Abstract
Purpose
To compare surgical results between conventional intraocular lens (IOL) implantation using an ophthalmic viscosurgical device (OVD) and IOL implantation using a balanced salt solution (BSS) after irrigation/aspiration (I/A) of the lens cortex.
Methods
A randomized prospective study was conducted on 62 patients who underwent cataract surgery. Following completion of conventional I/A of the lens cortex, we divided patients into two groups according to whether or not BSS was used. In group A (n = 31), the anterior chamber and the capsular bag were completely filled with an OVD before IOL implantation. On the other hand, in group B (n = 31), BSS was irrigated into the anterior chamber through a previous side port during IOL implantation. Surgical results were compared between the two groups.
Results
In both groups, IOP peaked six hours after surgery. The occurrence of an IOP spike by postoperative day one was observed in six cases (6 / 31) in group A and in no cases (0 / 31) in group B, a difference that was statistically significant (p = 0.024). The values of endothelial cell density, central corneal thickness, anterior chamber inflammation, myopic shift, and posterior capsule opacification were not significantly different between the two groups.
Conclusions
Compared with the use of OVD for IOL implantation, use of BSS during IOL implantation resulted in reductions in postoperative IOP spike and OVD removal time.
Keywords: Balanced salt solution, Endothelial cell density, Intraocular pressure spike, Ophthalmic viscosurgical device
Phacoemulsification (phaco) with the use of an ophthalmic viscosurgical device (OVD) is the preferred technique for use in modern cataract surgery. Surgical benefits of OVD include maintenance of the anterior chamber, protection of the corneal endothelium, and facilitation of intraocular lens implantation [1-3]. However, the OVD may cause an increase in intraocular pressure (IOP) and inflammation in the immediate postoperative period, which may result in further endothelial cell loss [4-8]. Therefore, to prevent these complications, the OVD should be removed via thorough aspiration after intraocular lens (IOL) implantation; however, an OVD located in the ciliary sulcus or behind the IOL may not be able to be easily removed. To solve these problems, following irrigation/aspiration (I/A) of the lens cortex, we implanted the IOL using balanced salt solution (BSS) instead of filling the anterior chamber and the capsular bag with an OVD. The aim of this study was to evaluate and compare clinical results between the OVD group (group A) and the BSS group (group B) during the postoperative period.
Materials and Methods
A randomized prospective study was conducted in Eulji General Hospital between March 2009 and September 2009 on 62 eyes of 62 patients with senile cataracts. Informed consent was obtained from all patients, and the study was approved by the Institutional Review Board of Eulji Medical Center. All patients underwent a detailed preoperative ophthalmic examination. Inclusion criteria were between 40 and 80 years and senile cataract greater than NO2 or NC2 according to the Lens Opacities Classification System III classification. Exclusion criteria included cases with a small pupil, extremely shallow chamber, compromised endothelial cell function, corneal disorder, complicated cataract, glaucoma, pseudoexfoliation, severe myopia, and a previous history of laser-assisted in situ keratomileusis. Other intraoperative exclusion criteria were a total surgical time of more than 30 minutes and a total phaco time of more than 90 seconds. Patients who had a posterior capsule rupture or those who were converted to an extracapsular cataract extraction during the procedure were excluded from subsequent analysis. Preoperative evaluation of patients included age, gender, and presence of any systemic diseases, such as hypertension or diabetes mellitus, best corrected visual acuity (BCVA), and IOP estimated using a Goldmann's applanation tonometer. Keratometry was performed using a Canon RK-5 autorefractor keratometer (Canon, Tochigiken, Japan), while the axial length and the anterior chamber depth were measured using a Hi-Scan standard A-scan machine (Optikon 2000, Rome, Italy). The power of the IOL was calculated in all of the patients using the SRK-II formula. Selected patients were divided into two groups: group A (OVD-used group) and group B (BSS-used group). The BCVAs were converted to the logarithms of the minimum angle of resolution (logMAR).
Surgery was performed using a Stellaris (Bausch & Lomb, Rochester, NY, USA) phaco machine by a single surgeon. Under topical anesthesia using 4% lidocaine, a side port was created at 11 o'clock limbus for the right eye (5 o'clock in the left eye) using a no. 7513 blade. A clear corneal incision in the temporal limbus was made with a 2.8 mm microsurgical knife (Kai Industries Co., Seki, Japan). The anterior chamber was filled with OVD (Amvisc Plus), and then a continuous curvilinear capsulorrhexis measuring approximately 5.0 mm to 5.5 mm in diameter was created using a 30-gauge needle with a curved tip and a capsular forceps. Hydrodissection and hydrodelineation were performed to achieve free rotation of the nucleus.
Following in-the-bag phaco of the nucleus using the divide and conquer technique, the cortex was removed using I/A. In group A, the anterior chamber and the capsular bag were completely filled with Amvisc Plus, and the IOL was then implanted into the capsular bag. In group B, the anterior chamber and the capsular bag were not filled with OVD; however, BSS was used for maintenance of the anterior chamber shape. The surgeon evaluated the patency of a 27-gauge Amvisc Plus needle connected to a three-way irrigation line with a 140 cm irrigation bottle height. After a small amount of OVD (about 0.05 mL) was placed on an IOL injector cartridge, the surgeon advanced the tip of the IOL injector into the anterior chamber through a temporal wound using his right hand. After a slight deepening of the anterior chamber by slight pressure from the injector, the surgeon inserted the 27-gauge Amvisc Plus needle into the anterior chamber through a side port, using the left hand for continuous irrigation with BSS, while maintaining irrigation using the phaco machine foot switch (Fig. 1A and 1B). The IOL, a one-piece, foldable, hydrophilic acrylic, aspheric Teklens II lens (Tekia Inc., Irvine, CA, USA), was then fully inserted into the capsular bag. Following IOL implantation in both groups, the IOL was centered using the IOL rotator, and residual Amvisc Plus was removed as thoroughly as possible. In all cases, the corneal wound was hydrated at the conclusion of the surgery. Subconjunctival injection consisting of 8 mg/0.2 mL triamcinolone acetonide was administered.
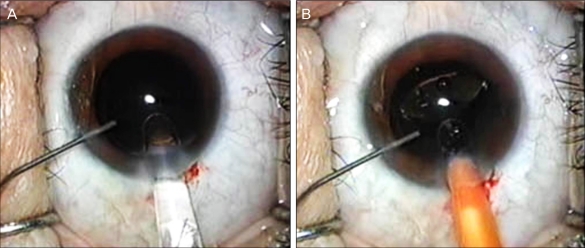
Fig. 1.
(A) Following irrigation/aspiration of the cortex, the anterior chamber is irrigated with balanced salt solution (BSS) through a side port using a 27-gauge Amvisc Plus needle before intraocular lens implantation. (B) Insertion of the intraocular lens into the capsular bag while the anterior chamber is maintained with BSS.
The IOP, endothelial cell density (ECD), central corneal thickness (CCT), anterior chamber inflammation, myopic shift, posterior capsule opacification (PCO), OVD removal time, and facilitation of IOL implantation were measured and compared between the two groups. Postoperative IOP was measured using a Goldmann's applanation tonometer at six hours, one day, one month, three months, and six months post operation. An IOP spike was defined as an increase greater than 30 mmHg by postoperative day one. Specular microscopy using the Topcon SP-2000P (Topcon Co., Tokyo, Japan) was performed preoperatively and three months after surgery. CCT was measured via ultrasound pachymetry using a Pocket pachymeter (Quantel Medical Inc., Clermont-Ferrand, France) preoperatively, and one day, one week, and three months after surgery. For evaluation of postoperative inflammation, the numbers of anterior chamber cells were graded with a 2 mm long and 1 mm wide slit beam with maximal light intensity and magnification. The findings were recorded as grade 0 if there was less than one cell in the field; grade 1+ if there were between 2 and 15 cells; grade 2+ if there were between 16 and 25 cells; grade 3+ if there were between 26 and 50 cells; and grade 4+ if there were more than 50 cells. Refractive myopic shift was defined as a spherical equivalent (SE) difference greater than -1.5 diopter compared to aiming SE at postoperative three months, as determined with a Canon RK-5 autorefractor keratometer. PCO was assessed with the pupils dilated by one author who was blinded to the surgical method used. Slitlamp photography of the IOL and posterior capsule were performed using retroillumination. PCO was defined as lens epithelial cell migration onto the visual axis with a BCVA reduction of more than two lines on the Snellen chart. Also, the time needed for complete removal of the OVD from the chamber using the I/A handpiece was measured. For removal of the OVD, the flow rate was fixed to 40 mL/min and the vacuum was set to 400 mmHg. The surgeon subjectively assessed facilitation of IOL implantation using a three point scale of 1 = poor; 2 = average; 3 = good. All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Randomization was performed preoperatively using a statistical random table.
Results
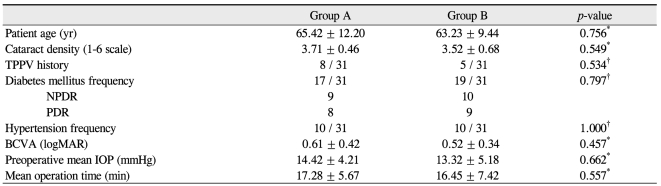
Table 1 shows the baseline information of the patients who participated in this study. The mean age was 65.42 ± 12.20 years in group A and 63.23 ± 9.44 years in group B, and the corresponding mean cataract densities were 3.71 ± 0.46 and 3.52 ± 0.68. Preoperative mean IOP was 14.42 ± 4.21 mmHg in group A and 13.32 ± 5.18 in group B, and the preoperative mean BCVA (logMAR) was 0.61 ± 0.42 in group A and 0.52 ± 0.34 in group B. No statistically significant differences in age, cataract density, preoperative IOP, or preoperative BCVA were observed between the two groups before surgery.
Table 1.
Clinical characteristics of the study subjects
TPPV = trans pars plana vitrectomy; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; BCVA = best corrected visual acuity; logMAR = logarithms of the minimum angle of resolution; IOP = intraocular pressure.
*Mann-Whitney U-test; †Fisher's exact test.
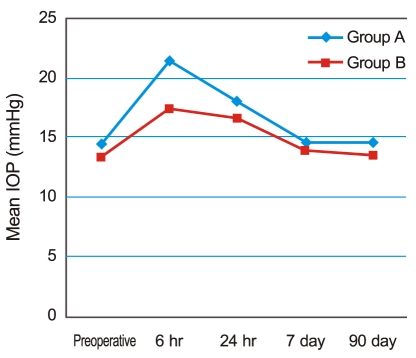
Fig. 2 shows the change in IOP over time. In both groups, IOP peaked six hours after surgery, with a mean IOP of 21.43 ± 6.41 mmHg in group A and 17.41 ± 5.12 mmHg in group B. The difference was significant (p = 0.034, according to the Mann-Whitney U-test). The IOP then gradually decreased to preoperative levels by seven days postoperative. Six IOP spikes were observed in group A (6 / 31) one day after surgery, although no cases (0 / 31) were observed in group B, another significant difference (p = 0.024) (Table 2).
Fig. 2.
The change in mean intraocular pressure (IOP) over time. In both groups, IOP peaked six hours after surgery, with a mean of 21.43 ± 6.41 mmHg in group A and 17.41 ± 5.12 mmHg in group B. The difference between the two groups was significant (p = 0.034). IOP then showed a gradual decrease to preoperative levels by seven days postoperative.
Table 2.
Frequency of intraocular pressure spikes by postoperative day one
*Calculated using Fisher's exact test.
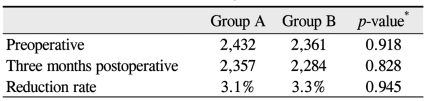
Table 3 shows ECD preoperative and three months postoperative. Reduction rates of ECD three months after surgery were 3.1% in group A and 3.3% in group B; this difference was not significant (p = 0.945). In group A, the mean postoperative BCVAs (logMAR) were 0.35 ± 0.42, 0.27 ± 0.31, and 0.25 ± 0.35 at one day, one month, and three months, respectively. In group B, the respective mean postoperative BCVAs (logMAR) were 0.33 ± 0.37, 0.24 ± 0.31, and 0.26 ± 0.36. None of these differences were significant (p = 0.643, 0.511, and 0.836, respectively, according to the Mann-Whitney U-test).
Table 3.
Endothelial cell density (cells/mm2)
*Calculated using the Mann-Whitney U-test.
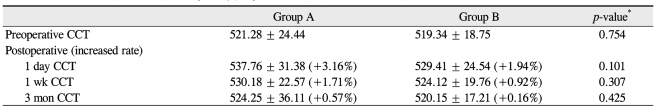
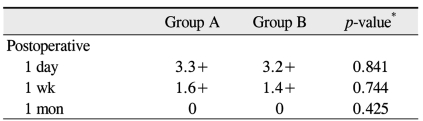
Postoperative CCT changes in group A were +3.16%, +1.71%, and +0.57% at day one, one week, and three months, respectively. In group B, the postoperative CCT changes were +1.94%, +0.92%, and +0.16%, respectively. None of these CCT changes were significantly different (p = 0.101, 0.307, and 0.425) (Table 4). At one day, one week, and one month postoperative, the mean numbers anterior chamber cells in group A were 3.3+, 1.6+, and 0 and were 3.2+, 1.4+, and 0 in group B, respectively. None of these anterior chamber cell numbers were significantly different (p = 0.841, 0.744, and 0.425) (Table 5).
Table 4.
Central corneal thickness (CCT, µm) in both groups
*Calculated using the Mann-Whitney U-test.
Table 5.
Anterior chamber cells in both groups at the postoperative periods
*Calculated using the Mann-Whitney U-test.
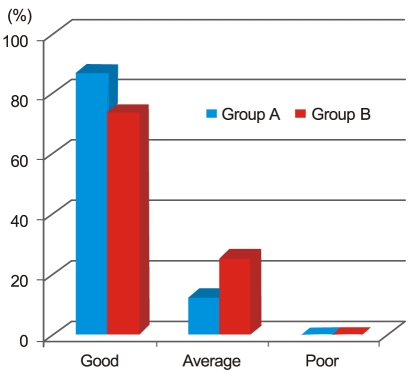
Three months post operation, there were four refractive myopic shifts (4 / 31) of more than -1.5 diopter in group A, while no refractive myopic shift (0 / 31) occurred in group B (p = 0.113). Six months postoperative, PCO occurred in three cases (3 / 31) in group A and in no case (0 / 31) in group B (p = 0.238) (Table 6). Fig. 3 shows the assessment of facilitation of IOL implantation. In group A, 'good', 'average', and 'poor' ratings were assigned to 87% (27 / 31), 13% (4 / 31), and 0% (0 / 31) of the eyes, respectively. In group B, these proportions were 74% (23 / 31), 26% (8 / 31), and 0% (0 / 31), respectively (p = 0.202). We measured the removal times for Amvisc Plus in group A and group B and calculated a mean OVD removal time of 50.42 ± 3.83 seconds in group A and that of 8.29 ± 4.40 seconds in group B (Fig. 4). Significantly less time was required for complete removal of Amvisc Plus in group B compared to that in group A (p ≤ 0.001).
Table 6.
Frequencies of myopic shift and posterior capsule opacification
*Calculated using Fisher's exact test.
Fig. 3.
Assessment of the facilitation of intraocular lens implantation. In group A, the ratings 'good', 'average', and 'poor' were assigned in 87% (27 / 31), 13% (4 / 31), and 0% (0 / 31) of cases, respectively, while those in group B were 74% (23 / 31), 26% (8 / 31), and 0% (0 / 31) (p = 0.202 according to the Mann-Whitney U-test).
Fig. 4.
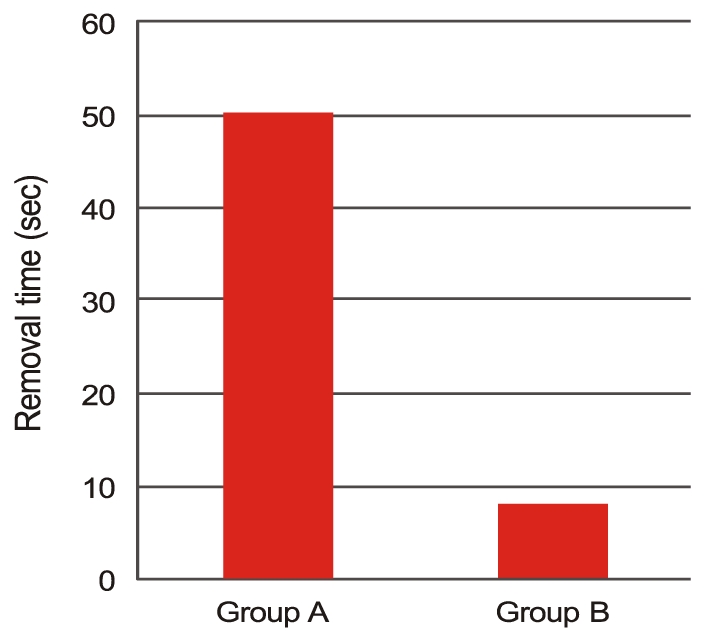
Mean ophthalmic viscosurgical device removal time was 50.42 ± 3.83 seconds in group A and 8.29 ± 4.40 seconds in group B, illustrating that the time to complete removal of Amvisc Plus was significantly less in group B (p ≤ 0.001 according to the Mann-Whitney U-test).
Discussion
Viscoelastic materials can be characterized according to their chemical composition (e.g., sodium hyaluronate, chondroitin sulfate, hydroxypropyl methylcellulose), physical properties (e.g., molecular weight, viscosity, cohesive versus dispersive), or both. One of the various OVDs, Amvisc Plus, which was used in this study, is a highly viscous, 132,000 cP, cohesive viscoelastic agent containing 1.6% high molecular weight sodium hyaluronate. Due to lubrication and transparency, this OVD facilitates IOL implantation through the maintenance of anterior chamber depth and visibility, minimizing interactions between tissues. However, because the postoperative IOP may increase due to residual Amvisc Plus, complete removal from the anterior chamber and the capsular bag should be assured at the conclusion of surgery to prevent or minimize post-operative IOP increase.
The viscoelastic substance remaining in the eye may cause mechanical obstruction of the trabecular meshwork and is a major reason for early postoperative IOP increase [9]. In 1983, Berson et al. [9] reported that sodium hyaluronate caused a substantial decrease (55% to 60%) in the outflow of aqueous humor when injected into the anterior chamber. Subsequently, it has become well accepted that retained viscoelastic materials inhibit aqueous outflow and result in increased IOP. Arshinoff [10] has published multiple studies comparing different viscoelastic materials and concluded that, if not completely removed, all types of OVD will cause postoperative increases in IOP. Koçak-Altintas et al. [11] also reported a higher incidence of postoperative IOP increase associated with use of higher viscosity OVD.
Thorough removal of viscoelastic substances is vital for avoidance of a postoperative IOP increase. However, complete removal of the OVD behind the IOL is known to be difficult. Several surgical techniques for removal of viscoelastic substances, particularly from behind the IOL, have been described [12-14]; however, complete avoidance of a postoperative IOP increase has not been achieved with any technique.
Nayak and Jain [15] reported that continuous anterior chamber infusion using an anterior chamber maintainer and omission of OVD during phaco and IOL implantation did not cause a significant difference in corneal swelling or endothelial cell loss in the immediate postoperative period up to one month.
In our study, we used OVD for facilitation of continuous curvilinear capsulorrhexis and protection of the endothelium. Following removal of the lens nucleus via phaco and I/A of the cortex, we implanted the IOL using only BSS through a previous side port. As a result, we expected to reduce the risk for high postoperative IOP due to residual OVD. A lower mean IOP six hours after surgery, as well as the lower frequency of IOP spike by postoperative day one, indicates that our method did reduce the risk for elevated postoperative IOP.
Our results suggest that the proposed method will be more useful in vitrectomized eyes with cataracts, a situation known to involve higher risks and complications compared to those in nonvitrectomized eyes. Challenges to removal of OVD behind the IOL in vitrectomized eyes include greater fluctuation of the anterior chamber, intraoperative miosis, an excessively mobile posterior capsule [16] due to weakened zonules, and loss of vitreous support [17]. In group A, four of eight eyes (50.0%) that had undergone previous vitrectomy experienced an IOP spike by postoperative day one, while IOP spike occurred in two of 23 eyes (8.7%) that had not undergone vitrectomy (p = 0.026) (Table 7).
Table 7.
Comparison of intraocular pressure spike frequencies by postoperative day one between 'vitrectomized eyes' and 'not vitrectomized eyes'
*Calculated using Fisher's exact test.
Complete IOL fixation on the posterior capsule is another advantage of our method. Enhancement of the IOL optic barrier effect is one of the goals of in-the-bag fixation. If accurately implanted into the capsular bag, the lens forms a barrier to central migration of lens epithelial cells by near complete contact of the IOL optic to the posterior capsule so that cells rarely reach the center of the posterior capsule [18]. However, the IOL can be positioned more anterior to the aiming location if there is residual OVD behind the IOL. This creates potential space between the posterior surface of the IOL and the posterior capsule and induces greater PCO. Here, we identified four patients with a myopic shift of greater than -1.5 diopter, although the myopic shift was not significant (p = 0.113). Within six months post operation, PCO had occurred in three of these patients (3 / 4).
Because the proposed methodology only utilizes approximately 0.05 mL of OVD for IOL loading in the injector cartridge, which is inserted into only the anterior chamber, the residual OVD was easily and completely removed using I/A. Thus, compared to conventional cataract surgery, not only was there significantly less time required for the overall procedure but removal of OVD in the anterior chamber was simplified.
There are several drawbacks to our methodology. First, when the IOL is inserted into the anterior chamber, the surgeon must hold the IOL injector with his right hand, while simultaneously irrigating the needle with his left hand. Improper performance of the procedure may lead to complications, such as sulcus implantation or corneal wrinkling. Although we initially experienced inappropriate insertion in two cases (2 / 31), it was corrected immediately using an IOL rotator. After correction, we did not notice a difference with regard to facilitation of IOL implantation between the two groups. Second, because the 27-gauge Amvisc Plus needle has a small diameter (about 0.3 mm), its flow rate (about 1.5 mL/min) is not sufficient to produce a full anterior chamber depth, even though the maximal bottle height was 140 cm. However, in spite of this condition, we did not experience posterior capsule rupture or zonular dialysis, and IOL implantation into the capsular bag was successful in all cases. Third, although we only used high viscosity cohesive Amvisc Plus in this study, other OVDs may yield different results. Further and larger studies using the same method with other OVDs are required. Lastly, we could not guarantee that the OVD was completely removed from the anterior chamber because of its lack of visibility.
The safety of cataract surgery has improved markedly with advancements in surgical techniques, equipment, and OVDs. However, OVD-related complications, such as IOP spikes and inflammation, may occur. Following I/A, our method uses irrigating solution and a 27-gauge Amvisc Plus needle instead of additional OVD. In conclusion, our technique can reduce the risk for postoperative high IOP due to residual OVD, especially in vitrectomized eyes, as well as the unwanted surgical costs incurred by additional OVD. Thus, BSS-based IOL implantation may be an alternative new method to replace OVD-based IOL implantation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tognetto D, Cecchini P, Ravalico G. Survey of ophthalmic viscosurgical devices. Curr Opin Ophthalmol. 2004;15:29–32. doi: 10.1097/00055735-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Lee WM, Choi SH, et al. Clinical evaluation of domestic hyal 2000(R) as a viscoelastic substance in phacoemulsification with posterior chamver intraocular lens. J Korean Ophthalmol Soc. 1998;39:2064–2073. [Google Scholar]
- 3.Park YK, Kim KS. The changes of corneal endothelial morphology after phacoemulsification by using healon gv of viscoat. J Korean Ophthalmol Soc. 1998;39:1729–1734. [Google Scholar]
- 4.Oxford Cataract Treatment and Evaluation Team (OCTET) Long-term corneal endothelial cell loss after cataract surgery. Results of a randomized controlled trial. Arch Ophthalmol. 1986;104:1170–1175. [PubMed] [Google Scholar]
- 5.Ruiz RS, Wilson CA, Musgrove KH, Prager TC. Management of increased intraocular pressure after cataract extraction. Am J Ophthalmol. 1987;103:487–491. doi: 10.1016/s0002-9394(14)74269-2. [DOI] [PubMed] [Google Scholar]
- 6.Bömer TG, Lagrèze WD, Funk J. Intraocular pressure rise after phacoemulsification with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience. Br J Ophthalmol. 1995;79:809–813. doi: 10.1136/bjo.79.9.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann R, Brint S, Stewart R, et al. Clinical comparison of Provisc and Healon in cataract surgery. J Cataract Refract Surg. 1995;21:543–547. doi: 10.1016/s0886-3350(13)80214-3. [DOI] [PubMed] [Google Scholar]
- 8.Arshinoff SA, Hofman I. Prospective, randomized trial comparing Micro Visc Plus and Healon GV in routine phacoemulsification. J Cataract Refract Surg. 1998;24:814–820. doi: 10.1016/s0886-3350(98)80137-5. [DOI] [PubMed] [Google Scholar]
- 9.Berson FG, Patterson MM, Epstein DL. Obstruction of aqueous outflow by sodium hyaluronate in enucleated human eyes. Am J Ophthalmol. 1983;95:668–672. doi: 10.1016/0002-9394(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 10.Arshinoff S. Postoperative intraocular pressure spikes. J Cataract Refract Surg. 2004;30:733–734. doi: 10.1016/j.jcrs.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 11.Koçak-Altintas AG, Anayol MA, Cakmak HB, Simsek S. Effects of topical dorzolamide on IOP after phacoemulsification with different types of ophthalmic viscosurgical devices. Eur J Ophthalmol. 2007;17:38–44. doi: 10.1177/112067210701700106. [DOI] [PubMed] [Google Scholar]
- 12.Wedrich A, Menapace R. Intraocular pressure following small-incision cataract surgery and polyHEMA posterior chamber lens implantation. A comparison between acetylcholine and carbachol. J Cataract Refract Surg. 1992;18:500–505. doi: 10.1016/s0886-3350(13)80106-x. [DOI] [PubMed] [Google Scholar]
- 13.Kohnen T, von Ehr M, Schütte E, Koch DD. Evaluation of intraocular pressure with Healon and Healon GV in sutureless cataract surgery with foldable lens implantation. J Cataract Refract Surg. 1996;22:227–237. doi: 10.1016/s0886-3350(96)80224-0. [DOI] [PubMed] [Google Scholar]
- 14.Jacobi PC, Engels B, Dietlein TS, Krieglstein GK. Effect of trabecular aspiration on early intraocular pressure rise after cataract surgery. J Cataract Refract Surg. 1997;23:923–929. doi: 10.1016/s0886-3350(97)80254-4. [DOI] [PubMed] [Google Scholar]
- 15.Nayak BK, Jain EK. Comparison of corneal endothelial cell loss during phacoemulsification using continuous anterior chamber infusion versus those using ophthalmic viscosurgical device: randomized controlled trial. Indian J Ophthalmol. 2009;57:99–103. doi: 10.4103/0301-4738.45500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinter SM, Sugar A. Phacoemulsification in eyes with past pars plana vitrectomy: case-control study. J Cataract Refract Surg. 1999;25:556–561. doi: 10.1016/s0886-3350(99)80055-8. [DOI] [PubMed] [Google Scholar]
- 17.Smiddy WE, Stark WJ, Michels RG, et al. Cataract extraction after vitrectomy. Ophthalmology. 1987;94:483–487. doi: 10.1016/s0161-6420(87)33420-7. [DOI] [PubMed] [Google Scholar]
- 18.Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]