Abstract
Within the embryonic lung, intrinsic nerve ganglia, which innervate airway smooth muscle, are required for normal lung development and function. We studied the development of neural crest-derived intrinsic neurons within the embryonic mouse lung by crossing Wnt1-Cre mice with R26R-EYFP reporter mice to generate double transgenic mice that express yellow fluorescent protein (YFP) in all neural crest cells (NCCs) and their derivatives. In addition to utilizing conventional immunohistochemistry on frozen lung sections, the complex organization of lung innervation was visualized in three dimensions by combining the genetic labelling of NCCs with optical projection tomography, a novel imaging technique that is particularly useful for the 3D examination of developing organs within embryos. YFP-positive NCCs migrated into the mouse lung from the oesophagus region at embryonic day 10.5. These cells subsequently accumulated around the bronchi and epithelial tubules of the lung and, as shown by 3D lung reconstructions with optical projection tomography imaging, formed an extensive, branching network in association with the developing airways. YFP-positive cells also colonized lung maintained in organotypic culture, and responded in a chemoattractive manner to the proto-oncogene, rearranged during transfection (RET) ligand, glial-cell-line-derived neurotrophic factor (GDNF), suggesting that the RET signalling pathway is involved in neuronal development within the lung. However, when the lungs of Ret−/− and Gfrα1−/− embryos, deficient in the RET receptor and GDNF family receptor α 1 (GFRα1) co-receptor respectively, were examined, no major differences in the extent of lung innervation were observed. Our findings demonstrate that intrinsic neurons of the mouse lung are derived from NCCs and that, although implicated in the development of these cells, the role of the RET signalling pathway requires further investigation.
Keywords: intrinsic lung neurons, lung development, neural crest cells, optical projection tomography, RET signalling, Wnt1-Cre mice
Introduction
The formation of intrinsic nerve ganglia, consisting of neuronal and glial cells, is a recognized component of lung development during embryogenesis. Neurons within these ganglia function to innervate the airway smooth muscle (Sparrow et al. 1999; Sparrow & Lamb, 2003), the activity of which is characterized by rhythmic contractions throughout the prenatal period thought to be critical for lung growth and maturation (Schittny et al. 2000). We have recently shown, in chick and human embryos, that intrinsic lung neurons are derived from the neural crest (Burns & Delalande, 2005; Burns et al. 2008). Neural crest cells (NCCs) are a transient population of multipotent cells that arise following closure of the neural tube. They migrate extensively throughout the embryo and give rise to a wide variety of cell types including melanocytes, the skeletal and connective tissues of the face, and neurons and glia of the sensory, sympathetic, and enteric nervous systems (ENS) (Kalcheim & Le Douarin, 1999). In order to form the ENS, the intrinsic innervation of the gastrointestinal tract, vagal (hindbrain) NCCs migrate into the foregut and colonize the entire length of the gut in an oral-to-anal direction (Le Douarin & Teillet, 1973; Burns & Le Douarin, 2001; Burns, 2005). Using interspecies quail–chick grafting to permanently fate-map NCCs in the chick embryo, we demonstrated that a subpopulation of vagal NCCs migrates from the foregut into the lung buds where they subsequently form ganglia containing neurons and glial cells (Burns & Delalande, 2005; Burns et al. 2008). More recently, we also demonstrated that intrinsic ganglia in the embryonic and fetal human lung are also neural crest-derived (Burns et al. 2008), as neural tissue that develops around the bronchi and epithelial tubules in close association with airway smooth muscle co-localizes with p75NTR, a specific marker for NCCs (Barlow et al. 2008).
The aim of the current study was to employ a genetic approach to fate-map, analyse, and image in three dimensions NCC development within the lungs of mouse embryos. We generated a double transgenic mouse line constitutively expressing yellow fluorescent protein (YFP) in all NCCs and their derivatives. We crossed the Wnt1-Cre mouse with a R26R-EYFP reporter mouse containing a YFP coding sequence preceded by a floxed STOP cassette in the ROSA26 locus (Srinivas et al. 2001). In Wnt-1-expressing neural crest precursor cells of the resulting embryos, the Cre recombinase gene was transcribed, leading to production of the Cre enzyme that excises the STOP cassette allowing transcription of the YFP gene and subsequent production of YFP protein (Cassiman et al. 2006). YFP can be detected in NCCs using either direct epifluorescence or by immunohistochemical staining using an anti-green fluorescence protein antibody, which is also specific for YFP.
In order to explore the complexity of lung innervation in three dimensions, we combined the genetic labelling approach for NCCs with optical projection tomography (OPT). This novel imaging technique is particularly suited for reconstructing vertebrate embryos and/or for examining the 3D anatomy of developing organs within embryos (Sharpe, 2003, 2004). Computer graphics technology allows OPT-generated images to be rotated, virtually sectioned through several axes, and explored, and movies can be produced of the resulting series of views.
In order to gain insight into the signalling mechanisms that direct NCCs to migrate from the foregut into the lungs, we investigated the role of the RET signalling pathway (Manie et al. 2001), a strong candidate for influencing NCC development within the lung. RET signalling is already known to be necessary for the migration of NCCs within the gut; loss of RET signalling results in gut aganglionosis in mice (Schuchardt et al. 1994) and the RET gene is the main gene implicated in the congenital aganglionic gut disorder Hirschsprung's disease in humans (Lantieri et al. 2006; Amiel et al. 2008; Tam & Garcia-Barcelo, 2009). Within the gut, the RET receptor and GFRα1 co-receptor are expressed on NCCs, and the ligand, glial-cell-line-derived neurotrophic factor (GDNF), which is expressed within the gut mesenchyme, has been shown to be a chemoattractant for NCCs (Young et al. 2001). In the lung, RET expression has been described in NCC-derived presumptive neurons in the chick (Burns & Delalande, 2005), and GFRα1 expression within lung ganglia in the human embryo (Burns et al. 2008). In lung explants, RET-expressing NCCs have been shown to migrate in a directional manner in response to GDNF (Tollet et al. 2002). Although no specific deficiency in intrinsic lung ganglia has been described in Ret−/− mice, in addition to gut aganglionosis, these mutant mice also have a depressed ventilatory response to inhaled CO2 (Burton et al. 1997). In humans, a significant percentage of patients with the developmental disorder congenital central hypoventilation syndrome, where there is an absence of adequate autonomic control of respiration with decreased sensitivity to hypoxia and hypercapnia (Chen & Keens, 2004), have Hirschsprung's disease and mutations in RET (Croaker et al. 1998; Sakai et al. 2001; Fitze et al. 2003). Taken together, these studies imply that RET signalling could be of critical importance for the normal development of lung innervation.
To investigate the role of the RET signalling pathway in intrinsic lung neuron development, we utilized an organotypic culture system, as previously described by Tollet et al. (2002), to test the ability of YFP-positive NCCs to chemotactically respond to the RET ligand GDNF. We also analysed the lungs of mouse embryos deficient in RET and GFRα1 to determine whether the neural crest-derived intrinsic innervation was compromised in the absence of these receptor components of the RET pathway.
Here we show that YFP-positive NCCs begin to migrate into the mouse lung from the oesophagus region at embryonic day (E)10.5. Over the following days, these cells accumulate around the bronchi and epithelial tubules of the lung and, as shown by 3D lung reconstructions with OPT imaging, form an extensive, branching network in association with the developing airways. We also demonstrate that YFP cells colonize the lung maintained in organotypic culture, and respond in a chemoattractive manner to GDNF, suggesting that the RET signalling pathway is involved in intrinsic lung development. However, contrary to this idea, when the lungs of Ret−/− and Gfrα1−/− embryos were examined by immunohistochemistry on sections, and Ret−/− lungs were examined by OPT and wholemount immunohistochemistry, we did not detect any apparent differences in the extent of lung innervation compared with wild-type littermates. This suggests that the role of the RET signalling pathway in the development of the intrinsic innervation of the lung requires further investigation.
Materials and methods
Animals
Wnt1-Cre mice, expressing Cre recombinase enzyme under the control of a Wnt-1 promoter/enhancer in NCCs, were crossed with R26R-EYFP reporter mice (obtained from The Jackson Laboratory), carrying a YFP sequence preceded by a floxed STOP cassette in the ROSA26 locus as previously described (Srinivas et al. 2001). The resulting Wnt1-Cre;R26R-EYFP offspring are hereafter designated Wnt1-Cre;YFP. The day after mating, when the plug was confirmed, was taken as E0.5. Wnt1-Cre;YFP mice were initially provided by Dr Miles Epstein (University of Wisconsin, USA). Ret and Gfrα1 homozygous, heterozygous and wild-type littermate mouse embryos were kindly provided by Dr Vassilis Pachnis (National Institute of Medical Research, UK) and Ret mutant mice were supplied by Dr Hideki Enomoto (RIKEN Center for Developmental Biology, Kobe, Japan) (Enomoto et al. 2001). Mice were genotyped by polymerase chain reaction of DNA samples prepared from tail tips, yolk sacs or whole embryos. Primer sequences and polymerase chain reaction protocols are available on request. Animals were killed by Schedule 1 methods in accordance with UK Home Office regulations and licences.
Immunohistochemistry for wholemounts and tissue sections
Wholemount embryos or dissected lung tissues were fixed for 1–2 h in 4% paraformaldehyde in phosphate-buffered saline (PBS) and then rinsed three times in PBS at room temperature and processed as previously described (Barlow et al. 2008). Briefly, antibody blocking solution (10% sheep serum, 1% Triton X-100 in PBS) was applied for 1 h at room temperature and then samples were rinsed extensively in PBS and incubated in primary antibody diluted in antibody blocking solution overnight at 4 °C. The following antibodies, all raised in rabbit, were used: anti-βIII tubulin (also called TuJ1, 1 : 1000, Covance), anti-green fluorescence protein (1 : 250, Invitrogen) and anti-smooth muscle actin (SMA) (1 : 100, AMS Biotechnology). The next day, samples were washed in three changes of PBS for a total of 1 h and then incubated with fluorescently tagged secondary antibodies (either anti-rabbit Alexa Fluor 488, 1 : 500, or anti-rabbit Alexa Fluor 568, 1 : 500; Invitrogen) for 4 h at room temperature. Samples were washed for 1 h before observation under a Leica MZFLIII dissecting fluorescent microscope or mounting under a coverslip using Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Fluorescent microscopy was carried out on a Zeiss Axioskop fluorescent microscope fitted with a Leica DC500 digital colour camera.
For immunolabelling of cryosections, embryos or dissected lung tissues were fixed in 4% paraformaldehyde for 3–4 h and prepared as previously described (Barlow et al. 2008). Frozen sections were cut at 10–12 mm using a Leica CM1900 cryostat at −22 °C. Following blocking in PBS containing 0.1% Triton X, 1% bovine serum albumin and 0.15% glycine, sections were incubated in primary antibodies as above for 4 h at room temperature. Slides were then washed in PBS and incubated with fluorescently tagged secondary antibodies, as above, for 1.5 h at room temperature. Following further washing in PBS, the slides were mounted with Vectashield Hard Set containing DAPI (Vector Laboratories) and examined using a Zeiss Axioskop microscope as above.
Wholemount immunohistochemistry for optical projection tomography
Mouse embryos or dissected lungs were fixed in 4% paraformaldehyde for 0.5–1.5 h depending on size. Following washing in Tris-buffered saline containing 1% Tween (TBST), samples were incubated in antibody block (TBST plus 10% sheep serum) overnight at 4 °C on a rotator. After blocking, primary antibody (anti-βIII tubulin diluted 1 : 1000) was applied overnight at 4 °C. Samples were washed six times for 30 min in TBST volumes > 5 mL, and then overnight in TBST at 4 °C. The secondary antibody, Alexa 568, was diluted in block solution and applied overnight at room temperature before finally washing in TBST. Following labelling, samples were mounted in agarose. Low melting point agarose (1%) was made and then filtered to remove impurities, and the samples were embedded in low melting point agarose and cooled to 4 °C. A square block with the specimen at its centre was cut and attached to a magnetic chuck using superglue. The chuck and agarose were dehydrated in methanol for 3 days, with the methanol being changed once per day. The samples were then scanned under UV and visible light using a Bioptonics OPT 3001 scanner and the images were reconstructed using proprietary NRecon software (Bioptonics, MRC Technology, Edinburgh, UK).
Organotypic lung culture
Lungs were harvested from mouse embryos and washed in sterile PBS. At E13.5 and beyond, the lung lobes were separated from each other and cultured separately due to their size. Under a tissue culture hood, 1 mL of culture medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 1% penicillin/streptomycin) was put into one well of a six-well culture plate. A membrane insert (Millipore) was then placed on top of the medium. Samples were gently transferred onto the membrane inserts using spatulas. A maximum of five lobes were placed on one insert. Lungs were then incubated at 37 °C in a 5% CO2 atmosphere. To test the ability of neural crest-derived tissue to migrate towards potential chemoattractants, agarose beads (Affi-Gel Blue Gel, Bio-Rad) impregnated with GDNF were prepared on the day of lung harvest. Agarose beads stored in sodium azide were washed in PBS eight times for 15 min. GDNF (2 mL) in a 0.5 mg μL−1 solution was added to 2 mL of beads in PBS and left in the fridge for at least 2 h before use. Control beads were prepared in the same way, but without the addition of GDNF. The beads were placed on the lung lobes using a mouth pipette and a hand-drawn glass needle (1–4 beads per lung lobe). Cultured lungs were imaged using a Zeiss Axiovert microscope contained in a chamber at 37 °C. Images of each lung were taken at regular intervals using Velocity software (Improvision, PerkinElmer, Waltham, MA, USA) under bright-field and fluorescent microscopy at ×10 and ×20 magnification.
Results
Spatiotemporal colonization of the developing lung by neural crest cells
Homozygous and heterozygous Wnt1-Cre;YFP mouse embryos strongly expressed YFP in Wnt1-positive NCCs at all stages examined (E10–postnatal day 0). Neural crest-derived tissues fluoresced brightly either upon wholemount examination, or following dissection, cryosectioning and immunostaining using an anti-green fluorescence protein antibody, which is also specific for YFP (data not shown). Positively labelled tissues included presumptive skeletal components of the head, and cells of the autonomic nervous system, including neurons of the ENS and intrinsic lung ganglia.
At E10.5, sections of Wnt1-Cre;YFP embryos revealed YFP-positive NCCs within the dorsal neural tube, dorsal root ganglia, migrating towards the developing heart, and in large numbers in the pharyngeal region (Fig. 1A–C). At this stage of development, the foregut has separated into the oesophagus and trachea, and the primitive lung buds have begun to form. Within the foregut, the majority of NCCs are associated with the oesophagus, although occasional YFP cells are present within the trachea and primitive lung buds (Fig. 1B,C). Similar distributions of NCCs are apparent within sections of Wnt1-Cre;YFP embryos at E11.5. At this stage, numerous YFP cells are grouped around the proximal foregut and are present with the gut. YFP-positive cells are more numerous within the lung buds, which have increased in size (Fig. 1H,I). NCCs within the lung buds frequently appear as interconnected chains of cells although individual cells, not connected with other YFP-positive cells, are also present (Fig. 1H). At E12.5 (not shown) and E13.5, as colonization of the lung buds by NCCs continues, YFP-positive cells frequently occur in close association with the primitive branching bronchi and epithelial tubules (Fig. 1K,L). These NCC-derived neurons and nerve fibres, as confirmed with double immunolabelling with YFP and the neural marker TuJ1 (Fig. 1M–O), appear to encircle the branching airways and epithelial tubules when examined in transverse section (Fig. 1L). This close association between NCC-derived tissue and the airways continues as the lungs develop and mature up to birth (Fig. 2), the latest stage examined in this study. Throughout this time period, YFP-positive NCCs coalesce to form small ganglia that lie underneath the airway epithelium (Fig. 2, also see Figs 7 and 8).
Fig. 1.
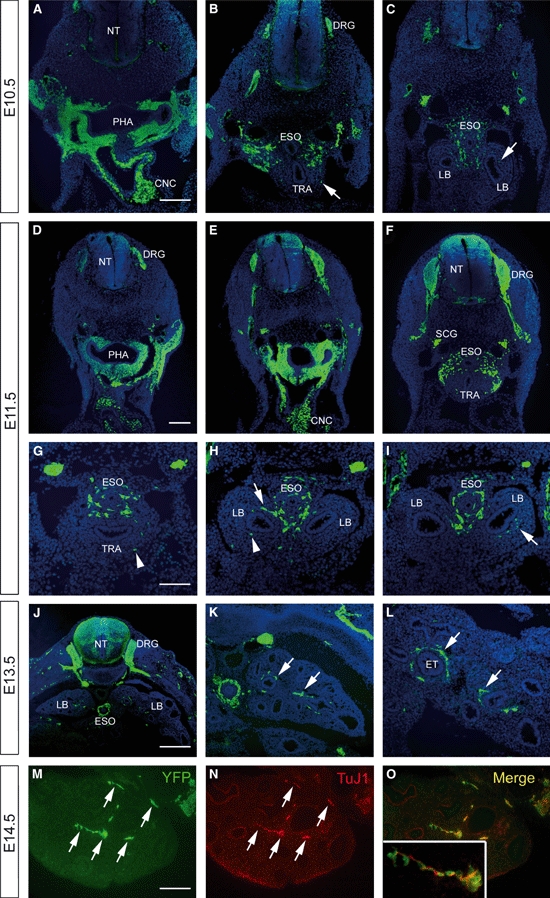
Sections of Wnt1-Cre;YFP embryos immunostained using anti-green fluorescence protein. (A–C) Embryonic day (E)10.5 embryo showing positively labelled neural crest cells within the dorsal neural tube (NT), dorsal root ganglia (DRG) and in the pharyngeal region. Occasional yellow fluorescent protein (YFP) cells (arrows) are present within the trachea (TRA) and lung buds (LBs). (D–I) At E11.5, interconnected (arrows) and individual (arrowheads) YFP cells are present within the TRA and LBs. (J–L) E13.5 embryo showing more numerous YFP cells within the developing lungs. The neural crest-derived tissue is closely associated with the epithelial tubules (ETs) (arrows). (M–O) To demonstrate that YFP+ cells develop into neurons, YFP/TuJ1 double immunolabelling was performed. The majority of YFP+ tissue (M) was also positive for the neural marker, TuJ1 (N) and clearly overlapped (inset, O). PHA, pharynx; CNC, cardiac neural crest; SCG, sympathetic chain ganglia; ESO, oesophagus. Scale bars: 200 μm in A–F; 100 μm in G–I; 400 μm in J; 200 μm in K; 100 μm in L–O.
Fig. 2.
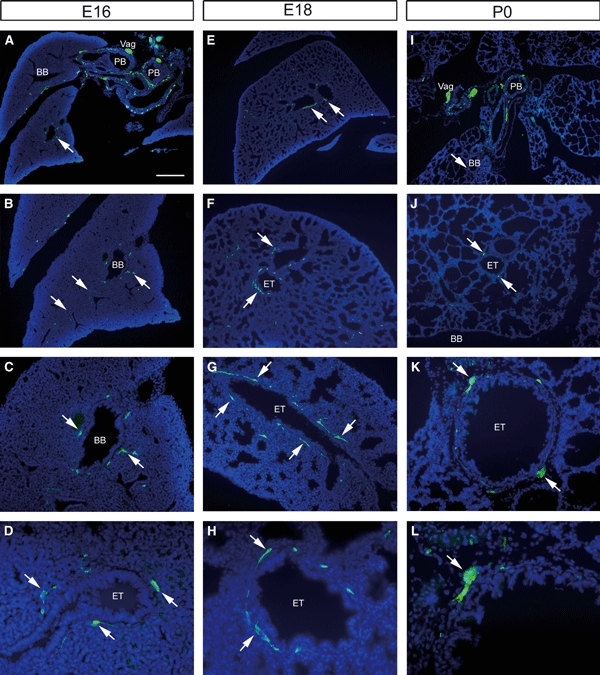
Sections of Wnt1-Cre;YFP embryos immunostained using anti-green fluorescence protein. (A–D) Embryonic day (E)16, (E–H) E18 and (I–L) postnatal day 0 mice. Extensive yellow fluorescent protein (YFP)-positive tissue is present close to the primary bronchi (PB) (A,I). Within the lung parenchyma, YFP tissue (arrows) occurs in close association with the branching bronchi (BB) and epithelial tubules (ETs) at all stages. This association is apparent in tubules sectioned either longitudinally (G) or transversely (K). Vag, vagus nerve. Scale bar: 400 μm in A,E,I; 200 μm in B,F,J; 100 μm in C,G,K; 50 μm in D,H,L.
Fig. 7.
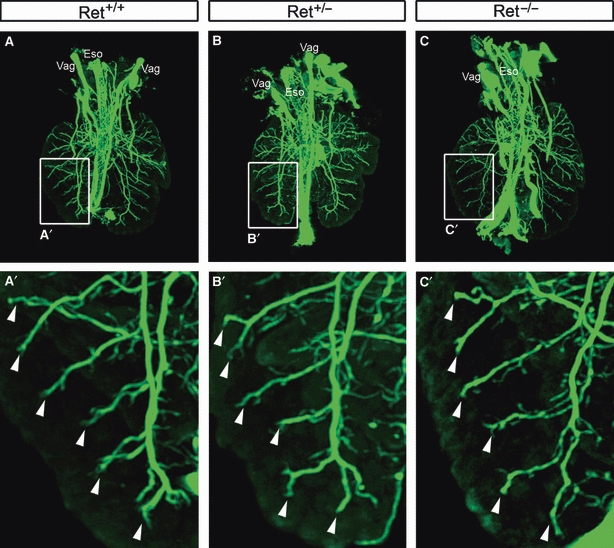
3D optical projection tomography reconstructions of lungs from embryonic day 14.5 Ret mutant and control littermate embryos stained with the neural marker TuJ1. The distribution of TuJ1-positive neural tissue is strikingly similar in Ret+/+ (A), Ret+/− (B) and Ret−/− (C) lungs. The boxed area in (A–C) is shown at higher magnification in the corresponding panels underneath. A consistent pattern of TuJ1 staining is present in the primary bronchus and branching bronchi (arrowheads). Vag, vagus nerve; Eso, oesophagus.
Fig. 8.

TuJ1 wholemount staining of the left lung from embryonic day (E)14.5 Ret+/+ and Ret−/− embryos. Similar TuJ1 staining patterns are present in neural tissue associated with primary bronchus (PB) and branching bronchi (arrowheads) in wild-type (A,B) and Ret−/− (E,F) lung. The boxed areas in (A,B,E,F) are shown at higher magnification in the corresponding panels underneath. (C,G) High magnification of nerve fibres, and associated TuJ1-positive neurons (arrows), projecting towards the lung periphery. (D,H) Confocal micrographs of major lung ganglia containing numerous neurons. Scale bar: 500 μm in A,E; 250 μm in B,F; 50 μm in C,G.
Optical projection tomography of intrinsic lung innervation
The information above, concerning NCC colonization of the developing lung, was obtained using conventional tissue sectioning and immunohistochemical staining techniques. Although this method provides valuable information concerning the establishment of neural crest-derived tissue within the developing lung, the inherent drawback of such an approach is the lack of information regarding the 3D distribution of neural tissue within a relatively large organ such as the lung. In order to obtain a more complete picture of lung innervations, we carried out wholemount YFP immunohistochemical staining of lungs obtained from Wnt1-Cre;YFP embryos at E14.5, followed by OPT. This approach generated data sets that were rendered in 3D to show the distribution of YFP-positive neural crest-derived tissue within the lung. 3D reconstructions showed intense YFP staining within the trachea, oesophagus and adjacent vagus nerves (Figs 3 and 4). Within the lungs, large nerve trunks were present along the major airways, with a finer branching network, corresponding to the shape of the branching airways, occurring in the more distal lung (Fig. 3D). In addition to the 3D reconstructions, virtual sections through the lung (Fig. 3C) demonstrated distributions of YFP cells consistent with those seen in actual immunohistochemical sections such as those in Figs 1 and 2. The 3D reconstructions, either viewed as movies (Movie S1) or rotated in a series of steps, allowed lungs to be viewed from the dorsal and ventral sides (Fig. 4). These views further highlighted the dense accumulation of neural tissue close to the primary bronchi, and sparser distribution in the distal airways towards the periphery of the lung.
Fig. 3.
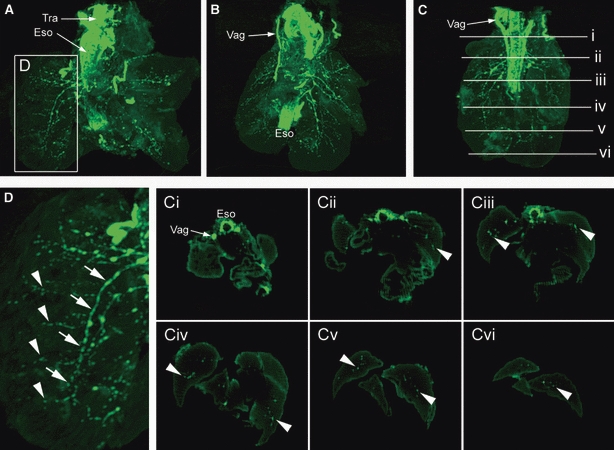
Lungs from embryonic day 14.5 Wnt1-Cre;YFP embryos immunostained with anti-green fluorescence protein and imaged using optical projection tomography (OPT). (A–C) The lungs from three different embryos, which all have similar staining patterns. The trachea (Tra), oesophagus (Eso) and vagus nerves (Vag) are intensely fluorescent, with a branching innervation apparent in the lung lobes. Staining is more intense in the region of the primary bronchi, and less intense in the lung periphery. (D) High magnification of the lung lobe indicated in (A) shows major nerve trunks (arrows) running parallel to the long axis of the airway, and finer fibres (arrowheads) travelling along the branching airways. Virtual transverse sections through the OPT lungs, at the levels indicated in (C), enable visualization of the Eso and Vag (Ci), and the identification of small groups of cells (arrowheads) within the lung lobes (Cii–Cvi).
Fig. 4.
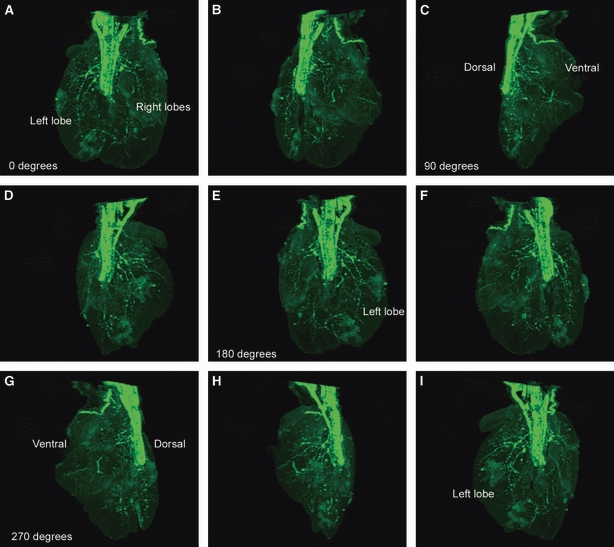
3D optical projection tomography reconstruction of lungs from embryonic day 14.5 Wnt1-Cre;YFP embryo rotated through a series of steps totalling over 300° (A–I). (A) Dorsal view of lungs showing intense yellow fluorescent protein staining in trachea and oesophagus. The proximal lung has a dense network of neural tissue, with sparse fibres in the distal lung. Lung innervation is more intense in the dorsal lung compared with the ventral lung (C,G).
Development and growth of lungs in organotypic culture
In order to establish and validate the lung culture protocol originally described by Tollet et al. (2002), mouse lung lobes from E13.5 Wnt1-Cre;YFP and wild-type embryos were isolated and cultured for up to 5 days, and immunostained after each day of culture with anti-YFP, TuJ1 and/or SMA antibodies (n = at least 6). Lungs survived and increased in size throughout this culture period, and underwent an extensive branching morphogenesis process consistent with normal lung development (Fig. 5A). Within cultured lungs, YFP-positive neural crest-derived cells migrated along the major airways, coalesced to form small ganglia, and appeared to overlie the airway smooth muscle that encircled the branching airways and epithelial tubules (Fig. 5). Cryosections of cultured lungs showed an apparently normal, although slightly flattened, morphology with SMA surrounding the epithelial tubules and YFP-positive nerve cells and fibres occurring adjacent to the SMA, on the side away from the airway epithelium as previously reported in human embryos (Burns et al. 2008). These findings thus validated the lung organotypic culture protocol as a model system for further investigations.
Fig. 5.
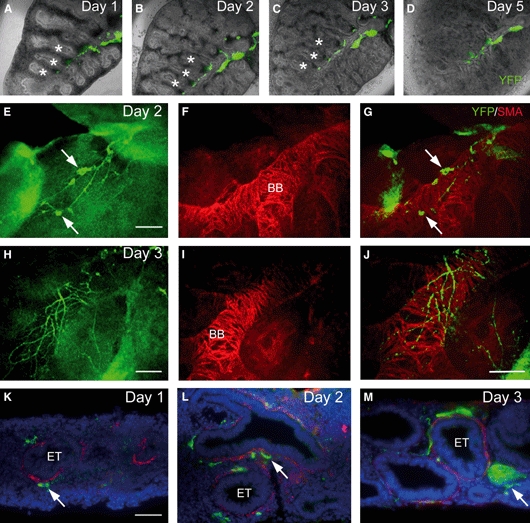
Development and growth of lungs in organotypic culture. (A–D) Embryonic day 13.5 lung from Wnt1-Cre;YFP embryo, cultured for 5 days. The primitive segmental bronchial tree (asterisks) undergoes serial branching morphogenesis during the culture period. Yellow fluorescent protein (YFP)-positive neural crest-derived tissue (green) is associated with the primary bronchus and branching bronchi (BB). (E–J) Wholemount cultured lungs immunostained with anti-green fluorescence protein (showing YFP fluorescence) and anti-smooth muscle actin (SMA). YFP-positive nerve ganglia (arrows) and interconnected nerve fibres overlie the SMA-positive fibres that circumferentially surround the BB and epithelial tubules (ETs). (G and J) Overlays with green background subtracted to highlight neural tissue. (K–M) Cryostat sections of lungs grown in culture. The ETs, which maintain their morphology throughout the culture period, are surrounded by thin layers of SMA (red staining). YFP-positive nerve ganglia (arrows) and nerve fibres occur in close association with the outer surface of the SMA. DAPI staining in blue. Scale bars: 100 μm in E–J; 50 μm in K–M.
Glial-cell-line-derived neurotrophic factor (GDNF), the ligand for the RET receptor tyrosine kinase (Manie et al. 2001), is a known chemoattractant for NCCs (Young et al. 2001; Tollet et al. 2002). In order to test the effects of GDNF on NCC migration within the developing lung, E13.5 Wnt1-Cre;YFP lungs were cultured for up to 5 days in the presence of GDNF-impregnated beads that were placed at various locations within the lung (Fig. 6A) (n = at least 6). As early as 1 day in culture, YFP-positive cells appeared to deviate from their normal migration route and migrate towards GDNF beads (Fig. 6B). After 3 days, YFP-positive cells had clearly accumulated around GDNF beads (Fig. 6C). After 5 days in culture, immunostaining with the neuronal marker TuJ1 revealed an extensive accumulation of neuronal cell bodies and nerve fibres totally surrounding GDNF beads (Fig. 6D–F). In control experiments, using beads prepared in the same way, but without the addition of GDNF, there was no positive chemoattractive effect and YFP-positive cells did not migrate towards or accumulate around beads (Fig. 6G–I).
Fig. 6.
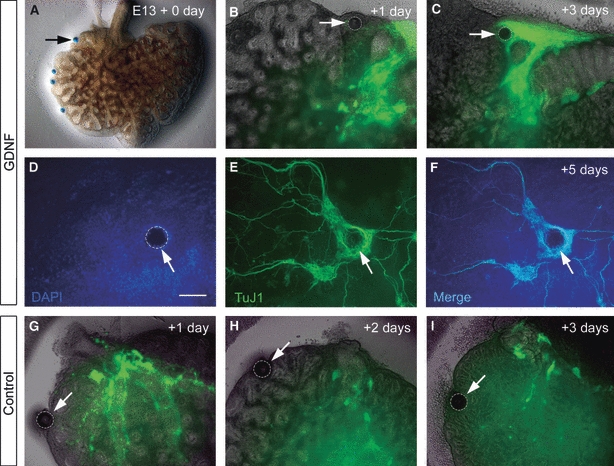
Neural crest cells are attracted to glial-cell-line-derived neurotrophic factor (GDNF) in cultured lungs. (A–C) Embryonic day (E)13.5 lung cultured for 3 days. The GDNF-soaked bead in (A) is highlighted in (B) and (C). After 1 day in culture, yellow fluorescent protein (YFP)-positive tissue begins to migrate towards the GDNF bead. After 3 days in culture (C), YFP tissue almost surrounds the bead. (D–F) DAPI (blue)-stained lung containing GDNF-soaked bead (arrow) after 5 days in culture. Immunostaining for TuJ1 shows a dense accumulation of TuJ1-positive neurons and nerve fibres surrounding the bead. (G–I) Control lung culture containing bead (arrow) without the addition of GDNF. YFP-positive cells do not migrate towards or surround the bead after 3 days in culture. Scale bar: 100 μm in D–F.
Analysis of lungs of mutant mouse deficient in RET receptors
As NCCs within the lung respond in a chemoattractive manner to the RET ligand GDNF by migrating towards and accumulating around GDNF beads, this strongly implies that the RET signalling pathway plays a role in controlling NCC development within the lung. To further explore this idea, we analysed, using the early neuronal marker TuJ1, the lungs of mouse embryos deficient in the RET receptor (Ret−/−) and the RET co-receptor GFRα1 (Gfrα1−/−) (n ≥ 3 per genotype). Mutant embryos and wildtype littermates were examined at E14.5, a stage at which neural crest-derived tissue within the lung is normally prevalent (see Figs 3 and 4). In Ret−/−, Gfrα1−/− and wild-type littermates, large calibre nerve trunks, the presumptive vagus nerves, were present adjacent to the oesophagus. Within the lungs, which appeared grossly normal in Ret−/− and Gfrα1−/− mice, TuJ1-positive nerve ganglia and fine nerve fibres were found in stereotypical locations, i.e. surrounding epithelial tubules in transverse section similar to controls (Figs S1 and S2). To determine if more subtle differences in lung innervation were present in Ret mutants (which were not apparent in histological sections), we used OPT reconstructions to examine entire lungs from Ret+/+, Ret+/− and Ret−/− embryos (Fig. 7; Movies S2–S4). OPT from these three genotypes revealed a remarkably similar distribution of TuJ1-positive neural tissue throughout the lungs, even at the level of the branching bronchi in the distal lung (Fig. 7). We also examined the TuJ1-positive neural tissue within wholemount lung preparations from Ret+/+ and Ret−/− embryos (Fig. 8). Again, the distribution of neural tissue within the lung was similar between wild-type and Ret−/− embryos, with nerve ganglia occurring in close association with the primary bronchus in the proximal lung (Fig. 8A,B). Confocal microscopy revealed these ganglia to contain numerous nerve cell bodies (Fig. 8D,H). Also, smaller ganglia, containing only a few neurons, were associated with fine nerve fibres projecting towards the lung periphery (Fig. 8C,G). When the numbers of TuJ1-positive neurons were counted in the larger ganglia of the left lung, Ret+/+ and Ret−/− tissues had a total of 160.5 (mean 16.1, SE 7.4) and 112.5 (mean 12.5, SE 4.6) neurons, respectively (n = 9 ganglia for each genotype, with two animals examined per genotype) (P = 0.55, Student's t-test). Thus, overall, we did not detect any significant differences in the amount or distribution of TuJ1-positive neural tissue and neurons within the lungs of Ret−/− mice using a range of approaches including immunohistochemistry on sections, OPT, wholemount immunohistochemistry and quantification.
Discussion
The Wnt1-Cre;YFP double transgenic mouse line, described by Cassiman et al. (2006), is a powerful tool for the analysis of NCC migration and differentiation in mammals. Once YFP expression has been activated by the expression of Cre under the control of the NCC-specific Wnt1 promoter, it is expressed in all NCCs and their progeny. In this study, the YFP marker allowed NCCs and their derivatives to be traced from the onset of Wnt1 expression at E8 into early postnatal stages, even after these cells have stopped expressing Wnt1. YFP was consistently expressed in all known neural crest-derived populations including the facial skeleton, cardiac outflow tract, sympathetic chain and dorsal root ganglia, and ENS. As previously reported, co-expression of both the Wnt1-Cre and YFP loci had no apparent adverse effects on embryonic development, and YFP-expressing NCCs exhibited normal spatiotemporal development patterns (Cassiman et al. 2006).
Although a number of previous studies have used similar reporter mice to label neural crest derivatives in a genetic approach, the presence of positively labelled NCCs within the lung was not reported (Yamauchi et al. 1999; Jiang et al. 2000; Pietri et al. 2003; Cassiman et al. 2006; Pilon et al. 2008). In these investigations it is possible that the lungs were not specifically examined or that NCCs within the lung may have been overlooked. However, in a study by Deal et al. (2006) where a Sox10GeoBAC transgene was generated that closely approximates the expression of Sox10 (an essential transcription factor required for NCC development), Sox10GeoBAC reporter expression, as demonstrated as β-galactosidase, was apparent in cells and branching nerve fibres within the embryonic mouse lung. Co-localization of β-galactosidase immunoreactivity with the peripheral glial marker brain fatty acid binding protein indicated that these cells were glial cells (Deal et al. 2006), thus confirming that the NCCs that migrate into the mouse lung, in addition to neurons, also differentiate into glia as previously described in the chick and human embryo (Burns & Delalande, 2005; Burns et al. 2008).
Here we showed that YFP-positive NCCs are first apparent within the lungs of the embryonic mouse at E10.5. At this stage, numerous NCCs are present within the bifurcating foregut, and occasional YFP-positive cells can be observed within the primitive lung buds. Studies on the early development of the intrinsic innervation of the lung have previously been carried out by Tollet et al. (2001). Using wholemount staining with the neural crest marker p75NTR and the neuronal marker protein gene product 9.5, these authors showed the vagus nerves, and associated NCCs, projecting into the lung buds at E11, the earliest stage examined in their study. In addition, other p75NTR-positive cells, not associated with the vagus nerves, were observed within the lungs at this stage, which, based on our findings in Wnt1-Cre;YFP mouse embryos, are likely to be vagal NCCs that migrate into the lungs from the foregut earlier in development. Tollet et al. (2001) went on to describe the continued migration of NCCs along the vagus nerves into the lungs until at least E13, implying that, in addition to migration from the foregut into the lung buds, NCCs may subsequently enter the lungs via migration along the vagus nerves. Antibody staining, quail–chick grafting and transgenic mouse labelling techniques are not able to distinguish between NCCs that have migrated into the lung directly from the foregut or along vagal nerve fibres that project into the lung as all of these techniques label the same (vagal neural crest) cell populations. However, in the current study, and in our previous investigations in the chick embryo (Burns & Delalande, 2005), we observed NCCs within the primitive lung buds prior to the appearance of branches of the vagus nerve within the lungs. Nevertheless, NCCs may subsequently migrate into the lungs along vagus nerve fibres and contribute to the intrinsic neuronal population. It will be interesting to address, in future studies, whether NCCs that migrate from the foregut into the lung, and from the vagus nerve into the lung, utilize different signalling pathways and/or give rise to different cell types within the lung.
Optical projection tomography, when combined with YFP immunostaining in Wnt1-Cre;YFP mice, proved to be a unique and novel method for generating 3D information on the intrinsic innervation of the lung. In Wnt1-Cre;YFP embryos, YFP has the benefit of marking cell projections as well as cell bodies, thus allowing the interconnecting processes of the developing nervous system to be imaged. This represents an advantage over the quail–chick interspecies grafting technique previously employed to track NCCs in the chick embryo (Burns & Le Douarin, 1998, 2001; Burns & Delalande, 2005). Using this NCC tracing method, only cell nuclei are marked by the quail cell specific perinuclear antibody, thus making possible OPT reconstructions of quail cell-labelled NCCs less informative. In the current study, 3D reconstructions by OPT imaging provided high-resolution detail not only of the nerve fibres adjacent to the primary airways but also of the fine calibre fibres projecting along the smaller branching airways. Virtual sections through OPT reconstructions were in keeping with the distribution of YFP-positive tissues obtained using cryostat sections, thus confirming the efficacy of this imaging technique. The use of OPT as an imaging tool for developmental biologists is rapidly increasing and a number of other groups have recently employed OTP imaging to visualize patterns of gene expression in the lungs of mouse embryos, with levels of detail not possible with conventional techniques. For example, the group led by Puri have examined Shh, Foxf1 and Fgf10 gene expression patterns in the mouse foregut and lung buds at several stages of development in normal mice and in mouse models of foregut/lung malformation and disease (Sato et al. 2008, 2009; Hajduk et al. 2010). Also, Miller et al. (2007) have used OPT to describe the expression of Plxdc2, a relatively uncharacterized transmembrane protein, in the central nervous system and other tissues, including the lungs, of embryonic mice. The use of OPT as an imaging tool will undoubtedly continue to rise as this method has already demonstrated its importance for visualizing gene expression in an in-toto context that can be applied to genetic or teratogenic models of congenital malformations (Sato et al. 2009; Hajduk et al. 2010).
To investigate the role of the RET signalling pathway in lung colonization by NCCs, and subsequent development of the intrinsic innervation, we utilized an organotypic lung culture system, originally described by Tollet et al. (2001, 2002) to test the chemoattractive properties of the RET ligand, GDNF, and then went on to analyse the lungs of mice deficient in the RET receptor (Ret−/−) and GFRα1 co-receptor (Gfrα1−/−). In lungs that were cultured over a 5-day period, the lobes enlarged and the airways lengthened, branched successively and were surrounded by smooth muscle. YFP-positive neural tissue developed along and around the airways and formed ganglia adjacent to the airway smooth muscle. As previously reported (Tollet et al. 2001), spontaneous narrowing of the airways occurred, showing that the airway neuromusculature was functionally active throughout the culture period. When GDNF-soaked beads were placed within cultured lungs, YFP-positive tissue migrated towards and then totally surrounded the GDNF beads. In their lung culture experiments, Tollet et al. (2002) reported similar findings; the area covered by p75NTR-positive nerves outside the explants increased dramatically in the presence of GDNF, and protein gene product 9.5-positive nerves concentrated around GDNF beads after 5 days in culture. Together, these findings suggest that RET signalling, acting through the RET receptor and the ligand GDNF, is required for NCC development in the lung. This idea is further supported by recent findings showing that in E18.5 Ret−/− embryos, although the overall TuJ1-positive innervation density is similar to controls, the numbers of TuJ1-immunopositive neurons are reduced in the trachea and primary bronchi (Langsdorf et al. 2010). However, in our current study, where we analysed the lungs of E14.5 mice using immunohistochemistry on sections (Ret−/− and Gfrα1−/−), and with OPT and wholemount immunohistochemistry (Ret−/−), we did not detect a marked deficit in the extent of innervation throughout the mutant lungs, and numbers of neurons within Ret−/− lung ganglia were not significantly reduced compared with controls. Thus, although both studies [our study and that of Langsdorf et al. (2010)] found the overall lung innervation density in Ret−/− mice and controls to be similar, the reasons for our different findings concerning numbers of neurons in Ret−/− lungs are not clear. The discrepancy could be due to the different developmental stages and/or regions examined in both studies. Although it is well established that Ret−/− and Gfrα1−/− mutants have enteric NCC defects resulting in gut aganglionosis, NCCs have been reported in reduced numbers within the oesophagus and rostral stomach of these mice (Durbec et al. 1996; Cacalano et al. 1998; Yan et al. 2004). Thus, if there is no significant loss of intrinsic lung innervation in the Ret−/− and Gfrα1−/− mutants, this supports the idea that NCCs in the lung migrate from the oesophagus, but possibly in a RET-independent manner (Durbec et al. 1996). However, RET signalling is complex and our work so far may only have provided limited insight into the actions of some components of this pathway. RET signalling occurs through a multisubunit complex comprising RET in conjunction with any one of four glycosyl phosphatidyl inositol-linked co-receptors (GFRα1–4). GFRα1, GFRα2, GFRα3 and GFRα4 interact preferentially with four distinct ligands, GDNF, neurturin, artemin and persephin, but there is a substantial degree of cross-specificity between the different GFRα subfamily members (Manie et al. 2001). In our previous studies, although we have shown that GDNF does not appear to be expressed in the lungs, GFRα1 is expressed in the lung mesenchyme (Burns & Delalande, 2005; Burns et al. 2008). Previous studies have suggested that GFRα1 may be able to activate signalling by binding to RET directly or by binding to other RET ligand(s) (Manie et al. 2001). Thus, in the development of lung innervation, there may be redundancy in RET, its co-receptors and/or ligands making the interpretation of results difficult. In future studies, it will be important to examine the expression of RET family co-receptors and ligands within the lung in detail, and perhaps use double mouse mutants or double gene knockdown experiments, to try to gain further insight into the roles of this complex signalling pathway in the development of lung innervation.
In summary, we have used a unique and novel combination of Wnt1-Cre;YFP mice to definitively label and trace NCCs and their progeny, and OPT imaging to generate 3D information on the NCC-derived intrinsic innervation of the mammalian lung. These techniques have allowed the clearest visualization of the intrinsic neural network within the developing lung and may provide the basis for subsequent studies of lung innervation defects in mouse mutants. The authors thank Catia Sousa, Cranfield Health, Cranfield, UK for assistance with OPT.
Acknowledgments
The RET and GFRα1 mutant mice were supplied by Dr Vassilis Pachnis (MRC National Institute of Medical Research, Mill Hill, UK). Additional RET mutant mice were supplied by Dr Hideki Enomoto (RIKEN Center for Developmental Biology, Kobe, Japan). Wnt1-Cre;YFP mice were initially supplied by Dr Miles Epstein (University of Wisconsin, USA). L.J.F. is supported by a PhD studentship from The Anatomical Society of Great Britain and Ireland.
Supporting Information
Movie S1. Movie showing rotating 3D optical projection tomography reconstruction of lungs from E14.5 Wnt1-Cre;YFP embryo.
Movie S2. Movie showing rotating 3D optical projection tomography reconstruction of lungs from E14.5 Ret+/+ embryo stained with the neural marker TuJ1.
Movie S3. Movie showing rotating 3D optical projection tomography reconstruction of lungs from E14.5 Ret+/− embryo stained with the neural marker TuJ1.
Movie S4. Movie showing rotating 3D optical projection tomography reconstruction of lungs from E14.5 Ret−/− embryo stained with the neural marker TuJ1.
Fig. S1. Comparison of lung innervation in control and Ret−/− mice.
Fig. S2. Comparison of lung innervation in control and Gfrα1−/− mice.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, et al. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. doi: 10.1387/ijdb.041935ab. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Delalande JM. Neural crest cell origin for intrinsic ganglia of the developing chicken lung. Dev Biol. 2005;277:63–79. doi: 10.1016/j.ydbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Thapar N, Barlow AJ. Development of the neural crest-derived intrinsic innervation of the human lung. Am J Respir Cell Mol Biol. 2008;38:269–275. doi: 10.1165/rcmb.2007-0246OC. [DOI] [PubMed] [Google Scholar]
- Burton MD, Kawashima A, Brayer JA, et al. RET proto-oncogene is important for the development of respiratory CO2 sensitivity. J Auton Nerv Syst. 1997;63:137–143. doi: 10.1016/s0165-1838(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiman D, Barlow A, Vander Borght S, et al. Hepatic stellate cells do not derive from the neural crest. J Hepatol. 2006;44:1098–1104. doi: 10.1016/j.jhep.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Chen ML, Keens TG. Congenital central hypoventilation syndrome: not just another rare disorder. Paediatr Respir Rev. 2004;5:182–189. doi: 10.1016/j.prrv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Croaker GD, Shi E, Simpson E, et al. Congenital central hypoventilation syndrome and Hirschsprung's disease. Arch Dis Child. 1998;78:316–322. doi: 10.1136/adc.78.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal KK, Cantrell VA, Chandler RL, et al. Distant regulatory elements in a Sox10-beta GEO BAC transgene are required for expression of Sox10 in the enteric nervous system and other neural crest-derived tissues. Dev Dyn. 2006;235:1413–1432. doi: 10.1002/dvdy.20769. [DOI] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, et al. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Fitze G, Paditz E, Schlafke M, et al. Association of germline mutations and polymorphisms of the RET proto-oncogene with idiopathic congenital central hypoventilation syndrome in 33 patients. J Med Genet. 2003;40:E10. doi: 10.1136/jmg.40.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk P, Murphy P, Puri P. Fgf10 gene expression is delayed in the embryonic lung mesenchyme in the adriamycin mouse model. Pediatr Surg Int. 2010;26:23–27. doi: 10.1007/s00383-009-2519-3. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Le Douarin NM. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Langsdorf A, Radzikinas K, Kroten A, et al. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0462OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantieri F, Griseri P, Ceccherini I. Molecular mechanisms of RET-induced Hirschsprung pathogenesis. Ann Med. 2006;38:11–19. doi: 10.1080/07853890500442758. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Manie S, Santoro M, Fusco A, et al. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 2001;17:580–589. doi: 10.1016/s0168-9525(01)02420-9. [DOI] [PubMed] [Google Scholar]
- Miller SF, Summerhurst K, Runker AE, et al. Expression of Plxdc2/TEM7R in the developing nervous system of the mouse. Gene Expr Patterns. 2007;7:635–644. doi: 10.1016/j.modgep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, et al. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Pilon N, Raiwet D, Viger RS, et al. Novel pre- and post-gastrulation expression of Gata4 within cells of the inner cell mass and migratory neural crest cells. Dev Dyn. 2008;237:1133–1143. doi: 10.1002/dvdy.21496. [DOI] [PubMed] [Google Scholar]
- Sakai T, Wakizaka A, Nirasawa Y. Congenital central hypoventilation syndrome associated with Hirschsprung's disease: mutation analysis of the RET and endothelin-signaling pathways. Eur J Pediatr Surg. 2001;11:335–337. doi: 10.1055/s-2001-18552. [DOI] [PubMed] [Google Scholar]
- Sato H, Murphy P, Giles S, et al. Visualizing expression patterns of Shh and Foxf1 genes in the foregut and lung buds by optical projection tomography. Pediatr Surg Int. 2008;24:3–11. doi: 10.1007/s00383-007-2036-1. [DOI] [PubMed] [Google Scholar]
- Sato H, Murphy P, Hajduk P, et al. Sonic hedgehog gene expression in nitrofen induced hypoplastic lungs in mice. Pediatr Surg Int. 2009;25:967–971. doi: 10.1007/s00383-009-2452-5. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Miserocchi G, Sparrow MP. Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol. 2000;23:11–18. doi: 10.1165/ajrcmb.23.1.3926. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Sharpe J. Optical projection tomography as a new tool for studying embryo anatomy. J Anat. 2003;202:175–181. doi: 10.1046/j.1469-7580.2003.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. Optical projection tomography. Annu Rev Biomed Eng. 2004;6:209–228. doi: 10.1146/annurev.bioeng.6.040803.140210. [DOI] [PubMed] [Google Scholar]
- Sparrow MP, Lamb JP. Ontogeny of airway smooth muscle: structure, innervation, myogenesis and function in the fetal lung. Respir Physiol Neurobiol. 2003;137:361–372. doi: 10.1016/s1569-9048(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Sparrow MP, Weichselbaum M, McCray PB. Development of the innervation and airway smooth muscle in human fetal lung. Am J Respir Cell Mol Biol. 1999;20:550–560. doi: 10.1165/ajrcmb.20.4.3385. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PK, Garcia-Barcelo M. Genetic basis of Hirschsprung's disease. Pediatr Surg Int. 2009;25:543–558. doi: 10.1007/s00383-009-2402-2. [DOI] [PubMed] [Google Scholar]
- Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn. 2001;221:48–60. doi: 10.1002/dvdy.1124. [DOI] [PubMed] [Google Scholar]
- Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: a role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol. 2002;26:420–429. doi: 10.1165/ajrcmb.26.4.4713. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- Yan H, Bergner AJ, Enomoto H, et al. Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent. Dev Biol. 2004;272:118–133. doi: 10.1016/j.ydbio.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Farlie PG, et al. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


