman, et al. 2008).
Figure 1.
Schematic of structure and function of tyrosine kinase receptor and its small molecule inhibitors
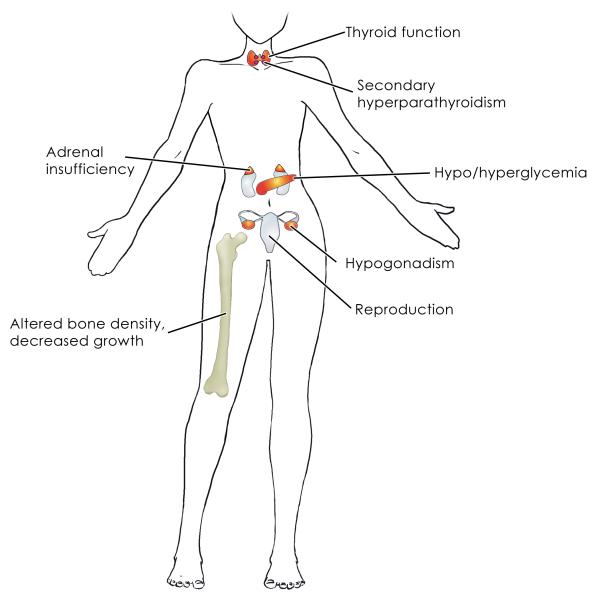
TKIs are administered orally and cause a number of side effects including fatigue, hypertension, rash, impaired wound healing, myelosuppression, and diarrhea (Widakowich, et al. 2007). The overall toxicity of TKIs, while less life-threatening than conventional cytotoxic chemotherapy, nevertheless is common and may require dose reduction. Recently proposed endocrine-related side effects of these agents include alterations in thyroid function, bone metabolism, linear growth, gonadal function, fetal development, and glucose metabolism, and adrenal function (Figure 2).
Figure 2.
Endocrine organs involved in side effects of tyrosine kinase inhibitors
THYROID FUNCTION
The increased use of TKIs has raised the awareness of adverse affects on thyroid hormone function and/or thyroid hormone metabolism, as outlined in Table 2. As a result of these studies, it is now recommended that all patients starting therapy with TKIs have their thyroid function tested prospectively (Sherman 2009b; Torino, et al. 2009). Other types of anti-cancer therapies have also been associated with thyroid dysfunction, including radiation to the pituitary and thyroid, and treatment with synthetic retinoid X receptor (RXR) agonist autoantibodies (Darzy and Shalet 2009; Garcia-Serra, et al. 2005; Sherman, et al. 1999). Hypothyroidism has also been reported after therapy with the angiogenesis inhibitor thalidomide (Badros, et al. 2002). Therapy with interleukin-2 (IL-2) and interferon-alpha (IFN-) have been reported to induce thyroid dysfunction and thyroid autoantibodies (Weijl, et al. 1993).
Table 2.
Studies reporting hypothyroidism in patients receiving TKI therapy
| Reference | Tumor type | TKI used | Subjects with TFTs (n) |
Hypothyroidism (%) |
|---|---|---|---|---|
| (Desai et al. 2006) | GIST | sunitinib | 42 | 36% |
| (Rini et al. 2007) | RCC | sunitinib | 66 | 85% |
| (Schoeffski 2006) | RCC and GIST | sunitinib | 33 | 37% prospectively 57% retrospectively |
| (Wong et al. 2007) | GIST | sunitinib | 40 | 53% |
| (Martorella AJ 2006) | RCC | sunitinib | 39 | 20% |
| (Sabatier 2009) | RCC | sunitinib | 54 | 68% |
| (Shaheen 2006) | RCC | sunitinib | 55 | 73% |
| (Mannavola et al. 2007) | GIST | sunitinib | 24 | 46% |
| (Chu, et al. 2007) | GIST | sunitinib | 36 | 14% |
| (Wolter et al. 2008) | RCC and GIST | sunitinib | 59 | 27% |
| (Tamaskar, et al. 2008) | RCC | sorafenib | 39 | 18% |
| (de Groot et al. 2005) | GIST and MTC | imatinib | 11 | 73% |
| (de Groot, et al. 2007) | MTC | imatinib | 15 | 60% |
| (Sherman et al. 2008) | DTC | motesanib | 93 | 22% |
| (Dora et al. 2008) | CML | imatinib | 68 | 0% |
The effect of TKIs in thyroid function was first noted in 2005 with the use of imatinib, which inhibits the TK activity of the ABL, c-KIT, and PDGRA kinases. In 8 MTC patients treated with imatinib, all of whom had previously undergone thyroidectomy, marked elevations in TSH up to a mean of 384% + 228% of the upper limit were noted.(de Groot, et al. 2005). Free T4 was decreased but generally remained within the reference range. Despite doubling the average dosage of levothyroxine, thyroid functions were normalized in only 3 out of 8 patients. The authors described associated symptoms that could be considered consistent with hypothyroidism, including fatigue and edema (47). However, the authors pointed out that it was difficult to distinguish whether these were caused by hypothyroidism, other underlying disease, or imatinib itself. Interestingly, in this initial description of thyroid dysfunction associated with TKIs, the patients had no thyroid glands (since they had undergone thyroidectomies for their tumors), therefore “thyroid dysfunction” could be better described as “alterations in TSH and free T4” or “worsening post-surgical hypothyroidism,” because the thyroid gland itself could not be implicated in the pathophysiology. One study evaluating the thyroid function of 68 patients with CML and intact thyroid glands who received imatinib did not find any adverse effect on thyroid function, suggesting that perhaps the effect of imatinib was unique to patients surgically rendered athyroid (Dora, et al. 2008). Motesanib, a TKI with inhibitory effects on VEGFR, PDGFRA, c-kit and RET, was also associated with marked exacerbation of post-surgical hypothyroidism in a phase II thyroid cancer trial. In this study, 60-70% of patients exhibited elevated TSH values, with 22% of patients defined as having clinical hypothyroidism, and a mean levothyroxine dosage increase of 30% was required to maintain normal TSH levels (Pacini, et al. 2007).
A number of other studies have followed thyroid functions in patients receiving TKIs who have intact thyroid glands (Table 2). Sorafenib inhibits the kinase activity of RAF/MEK/ERK VEGFR2 and PDGFR. Tamaskar et al. studied the incidence of thyroid function alterations in patients with metastatic renal cell cancer (RCC): hypothyroidism developed in 7 of 39 patients (18% ), and was first observed 2 – 4 months after initiation of sorafenib.
The TKI most frequently associated with hypothyroidism is sunitinib, which targets VEGFRs, PDGFRs, KIT, and RET. Desai et al. prospectively obtained TFTs in 42 patients with GIST who were receiving sunitinib (Desai, et al. 2006). Abnormal elevations in TSH were seen in 26 patients (62%), while persistent hypothyroidism was documented in 15 patients (36%). The average length of time to first peak elevation of TSH was 50 weeks of therapy (range 12-94 weeks). The investigators were able to normalize TSH in all patients with levothyroxine therapy. The authors suggested destructive thyroiditis as a possible mechanism of sunitinib-induced hypothyroidism, as 6 out of 15 patients with hypothyroidism had a TSH level below 0.5 mcIU/mL prior to developing hypothyroidism. In addition, two patients were found to have atrophic thyroid tissue on ultrasound, thought to be consistent with thyroiditis. Rini et al. evaluated thyroid abnormalities in 66 patients with RCC treated with sunitinib (Rini, et al. 2007). The results of this study are complicated by the fact that 30 of the patients received treatment with cytokine-based therapy (Weijl et al. 1993). In total, 56 of the 66 patients (85%) developed hypothyroidism; symptoms that can be associated with hypothyroidism, such as cold intolerance, fatigue, edema, dry skin, and thinning hair, were seen in 47 out of 66 patients (84%). This study also proposed thyroiditis as a possible mechanism for the observed abnormalities as thyroglobulin antibodies were found in 13 out of the 44 patients in whom they were measured. Two other reports have also demonstrated sunitinib-induced hypothyroidism associated with destructive thyroiditis (Faris, et al. 2007; Grossmann, et al. 2008). However, a number of other studies have shown recovery of TSH values back to the normal range following treatment with sunitinib, which does not support the hypothesis of destructive thyroiditis (Grossmann et al. 2008; Wolter, et al. 2008).
Schoeffeski et al measured thyroid functions in 19 patients with RCC and GIST receiving sunitinib (Schoeffski 2006). Of these patients, 7 out of 19 (37%) showed elevated TSH during treatment while 8 out of the 14 (57%) patients went on to develop hypothyroidism after a median duration of 44 weeks. Wong et al. evaluated the prevalence of hypothyroidism in a cohort of 40 patients with GIST, 53% of whom exhibited hypothyroidism after a median of 5 months of treatment with sunitinib (Wong, et al. 2007). This study was limited by the lack of baseline thyroid function data in the majority of patients, making it difficult to determine the true underlying incidence of thyroid dysfunction.
Martorella et al. reported a 20% incidence of hypothyroidism in a group of 39 patients with RCC treated with sunitinib; however, a complicating factor is that all patients had prior treatment with interleukin-2 (Martorella AJ 2006). Shaheen et al. evaluated TFTs in 55 patients with RCC, 73% of whom developed hypothyroidism (Shaheen 2006). Sabatier et al. performed a prospective observational analysis of hypothyroidism during sunitinib therapy in metastatic RCC, and found that 68% of the 54 patients developed hypothyroidism. In a study by Mannavola et al, 46% of patients with GIST on sunitinib treatment developed hypothyroidism requiring therapy with levothyroxine, while 25% exhibited transient TSH elevation (Mannavola, et al. 2007). Thyroid ultrasound scans and iodine-123 (123I) thyroidal uptake were performed at the end of several periods of sunitinib treatment. They found that 123I uptake was significantly reduced at the end of treatment periods, with partial or total normalization when therapy with TKIs was discontinued. The authors suggested that the underlying mechanism of sunitinib-induced thyroid dysfunction was impaired iodine uptake.
Two cases of RCC patients diagnosed with a nodular thyroid gland were observed to have marked shrinkage of the thyroid gland during treatment with sunitinib (Rogiers, et al. 2010). Thyroid gland volume reduction was measured via computed tomography scan, showing progression to near complete disappearance of the gland in one of the two patients. The authors hypothesize that TKI-induced thyroid function may be due to capillary regression induced by VEGF inhibition. A recent case report described a patient with RCC who developed hypothyroidism while on sunitinib and was found to develop an atrophic thyroid with marked reduction of vascularity (Makita, et al. 2010). However, disputing these findings is a study by Mannavola et al. who performed thyroid ultrasounds on 11 patients both before and during sunitinib treatment and did not detect changes in thyroid gland volume (Mannavola et al. 2007).
The mechanism by which TKIs cause thyroid dysfunction remains unclear. A number of in-vitro and animal studies have been performed to try and characterize the mechanism of TKI-induced hypothyroidism. Wong et al. performed in vitro assays to measure the effect of sunitinib on peroxidase activity, and found that sunitinib had antiperoxidase activity 25-30% as potent as propothiouracil (Wong et al. 2007). They propose that sunitinib acts directly on the thyroid gland via inhibition of peroxidase activity and thyroid hormone synthesis. Salem et al. evaluated the pathologic mechanism of sunitinib-induced hypothyroidism in rat thyroid cell cultures, and found that incubation with sunitinib for 24 hours caused a dose-related increase of 125I-iodide uptake, suggesting that inhibition of iodine uptake is unlikely to be the mechanism of sunitinib-induced hypothyroidism (Salem, et al. 2008).
The definition of hypothyroidism was somewhat variable in the studies reviewed, and some studies lacked complete TFT data in all patients. Postulated mechanisms are presented in Table 3. Interestingly, alterations in thyroid function tests have also been observed in clinical trials of thyroid cancer patients who have undergone thyroidectomy. This would certainly argue against a direct role of the thyroid gland in the mechanism of the effect of TKIs on TSH levels. Thus, some of the proposed mechanisms to explain the elevated TSH cannot explain the observed alterations of TFTs in studies of post-thyroidectomy patients. One mechanism to explain worsening TSH elevation in post-thyroidectomy patients would be an indirect affect of sunitinib on the metabolism of thyroid hormone, or with thyroid hormone action at the pituitary level. It is plausible that the different types of TKIs have more than one mechanism affecting thyroid functions, but it remains more likely that there is a universal drug class effect of these medications that has yet to be clarified.
Table 3.
| Proposed mechanisms of TKI induced hypothyroidism |
|---|
|
Unanswered questions remain regarding the optimal management of TKI-induced hypothyroidism. Baseline thyroid function tests should be performed before the initiation of TKI therapy and TFTs should be frequently monitored during treatment with TKIs. Levothyroxine treatment should be started when clinical hypothyroidism develops. However, the management of asymptomatic subclinical hypothyroidism (TSH 5-10 mcIU/mL) is uncertain in cancer patients in whom symptoms of hypothyroidism, such as fatigue, might overlap with symptoms of the malignancy and its treatment. Individuals with elevated TSH can be effectively managed with thyroid hormone replacement, such that hypothyroidism alone is not an indication for dose reduction or discontinuation of TKI therapy (Torino et al. 2009). Additional prospective clinical trials are necessary to investigate this important endocrine side effect and to determine the underlying molecular mechanism of TKI-related hypothyroidism.
ALTERED BONE DENSITY AND SECONDARY HYPERPARATHYROIDISM
A number of recent studies have demonstrated altered bone and mineral metabolism in patients receiving imatinib, however to date this affect has not been reported in other TKIs (Berman, et al. 2006; Fitter, et al. 2008; Grey, et al. 2006; Osorio, et al. 2007). As patients taking TKIs often continue the treatment indefinitely, clinicians need to be aware of the potential long-term effects of these agents on the skeleton. A 2-year prospective clinical study of the biochemical and skeletal effects of imatinib revealed secondary hyperparathyroidism and decreased bone turnover during prolonged treatment with imatinib (O’Sullivan, et al. 2009). In 9 patients with bcr-abl positive CML, therapy with imatinib was associated with a biphasic change in bone turnover, with an initial stimulation of bone formation followed by a period of suppression of bone resorption and formation. Bone mineral density in the cohort studied was stable or increased during the first 2 years of therapy, along with the development of mild secondary hyperparathyroidism (O’Sullivan et al. 2009). Two additional studies have demonstrated increased cortical bone mineralization in CML patients treated with imatinib (Fitter et al. 2008; Jonsson, et al. 2008).
The effects of TKIs on bone mineral metabolism and bone remodeling are hypothesized to be due to unspecific inhibition of tyrosine kinases expressed by osteoclasts and osteoblasts, such as c-KIT and PDGFRA (Berman et al. 2006). In vitro studies have shown that the TKI dasatinib can cause dysregulation of bone remodeling via inhibition of osteoclasts (Vandyke, et al. 2010). Additional in vitro studies revealed that imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and osteoclastogenesis (O’Sullivan, et al. 2007).
LINEAR GROWTH
Due to the small numbers of pediatric patients receiving single drug therapy with TKIs, it has not been feasible to conduct large-scale clinical trials to gain information about the effects of long-term therapy with TKIs on linear growth in childhood and adolescence. Angiogenesis is controlled in part by soluble factors such as VEGF and PDGF. Imatinib was shown to inhibit PDGF-induced cell proliferation and activity in chondrocyte cultures in vitro (Vandyke, et al. 2009). In mouse models, VEGF has been linked to key steps in cartilage remodeling, ossification, and angiogenesis during endochondral bone formation (Gerber, et al. 1999). Recent in vivo studies in rats showed narrowing of the growth plate at the proximal tibia in imatinib-treated animals (Vandyke et al. 2009). In a mouse model, imatinib treatment had an anti-resorptive effects on osteoclasts that impaired the length of tubular bone, particularly in prepubertal animals (Suttorp M 2008). These studies have raised concern for the potential effects of TKIs on longitudinal growth in children.
Three recently published case studies report decelerated growth in pre-pubertal CML patients undergoing imatinib therapy (Kimoto, et al. 2009; Mariani, et al. 2008; Schmid, et al. 2009). In one such case, the authors reported an 11-year-old boy who experienced a dramatic reduction in growth rate from 4.3 to 1.5 cm per year after initiating imatinib therapy (Mariani et al. 2008). In another case, a 6-year-old girl had significantly decreased growth velocity after being started on imatinib therapy for CML; the same patient returned to an increased growth rate after cessation of imatinib: during 4 years of imatinib therapy, the patient’s height SD score decreased from −0.7 to −2.7 (Kimoto et al. 2009). Finally, a third case reported a 5-year-old-girl whose height fell from the 74th percentile to the 9th percentile after three years of treatment with imatinib (Schmid et al. 2009). Additional larger scale clinical studies are necessary to draw meaningful conclusions about the effect of imatinib and other TKIs on linear growth in children.
HYPOGONADISM AND OVARIAN INSUFFICIENCY
A number of case reports have presented a possible association between gynecomastia and TKI use. One described a 69-year-old patient with metastatic RCC who developed painful gynecomastia while on sunitinib therapy; this gynecomastia was partially reduced while off therapy and resumed when sunitinib was reinitiated (Ballardini, et al. 2009). Another report describes a patient with CML initiated on imatinib at age 11. Beginning at age 14, this patient had progressive increase of follicle-stimulating hormone (FSH) and a decrease of inhibin-B, along with the development of gynecomastia (Mariani et al. 2008). Another report described the development of gynecomastia in a 42-year-old male on imatinib for GIST(Kim, et al. 2005). In one case of gynecomastia in a 70 year old male after treatment with dasatinib for CML, tamoxifen was added as combination therapy with subsequent reduction in gynecomastia (Caocci, et al. 2008). Another study looked at testosterone levels in 38 men receiving imatinib for CML at baseline and during treatment. Seven cases of gynecomastia were noted (18% of patients), with associated significant decrease in testosterone concentrations (Gambacorti-Passerini, et al. 2003). The mechanism by which sunitinib and other TKIs may induce gynecomastia is unknown, although c-KIT and PRGFRA are expressed in the testis and are involved in the production of testosterone (Basciani, et al. 2002). Imatinib has been shown to inhibit Leydig cell tumor growth in rat models, and this may be due to inhibition of proliferation and ligand-stimulated phosphorylation of PDGFRA and c-KIT (Basciani, et al. 2005).
One case of primary ovarian insufficiency during imatinib therapy was recently reported, (Christopoulos, et al. 2008); however the report was later challenged as being overly speculative (Malozowski, et al. 2008). In animal models, fertility in female rats has not been adversely affected by imatinib, and experience with the drug has shown pregnancies in women taking TKIs (Hensley and Ford 2003; Robinson, et al. 2007). Future long-term evaluation of the effects of TKIs on ovarian function and fertility are required
PREGNANCY AND TKIs
As the use of TKIs becomes more widespread, the safety of these agents during pregnancy has been called into question in light of the potential risks to the fetus. Little data exists on the effect of TKIs on fetal growth and development, and current recommendations call for the use of effective contraception in young women undergoing treatment. However, in cases where alternative therapy or stopping therapy are not acceptable alternatives, these agents have been used during pregnancy, and some reports are available on outcomes of pregnancies in women taking TKIs. A review on the use of targeted treatment with TKIs during pregnancy was recently published (Robinson et al. 2007). Imatinib has been linked with low birth weight, and both erlotinib and lapatinib have been associated with oligohydramnios, necessitating the close ultrasound follow-up of growth and amniotic-fluid index in these patients.
ADRENAL INSUFFICIENCY
Although overt adrenal insufficiency has not been reported in patients receiving TKIS, adrenal damage was observed in animal studies (Pfizer). Adrenal toxicity was reported in repeat dose studies performed in rats and monkeys at plasma exposures as low as 0.7 times the area under the curve (AUC) observed in clinical studies. A number of histological changes of the adrenal gland were noted, including hemorrhage, necrosis, congestion, hypertrophy and inflammation. In clinical studies, CT/MRI obtained in 336 patients after exposure to one or more cycles of sunitinib failed to demonstrate evidence of adrenal hemorrhage or necrosis. Adrenocorticotropin (ACTH) stimulation testing was performed in approximately 400 patients across multiple clinical trials of sunitinib, with only one patient found to have consistently abnormal test results during treatment. Eleven additional patients with normal baseline testing had abnormalities in the final test performed, with peak cortisol levels of 12-16.4 mcg/dL (normal >18 mcg/dL) following stimulation. None of these patients were reported to have clinical evidence of adrenal insufficiency (Pfizer).
The hypothalamic-pituitary-adrenal (HPA) axis was evaluated in 25 patients with CML treated with imatinib using glucagon stimulation testing as well as low dose (1 microgram) ACTH testing. Twelve (48%) of patients were defined as HPA deficient in this study (defined as a peak serum cortisol level <18 micrograms/dL measured 30 minutes after intravenous delivery of 1 microgram of ACTH), indicating an increased prevalence of subclinical glucocorticoid deficiency in patients receiving imatinib (Bilgir, et al. 2010).The Food and Drug Administration drug approval summary cautions that although no overt clinically important adrenal suppression has been observed in patients taking sunitinib, subclinical toxicity may be unmasked by physiologic stress; therefore monitoring for adrenal insufficiency is recommended in patients undergoing stressors such as surgery, trauma or severe infection (Rock, et al. 2007).
GLUCOSE METABOLISM
Increasing evidence suggests that TKIs influence glucose metabolism; both elevated and decreased blood glucose levels have been attributed to TKIs. The mechanism by which TKIs alter blood glucose levels is not known. Of the 6 FDA approved TKIs, 3 of these agents, imatinib, sunitinib, and nilotinib have all been associated with apparently opposite effects on glucose metabolism.
Imatinib has been associated with the adverse reaction of hyperglycemia in 0.1-1% of patients as reported in the adjuvant GIST trial (Novartis). However, a number of reports in the literature have cited glucose-lowering effects thought to be associated with imatinib. A case of regression of long-standing type 2 diabetes during treatment of CML with imatinib was first reported in 2005 (Veneri, et al. 2005). The authors postulated that inhibition of phosphorylation by imatinib may serve to improve insulin sensitivity. Another report revealed improvement of fasting blood glucose levels in 6 out of 7 diabetic CML patients being treated with imatinib, allowing reduction of insulin dosage or oral anti-diabetes therapy (Breccia, et al. 2004). Two patients with GIST and hypoglycemia were reported in whom imatinib is likely to have contributed to the severity of the hypoglycemia (Hamberg, et al. 2006). In vitro studies have shown that imatinib enhances β-cell survival, potentially contributing to the glucose-lowering effects observed thus far with the use of this TKI (Hagerkvist, et al. 2007).
The prescribing information for sunitinib reports the incidence of hypoglycemia in 73 out of 375 patients (19%) and hyperglycemia in 58 out of 375 patients (15%) (Pfizer). Proposed mechanisms include regression of pancreatic islets, modulation of IGF-1 signaling, or decreased glucose uptake. In metastatic renal carcinoma, hyperglycemia has been reported as a toxicity associated with the use of sunitinib in 15% of cases (Guevremont, et al. 2009). Blood glucose level variations associated with sunitinib therapy were retrospectively reviewed in nineteen diabetic patients treated for RCC. All patients had a decrease in blood glucose level after 4 weeks of treatment (Billemont, et al. 2008).
Updated data from the phase II trial of nilotinib for patients with CML listed hyperglycemia as a grade 3/4 toxicity associated with 12% of patients taking this agent (Deremer, et al. 2008). The prescribing information for nilotinib reports increased blood glucose as a common adverse reaction, occurring in less than 5% of patients (Novartis). The increased reports of altered glucose levels in patients receiving TKIs are difficult to interpret, given that some agents are associated with both hyper and hypoglycemia. In diabetic patients, careful assessment of glycemic control while on TKIs is recommended. Monitoring hemoglobin A1C and blood glucose levels periodically for non-diabetic patients while on treatment, as well as advising patients to report any excessive thirst or polyuria, are both reasonable recommendations.
CONCLUSIONS
Clinicians must be familiar with the recognition and management of endocrine-related side effects associated with TKIs. While an association between TKIs and TSH and PTH elevation has been established, the etiology behind these associations remains to be elucidated. Both the TSH and thryrotropin-releasing hormone (TRH) receptors are members of the G protein-coupled receptor (GPCR) super-family, while the thyroid hormone receptor is a nuclear receptor; thus, none of the thyroid signaling pathway receptors belongs to the TK class. The same can be said for the parathyroid hormone receptor 1 (PTH1R), which is yet another GPCR regulating calcium ion homeostasis through activation of adenylate cyclase and phospholipase C. In addition, the calcium-sensing receptor (CaSR) is another GPCR that acts by sensing extracellular levels of calcium and controls calcium homeostasis by regulating the release of PTH. Although these signaling cascades were once thought to be discrete, recent insights show integrated crosstalk between certain RTK and G-protein coupled receptors, whereby these signaling pathways merge to form complex signaling networks (Gavi, et al. 2006). This “cross talk” may have implications when considering drug side effects. A recent review highlighted examples of TK activation modifying the function of GPCR (Gavi et al. 2006). One prominent example of this “cross talk” is by both the insulin receptor and the b2 adrenergic receptor, whereby insulin receptor activation promotes phosphorylation of the b2-AR both directly and indirectly; this well studied example shows how insulin can counter regulate catecholamine action in the regulation of glucose metabolism. TKs modulate diverse GPCR and thus impact physiological function. The TK and GPCR signaling pathways both converge on the MAP kinase cascade. Cross talk between these two types of receptors may occur both at the protein-protein interaction level and also downstream in the respective signaling cascades, potentially providing a mechanistic basis for these endocrine-related side effects of TKIs. Further investigation is required to elucidate the exact molecular mechanisms underlying endocrine dysfunction in patients receiving TKIs.
ACKNOWLEDGEMENTS
This work was made possible by the intramural research division of the National Institutes of Child Health and Human Development We would like to thank Nichole Jonas and Jeremy Swan for their assistance with graphic design
Funding: Supported by the Intramural research division of the Eunice Kennedy Shriver National Institute of Child Health and Human Development
Footnotes
Disclosure statement: We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
REFERENCES
- Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–1257. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- Badros AZ, Siegel E, Bodenner D, Zangari M, Zeldis J, Barlogie B, Tricot G. Hypothyroidism in patients with multiple myeloma following treatment with thalidomide. Am J Med. 2002;112:412–413. doi: 10.1016/s0002-9343(01)01137-8. [DOI] [PubMed] [Google Scholar]
- Ballardini P, Margutti G, Aliberti C, Manfredini R. Onset of male gynaecomastia in a patient treated with sunitinib for metastatic renal cell carcinoma. Clin Drug Investig. 2009;29:487–490. doi: 10.2165/00044011-200929070-00007. [DOI] [PubMed] [Google Scholar]
- Basciani S, Brama M, Mariani S, De Luca G, Arizzi M, Vesci L, Pisano C, Dolci S, Spera G, Gnessi L. Imatinib mesylate inhibits Leydig cell tumor growth: evidence for in vitro and in vivo activity. Cancer Res. 2005;65:1897–1903. doi: 10.1158/0008-5472.CAN-04-2181. [DOI] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Arizzi M, Ulisse S, Rucci N, Jannini EA, Rocca C Della, Manicone A, Carani C, Spera G, et al. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-alpha and -beta during human testicular development and disease. J Clin Endocrinol Metab. 2002;87:2310–2319. doi: 10.1210/jcem.87.5.8476. [DOI] [PubMed] [Google Scholar]
- Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, Wilson BA, Heller G, Sauter NP. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- Bilgir O, Kebapcilar L, Bilgir F, Sari I, Oner P, Karaca B, Alacacioglu I. Is there any relationship between imatinib mesylate medication and hypothalamic-pituitary-adrenal axis dysfunction? Int J Clin Pract. 2010;64:45–50. doi: 10.1111/j.1742-1241.2008.01856.x. [DOI] [PubMed] [Google Scholar]
- Billemont B, Medioni J, Taillade L, Helley D, Meric JB, Rixe O, Oudard S. Blood glucose levels in patients with metastatic renal cell carcinoma treated with sunitinib. Br J Cancer. 2008;99:1380–1382. doi: 10.1038/sj.bjc.6604709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breccia M, Muscaritoli M, Aversa Z, Mandelli F, Alimena G. Imatinib mesylate may improve fasting blood glucose in diabetic Ph+ chronic myelogenous leukemia patients responsive to treatment. J Clin Oncol. 2004;22:4653–4655. doi: 10.1200/JCO.2004.04.217. [DOI] [PubMed] [Google Scholar]
- Caocci G, Atzeni S, Orru N, Azzena L, Martorana L, Littera R, Ledda A, La Nasa G. Gynecomastia in a male after dasatinib treatment for chronic myeloid leukemia. Leukemia. 2008;22:2127–2128. doi: 10.1038/leu.2008.106. [DOI] [PubMed] [Google Scholar]
- Christopoulos C, Dimakopoulou V, Rotas E. Primary ovarian insufficiency associated with imatinib therapy. N Engl J Med. 2008;358:1079–1080. doi: 10.1056/NEJMc0707841. [DOI] [PubMed] [Google Scholar]
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007;148:936–941. doi: 10.1210/en.2006-0921. [DOI] [PubMed] [Google Scholar]
- Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009;12:40–50. doi: 10.1007/s11102-008-0088-4. [DOI] [PubMed] [Google Scholar]
- Daub H. Kinase inhibitors: narrowing down the real targets. Nat Chem Biol. 2010;6:249–250. doi: 10.1038/nchembio.336. [DOI] [PubMed] [Google Scholar]
- de Groot JW, Zonnenberg BA, Plukker JT, van Der Graaf WT, Links TP. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther. 2005;78:433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, de Vries MM, Links TP, Lips CJ, Voest EE. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3466–3469. doi: 10.1210/jc.2007-0649. [DOI] [PubMed] [Google Scholar]
- Deremer DL, Ustun C, Natarajan K. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther. 2008;30:1956–1975. doi: 10.1016/j.clinthera.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, Morgan JA, Dychter SS, Larsen PR, Demetri GD, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- Dora JM, Leie MA, Netto B, Fogliatto LM, Silla L, Torres F, Maia AL. Lack of imatinib-induced thyroid dysfunction in a cohort of non-thyroidectomized patients. Eur J Endocrinol. 2008;158:771–772. doi: 10.1530/EJE-08-0006. [DOI] [PubMed] [Google Scholar]
- Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Faris JE, Moore AF, Daniels GH. Sunitinib (sutent)-induced thyrotoxicosis due to destructive thyroiditis: a case report. Thyroid. 2007;17:1147–1149. doi: 10.1089/thy.2007.0104. [DOI] [PubMed] [Google Scholar]
- Fitter S, Dewar AL, Kostakis P, To LB, Hughes TP, Roberts MM, Lynch K, Vernon-Roberts B, Zannettino AC. Long-term imatinib therapy promotes bone formation in CML patients. Blood. 2008;111:2538–2547. doi: 10.1182/blood-2007-07-104281. [DOI] [PubMed] [Google Scholar]
- Fox E, Widemann B, Whitcomb PO, Aikin A, Dombi E, Lodish M, Stratakis CA, Steinberg S, Wells SA, Jr., Balis FM. Phase I/II trial of vandetanib in children and adolescents with hereditary medullary thyroid carcinoma. American Society of Clinical Oncology 2009 Annual Meeting.2009. [Google Scholar]
- Gambacorti-Passerini C, Tornaghi L, Cavagnini F, Rossi P, Pecori-Giraldi F, Mariani L, Cambiaghi N, Pogliani E, Corneo G, Gnessi L. Gynaecomastia in men with chronic myeloid leukaemia after imatinib. Lancet. 2003;361:1954–1956. doi: 10.1016/S0140-6736(03)13554-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Serra A, Amdur RJ, Morris CG, Mazzaferri E, Mendenhall WM. Thyroid function should be monitored following radiotherapy to the low neck. Am J Clin Oncol. 2005;28:255–258. doi: 10.1097/01.coc.0000145985.64640.ac. [DOI] [PubMed] [Google Scholar]
- Gavi S, Shumay E, Wang HY, Malbon CC. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab. 2006;17:48–54. doi: 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Grey A, O’Sullivan S, Reid IR, Browett P. Imatinib mesylate, increased bone formation, and secondary hyperparathyroidism. N Engl J Med. 2006;355:2494–2495. doi: 10.1056/NEJMc062388. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Premaratne E, Desai J, Davis ID. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol (Oxf) 2008;69:669–672. doi: 10.1111/j.1365-2265.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- Guevremont C, Alasker A, Karakiewicz PI. Management of sorafenib, sunitinib, and temsirolimus toxicity in metastatic renal cell carcinoma. Curr Opin Support Palliat Care. 2009;3:170–179. doi: 10.1097/SPC.0b013e32832e4681. [DOI] [PubMed] [Google Scholar]
- Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O’Dwyer PJ, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. FASEB J. 2007;21:618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- Hamberg P, de Jong FA, Boonstra JG, van Doorn J, Verweij J, Sleijfer S. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30–31. doi: 10.1200/JCO.2006.06.5318. [DOI] [PubMed] [Google Scholar]
- Hensley ML, Ford JM. Imatinib treatment: specific issues related to safety, fertility, and pregnancy. Semin Hematol. 2003;40:21–25. doi: 10.1053/shem.2003.50038. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Cortes JE, Giles FJ, O’Brien S, Kantarjian HM. Current and emerging treatment options in chronic myeloid leukemia. Cancer. 2007;109:2171–2181. doi: 10.1002/cncr.22661. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Olsson B, Ohlsson C, Lorentzon M, Mellstrom D, Wadenvik H. Increased cortical bone mineralization in imatinib treated patients with chronic myelogenous leukemia. Haematologica. 2008;93:1101–1103. doi: 10.3324/haematol.12373. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kim H, Chang HM, Ryu MH, Kim TW, Sohn HJ, Kim SE, Kang HJ, Park S, Lee JS, Kang YK. Concurrent male gynecomastia and testicular hydrocele after imatinib mesylate treatment of a gastrointestinal stromal tumor. J Korean Med Sci. 2005;20:512–515. doi: 10.3346/jkms.2005.20.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto T, Inoue M, Kawa K. Growth deceleration in a girl treated with imatinib. Int J Hematol. 2009;89:251–252. doi: 10.1007/s12185-008-0251-8. [DOI] [PubMed] [Google Scholar]
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr., Vasko VV, Saji M, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- Lodish MB, Stratakis CA. RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer. Expert Rev Anticancer Ther. 2008;8:625–632. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N, Miyakawa M, Fujita T, Iiri T. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid. 2010;20:323–326. doi: 10.1089/thy.2009.0414. [DOI] [PubMed] [Google Scholar]
- Malozowski S, Nelson L, Calis KA. More on ovarian insufficiency with imatinib. N Engl J Med. 2008;358:2648. doi: 10.1056/NEJMc080707. author reply 2648-2649. [DOI] [PubMed] [Google Scholar]
- Mannavola D, Coco P, Vannucchi G, Bertuelli R, Carletto M, Casali PG, Beck-Peccoz P, Fugazzola L. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- Mariani S, Giona F, Basciani S, Brama M, Gnessi L. Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet. 2008;372:111–112. doi: 10.1016/S0140-6736(08)61023-5. [DOI] [PubMed] [Google Scholar]
- Martorella AJOG, Hann LE, Motzer RJ, Robbins RJ. Receptor kinase (RTK) inhibitor SU11248 may cause hypothyroidism in a select group of patients with metastatic renal cell carcinoma (RCC). 88th Annual Meeting of the Endocrine Society; Boston, MA, USA. 2006. [Google Scholar]
- Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. Novartis Gleevac package insert revised 02/2010 Novartis Package insert for Tasigna (nilotinib).
- O’Sullivan S, Horne A, Wattie D, Porteous F, Callon K, Gamble G, Ebeling P, Browett P, Grey A. Decreased bone turnover despite persistent secondary hyperparathyroidism during prolonged treatment with imatinib. J Clin Endocrinol Metab. 2009;94:1131–1136. doi: 10.1210/jc.2008-2324. [DOI] [PubMed] [Google Scholar]
- O’Sullivan S, Naot D, Callon K, Porteous F, Horne A, Wattie D, Watson M, Cornish J, Browett P, Grey A. Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms. J Bone Miner Res. 2007;22:1679–1689. doi: 10.1359/jbmr.070719. [DOI] [PubMed] [Google Scholar]
- Osorio S, Noblejas AG, Duran A, Steegmann JL. Imatinib mesylate induces hypophosphatemia in patients with chronic myeloid leukemia in late chronic phase, and this effect is associated with response. Am J Hematol. 2007;82:394–395. doi: 10.1002/ajh.20778. [DOI] [PubMed] [Google Scholar]
- Pacini F, Sherman S, Schlumberger M, Elisei R, Wirth L, Bastholt L, Martins R, Hofmann M, Locati L, Eschenberg M. Exacerbation of Postsurgical Hypothyroidism during Treatment of Advanced Differentiated (DTC) or Medullary (MTC) Thyroid Carcinoma with AMG 706. Horm Res. 2007:29. JPD. DS. Pfizer Package insert Sutent.
- Raymond E. American Society of Clinical Oncology. Chicago, IL: 2010. Updated results of the phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of advanced pancreatic neuroendocrine tumors (NET) [Google Scholar]
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, Reddy S, Dreicer R, Bukowski RM. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- Robinson AA, Watson WJ, Leslie KK. Targeted treatment using monoclonal antibodies and tyrosine-kinase inhibitors in pregnancy. Lancet Oncol. 2007;8:738–743. doi: 10.1016/S1470-2045(07)70242-5. [DOI] [PubMed] [Google Scholar]
- Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- Rogiers A, Wolter P, de Beeck K Op, Thijs M, Decallonne B, Schoffski P. Shrinkage of thyroid volume in sunitinib-treated patients with renal-cell carcinoma: a potential marker of irreversible thyroid dysfunction? Thyroid. 2010;20:317–322. doi: 10.1089/thy.2009.0125. [DOI] [PubMed] [Google Scholar]
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- Sabatier R, Gravis G, Deville J, Salem N, Brunelle S, Walz J, Marcy M, Narbonne H, Viens P, Bladou F, Institut Paoli Calmettes, Marseille, France. CHU Marseille, Hopital La Timone, Marseille, France. CHU Marseille, hopital Sainte Marguerite, Marseille, France Hypothyroidism and survival during sunitinib therapy in metastatic renal cell cancer: A prospective observational analysis. 2009 Genitourinary Cancers Symposium, ASCO.2009. [Google Scholar]
- Salem AK, Fenton MS, Marion KM, Hershman JM. Effect of sunitinib on growth and function of FRTL-5 thyroid cells. Thyroid. 2008;18:631–635. doi: 10.1089/thy.2007.0336. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- Schmid H, Jaeger BA, Lohse J, Suttorp M. Longitudinal growth retardation in a prepuberal girl with chronic myeloid leukemia on long-term treatment with imatinib. Haematologica. 2009;94:1177–1179. doi: 10.3324/haematol.2009.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffski P, Wolter P, Himpe U, Dychter SS, Baum C, Prenen H, Wildiers H, Bex M, Dumez H. Sunitinib-related thyroid dysfunction: A single-center retrospective and prospective evaluation. J. Clin. Oncol ASCO Meeting Abstracts. 2006;24:3092. [Google Scholar]
- Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway. Clin Cancer Res. 2008;14:3651–3656. doi: 10.1158/1078-0432.CCR-08-0333. [DOI] [PubMed] [Google Scholar]
- Shaheen PE, Tamaskar IR, Salas RN, Rini BI, Garcia J, Wood L, Dreicer R, Bukowski RM. ASCO. 2006. Thyroid function tests (TFTs) abnormalities in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with sunitinib. [Google Scholar]
- Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab. 2009a;94:1493–1499. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- Sherman SI. Tyrosine kinase inhibitors and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009b;23:713–722. doi: 10.1016/j.beem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, Duvic M. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340:1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- Suttorp MBJ, Vaitl J, Mosch B, Pursche S, Jung R. Side effects on the heart and skeleton of growing mice attributed to chronic imatinib exposure. 2008. p. 402.
- Tamaskar I, Bukowski R, Elson P, Ioachimescu AG, Wood L, Dreicer R, Mekhail T, Garcia J, Rini BI. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann Oncol. 2008;19:265–268. doi: 10.1093/annonc/mdm483. [DOI] [PubMed] [Google Scholar]
- Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521–530. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- Vandyke K, Dewar AL, Diamond P, Fitter S, Schultz CG, Sims NA, Zannettino AC. The tyrosine kinase inhibitor dasatinib dysregulates bone remodelling through inhibition of osteoclasts in vivo. J Bone Miner Res. 2010 doi: 10.1002/jbmr.85. [DOI] [PubMed] [Google Scholar]
- Vandyke K, Dewar AL, Fitter S, Menicanin D, To LB, Hughes TP, Zannettino AC. Imatinib mesylate causes growth plate closure in vivo. Leukemia. 2009;23:2155–2159. doi: 10.1038/leu.2009.150. [DOI] [PubMed] [Google Scholar]
- Veneri D, Franchini M, Bonora E. Imatinib and regression of type 2 diabetes. N Engl J Med. 2005;352:1049–1050. doi: 10.1056/NEJM200503103521023. [DOI] [PubMed] [Google Scholar]
- Weijl NI, Van der Harst D, Brand A, Kooy Y, Van Luxemburg S, Schroder J, Lentjes E, Van Rood JJ, Cleton FJ, Osanto S. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. J Clin Oncol. 1993;11:1376–1383. doi: 10.1200/JCO.1993.11.7.1376. [DOI] [PubMed] [Google Scholar]
- Wells SA, Jr., Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28:767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Jr., Santoro M. Targeting the RET pathway in thyroid cancer. Clin Cancer Res. 2009;15:7119–7123. doi: 10.1158/1078-0432.CCR-08-2742. [DOI] [PubMed] [Google Scholar]
- Widakowich C, de Castro G, Jr., de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12:1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- Wolter P, Stefan C, Decallonne B, Dumez H, Bex M, Carmeliet P, Schoffski P. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer. 2008;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Rosen LS, Mulay M, Vanvugt A, Dinolfo M, Tomoda C, Sugawara M, Hershman JM. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]