Abstract
Purpose
To investigate the effect of yellow tinted intraocular lenses (IOLs), implanted in the bag after phacoemulsification, on the result of frequency doubling technique (FDT) perimetry.
Methods
For 68 eyes of 52 patients, an IOL was implanted in the bag after phacoemulsification. The patients were categorized into three groups according to the type of implanted IOLs used. IOLs were selected randomly among two types of yellow IOLs (Acrysof SN60WF IOL, Hoya YA60BBR IOL) and a clear IOL was used as a control. A FDT Humphrey matrix was performed 2 months after the surgery. The mean deviation (MD) and pattern standard deviation (PSD) among these three groups was analyzed using Mann-Whitney U-test.
Results
Two months after the procedure, there was no significant difference between each of the three groups: the clear IOL and Hoya YA60BBR IOL (MD, p = 0.21; PSD, p = 0.27), the clear IOL and Alcon SN60WF IOL (MD, p = 0.11; PSD, p = 0.22), and the Hoya YA60BBR IOL and Alcon SN60WF IOL (MD, p = 0.33; PSD, p = 0.56).
Conclusions
When interpreting the result of the FDT after cataract surgery, the color and type of IOLs used should not be considered.
Keywords: Frequency doubling perimetry, Yellow tinted intraocular lenses
The cornea absorbs light with a wavelength below 295 nm and the crystalline lens absorbs light with a wavelength below 400 nm. Therefore, the human retina is protected from short-wavelength light. After cataract surgery with the implantation of a clear intraocular lens (IOL), the retina is now exposed to short-wavelength light. Consequently, yellow intraocular lenses have emerged as a blue blocker. From recent studies, it has been revealed that blue-light filtering IOLs may have protective effects against exudative age related macular degeneration [1].
Frequency doubling technique (FDT) perimetry is a well-known procedure used for glaucoma screening. Threshold tests potentially may identify glaucomatous defects sooner than other methods. The FDT stimulus predominantly stimulates the Magnocellular pathway, which is primarily involved in motion and flicker detection and represents about 10% of all retinal ganglion cells. The FDT perimeter (Welch Allyn, Skaneateles Falls, NY; Humphrey Instruments, San Leandro, CA, USA) uses a vertical sine wave grating of low spatial frequency (0.25-0.50 cycle/degree) that undergoes counterphase flickering at a high-temporal frequency (12-25 Hz). The contrast of the stimulus is modified in each location to calculate threshold sensitivity [2]. Some reports demonstrated the effect of cataracts on FDT perimetry, suggesting a significant improvement in mean deviation (MD) without a significant change in pattern standard deviation (PSD) after cataract surgery with clear IOLs [3,4]. In this study, we compared the effect of IOLs between each of the three groups (the clear IOL, Alcon Acrysof IQ SN60WF [Alcon, Fort Worth, TX, USA], and Hoya YA60BBR [Hoya Co., Tokyo, Japan]) with the result of FDT perimetry.
Materials and Methods
We prospectively included 68 eyes of 52 patients who underwent cataract surgery and intraocular lens implantation with otherwise normal eye findings. Informed consent was obtained from each patient before enrollment in the study. All the procedures conformed to the tenets of the Declaration of Helsinki.
Fifty-two patients had cataract surgery under similar preoperative conditions using the same phacoemulsification technique with the Infinity machine and Ozil system (Alcon). All the surgeries were performed without complications by the same surgeon (CKJ). The operated eye was randomized to receive an AcrySof IQ IOL (Alcon), a Hoya YA60BBR IOL, or a conventional clear IOL (OII Biovue3; BioVue, Ontario, CA, USA) in the capsular bag. Postoperatively, all patients were given gatifloxacin 0.3% (Gatiflo, Handok pharmaceuticals, Seoul, Korea) and prednisolone acetate 1% (Pred Forte; Allergan, Irvine, CA, USA), 4 times a day for 30 days. After 30 days, the application of the two eyedrops was decreased to by twice a day for 30 days.
Patients with diabetes mellitus, poor cooperation, glaucoma (diagnosed by intraocular pressure, visual field exam, optic nerve morphology, and retinal nerve fiber layer findings), complicated cataracts and any negative events resulting from cataract surgery were excluded from the study.
Routine postoperative ocular evaluations (visual acuity, refraction, IOP measurements, etc.) were carried out at each visit to avoid the bias of low visual acuity on perimetry. FDT perimetry was performed with a FDT Humphrey matrix (Carl Zeiss Meditec, Dublin, CA, USA). The FDT 24-2 threshold test was performed following the same procedure for eyes with clear or yellow IOL implantations with the refractive error fully corrected, two months after the operation. The global indices obtained by FDT were MD and PSD. The data was analyzed with the nonparametric Mann-Whitney U-test or Kruksal-Wallis test using a 5% significance level by SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
Results
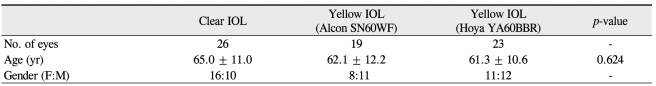
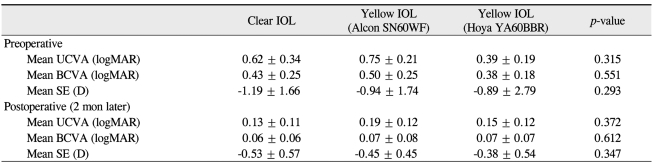
A total of 52 people were enrolled in the study (28 men and 24 women). The mean age was 63.2 ± 11.5 years old (range, 52 to 78 years). Demographics are shown in Table 1. There were no significant differences among three IOL groups with respect to visual acuity and spherical equivalence in the preoperative and postoperative phase (Table 2).
Table 1.
Demographic characteristics of participants
Each lens was analyzed by the Kruksal-Wallis test (p < 0.05).
IOL = intraocular lens.
Table 2.
Mean logarithm of the minimum angle of resolution (logMAR) visual acuity and spherical equivalent data examined in preoperative and postoperative phases.
Values are presented as mean ± SD.
Each lens was analyzed by the Kruksal-Wallis test (p < 0.05).
UCVA = uncorrected visual acuity; BCVA = best corrected visual acuity, SE = spherical equivalent.
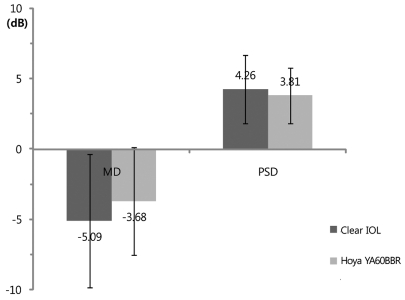
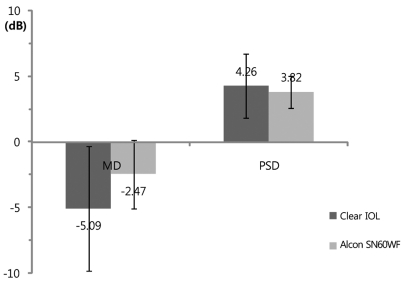
The results of FDT perimetry testing are shown in Figs. 1, 2, and 3. There were no statistically significant differences for MD and PSD between the clear IOL and Hoya YA60BBR IOL groups (MD: clear IOL, -5.09 ± 4.72 Db; Hoya YA60BBR IOL, -3.68 ± 3.83 dB; p = 0.21 / PSD: clear IOL, 4.26 ± 2.45 dB; Hoya YA60BBR IOL, 3.81 ± 1.99; p = 0.27, Mann-Whitney U-test). There were no statistically significant differences for MD and PSD the clear IOL and Alcon SN60WF IOL groups (MD: clear IOL, -5.09 ± 4.72 dB; Alcon SN60WF IOL, -2.47 ± 2.61 dB; p = 0.11 / PSD: clear IOL, 4.26 ± 2.45 dB; Alcon SN60WF IOL, 3.28 ± 1.23 dB; p = 0.22, Mann-Whitney U-test). No statistically significant differences were observed between the two types of yellow IOL groups (MD: Hoya YA60BBR, -3.68 ± 3.83 dB; Alcon SN60WF, -2.47 ± 2.61 dB; p = 0.33 / PSD: Hoya YA60BBR, 3.81 ± 1.99 dB; Alcon SN60WF, 3.28 ± 1.23; p = 0.56, Mann-Whitney U-test).
Fig. 1.
Postoperative mean deviation (MD) and pattern standard deviation (PSD) of the frequency doubling perimetry result. There was no significant difference in MD between the clear and Hoya YA60BBR intraocular lens (IOL) (Mann-Whitney U-test, p = 0.21). There was no significant difference in PSD between clear and Hoya YA60BBR IOLs (Mann-Whitney U-test, p = 0.27).
Fig. 2.
Postoperative mean deviation (MD) and pattern standard deviation (PSD) of the frequency doubling perimetry result. There was no significant difference in MD between the clear and Alcon SN60WF intraocular lenses (IOLs) (Mann-Whitney U-test, p = 0.11). There was no significant difference in PSD between the clear and Alcon SN60WF IOLs (Mann-Whitney U-test, p = 0.22).
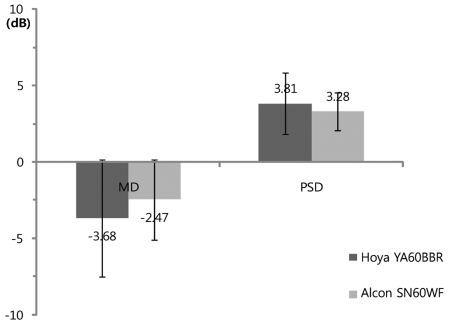
Fig. 3.
Postoperative mean deviation (MD) and pattern standard deviation (PSD) of the frequency doubling perimetry result. There was no significant difference in MD between the Hoya YA60BBR and Alcon SN60WF intraocular lenses (IOLs) (Mann-Whitney U-test, p = 0.33). There was no significant difference in PSD between the Hoya YA60BBR and Alcon SN60WF IOLs (Mann-Whitney U-test, p = 0.56).
Discussion
FDT perimetry is barely affected by refractive errors. Therefore, it is more useful for glaucoma screening than the standard automated perimetry. In this study, FDT perimetry was used for comparing the effect of the colors of IOLs. No significant difference of postoperative visual acuity and spherical equivalence between each of three groups place greater accuracy on the results from FDT perimetry in this study.
Another report by Ueda et al. [5] showed that there was no significant difference between clear and yellow IOLs for either MD or PSD. They compared the Hoya VA60BB (clear IOL) with Hoya YA60BB (yellow IOL). The results from FDT perimetry were also not influenced by the color of the IOL. In this study, the results from FDT perimerty of the conventional clear IOL and yellow tinted IOL were not significantly different.
The two yellow tinted IOLs used in this study were Hoya YA60BBR and Alcon SN60WF. They are similar in many aspects. Both of the IOLs have hydrophobic acrylic optics with similar size (optic diameter 6 mm) and similar refractive index (YA60BBR, 1.51; SN60WF, 1.55). These blue-blockers absorb short wavelength light for retinal protection, but also absorb different ranges of wavelength. Hoya YA60BBR mainly absorbs lights in the range of 400-450 nm, while Alcon SN60WF absorbs light in the 400-500 nm wavelength range [6,7]. However, in this study, the different spectral transmittance between the two types of yellow tinted IOLs did not affect the results of FDT perimetry. Therefore, FDT perimetry results after cataract surgery can be interpretated without giving regard to the type of yellow tinted IOL used.
FDT perimetry predominantly stimulates the magnocellular pathway by using frequency doubling illusions. Rod cells may transit signals to the lateral geniculate body through the magnocellular pathway of ganglion cells. This theory was suggested by three articles. As repoted by Lee et al. [8], rod inputs were much more apparent in magnocellular pathway cells. Purpura et al. [9] stated that the Magnocellular pathway is the predominant conveyor of information of spatial contrast to the visual cortex in the mesopic and scotopic illuminations. Sun et al. [10] reported that rod threshold areas are inferred to be mediated by the magnocellular pathway. As a result, we can assume that a frequency doubling illusion predominantly stimulates rod cells. Sensitivity of cones and rods varies in some conditions. First, the illuminance level determines the level of activity of photoreceptors. Rods are more active photoreceptors in scotopic conditions. Secondly, rod cells are more sensitive than cone cells in short wavelength light. Stabell and Stabell [11] suggested that the "rod color" is blue. Therefore, if a short wavelength blue ray light was blocked by the yellow tinted IOL, we can assume that the result of FDT perimetry would be altered.
There was no significant difference in the result of FDT perimetry between conventional clear and yellow tinted IOLs. According to a previous report by Ueda et al. [5], this lack of a significant difference was explained through findings from a report by Antonio and associates that compared clear IOLs with yellow IOLs with respect to contrast sensitivity. At different spatial frequencies, contrast sensitivity exhibits similar values in patients implanted with clear and yellow tinted IOLs. The contrast sensitivity test predominantly activates the cone pathway, so this process may be mainly mediated by P-cell pathway. This is owed to the test condition: which consists of photopic conditions and requirements of the ability for high spatial frequency and fine visual discrimination. Although FDT perimetry determines contrast sensitivity for detecting frequency doubling stimulus, it is not sufficient to completely explain the process. Frequency doubling illusion has low spatial and high temporal frequency, so it selectively stimulates the magnocellular pathway.
There are two considerations to explain why there are no significant differences in the result of FDT perimetry. First, the rod pathway has convergence pattern. In the case of the rod system, the convergence of preceding neurons to the ganglion cell is enormous and consist of 75,000 rods going through 5,000 rod bipolar cells to 250 AII amacrine cells to a single alpha ganglion cell [12]. Though the sensitivity of the rod cells are decreased due to the blocking short wavelength light, some population of rods can activate this pathway and transmit signals to the magnocellular pathway. Second, the testing condition of FDT perimetry is not a scotopic condition. In FDT perimetry, ambient room illumination was dim, and background luminance of the monitor was 45 cd/m2. Therefore, it is a mesopic condition. In scotopic conditions, only rod cells are active photoreceptors. Cone cells are also active photoreceptors in mesopic conditions. This means that cone cells are able to mediate visual signals in FDT perimetry.
This study has several limitations that included the absence of preoperative FDT perimetry data, the small number of cases, and the absence of concurrent standard automated perimetry. A larger number of cases, more than 100 cases in each group, could make a difference in the findings between each group, or confirm the result seen in this study. Therefore, further studies are needed to make up for these points in the future.
In conclusion, there were no significant changes in the result of FDT perimetry after cataract surgery between clear and yellow IOLs. When interpreting the results of FDT perimetry, the color and type of IOLs do not need to be taken into account.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Yanagi Y, Inoue Y, Iriyama A, Jang WD. Effects of yellow intraocular lenses on light-induced upregulation of vascular endothelial growth factor. J Cataract Refract Surg. 2006;32:1540–1544. doi: 10.1016/j.jcrs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Fogagnolo P, Rossetti L, Ranno S, et al. Short-wavelength automated perimetry and frequency-doubling technology perimetry in glaucoma. Prog Brain Res. 2008;173:101–124. doi: 10.1016/S0079-6123(08)01108-4. [DOI] [PubMed] [Google Scholar]
- 3.Tanna AP, Abraham C, Lai J, Shen J. Impact of cataract on the results of frequency-doubling technology perimetry. Ophthalmology. 2004;111:1504–1507. doi: 10.1016/j.ophtha.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Kook MS, Yang SJ, Kim S, et al. Effect of cataract extraction on frequency doubling technology perimetry. Am J Ophthalmol. 2004;138:85–90. doi: 10.1016/j.ajo.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Ueda T, Ota T, Yukawa E, Hara Y. Frequency doubling technology perimetry after clear and yellow intraocular lens implantation. Am J Ophthalmol. 2006;142:856–858. doi: 10.1016/j.ajo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Ernest PH. Light-transmission-spectrum comparison of foldable intraocular lenses. J Cataract Refract Surg. 2004;30:1755–1758. doi: 10.1016/j.jcrs.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Brockmann C, Schulz M, Laube T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J Cataract Refract Surg. 2008;34:1161–1166. doi: 10.1016/j.jcrs.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Lee BB, Smith VC, Pokorny J, Kremers J. Rod inputs to macaque ganglion cells. Vision Res. 1997;37:2813–2828. doi: 10.1016/s0042-6989(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 9.Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci U S A. 1988;85:4534–4537. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H, Pokorny J, Smith VC. Rod-cone interactions assessed in inferred magnocellular and parvocellular postreceptoral pathways. J Vis. 2001;1:42–54. doi: 10.1167/1.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Stabell U, Stabell B. Mechanisms of chromatic rod vision in scotopic illumination. Vision Res. 1994;34:1019–1027. doi: 10.1016/0042-6989(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 12.Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–642. doi: 10.1523/JNEUROSCI.08-02-00623.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]