Abstract
Objective:
Consistent with the cognitive reserve hypothesis, higher education and vocabulary help persons with Alzheimer disease (AD) and multiple sclerosis (MS) better withstand neuropathology before developing cognitive impairment. Also, premorbid cognitive leisure (e.g., reading, hobbies) is an independent source of cognitive reserve for elders with AD, but there is no research on the contribution of leisure activity to cognition in MS. We investigated whether premorbid cognitive leisure protects patients with MS from cognitive impairment.
Methods:
Premorbid cognitive leisure was surveyed in 36 patients with MS. Neurologic disease severity was estimated with brain atrophy, measured as third ventricle width on high-resolution MRI. Cognitive status was measured with a composite score of processing speed and memory.
Results:
Controlling for brain atrophy, premorbid cognitive leisure was positively associated with current cognitive status (r p = 0.49, p < 0.01), even when controlling for vocabulary (r p = 0.39, p < 0.05) and education (r p = 0.47, p < 0.01). Also, premorbid cognitive leisure was unrelated to brain atrophy (r = 0.03, p > 0.5), but a positive partial correlation between leisure and atrophy emerged when controlling for cognitive status (r p = 0.37, p < 0.05), which remained when also controlling for vocabulary (r p = 0.34, p < 0.05) and education (r p = 0.35, p < 0.05).
Conclusions:
Premorbid cognitive leisure contributes to cognitive status in patients with MS independently of vocabulary and education. Also, patients with MS who engaged in more cognitive leisure were able to withstand more severe brain atrophy at a given cognitive status. Premorbid cognitive leisure is supported as an independent source of cognitive reserve in patients with MS.
GLOSSARY
- AD
= Alzheimer disease;
- MS
= multiple sclerosis;
- TVW
= third ventricle width.
The cognitive reserve hypothesis posits that lifetime intellectual enrichment lessens the negative impact of neurologic disease on cognitive status.1,2 That is, persons with higher intellectual enrichment are able to withstand more severe neuropathology before developing cognitive impairment or dementia. The cognitive reserve hypothesis has been well-supported in Alzheimer disease (AD),2 and there is a growing literature on cognitive reserve in multiple sclerosis (MS).3–5 Although intellectual enrichment is usually estimated with proxies such as educational attainment or vocabulary knowledge,1–5 researchers have also shown that premorbid cognitive leisure activity (e.g., reading books, playing cards) independently protects elders from dementia.6–9 The current research investigates whether premorbid cognitive leisure activity protects persons with MS from cognitive decline.
METHODS
Subject enrollment.
Subjects were 36 persons with MS10 (31 women) with no exacerbation in the last 4 weeks, no current corticosteroid use, and no history of serious psychiatric illness, substance abuse, learning disability, or other neurologic condition. Mean age was 45.1 ± 7.1 years (range 27–54). Disease duration was 9.2 ± 6.0 years, with MS courses including relapsing-remitting (n = 28), secondary progressive (n = 6), and primary progressive (n = 2). Subjects retrospectively reported cognitive leisure activity in their early 20s before they developed MS. As such, only subjects who were diagnosed after age 25 years were enrolled. Educational attainment was 16.0 ± 2.3 years, and vocabulary knowledge estimated with the Wechsler Abbreviated Scale of Intelligence was within normal limits (Vocabulary mean T score = 52.9 ± 7.7).
Standard protocol approvals, registrations, and patient consents.
Institutional review boards responsible for ethical standards at UMDNJ and the Kessler Foundation Research Center approved this study. Written informed consent was obtained from all subjects prior to participation.
Brain atrophy.
Brain atrophy was estimated with third ventricle width (TVW), which has been identified as the best neuroanatomic predictor of cognitive status in patients with MS.11 Consistent with established procedures,3–5,11 TVW was defined as the distance in millimeters between the left and right boundaries of the third ventricle as imaged in the axial plane of high-resolution 3-dimensional images of the brain acquired from magnetization-prepared rapid gradient echo scans performed in a 3.0 T Siemens Allegra scanner. Detailed procedures are provided elsewhere,3 with high interrater and intrarater reliabilities (r >0.96). Mean TVW was 5.0 ± 2.1 mm.
Cognitive leisure activity.
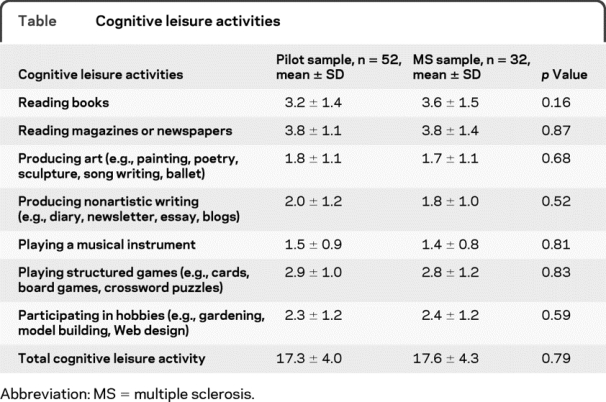
Subjects completed a questionnaire to quantify participation in cognitive leisure activities during their early 20s before they developed MS. We focused on premorbid cognitive leisure activity to ensure that our estimate of leisure activity was independent of neurologic disease progression, which is consistent with investigations in AD.6–9 As the first investigation of cognitive leisure in MS, we limited our survey to a single premorbid age range (early 20s) to best control for age-related differences in lifestyle that may impact leisure activity (e.g., interpersonal differences in parenting responsibilities during early 30s). Seven items were chosen based on similar studies examining cognitive leisure in aging/AD (table).6–9 Consistent with previous methods,9 frequency of participation was endorsed as 1) once/less per year, 2) several times per year, 3) several times per month, 4) several times per week, or 5) daily. Pilot testing of this questionnaire was conducted with 52 healthy persons (mean age 41.4 ± 11.1 years) who were asked to retrospectively report on leisure activity in their early 20s. Responses by persons with MS in the current sample matched those of pilot healthy subjects (table), which is consistent with our aim of estimating cognitive leisure activity before the onset of MS. The leisure questionnaire showed very good test–retest reliability after 2 weeks in a subset of 16 pilot subjects (r = 0.95, p < 0.001).
Table Cognitive leisure activities
Although many patients with MS have memory impairment, such memory problems are characterized by deficits in new learning/anterograde memory rather than dysfunctional remote autobiographical memory.12 In fact, research using the Autobiographical Memory Interview found little to no difference in autobiographical memory accuracy between patients with MS and healthy persons.13 Moreover, the consistency between autobiographical memory and collateral reports was essentially perfect for both patients with MS and healthy persons.13 Given this, there is no reason to doubt the accuracy of autobiographical reports by patients with MS regarding their early-life cognitive leisure activity.
Cognitive status.
Slowed processing speed and learning/memory problems are the most prevalent cognitive deficits among persons with MS.12 We assessed processing speed with the Symbol Digit Modalities Test, and learning/memory with the total learning score of the open-trial Selective Reminding Test.4 Performance on tasks was converted to sample-based z scores, which were then averaged into a single cognitive status score.
Statistical analyses.
To investigate the association between premorbid cognitive leisure and current cognitive status, we performed a partial correlation between these variables controlling for brain atrophy. We repeated this correlation controlling for vocabulary knowledge and education to determine whether cognitive leisure makes an independent contribution to cognitive status in patients with MS.
The cognitive reserve hypothesis posits that persons with greater intellectual enrichment are able to withstand more severe neuropathology before developing cognitive impairment.1,2 As such, at a given cognitive status (e.g., dementia), persons with greater intellectual enrichment possess more severe neuropathology than persons with lesser enrichment, because more severe neuropathology is required for persons with higher enrichment to have comparable cognitive decline. To demonstrate this statistically, previous research on cognitive reserve in aging and dementia has shown no direct relationship between intellectual enrichment and neuropathology, but a positive partial correlation between intellectual enrichment and neuropathology when controlling for current cognitive status.1,2,7,14,15 Consistent with this statistical approach, we first calculated the correlation between premorbid cognitive leisure activity and brain atrophy in patients with MS, followed by a partial correlation between cognitive leisure activity and brain atrophy controlling for current cognitive status. Similar to previous research, we expect no relationship between cognitive leisure and atrophy; however, a partial correlation should emerge when controlling for cognitive status. This procedure was repeated controlling for vocabulary knowledge and education.
RESULTS
There was a positive partial correlation between premorbid cognitive leisure and current cognitive status controlling for brain atrophy (r p = 0.49, p < 0.01). That is, patients with MS with higher premorbid cognitive leisure activity exhibit greater cognitive functioning. This correlation remained when also controlling for vocabulary knowledge (r p = 0.39, p < 0.05), education (r p = 0.47, p < 0.01), or vocabulary and education (r p = 0.38, p < 0.05), indicating that premorbid cognitive leisure activity makes an independent contribution to cognitive reserve in persons with MS (figure).
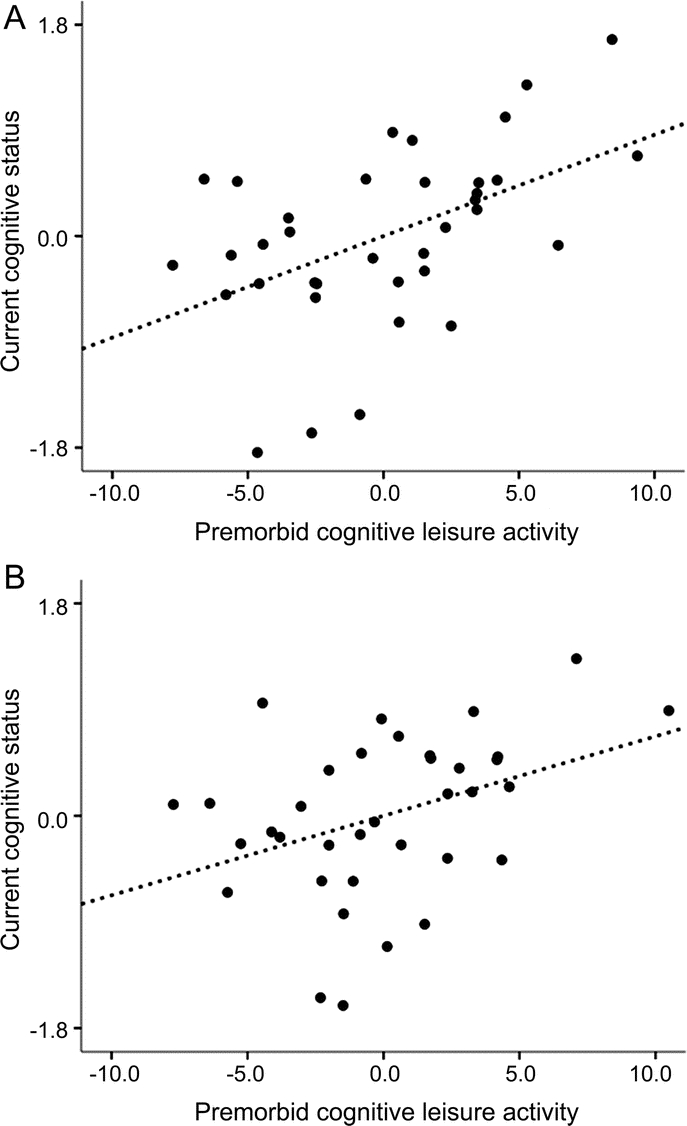
Figure Relationship between premorbid cognitive leisure activity and current cognitive status
(A) Controlling for brain atrophy, patients with multiple sclerosis (MS) who participated in more premorbid cognitive leisure activity demonstrated better current cognitive status (r p = 0.49, p < 0.01). (B) This positive relationship between premorbid cognitive leisure and current cognitive status remained when also controlling for vocabulary knowledge and educational attainment (r p = 0.38, p < 0.05). That is, premorbid cognitive leisure was identified as an independent contributor to cognitive reserve in patients with MS.
There was no relationship between premorbid cognitive leisure and brain atrophy (r = 0.03, p > 0.5); however, a positive partial correlation emerged with controlling for cognitive status (r p = 0.37, p < 0.05). That is, persons with greater premorbid cognitive leisure were able to withstand more severe MS neuropathology before showing cognitive impairment. Importantly, the protective effect of premorbid cognitive leisure remained when controlling for vocabulary knowledge (r p = 0.34, p < 0.05), education (r p = 0.35, p < 0.05), or vocabulary and education (r p = 0.34, p = 0.05).
DISCUSSION
Intellectual enrichment (estimated with education or vocabulary knowledge) lessens the negative impact of neurologic disease on cognition in persons with AD1,2 and MS.3,4 Research with elders has also shown that premorbid cognitive leisure protects against dementia.6–9 The current research demonstrates the independent contribution of premorbid cognitive leisure to cognitive status in persons with MS, with greater leisure activity linked to lesser cognitive dysfunction. Moreover, patients with MS who engaged in more premorbid cognitive leisure were able to withstand more severe brain atrophy before showing cognitive decline. To our knowledge, this research may be the first to document the protective impact of premorbid cognitive leisure activity in a neurologic sample other than aging/dementia.
The independent contribution of leisure activity to cognition in patients with MS supports the emerging notion that lifestyle choices can have a direct impact on the brain. Stern and colleagues2,16 posit that intellectual enrichment is related to the elaboration of synaptic networks, which results in greater cerebral efficiency. Indeed, environmental enrichment has been linked to neural plasticity and synaptogenesis in animals,17 and the popular “London taxi driver” study (among others) provided neuroanatomic support for the cognitive reserve hypothesis in humans, as the amount of time spent as a taxi driver was positively correlated with posterior hippocampal volumes (brain regions associated with spatial memory).18 Finally, a recent fMRI study linked cognitive reserve to cerebral efficiency in patients with MS, as patients with higher vocabulary knowledge showed greater maintenance of the brain's default network (resting state) and lesser recruitment of prefrontal cortex to perform cognitive tasks.5 That is, patients with MS with higher cognitive reserve required fewer cerebral resources to perform cognitive tasks, likely explaining the protective impact of reserve on cognition in patients with MS.3,4
Limitations of the current study include the modest sample size and single estimates of brain atrophy and cognitive status. Future research should investigate the impact of cognitive leisure across distinct cognitive domains. Occupational complexity has been supported as an independent source of cognitive reserve among elders,15 but occupational variables were not investigated in the current study. Future research should consider whether the degree and duration of cognitively stimulating occupations afford independent protection from cognitive decline, even if such careers are ended prematurely due to MS disease.
The current findings highlight the importance of premorbid cognitive leisure activity to current cognitive status. Future research is needed to investigate whether cognitive leisure activity initiated after a diagnosis of MS can bolster cognitive reserve against impairment. That is, would a regimen of cognitively simulating activity “prescribed” at the time of MS diagnosis protect persons with MS from future cognitive impairment? Although this question is yet to be answered directly, there appears to be adequate evidence in the cognitive reserve literature to encourage all persons to maintain a cognitively stimulating lifestyle.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. J.F. Sumowski.
DISCLOSURE
Dr. Sumowski receives research support from the NIH (NICHD HD060765 [PI]) and the National Multiple Sclerosis Society. Dr. Wylie receives research support from the NIH (NINDS R41 NS050007-03 [Co-I] and NIMH R01HD045798-Suppl [Co-I]) and the Kessler Foundation Research Center New Jersey Commission on Brain Injury Research. A. Gonnella reports no disclosures. Dr. Chiaravalloti receives research support from the NIH (NCMRR HD045798 [PI], NCMRR HD045798S [PI], NIDRR H133A070037 [PI], NIDRR H133G090078, and NIDRR H133P090009 [Project Director]). Dr. DeLuca serves on a scientific advisory board for Biogen Idec; serves as an Associate Editor for the Archives of Physical Medicine and Rehabilitation and on the editorial boards of Multiple Sclerosis, Rehabilitation Psychology, the Journal of Clinical and Experimental Neuropsychology, Neuro-psychoanalysis, and Neuropsychology Review; and receives research support from the NIH (NCMRR HD045798 [Co-I]) and the National Multiple Sclerosis Society.
Address correspondence and reprint requests to Dr. James F. Sumowski, Neuropsychology & Neuroscience Laboratory, Kessler Foundation Research Center, 300 Executive Drive, Suite 10, West Orange, NJ 07052 jsumowski@kesslerfoundation.org
Study funding: Supported by the National Multiple Sclerosis Society (RG3330A1/3 to N.C., MB0003 to J.D.) and the National Institutes of Health (HD045798 to N.C., HD060765 to J.F.S.).
Disclosure: Author disclosures are provided at the end of the article.
Received March 23, 2010. Accepted in final form June 16, 2010.
REFERENCES
- 1.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 2.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumowski JF, Chiaravalloti N, Wylie GR, DeLuca J. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc 2009;15:606–612. [DOI] [PubMed] [Google Scholar]
- 4.Sumowski JF, Wylie GR, Chiaravalloti N, DeLuca J. Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 2010;74:1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumowski JF, Wylie GR, DeLuca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010;133:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001;57:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarmeas N, Zarahn E, Anderson KE, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol 2003;60:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508–2516. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 11.Benedict RH, Weinstock-Guttman B, Fishman I, et al. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 2004;61:226–230. [DOI] [PubMed] [Google Scholar]
- 12.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 13.Paul RH, Blanco CR, Hames KA, Beatty WW. Autobiographical memory in multiple sclerosis. J Int Neuropsychol Soc 1997;3:246–251. [PubMed] [Google Scholar]
- 14.Brickman AM, Siedlecki KL, Muraskin J, et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol Aging Epub 2009 Nov 17. [DOI] [PMC free article] [PubMed]
- 15.Stern Y, Alexander GE, Prohovnik I, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology 1995;45:55–50. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Habeck C, Moeller J, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 2005;15:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 2000;1:191–198. [DOI] [PubMed] [Google Scholar]
- 18.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 2000;97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]


