Abstract
VGF mRNA and its precursor-derived products are selectively expressed in certain neurons and promptly respond to neurotrophins and to neural/electrical activity. Proteomic studies have previously revealed a reduction in some VGF peptides in the cerebrospinal fluid of patients affected by Alzheimer's disease and other conditions, suggesting their potential diagnostic and clinical significance. As the presence of VGF peptides within the human cortex has been somewhat elucidated, they were studied postmortem in the frontal, parietal, and temporal cortex areas of control subjects and patients affected by Parkinson's disease, and in parietal cortex samples from patients with Alzheimer's disease. We raised antibodies to the C-/N-terminal portions of the proVGF precursor protein, to the TPGH and TLQP sequences and to the neuroendocrine regulatory peptide (NERP)-1, all used for enzyme-linked immunosorbent assay coupled with gel chromatography and for immunohistochemistry. In the control brain samples, the levels of TPGH and C-terminus peptides were about 130–200 and 700–2000 pmol g−1, respectively, the N-terminus and NERP-1 peptides were less represented (about 10–30 and 4–20 pmol g−1, respectively), and the TLQP peptides were below detection limits. Upon gel chromatography, the VGF antisera mainly revealed small molecular weight forms (i.e. about 0.8–1.3 kDa), whereas VGF immunolocalisation was found within different types of neuron in rat and bovine brain cortices. In the Parkinson's disease samples, a clear-cut decrease was revealed in the parietal cortex only, exclusively for TPGH and NERP-1 peptides, whereas in the Alzheimer's disease samples, a reduction in all of the VGF peptides was shown. The results suggest the involvement of VGF in the physiological or pathophysiological mechanisms occurring in the parietal cortex of patients with Parkinson's and Alzheimer's diseases.
Keywords: Alzheimer's disease, human cortex, Parkinson's disease, VGF peptides
Introduction
The vgf gene encodes a polypeptide of 617 or 615 amino acids (in rat/mouse or human, respectively) (Canu et al. 1997; Salton et al. 2000; Levi et al. 2004), with a number of conserved stretches of basic amino acid residues representing potential or demonstrated processing sites for neuroendocrine prohormone convertases, such as PC1/3 and PC/2 (Trani et al. 2002).
The vgf gene contains cAMP-response element binding protein binding sites critical for brain-derived neurotrophic factor (BDNF)-induced VGF expression (Bozdagi et al. 2008), and it has been reported that VGF transcription is accompanied by translation within 3 h of BDNF exposure in neurons from hippocampal cultures (Alder et al. 2003). VGF mRNA is upregulated by neuronal activity, seizures, and lesions (Mahata et al. 1993; Snyder et al. 1998), and reduced in drug-free depressed patients (Cattaneo et al. 2010). The VGF precursor is processed during neuronal differentiation in vitro and localises into large dense core vesicles (Trani et al. 1995; Possenti et al. 1999).
In cells and tissues, proteolytic processing was shown to yield diverse peptides, some of which are of low molecular weight (MW) and are preferentially released by various stimuli (Possenti et al. 1989, 1999; Trani et al. 1995).
An endogenous peptide corresponding to the C-terminal 30-amino-acid segment of the human proVGF has been identified from the bovine posterior pituitary (peptide V) (Liu et al. 1994). Although only partially studied, some VGF peptides have already shown a diversity of specific neuronal bioactivities. The exogenous TLQP-62 peptide was shown to enhance synaptic activity (Alder et al. 2003), and induce neurogenesis (Thakker & Alder, 2009) as well as to affect cognitive mechanisms (Brouillette et al. 2007) and depression (Hunsberger et al. 2007).
The two recently recognised NERP-1 and NERP-2 peptides inhibit vasopressin release from the rat hypothalamus, in connection with the maintenance of water balance (Yamaguchi et al. 2007; Toshinai & Nakazato, 2009).
Several novel peptides derived from the VGF precursor were found to be changed in the cerebrospinal fluid (CSF) of patients affected by Alzheimer's disease (AD) and frontotemporal dementia (Carrette et al. 2003; Ruetschi et al. 2005; Selle et al. 2005). A peptide of ∼4 kDa was found to be decreased in the CSF of patients affected by amyotrophic lateral sclerosis; however, it was unchanged in patients with Parkinson's disease (PD) (Pasinetti et al. 2006).
Although VGF has been suggested as a potential diagnostic and prognostic biomarker, and is important for modulating neuronal activity, only a few studies (Haung et al. 2006) are available regarding the presence of VGF peptides in the human cortex. Hence, using enzyme-linked immunosorbent assay coupled with gel chromatography, we investigated the levels of the VGF peptides and their MW forms in the normal human cortex and, using immunohistochemistry, we studied the localisation of the same peptides in bovine and rat brains. Furthermore, as PD is characterised by a progressive loss of dopaminergic neurons in subcortical areas projecting to the brain cortex, cortices of cases of PD in parallel with AD brain samples were used to investigate possible VGF peptide level changes.
Materials and methods
Patients
The frontal, parietal and temporal cortex samples (Fig. 1) were taken from patients not affected by any neurological disease (controls; n = 8 for each cortex area; age 60–78 years; four females and four males), and from patients with PD (n = 6 for each cortex area; age 60–80 years; three females and three males). In addition, parietal cortices were also taken from patients with AD (n = 5; age 72–80 years; three females and two males). The diagnosis of PD was according to nigrostriatal neuronal degeneration, and parkinsonisms were not present in this study. We also collected brains of other patients affected by diverse neurological diseases (n = 6), with two cases each of amyotrophic lateral sclerosis, Pick's disease, and multiple sclerosis.
Fig. 1.
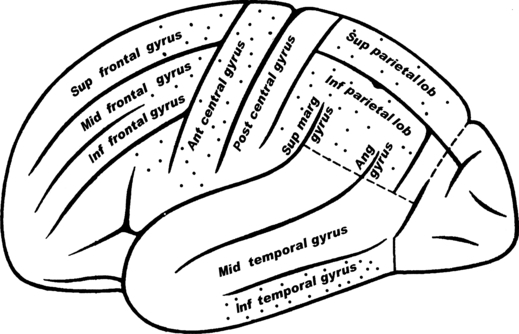
Anatomical diagram of the brain regions examined. Sagittal view of the human brain. Dots designate the specific part of each area taken for the VGF peptide quantification. Sup, superior; mid, middle; inf, inferior; ant, anterior; lob, lobule; sup marg gyrus, supramarginal gyrus; ang gyrus, angular gyrus.
Tissue samples
The human samples, taken postmortem (autolysis time about 9–10 h), were unfixed-frozen, and stored at −80 °C (by the Los Angeles Tissue Bank, http://www.loni.ucla.edu/uclabrainbank/index.html).
From each tissue sample (approximate size: 1.5–3 cm high × 1–2 cm deep), four pieces were cut across the four sides, then coarsely minced together with a scalpel and weighed (such a procedure was performed twice, and each time minced tissues were used as given below).
For enzyme-linked immunosorbent assay, the tissue samples were homogenised in phosphate-buffered saline (PBS) (0.01 m PO4, pH 7.2–7.4, 0.15 m NaCl, ∼10 mL g−1 tissue) containing a protease inhibitor cocktail (P8340; Sigma-Aldrich, Schnelldorf, Germany) by using an Ultra-Turrax homogeniser (Ika-Werke, Staufen, Germany), heated in a boiling water bath (10–15 min), and centrifuged (3000 g, 10–15 min). The supernatants were kept frozen until use (−20 °C or lower). The experimental design and procedures were approved by the relevant ethical committee at the University of Cagliari (protocol number 450/09/C.E.).
For immunohistochemistry, samples of the bovine frontal, parietal, and temporal cortices (n = 6, from a local abattoir) were taken as well as human samples (Fig. 1), kept at 0 °C (on ice) and paraformaldehyde fixed (40 g L−1, in 0.1 m PO4 buffer) for 3 h. Male rats (n = 6, Sprague-Dawley) were transcardially perfused with the same fixative, and the entire brains were removed from the skulls. All samples were rinsed in PBS containing 70 g L−1 sucrose and 0.1 g L−1 NaN3, set up within blocks using cryoembedding media (65–75 g L−1 polyvinyl alcohol, 40 mL L−1 polyetilen glycol 56–98, 10 mL Tween 20, 0.5–1 g L−1 NaN3) (Cocco et al. 2003), snap-frozen in melting freon cooled with liquid nitrogen, and stored in the vapour phase of a liquid nitrogen tank. The sections were prepared using a cold-knife cryomicrotome (Leica, Germany), collected on slides (Sigma, Milan, Italy) and stored at −80 °C.
VGF peptide antibodies
The antigens (Table 1) were conjugated with bovine thyroglobulin via an additional D-tyrosine, or keyhole limpet haemocyanin via an additional C-terminal cysteine (for the TLQP and N-terminus peptides only).
Table 1.
VGF peptides used for the rabbit and guinea pig immunisations.
| Antigen | Short name | Species | References |
|---|---|---|---|
| hVGF607–615 | proVGF C-term. | Rabbit | Brancia et al. (2005), Cocco et al. (2007) |
| VGF609–617 | proVGF C-term. | Rabbit | Ferri et al. (1995), Trani et al. (1995, 2002) |
| hVGF419–427 | TPGH | Rabbit | D’Amato et al. (2008) |
| rVGF422–430 | APGH | Rabbit | Rindi et al. (2007), D’Amato et al. (2008) |
| VGF298–306 | NERP-1 | Rabbit | Cocco et al. (2007), Rindi et al. (2007) |
| VGF23–31 | proVGF N-term. | Guinea pig | This study |
| rVGF556–565 | TLQP | Guinea pig | Cocco et al. (2007), D’Amato et al. (2008) |
h, human; r, rat; C-term., C-terminus; N-term., N-terminus.
At the C-terminal end of the human/bovine (Salton et al. 2000) and rat (Ferri et al. 1995) proVGF, the Arg613-Arg614-Pro615 and His615-Arg616-Pro617 sequences, respectively, are found. Hence, the C-terminus antisera were raised against the corresponding nonapeptides, whereas the N-terminus of proVGF (Sigma-Aldrich) corresponds to the human ‘APPGRPEA’ amino acid sequence (differing by a single amino acid from the bovine and rat sequences).
The TLQP peptides were isolated from rat brains and proved to be cleaved at the rat VGF553–555 (Arg553-Pro554-Arg555) processing site (Trani et al. 2002). Hence, the rat VGF556–565 peptide was synthesised and conjugated at its C-terminus (Brancia et al. 2005).
The human VGF419–427 (which is similar to the bovine sequence, accession no. XP_875466.2) and the rat VGF422–430 peptides contain at their C-terminus the ‘TPGH’ and ‘APGH’ amino acid sequences, respectively (Ferri et al. 1995; D’Amato et al. 2008), whereas the so-called ‘NERP-1’ peptide corresponds to the human VGF298–306 sequence (identical to the rat and bovine sequences) and contains an amide group at its C-terminus (Cocco et al. 2007).
All of the procedures were performed in accordance with the care and use of animals approved by the American Physiological Society and EEC Council Directive of November 1986 (86/609).
Enzyme-linked immunosorbent assay
For each assay characterisation, cross-reactions were addressed using a range of different peptides (Table 2).
Table 2.
VGF assay characterisation using peptides with extended/truncated amino acid sequences and/or containing additional arginine (R) as well as peptides corresponding to the rat amino acid sequences.
| VGF antiserum | Peptide | IC50 (pmol mL−1)* | Cross-reactivity (%) |
|---|---|---|---|
| hVGF C-term. | hVGF607–615 (IEHVLLRRP) | 0.5 | 100 |
| hVGF603–612 (...HVLL) | 59 | 0.8 | |
| rVGF609–617 | 132 | 0.4 | |
| hTPGH | hVGF419–427 (RSQEETPGH) | 0.2 | 100 |
| hVGF419–428 (………..TPGHR) | 50 | 1 | |
| rVGF422–430 (………..APGH) | 2.5 | 20 | |
| rVGF422–431 (………..APGHR) | > 1000 | < 0.05 | |
| NERP-1 | VGF298–306 (QQGLAQVEA-NH2) | 0.008 | 100 |
| VGF298–306 (………AQVEA) | > 1000 | < 0.0001 | |
| VGF298–306 (………AQVEAG) | > 1000 | < 0.0001 | |
| VGF N-term. | hVGF23–30 (APPGRPEA) | 0.1 | 100 |
| rVGF24–31 (……...RSDV) | > 1000 | < 0.005 | |
| rVGF4–240 | > 1000 | < 0.005 |
IC50, half-maximal inhibitory concentration; h, human; r, rat; C-term., C-terminus; N-term., N-terminus.
The HVLL peptide corresponds to the C-terminus peptide deprived of the three final amino acids, whereas for the NERP-1, peptides without amidation as well as with additional glycine (G) were used. All of the antisera used for the tissue VGF quantification showed 100% cross-reactivity with the corresponding peptides.
Picomoles per assay well (100 μL incubation volume).
Multiwell plates (Maxisorp, Milan, Italy) were coated with the corresponding synthetic peptides (each 5–50 nm, in carbonate–bicarbonate buffer, pH 9.6, overnight at 0–4 °C). Hence the plates were incubated (for 4 h at 20–25°C) with PBS–donkey serum (PBS containing 90 mL L−1 donkey and 0.2 g L−1 NaN3). Primary incubations were carried out in duplicate, including the relevant standards (0.005–500 nm) and the serially diluted human samples (100 μL per well incubation volume, in PBS–donkey; 4 h at room temperature with constant agitation). The PD and AD samples (or other neurological diseases) were always disposed in parallel with the controls into the wells.
After incubation (1 h) with the relevant biotinylated secondary antibodies (Jackson, West Grove, PA, USA) and the streptavidin–peroxidase conjugate (30 min) (Biospa, Milan, Italy), the wells were filled with tetramethylbenzidine substrate (100 μL per well incubation volume) (Kem-En-Tec Diagnostics, Taastrup, Denmark). On development, the reaction was stopped with HCl (1 m, 100 μL per well), and the optical density was measured at 450 nm using a multilabel plate reader (Chameleon; Hidex, Turku, Finland). Statistical analyses were carried out by one-way anova, followed by a t-test (StatistiXL software: http://www.statistxl.com).
Chromatography
Gel chromatographies were run for five brain cortices including three samples from control cases (one for each cortex area) and one each from the PD and AD parietal cortices. The extracts (∼2 mL) were loaded onto a Sephadex G-50s column (2 cm2 × 1 m), equilibrated with 50 mm ammonium bicarbonate and eluted with the same buffer. An MW marker kit (MWGF70; Sigma) was used for the column calibration. The collected fractions (3 mL) were reduced in volume using a vacufuge concentrator (Eppendorf, Milan, Italy), and analysed using enzyme-linked immunosorbent assay.
Immunohistochemistry
The bovine and rat sections were washed in PBS and incubated overnight with the VGF antisera diluted (1 : 2000–4000 for the C-terminus and 1 : 300–1000 for the others) in PBS containing 30 mL L−1 of donkey serum, 30 mL L−1 of bovine/rat serum and 0.2 g L−1 of NaN3. Donkey anti-rabbit or anti-guinea pig secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) conjugated with Cy3 were used to reveal the positive labelling. The routine controls included substitution of each antibody, in turn, with PBS. The slides, coverslipped with PBS–glycerol, were observed and photographed using BX41 and BX51 fluorescence microscopes (Olympus, Milan, Italy) equipped with Fuji S2 and S3 Pro digital cameras (Fujifilm, Milan, Italy).
Results
Enzyme-linked immunosorbent assay
We studied the levels of VGF peptides in the postmortem frontal, parietal and temporal cortex samples from the control and PD brains as well as the AD parietal cortex samples.
In the brain control cases (Fig. 2), the VGF peptide levels were of the order of approximately 130–200 and 700–2000 pmol g−1 for TPGH and C-terminus peptides, respectively, whereas the NERP-1 and N-terminus peptides were less abundant (about 4–20 and 10–30 pmol g−1, respectively). Conversely, the TLQP peptides were below detection limits in the samples studied; hence, they were not further pursued.
Fig. 2.
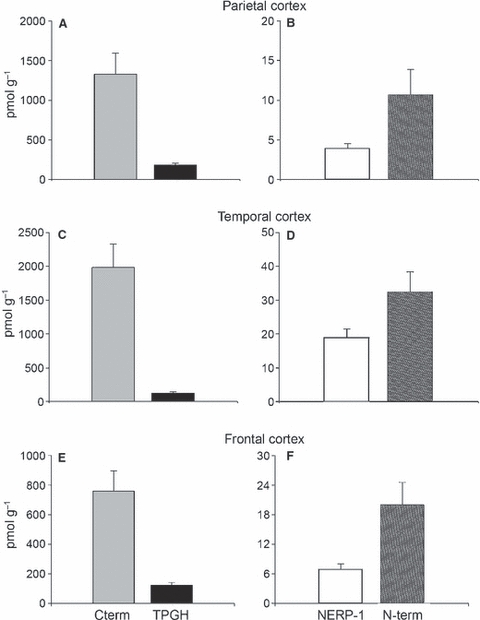
VGF peptide levels in the parietal, temporal, and frontal cortices of control cases. In the control cortices tested, the C-terminus (C-term) and TPGH peptides (A,C,E) were the most abundant, compared with the N-terminus (N-term) and NERP-1 peptides (B,D,F). The latter were largely present in the temporal cortex, compared with the other brain areas.
When the PD brain samples were compared in parallel assays, a clear-cut decrease was found exclusively for TPGH (Fig. 3B) and NERP-1 peptides (Fig. 3C) in the parietal cortex only, whereas none of the other cortex areas revealed changes using any of the VGF antisera (not shown).
Fig. 3.
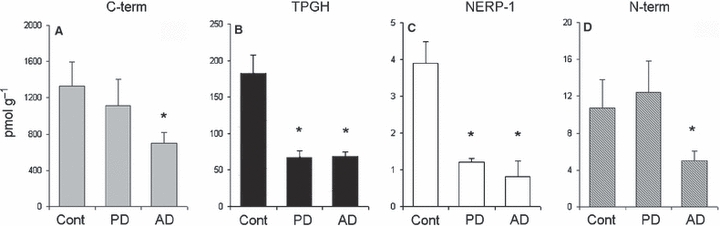
VGF peptide changes in the parietal cortices of Parkinson's disease (PD) and Alzheimer's disease (AD) cases. In the PD samples, a striking decrease was revealed exclusively for TPGH (B: 182 ± 25 and 66 ± 9 pmol g−1, mean ± SEM, P < 0.001, controls vs. PD, respectively) and NERP-1 peptides (C: 3.89 ± 0.59 and 1.2 ± 0.1 pmol g−1, mean ± SEM, P < 0.01 controls vs. PD, respectively), whereas the other peptides did not change (A,D). However, in the AD samples, a common decrease was shown using all of the VGF antisera. A clear-cut decrease was revealed for the C-terminus (C-term) (A: 1330 ± 261 and 700 ± 120 pmol g−1, mean ± SEM, controls vs. AD, respectively, P < 0.045), TPGH (B: 182 ± 25 and 68.2 ± 6.3, pmol g−1, mean ± SEM, controls vs. AD, respectively, P < 0.010), NERP-1 (C: 3.89 ± 0.59 and 0.8 ± 0.4, pmol g−1, mean ± SEM, controls vs. AD, respectively, P < 0.007) and N-terminus (N-term) (D: 10.7 ± 3.1 and 5 ± 1.1 pmol g−1, mean ± SEM, controls vs. AD, respectively, P < 0.002) compared with the controls. * = P.
Conversely, in the AD parietal cortices, a similar decrease was found for all of the VGF peptides studied (Fig. 3A–D).
None of the brain cortices collected from patients affected by the other conditions (amyotrophic lateral sclerosis, multiple sclerosis, and Pick's disease) revealed a decrease in any of the VGF peptides studied, compared with the controls (Supporting Information Table S1).
Chromatography
Gel chromatographies were run in order to analyse the MW forms recognised by each VGF antiserum.
In all of the normal cortex areas, using the C-terminus antiserum, only a peak of 0.8–1 kDa, corresponding to the estimated MW of the last C-terminal amino acids of the proVGF protein, was visible (Figs 4A,B and 5A, upper panel). However, the N-terminus antiserum mainly recognised a 20-kDa form and a small amount of either the 0.8–1- or 60-kDa fragments (Figs 4G,H and 5D, upper panel). As for the NERP-1 peptides, they were mainly present in small MW forms, i.e. 0.8–1 kDa in all of the cortex areas (Figs 4C,D and 5B, upper panel) and 1.5–2 kDa in the frontal cortex only (Fig. 4C). In the latter area, a very small amount of a 60-kDa form was also recognised by the VGF298–306 antiserum. Finally, through the TPGH assay, a form corresponding to 0.8–1 kDa was mainly found in all of the cortex areas (Figs 4E,F and 5C, upper panel), whereas a small amount of a second peak of ∼4 kDa was revealed in the temporal and frontal cortex areas only (Fig. 4E,F).
Fig. 4.
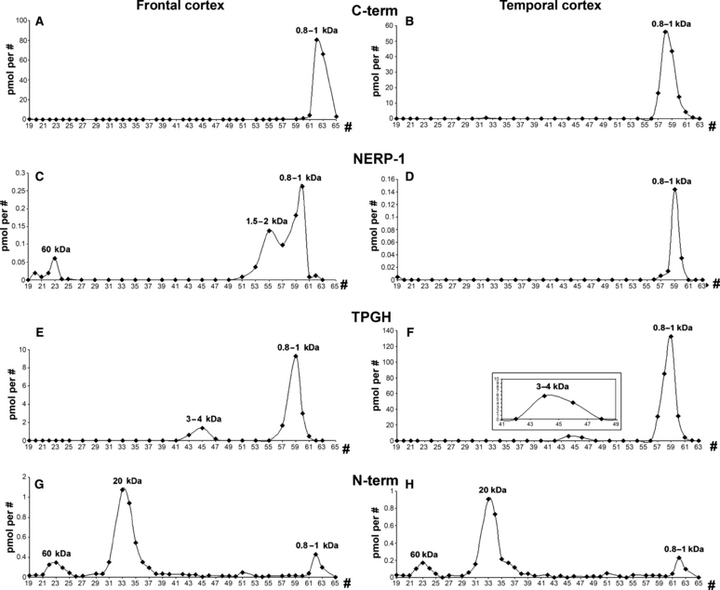
Chromatography of the frontal (A,C,E,G) and temporal (B,D,F,H) cortices. Control cases. In the frontal and temporal cortex areas, through the C-terminus (C-term) (A,B), NERP-1 (C,D) and TPGH (E,F) assays, a peak of around 0.8–1 kDa was mainly revealed, whereas a form of 1.5–2 kDa was exclusively labelled by the NERP-1 antiserum in the frontal cortex only (C), where the latter also revealed a peak of ∼60 kDa. Through the TPGH assay, lower levels of another molecular weight (MW) form were also revealed, i.e. a peak of ∼3–4 kDa in the frontal (E) and temporal (F) cortex areas. The N-terminus (N-term) antiserum primarily recognised a larger MW form of 20 kDa and only low levels of the 60- and 0.8–1-kDa forms in either the frontal (G) or temporal (H) cortex areas. pmol per #, picomoles/fraction.
Fig. 5.
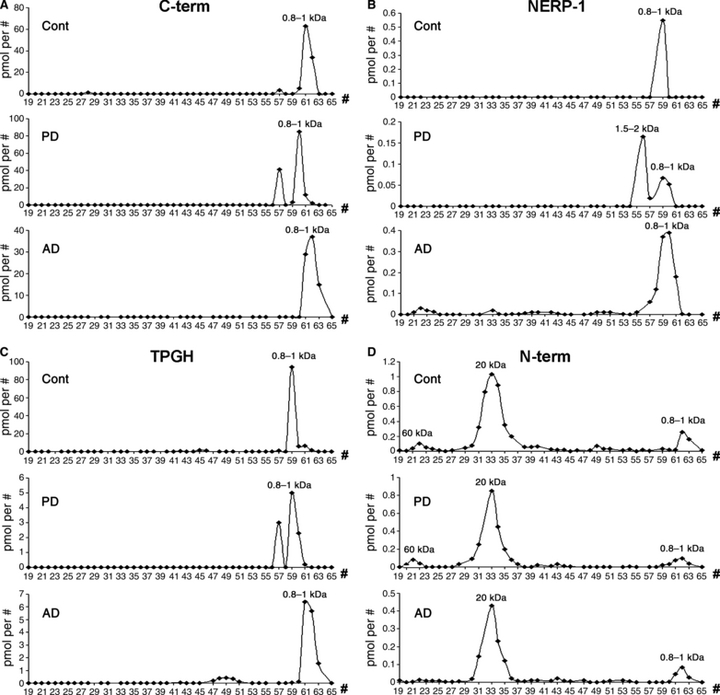
Chromatography of the control, Parkinson's disease (PD) and Alzheimer's disease (AD) parietal cortices. In the normal parietal cortex, a small molecular weight (MW) form of around 0.8–1 kDa was mainly revealed in the C-terminus (C-term), NERP-1, and TPGH assays (A–C, upper panels), whereas the N-terminus (N-term) antiserum mainly recognised a larger MW form of 20 kDa and only low levels of the 60- and 0.8–1-kDa forms (D, upper panel). Differences were not seen between PD (A–D, middle panels) and control (A–D, upper panels) samples for each of the VGF antisera. However, in the AD samples, using the N-term antiserum, a large reduction in the 60-kDa form (D, lower panel) was revealed compared with the control peak (D, upper panel). A form of the same MW was also labelled by the NERP-1 antiserum in AD only, but not in the PD or control case (B, lower panel). pmol per #, picomoles/fraction.
In the PD parietal cortex (Fig. 5A–D, middle panels), the MW peaks recognised by all of the antisera were similar to those revealed using the control cases (Fig. 5A–D, upper panels). The AD parietal cortex instead (Fig. 5A–D, lower panels) revealed a few differences concerning the 60-kDa MW peak, which was recognised to some extent by the NERP-1 antiserum (Fig. 5B, lower panel) and was not visible with the N-terminus assay (Fig. 5D, lower panel), contrary to the control cases (Fig. 5B,D, upper panels). Recovery after chromatography ranged between 80 and 95% for all of the peptides tested.
Immunohistochemistry
In connection with sequence similarities in the regions of the VGF peptides studied (see Materials and methods), the immunofluorescence detection was carried out with bovine (Fig. 6) and rat brains (Fig. 7).
Fig. 6.
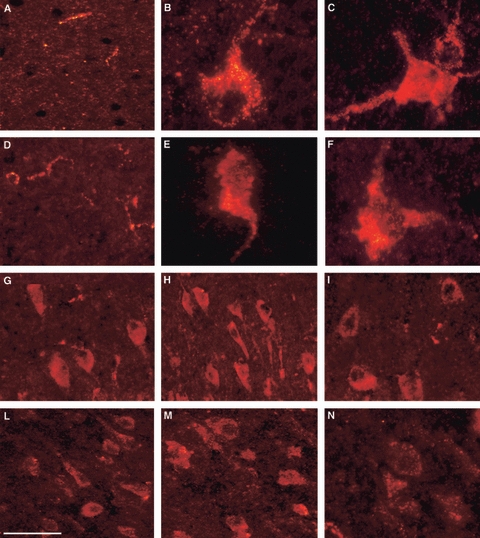
VGF peptide immunolocalisation in the bovine brain. VGF immunoreactivity (Cy3 red labelling) in frontal (A,D,G,L), parietal (B,E,H,M) and temporal (C,F,I,N) cortices. C-terminus immunoreactivity found in axons of the external layer (A) and in large perikarya with visible apical dendrites (B,C). NERP-1 antiserum labelling axons of the external layer (D), and perikarya with basal (E) and apical (F) dendrites. N-terminus (G–I) and TPGH (L–N) antisera-labelled groups of perikarya with different sizes and morphologies. Scale bar: 50 μm.
Fig. 7.
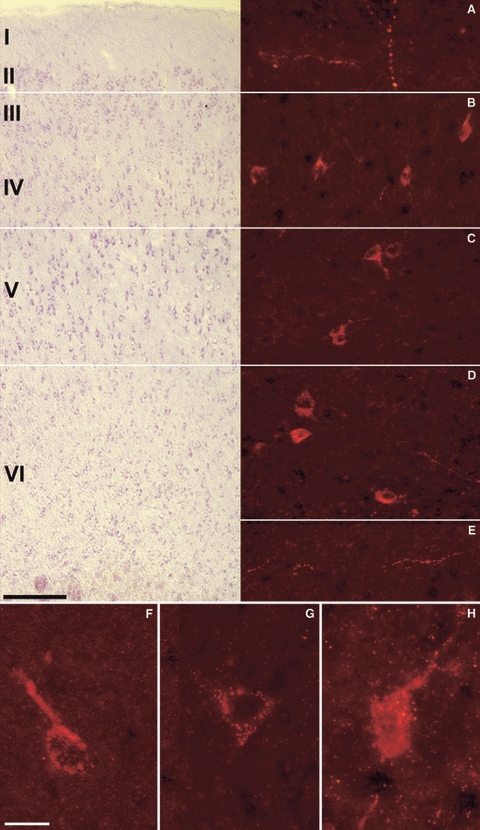
VGF peptide immunolocalisation in the rat brain. Immunoreactivity and cortical distribution of the C-terminus and NERP-1 peptides in the rat parietal cortex (Cy3 red labelling). (A–E) C-terminus staining. Roman numerals indicate cortical layers in cresyl violet sections (on the left), in parallel with immunofluorescence-stained sections. In all of the layers, the antiserum decorated either axons as perikarya found singly or in small groups, often with apical dendrites and sometimes disposed in a horizontal plane (B). Scale bars: 100 μm. (F–H) NERP-1 labelling was restricted to single perikarya with a granular staining and often with visible apical dendrites (F,H). Scale bar: 25 μm.
In either the bovine or rat brain sections (Figs 6A–C and 7A–E), the neurons labelled by the C-terminus antiserum were the most abundant and bright compared with those revealed by the other VGF antisera in all of the different cortex areas where the same antiserum decorated axons, neuron terminals, and perikarya distributed in virtually all of the layers.
In both species, C-terminus-positive perikarya were found singly or in small groups, often of large size with a visible apical dendrite, suggesting an abundant presence of pyramidal cells (for criteria see Conti et al. 1987).
As for the NERP-1 antiserum, perikarya with apical and/or basal dendrites were found labelled within all of the bovine (Fig. 6D–F) and rat (Fig. 7F–H) cortex areas. In the rat sections, they were mostly present with a granular staining, whereas positive axons were scarcely found but brightly stained in all of the bovine samples, mainly within the external cortex layer (Fig. 6D). The TPGH and N-terminus antisera stained groups of perikarya restricted to certain layers (probably III) in all of the bovine cortex samples (Fig. 6G–I, L–N respectively), whereas in those of the rat brain, only a small number of cells was stained (not shown).
Discussion
In the present study, we report novel outcomes describing the high degree of proVGF processing present in the human parietal, frontal, and temporal cortex areas. In the brain samples from controls, the C-terminus and TPGH peptides were the most abundant, whereas the N-terminus and NERP-1 peptides were present in minor concentrations.
In the parietal cortex of PD cases, but not in the other cortex areas, changes were seen for TPGH and NERP-1 peptides only, whereas in the AD cases of the same area, a similar decrease in all of the VGF peptides was revealed.
As the human unfixed tissues obtained from the tissue bank were not adequate for the immunohistochemical detection, we used bovine and rat tissues, in connection with sequence similarities in the regions of the VGF peptides that we studied. In both species, using our specific antisera, the positive staining found in different types of neurons suggests the involvement of the VGF peptides in diverse neuronal mechanisms of the brain cortex. Such results may be useful for future animal model investigations, as important tools supporting research on clinical treatments in PD and AD. The specific labelling of our antibodies is demonstrated by the experiments of characterisation shown in Table 2. Using the C-terminus antiserum, only a peak corresponding to the estimated MW of the last C-terminal amino acids of the proVGF protein was visible in all of the brain areas tested. In previous studies (Brancia et al. 2005; Cocco et al. 2007; D’Amato et al. 2008), the VGF C-terminus antiserum revealed a major peak close to the void volume, compatible with the expected migration of the VGF precursor. Hence, in the human cortex, the absence of the proVGF precursor protein could be due to a high processing resulting in heterogeneous populations of different MW cleaved peptides, mostly of small size, and may be related to different mechanisms. Such heterogeneity of the VGF peptides may be a consequence of proteolytic cleavage by a complex pattern of tissue-specific prohormone convertases.
The N-terminus antiserum instead revealed the presence of the 20-, 60-, and 0.8–1-kDa forms, different from the small N-terminal fragments of VGF found changed in the CSF of patients affected by frontotemporal dementia (Ruetschi et al. 2005), AD (Selle et al. 2005) and schizophrenia (Haung et al. 2006).
As for the TPGH assay, the same MW form of 0.8–1 kDa that we found in the human cortex was previously recognised by the corresponding antiserum in extracts of the mammalian adrenal gland (D’Amato et al. 2008) and stomach mucosa (Brancia C, Cocco C, D'Amato F, Noli B, Sanna F, Possenti R, Argiolas A, Ferri GL, unpublished data). Regarding the second form of ∼4 kDa revealed by the TPGH-VGF419–427 antiserum in the frontal and temporal cortex, a 4.8 kDa peak has been previously found (Pasinetti et al. 2006; Carrette et al. 2003) but only partial sequences of approximately 1500–2200 Da, related to the human VGF373–417 region, has been identified. Hence, the 4-kDa form revealed by our antiserum could at least partly overlap the original peptide(s).
Using the NERP-1 antiserum, a form of ∼30 kDa was revealed in the human pituitary and adrenal (Rindi et al. 2007) and in mammalian endocrine pancreas (Cocco et al. 2007). Recently, mass spectrometric analysis of brain extracts demonstrated the endogenous presence of another NERP-1 form of low MW in the rat hypothalamus, which has been found to regulate vasopressin release (Toshinai & Nakazato, 2009). Hence, different MW forms of NERP-1 peptides could be related to different areas, produced by diverse post-translational processing, and involved in different mechanisms.
The TPGH and NERP-1 peptides were distinctly reduced in the PD parietal cortex, but not in the frontal and temporal cortices. As little is known as to how VGF peptides respond and change in such neuronal disease conditions, our understanding would be useful for future investigations aimed at improving the knowledge of mechanisms involved in PD.
Interestingly, in previous neuroimaging studies, patients with PD showed neuronal overactivity in the parietal cortex associated with performance of sequential finger movements (Samuel et al. 1997; Ukmar et al. 2005). The parietal cortex has reciprocal connections with the premotor cortex and prefrontal cortex (Pandya & Yeterian, 1985) but does not receive inputs from the basal ganglia (Playford et al. 1992). Hence, it has been hypothesised that a neuronal switch able to facilitate the performance of complex finger movements could be present in the parietal cortex of patients with PD (Samuel et al. 1997). As the rat APGH and NERP-1 peptides were found to be induced in endocrine cells under physiological and pathological stimuli (Rindi et al. 2007), they might be involved in local adaptive neuronal mechanisms occurring in the PD parietal cortex; the significance of such modulation is presently unknown.
We found a reduction in all of the VGF peptides studied in the parietal cortex of AD samples, according to the VGF decrease in the CSF mentioned above. By longitudinal magnetic resonance imaging studies in subjects who progressed to AD within 4–5 years, the parietal cortex revealed a high rate of atrophy correlated with changes in clinical severity and decline in cognition (Foundas et al. 1997). Furthermore, in the latter brain area of patients with AD, a significant deficit in proBDNF protein was also found (Fahnestock et al. 2002), whereas an overlap in VGF and trk mRNA expression has been reported in the rat central nervous system (Snyder et al. 1997). Hence, it can be hypothesised that a decrease in VGF may be correlated to loss of neuronal products like BDNF, resulting in the failure of neuronal protective functions. Taken together, our findings suggest an important role of the VGF peptides in cortical circuits, with a significant impact for future investigations regarding PD and AD.
As the pathway of release into the CSF is still not known, further studies are required to address such issues, as well as animal model investigations.
Acknowledgments
This work was partly supported by research grants from MIUR FIRB (RBNE013XSJ_002 and RBNE01JKLF_002 to G.-L.F.) and the Ministry of Health, Italy.
C.B. and B.N. were sponsored by the ARS (Autonomous Region of Sardinia) through a grant financed with the ‘Sardinia PO FSE 2007-1013’ funds and provided according to the L.R. 7/2007 for the ‘Promotion of the Scientific Research and of the Technological Innovation in Sardinia’, whereas F.D.’A. was sponsored by the ARS, ‘Master and Back’ programme.
The authors acknowledge the Los Angeles Tissue Bank for all of the tissues used in this study, Prof. Valeria Sogos for supporting the work, and Valentina Maccioni for technical work.
Authors’ contribution
B.N., C.C., A.L. and F.D.’A. performed enzyme-linked immunosorbent assay and chromatography; C.C. and P.B. supervised the study; C.B. and C.C. performed immunohistochemistry; G.-L.F. raised the VGF antibodies; C.C. wrote the paper; and P.B. revised the paper.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. VGF peptide level ranges (pmol g−1) found using ELISA in the frontal, parietal, and temporal cortex samples of amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and Pick's disease.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alder J, Thakker-Varia S, Bangasser DA, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;34:10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Rich E, Tronel S, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28:9857–9869. doi: 10.1523/JNEUROSCI.3145-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancia C, Nicolussi P, Cappai P, et al. Differential expression and seasonal modulation of VGF peptides in sheep pituitary. J Endocrinol. 2005;186:97–107. doi: 10.1677/joe.1.05992. [DOI] [PubMed] [Google Scholar]
- Brouillette JY, During MJ, Quirion R. Hippocampal gene expression profiling reveals the possible involvement of Homer1 and GABAB receptors in scopolamine-induced amnesia. J Neurochem. 2007;6:1978–1989. doi: 10.1111/j.1471-4159.2007.04666.x. [DOI] [PubMed] [Google Scholar]
- Canu N, Possenti R, Ricco AS, et al. Cloning, structural organization analysis, and chromosomal assignment of the human gene for the neurosecretory protein VGF. Genomics. 1997;45:443–446. doi: 10.1006/geno.1997.4945. [DOI] [PubMed] [Google Scholar]
- Carrette O, Demalte I, Scherl A, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer's disease. Proteomics. 2003;8:1486–1494. doi: 10.1002/pmic.200300470. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Sesta A, Calabrese F, et al. The expression of VGF is reduced in leukocytes of depressed patients and is restored by effective antidepressant treatment. Neuropsychopharmacology. 2010;103:2010–2011. doi: 10.1038/npp.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco C, Melis GV, Ferri GL. Embedding media for cryomicrotome: an applicative reppraisal. Appl Immunohistochem Mol Morphol. 2003;11:274–280. doi: 10.1097/00129039-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Cocco C, Brancia C, Pirisi I, et al. VGF metabolic-related gene: distribution of its derived peptides in mammalian pancreatic islets. J Histochem Cytochem. 2007;55:619–628. doi: 10.1369/jhc.6A7040.2007. [DOI] [PubMed] [Google Scholar]
- Conti F, Aldo R, Peter P, et al. Glutamate-positive neurons in the somatic sensory cortex of rats and monkeys. J Neurosci. 1987;7:1887–1901. doi: 10.1523/JNEUROSCI.07-06-01887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato F, Noli B, Brancia C, et al. Differential distribution of VGF-derived peptides in the adrenal medulla and evidence for their selective modulation. J Endocrinol. 2008;197:359–369. doi: 10.1677/JOE-07-0346. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Garzon D, Holsinger RM, et al. Neurotrophic factors and Alzheimer's disease: are we focusing on the wrong molecule? J Neural Transm Suppl. 2002;62:241–252. doi: 10.1007/978-3-7091-6139-5_22. [DOI] [PubMed] [Google Scholar]
- Ferri GL, Gaudio RM, Cossu M, et al. The “VGF” protein in rat adenohypophysis: sex differences and changes during the estrous cycle and after gonadectomy. Endocrinology. 1995;136:2244–2251. doi: 10.1210/endo.136.5.7720674. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Mahoney SM, et al. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer's disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:81–89. [PubMed] [Google Scholar]
- Haung JT, Leweke FM, Oxley D, et al. Disease biomarkers in cerebrospinal fluid of patients with first-onset. PLoS Med. 2006;3:428. doi: 10.1371/journal.pmed.0030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, et al. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- Levi A, Ferri GL, Watson E, et al. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol. 2004;24:517–533. doi: 10.1023/B:CEMN.0000023627.79947.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-W, Andrews PC, Mershon JL, et al. Peptive V: a VGF-derived neuropeptide purified from bovine posterior pituitary. Endocrinology. 1994;135:2742–2748. doi: 10.1210/endo.135.6.7988466. [DOI] [PubMed] [Google Scholar]
- Mahata M, Hortnagl H, Mahata SK, et al. Messengers RNA levels of chromogranin B, secretogranin II, and VGF in rat brain after AF 64A-induced septohippocampal cholinergic lesions. J Neurochem. 1993;61:1648–1656. doi: 10.1111/j.1471-4159.1993.tb09799.x. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connection of cortical association areas. Cereb Cortex. 1985;4:3–61. [Google Scholar]
- Pasinetti GM, Ungar LH, Lange DJ, et al. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, et al. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Possenti R, Eldridge JD, Paterson BM, et al. A protein induced by NGF in PC 12 cells is stored in secretory vesicles and release through the regulated pathway. EMBO J. 1989;8:2217–2223. doi: 10.1002/j.1460-2075.1989.tb08345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possenti R, Rinaldi AM, Ferri GL, et al. Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology. 1999;140:3727–3735. doi: 10.1210/endo.140.8.6920. [DOI] [PubMed] [Google Scholar]
- Rindi G, Licini L, Necchi V, et al. Peptide products of the neurotrophin-inducible gene vgf are produced in human neuroendocrine cells from early development and increase in hyperplasia and neoplasia. J Clin Endocrinol Metab. 2007;92:2811–2815. doi: 10.1210/jc.2007-0035. [DOI] [PubMed] [Google Scholar]
- Ruetschi U, Zetterberga H, Podustd VN, et al. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196:273–281. doi: 10.1016/j.expneurol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Salton SRJ, Ferri GL, Hahm S, et al. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21:199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Selle H, Lamerz J, Buerger K, et al. Identification of novel biomarker candidates by differential peptidomics analysis of cerebrospinal fluid in Alzheimer's disease. Comb Chem High Throughput Screen. 2005;8:801–806. doi: 10.2174/138620705774962391. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Li J, Salton SR. Comparison of VGF and trk mRNA distributions in the developing and adult rat nervous systems. Brain Res Mol Brain Res. 1997;49:307–311. doi: 10.1016/s0169-328x(97)00216-7. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Cheng HV, Murray KD, et al. The messenger RNA encoding VGF, a neuronal peptide precursor, is rapidly regulated in the rat central nervous system by neuronal activity seizure and lesion. Neuroscience. 1998;828:7–19. doi: 10.1016/s0306-4522(97)00280-7. [DOI] [PubMed] [Google Scholar]
- Thakker VS, Alder J. Neuropeptides in depression: role of VGF. Behav Brain Res. 2009;197:262–278. doi: 10.1016/j.bbr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Nakazato M. Neuroendocrine regulatory peptide-1 and -2: novel bioactive peptides processed from VGF. Cell Mol Life Sci. 2009;66:1939–1945. doi: 10.1007/s00018-009-8796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trani E, Ciotti T, Rinaldi AM, et al. Tissue-specific processing of the neuroendocrine protein VGF. J Neurochem. 1995;65:2441–2449. doi: 10.1046/j.1471-4159.1995.65062441.x. [DOI] [PubMed] [Google Scholar]
- Trani E, Giorni A, Canu N, et al. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem. 2002;81:565–574. doi: 10.1046/j.1471-4159.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Furlan C, Moretti R, et al. Functional MRI in the assessment of cortical activation in subjects with Parkinson's disease. Neuroradiology. 2005;111:104–105. doi: 10.1007/s11547-006-0011-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Sasaki K, Satomi Y, et al. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J Biol Chem. 2007;282:26354–26360. doi: 10.1074/jbc.M701665200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


