Abstract
Purpose
The purpose of this study was to investigate the effects of contrast display exposure on neuronal directional and spatial frequency tuning. Neuronal responses were recorded from ninety-four neurons in cortical areas 17 and 18 in two adult cats.
Methods
A multi-channel microelectrode was implanted in cortical areas 17 and 18 of two paralyzed and anaesthetized cats. Various drifting sinusoidal grating contrast displays were presented to one of the cats' eyes in the visual field. Contour plots based on the neuronal responses to the drifting sinusoidal grating displays using various contrasts (i.e., 0.4, 0.7, and 1.0) and velocities (i.e., 4.6, 13.9, 23.1, 32.3, 41.5, 50.8, and 60.0 deg/sec) were plotted as a function of the spatial frequency and the direction associated with each velocity and contrast used.
Results
Five parameters were extracted from these contour plots: 1) optimum response, 2) preferred direction, 3) optimum spatial frequency, 4) directional tuning width, and 5) spatial frequency bandwidth. To determine the optimal velocity, each parameter was plotted against each of the specific display contrasts used, and a 'best fit' line was established. Response amplitudes were dependent on the type of contrast utilized; however, the spatial frequency and directional tuning properties were stable for the cortical neurons assessed.
Conclusions
The results of the presentation of different contrasts on neuronal directional and spatial frequency tuning are consistent with behavioral results when medium and high contrast displays are used.
Keywords: Contrast, Electrophysiology, Feline, Visual cortex, Visual stimulus
A major goal of vision science is to further the understanding of how cortical neurons respond to visual stimulation. Previous research has established that neurons in visual cortex areas 17 and 18 are sensitive to several properties of drifting sinusoidal grating displays, including: contrast [1,2], directional frequency [3,4], and spatial frequency [1,5-9]. Recordings from individual neurons have revealed that the preferred tuning parameters and frequencies of cortical neurons in these areas are relatively independent of the contrast display of the stimulus. Specifically research has shown that the optimal directions and bandwidths associated with orientation [10-14] and spatial frequency [12] remain constant across different contrast display levels. A broadening of the spatial frequency bandwidth has been shown to occur at high contrast levels [2,15]. However, these results were obtained using a limited range of parameters and/or a flashing stimulus. Thus, it remains unclear whether these results will apply across a wider range of parameters and contrast-based stimuli. In previous studies, directional tuning properties have been obtained using only a single, optimal, spatial frequency and a single velocity, and spatial frequency tuning properties were obtained using only the optimal direction and the optimal temporal frequency (i.e., 2 Hz or 4 Hz). Additionally, temporal frequency tuning properties have been obtained using only the optimal spatial frequency.
Stimulus parameters can be made to vary independently of one another. As such, the nature of the effect of the interaction of these various stimulus parameters on single cell neuronal responses remains unclear. To examine the nature of the effect of the interaction of these stimulus parameters, single neuron responses were recorded from the visual cortical areas 17 and 18 of two adult cats. Additionally, the effects of changing the stimulus contrast on the directional and spatial frequency tuning responses of the neurons were recorded simultaneously. A motion parameter was added to the stimulus, as a drifting stimulus is different than a stationary stimulus due to the added parameter of speed.
Materials and Methods
Animal preparation
All experimental neural responding procedures were approved by the Seoul National University Animal Care and Use Committee, and all in vivo experimental procedures were performed according to the standards of the Association for Research in Vision and Ophthalmology. All neural recordings were made in cortical areas 17 and 18 of two adult cats, weighing 3.1 and 3.4 kg (Hanlym Lab. Animal Co., Hwaseong, Korea). Atropine (0.05 mg) and dexamethasone (40 mg) were injected subcutaneously into each cat to reduce tracheal secretions and to minimize stress-based responding. The cats were initially anesthetized with Ketamine and Xylazine (15 mg/kg and 1.5 mg/kg, intramuscular injection). Endotracheal tubes were inserted into the trachea of each cat to allow for artificial respiration. Anesthesia was maintained using 2% to 3% isofluorane in O2 during each surgery. The concentration of isofluorane was reduced to 0.5% in O2 during the recording in each cat. The heads of the animals were secured using a stereotaxic device (Boardtech, Incheon, Korea) using ear and mouth bars as well as clamps to the orbital rim. Stainless steel pegs were implanted with screws in the frontal bones of both cats. Then, the stainless steel pegs were secured to the stereotaxic device. A craniotomy over the lateral gyrus of left hemispheres of both cats exposed the dura, which was surgically removed. After stabilization of anesthesia, the cats were immobilized with gallamine triethiodide (10 mg). The respiratory rates and tidal volumes of both cats were controlled with a respiratory pump (Clare ventilator, Victoria, Australia) to maintain end-tidal CO2 levels at 3.8% to 4.2%, and the body temperatures of both cats were maintained at 37.5℃. A continuous infusion of Ringer's solution, containing gallamine triethiodide (10 mg/kg/hr) and glucose (80 mg/kg/hr), was administered to each cat.
The eyes (conjunctival sacs) of both cats were irrigated with a 10% phenylephrine (Neosynephrine-Pos, Ursa pharm., Saarbrücken, Germany) and 0.1% atropine solution. The corneas of the eyes were protected with non-corrective contact lenses. The retinal coordinates were obtained with light to monitor the fixation location of both cats. Artificial pupils (5 mm diameter) were placed in front of the eyes of both cats. Trial lenses (usually between -2 and -3 diopters) were placed in front of the right eyes to correct for distance, and the left eyes were occluded.
Stimulus presentation
Stimuli were generated using Visionworks for Electrophysiology (Vision Research Graphics, Durham, NH, USA) on a 19-inch monitor (950NF; Samsung, Suwon, Korea), placed 57 cm in front of the cats' right eyes, using a 1024 × 768 pixel resolution and a 85-Hz refresh rate. When action potentials were observed in one or more neurons, preliminary tests were performed. Specifically, the approximate position of the cells' receptive field was obtained. The position was conducted manually by varying the X-Y position of an aperture drifting sinusoidal grating display on the monitor. Once the receptive field was isolated, the stimulus (15 deg × 15 deg) was fixed in a position restricting the number of cortical neurons (to between three and five) which were responding to the stimulus.
To measure the effects of various contrasts and velocities, directional and spatial frequency tuning selectivity was measured using drifting grating displays (described below). Drifting sinusoidal grating displays were placed to the right eye of each cat within a square aperture (15 deg × 15 deg). The spatial frequency and directional tuning properties of the neurons were examined using drifting sinusoidal grating displays with varying spatial frequencies, velocities, and directions [16-18]. The experimental protocol utilized a series of 10 spatial frequencies and 16 directions at seven velocities (i.e., a total number of stimuli, 1,120). At each spatial frequency (e.g., 0.05 cycle/deg) and orientation (67.5 deg), the stimulus was shown for 1 second at varying velocities (+60, +50.8, +41.5, +32.3, +23.1, +13.9, +4.6, -4.6, -13.9, -23.1, -32.3, -41.5, -50.8, and -60.0 deg/sec). This set of velocities was repeated for seven orientations (-22.5 deg intervals). Between each orientation set, a blank screen was presented for 3 seconds. After all orientations and velocities were presented, the sequence was repeated using nine spatial frequencies (0.27, 0.48, 0.70, 0.92, 1.13, 1.35, 1.57, 1.78, and 2.0 cycle/deg). In total, the stimulus set resulted in 1,120 responses and 80 resting discharges for each animal. The stimulus set was repeated two or four times and resulted in seven directional and spatial frequency tunings (Fig. 1). To investigate the effect of the contrast displays on the directional and spatial frequency tuning, the tuning test was conducted at three contrast levels (0.4, 0.7, and 1.0).
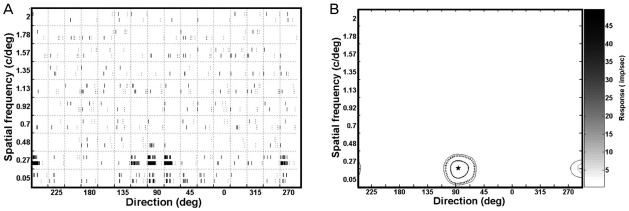
Fig. 1.
Quantification of directional / spatial frequency tuning plots (cell no. 0706-c97-u1-v2). The stimuli were sinusoidal gratings using 10 spatial frequencies (the y-axis) in 16 directions (22.5 deg interval, the x-axis) using a specific velocity (50.2 deg/sec) and contrast (100%). The resting discharge was collected during one second, prior to the presentation of the drifting sinusoidal gratings at each orientation. The horizontal axis ranged from 0 to 337.5 and represented 'direction', while the vertical axis ranged from 0.05 to 2.0 cycle/deg and represented 'spatial frequency'. (A) Raster plots of responses of an individual neuron in a joint directional and spatial frequency domain to drifting sinusoidal grating stimuli. Raster plots revealed responses to 160 different stimuli (combinations of 10 spatial frequencies and 16 directions). The horizontal axis represents 2 seconds (stimulation for 1 second and resting discharge for 1 second), while the vertical axis represents two trials. The response strength was calculated during one second. Mean firing rates (n=2) were averaged for each stimulus (i.e., 10 × 16 array). After adding the 17th array with the first array, the 10 × 17 array was processed further using the interp2 (cubic) function to produce a 201 × 341 array. A tuning contour plot showing the joint directional and spatial frequency domain is shown in (B). (B) The contour plot in a joint direction and spatial frequency domain. The contour plot at the 50% of maximum response (star), at the optimal direction (along the x-axis), and at the optimal spatial frequency (along the y-axis) are indicated by asterisks. Five values were calculated (optimal response, 51 impulses/sec; optimal direction, 94.6 deg; tuning width, 42.3 deg; optimal spatial frequency, 0.26 c/deg; bandwidth, 0.29 c/deg) based on the contour plot after measuring the horizontal distance (i.e., tuning width) and vertical distance (i.e., bandwidth) of the boundary.
Electrophysiological recording
Single and/or multiple units were recorded extracellularly from areas 17 and 18, using a Utah multielectrode array (UEA; Bionic Technologies, Salt Lake City, UT, USA). The UEA was implanted at a depth of 1.5 or 1.0 mm using a high-velocity inserter [19]. The device was implanted at the junction of the lateral and posterior lateral gyri as described by Tusa et al. [20].
Neuronal activity was amplified (5,000 ×), filtered (250-7, 500 Hz), and digitized (30 kHz sample rate, with 8 bits/sample, at a selectable, resolution of between 0.5-8 µV per bit), using a 100-channel data acquisition system (Bionic Technologies). At the end of the recording session, both cats were euthanized with a lethal dose of pentobarbital in excess of 100 mg/kg body weight intravenous injection.
Data analysis and presentation
Single unit responses were classified off-line using Matlab ver. 2.0 beta (Bionic Technologies). The responses of each neuron to the 160 stimuli (10 spatial frequencies × 16 directions × 1 velocity) per second are shown in a raster plot of the directional versus spatial frequency domains (Fig. 1A). The center (marked with a star) of the inner-most contour represents the optimal response, which is located at the point of optimal directional (along the x-axis) and spatial frequency (along the y-axis). The contour at 50% of the optimal response is indicated by an asterisk. The directional tuning bandwidth and spatial frequency bandwidth were extracted by measuring the horizontal distances and vertical distances to the boundary. The responses in the null direction were also calculated. A directionality index was computed as 1 minus the ratio of the response in the null direction to the response in the preferred direction. As a result of these calculations, seven values were extracted from the contour plot.
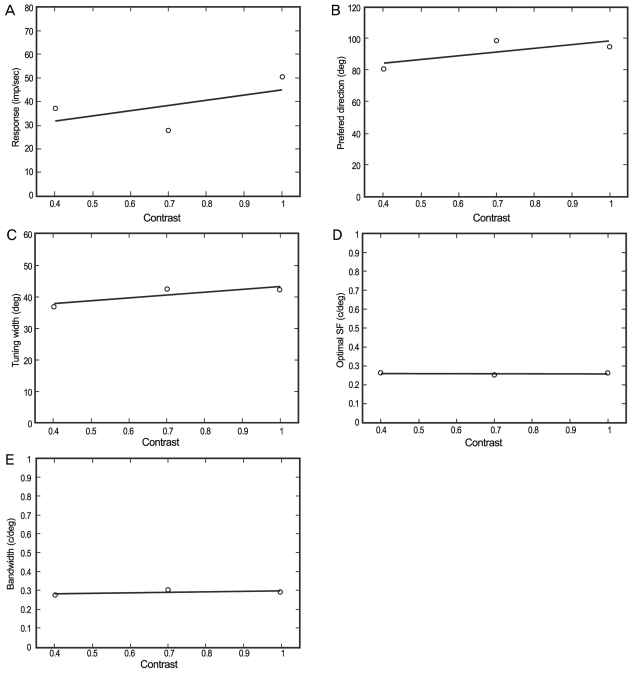
Each value (optimal response, optimal direction, optimal spatial frequency, tuning width, and band width) was plotted along the contrast (0.4, 0.7, and 1.0). A line of 'best fit' was calculated (Fig. 2), and the distribution of the slopes for these 'best fit' lines is shown for each parameter in Fig. 3.
Fig. 2.
Changes in five different parameters (A-E) at three contrast levels (0.4, 0.7, and 1.0). (A) Responses for the gratings. The slope of linear regression was fitted to the optimal responses as functions of the various contrasts. (B) Optimal directions. (C) Tuning widths. (D) Optimal spatial frequencies (SF). (E) Band widths.
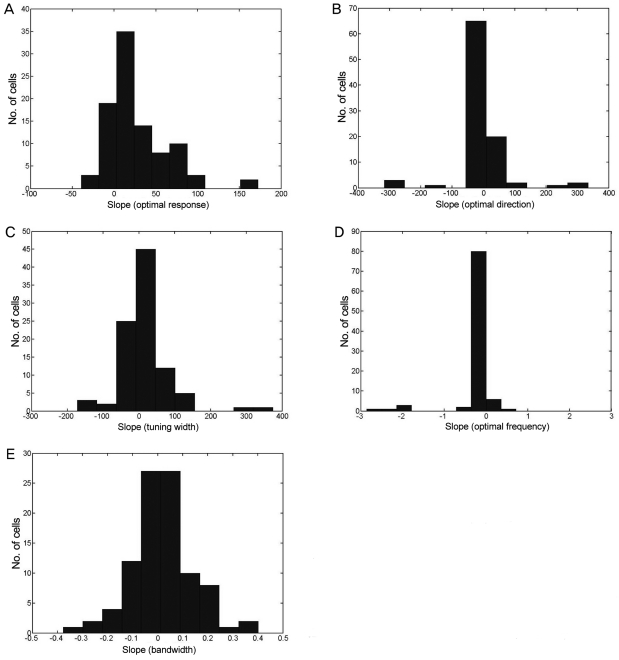
Fig. 3.
Distribution of slopes (n = 94) for the five parameters (A-E) in response to changes in the contrast. Most of these slopes (except the response strength, (A) are close to zero, indicating that these functions remained relatively constant as the contrast varied. (A) shows the distribution for the changes in the optimal responses at the optimal direction and optimal spatial frequency (mean, 27.86; SD, 36.69). (B) Shows the distribution of changes in the optimal direction (mean, -0.21; SD, 79.72). (C) Shows the distribution of the changes in the directional tuning width (mean, 14.18; SD, 72.55). (D) shows the distribution of the changes in the optimal spatial frequency (mean, -0.13; SD, 0.50). (E) Shows the distribution of the changes in the spatial frequency (mean, 0.02; SD, 0.13).
Results
Recordings were made from a total of 153 cells. Of these, 94 cells (61%) were selected for further analysis. The contour plots of these cells showed either one clear peak (direction selective) or two clear peaks (non-direction selective). Each of five parameters (optimal response, optimal direction, tuning width, optimal spatial frequency, and bandwidth) was tested to determine whether that parameter was affected by the contrast display. The slope of the relationship between the parameters was extracted by fitting a linear function (Fig. 2A-2E). The distributions of the slopes for all five parameters are shown in Fig. 3.
The responses of most of the cells increased linearly with increasing contrast (mean, 27.86; SD, 36.69) (Fig. 3A); however, some of the cells displayed a saturation effect. The optimal direction did not change for most cells (mean, -0.21; SD, 79.72) (Fig. 3B) across the various contrast displays. The tuning bandwidth was also stable (mean, 14.18; SD, 72.55) (Fig. 3C) across various contrast displays, as were the optimal spatial response frequencies (mean, -0.13; SD, 0.50) (Fig. 3D) and bandwidths (mean, 0.02; SD, 0.13) (Fig. 3E).
Discussion
The goal of this study was to examine the effects of various contrasts on the directional/spatial frequency tuning properties of neurons in the cat visual cortex. Previous research on the selectivity of striate cortical neurons has shown that neurons in this region respond to bars or edges [3]. Gratings (square or sine-wave) have also been used [4,6]. Light and dark bars divide striate cortical neurons into two main groups: simple and complex cells (depending on the discreteness or overlap of these sub-regions). Complex cells can be further divided into standard and special complex cells, depending on their length summation [21,22]. These types of classifications were not used in this experiment. The main objective of this experiment was to examine the effects of contrast displays on directional and spatial frequency tuning of neurons in the straital cortex. Additionally, length summation was not able to be tested using a multi-channel recording system. Orientation/directional tuning curves have been obtained by comparing the responses to stimuli of different orientations and directions. Gratings establish the cells' tuning to specific spatial frequency [1,5-9], as well as to orientation and the direction of movement [3,4]. Preferred orientations tend to remain invariant over time [23].
Visual cortical neurons are responsive to a particular visual stimulus during visual information processing. Previously, studies have recorded and analyzed the responses of a single neuron under a particular set of conditions. However, these methods lead to understanding of neuronal behavior under a single, specific condition (e.g., at the optimal direction or optimal spatial frequency). Recently, multi-electrodes have become available, including the Utah arrays [19] used in this study. The major benefits of using multi-electrodes, include: 1) allowing the placement of multiple electrodes at once, rather than individually; and 2) the ability to receive data from multiple sites at the same time. When multi-channel recording methods are used, stimulus parameters cannot be limited as each cell prefers different values. By using additional non-optimal stimuli, other neurons which prefer such stimuli can be recorded at the same time. It may take twice the time, but because more than 50 cells can be recorded simultaneously with the multi-channel recording system, it is more time effective. To get obtain and appropriate isolation of spikes from each channel, each micro-electrode should be advanced separately.
The results of this study show that neurons in visual cortex areas 17 and 18 of the cat exhibit contrast-invariant directional and spatial frequency tuning. The stability of both the tuning width and the bandwidth in response to varying contrasts has been established. However, previous work has suggested some level of variability associated with spatial frequency selectivity [2,15]. Previous work demonstrating the stability of these tuning properties has used experiments of different animals, and it was considered necessary to confirm these results in the same animal. Additionally, interactions between direction tuning selectivity and spatial frequency selectivity or velocity were also considered to be likely. Additionally, the effects of various contrasts on tuning properties were investigated using a drifting stimulus rather than a stationary stimulus.
Previous work has shown that humans recognize either an object's direction of movement or its spatial frequency with no effect of contrast [12]. The results of this study show a pattern of responses which is consistent with this; however, the relationship between the activity of these cells and the perceptions of the animal remain unclear. The saturation of responses in some cells observed in this study (minus and zero slopes in Fig. 3A) is consistent with previous results [10,24]. Direction selectivity has been shown to be dependent on contrast [25]. This was not observed in this study, and it may only occur at low levels of contrast (lower than 30%). In summary, these results are consistent with the proposal that both the directional tuning and spatial frequency tuning characteristics of cortical cells are stable when the contrast of the stimulus placed in the visual field is greater than 40%.
Acknowledgements
The author thanks M. A. Leem for her valuable technical assistance. This work was supported by Seoul National University and Research Institute of Veterinary Science. These animal-experiments were conducted according to the ethical procedures of Seoul National University (approval no. SNU-061106-2).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tolhurst DJ, Movshon JA. Spatial and temporal contrast sensitivity of striate cortical neurones. Nature. 1975;257:674–675. doi: 10.1038/257674a0. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- 3.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell FW, Cleland BG, Cooper GF, Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968;198:237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell FW, Cooper GF, Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969;203:223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffei L, Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973;13:1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- 7.Schiller PH, Finlay BL, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. III. Spatial frequency. J Neurophysiol. 1976;39:1334–1351. doi: 10.1152/jn.1976.39.6.1334. [DOI] [PubMed] [Google Scholar]
- 8.Movshon JA, Thompson ID, Tolhurst DJ. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22:545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- 10.Sclar G, Freeman RD. Orientation selectivity in the cat's striate cortex is invariant with stimulus contrast. Exp Brain Res. 1982;46:457–461. doi: 10.1007/BF00238641. [DOI] [PubMed] [Google Scholar]
- 11.Skottun BC, Bradley A, Ramoa AS. Effect of contrast on spatial frequency tuning of neurones in area 17 of cat's visual cortex. Exp Brain Res. 1986;63:431–435. doi: 10.1007/BF00236862. [DOI] [PubMed] [Google Scholar]
- 12.Skottun BC, Bradley A, Sclar G, et al. The effects of contrast on visual orientation and spatial frequency discrimination: a comparison of single cells and behavior. J Neurophysiol. 1987;57:773–786. doi: 10.1152/jn.1987.57.3.773. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JS, Lampl I, Gillespie DC, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. doi: 10.1126/science.290.5498.1968. [DOI] [PubMed] [Google Scholar]
- 14.Alitto HJ, Usrey WM. Influence of contrast on orientation and temporal frequency tuning in ferret primary visual cortex. J Neurophysiol. 2004;91:2797–2808. doi: 10.1152/jn.00943.2003. [DOI] [PubMed] [Google Scholar]
- 15.Sceniak MP, Hawken MJ, Shapley R. Contrast-dependent changes in spatial frequency tuning of macaque V1 neurons: effects of a changing receptive field size. J Neurophysiol. 2002;88:1363–1373. doi: 10.1152/jn.2002.88.3.1363. [DOI] [PubMed] [Google Scholar]
- 16.Jones JP, Stepnoski A, Palmer LA. The two-dimensional spectral structure of simple receptive fields in cat striate cortex. J Neurophysiol. 1987;58:1212–1232. doi: 10.1152/jn.1987.58.6.1212. [DOI] [PubMed] [Google Scholar]
- 17.Hammond P, Pomfrett CJ. Influence of spatial frequency on tuning and bias for orientation and direction in the cat's striate cortex. Vision Res. 1990;30:359–369. doi: 10.1016/0042-6989(90)90078-y. [DOI] [PubMed] [Google Scholar]
- 18.Park YH, Kim JN. Spatial frequency and velocity tunings of neurons in areas 17 and 18 of the cat using a 100 microelectrode array. Korean J Lab Anim Sci. 2004;20:26–30. [Google Scholar]
- 19.Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng. 1992;20:413–422. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 20.Tusa RJ, Palmer LA, Rosenquist AC. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978;177:213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert CD. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977;268:391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond P, Ahmed B. Length summation of complex cells in cat striate cortex: a reappraisal of the "special/standard" classification. Neuroscience. 1985;15:639–649. doi: 10.1016/0306-4522(85)90065-x. [DOI] [PubMed] [Google Scholar]
- 23.Henry GH, Bishop PO, Tupper RM, Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973;13:1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- 24.Dean AF. The relationship between response amplitude and contrast for cat striate cortical neurones. J Physiol. 1981;318:413–427. doi: 10.1113/jphysiol.1981.sp013875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson MR, Li B, Freeman RD. Direction selectivity of neurons in the striate cortex increases as stimulus contrast is decreased. J Neurophysiol. 2006;95:2705–2712. doi: 10.1152/jn.00885.2005. [DOI] [PubMed] [Google Scholar]