Abstract
Objectives:
Physical activity (PA) has been hypothesized to spare gray matter volume in late adulthood, but longitudinal data testing an association has been lacking. Here we tested whether PA would be associated with greater gray matter volume after a 9-year follow-up, a threshold could be identified for the amount of walking necessary to spare gray matter volume, and greater gray matter volume associated with PA would be associated with a reduced risk for cognitive impairment 13 years after the PA evaluation.
Methods:
In 299 adults (mean age 78 years) from the Cardiovascular Health Cognition Study, we examined the association between gray matter volume, PA, and cognitive impairment. Physical activity was quantified as the number of blocks walked over 1 week. High-resolution brain scans were acquired 9 years after the PA assessment on cognitively normal adults. White matter hyperintensities, ventricular grade, and other health variables at baseline were used as covariates. Clinical adjudication for cognitive impairment occurred 13 years after baseline.
Results:
Walking amounts ranged from 0 to 300 blocks (mean 56.3; SD 69.7). Greater PA predicted greater volumes of frontal, occipital, entorhinal, and hippocampal regions 9 years later. Walking 72 blocks was necessary to detect increased gray matter volume but walking more than 72 blocks did not spare additional volume. Greater gray matter volume with PA reduced the risk for cognitive impairment 2-fold.
Conclusion:
Greater amounts of walking are associated with greater gray matter volume, which is in turn associated with a reduced risk of cognitive impairment.
GLOSSARY
- 3MSE
= modified Mini-Mental State Examination;
- CHS-CS
= Cardiovascular Health Study Cognition Study;
- DSST
= Digit Symbol Substitution Test;
- GM
= gray matter;
- MCI
= mild cognitive impairment;
- OR
= odds ratio;
- PA
= physical activity;
- SPM
= Statistical Parametric Mapping;
- TIV
= total intracranial volume;
- VBM
= voxel-based morphometry;
- WM
= white matter.

e–Pub ahead of print
Gray matter (GM) volume shrinks in late adulthood, often preceding and leading to cognitive impairment.1 Participation in physical activity (PA) and exercise, however, has been hypothesized to protect against the deterioration of brain tissue, but this hypothesis has not been tested in longitudinal studies.2,3 Limited support for this hypothesis comes from cross-sectional neuroimaging research demonstrating that older adults who are more fit have greater GM volume in the prefrontal and temporal lobes,4–6 and larger hippocampal volumes,7 than their less fit peers. Randomized controlled trials over 6 months have also shown increased cortical volume in response to a moderate-intensity exercise regimen.8
Prospective longitudinal studies have identified physical inactivity as a risk factor for dementia,9–17 while others have found that greater PA predicts stable cognitive function over an 8-year period.18 This research suggests that participation in PA earlier in life might be predictive of less cortical shrinkage in late adulthood. Although a linear association between cognition and PA is likely, it is also possible that there is little benefit of additional PA past a certain threshold.17 Similarly, a minimal amount of PA may be necessary for any long-term protection on brain function to be detected.2,3,19
In this study, we investigated whether PA, assessed at baseline, predicted GM volume 9 years later. Because brain MRI measures were unavailable at baseline and the design was not a randomized intervention, we cannot conclude a causal association between PA and GM volume. However, an association between PA and brain volume could be clinically meaningful. Therefore, we also predicted that greater GM volume related to PA would be associated with a reduced risk of developing cognitive impairment. In addition, we tested whether a threshold could be identified to establish a minimal amount of PA necessary to protect against the loss of GM volume and the development of cognitive impairment. We hypothesized that after controlling for several baseline measures of health and function, those participants who were more physically active would have greater GM volume at follow-up than those less active, and that greater GM volume would be associated with a reduced risk of developing cognitive impairment.
METHODS
Participants.
Participants were part of the Pittsburgh component of the Cardiovascular Health Study Cognition Study (CHS-CS). The CHS-CS is derived from the larger multisite CHS, a population-based longitudinal study of coronary heart disease and stroke in individuals 65 and older. Details of the CHS design are described elsewhere.20,21 Participants were recruited from a Medicare database and sent a letter of invitation for participation and were enrolled if they were over 65 years of age, ambulatory, and noninstitutionalized. Baseline measures of PA were collected in 1989–1990. Low-resolution MRI were acquired 2–3 years after baseline. Acquisition of high-resolution MRI began in 1998, approximately 9 years after the baseline assessment of PA. Clinical adjudication for cognitive impairment and dementia occurred in the same year of, and 4 years after, the MRI (13 years after baseline). Detailed information about the clinical adjudication process has been previously described.22
Characteristics of the 1,479 CHS Pittsburgh participants have been described.20 Beginning in 1988–1989, participants completed the modified Mini-Mental State Examination (3MSE) and the Digit Symbol Substitution Test (DSST). From this sample, 924 met criteria (e.g., no pacemaker) for MRI. In 1998–1999, participants from the first MRI session were recruited for another MRI session. A total of 516 individuals completed this assessment. Of this number, 299 participants (mean age = 78; 182 female) met or surpassed all criteria for this study, including cognitively normal clinical status (identified by a detailed neurologic and neuropsychological assessment22), absence of strokes or neurologic diseases (e.g., Parkinson disease) at the time of the second scan, and valid white matter (WM) hyperintensity measures from the first MRI scan (figure 1). Therefore, all participants were cognitively normal at the time of the high-resolution MRI scan. At the time of adjudication for cognitive impairment 13 years after the baseline assessment, 116 were diagnosed with dementia or mild cognitive impairment (MCI). This number is consistent with reported incidence rates for cognitive impairment.23 For logistic regression analyses, individuals with either dementia or MCI were combined into a single group that we label here as cognitive impairment.
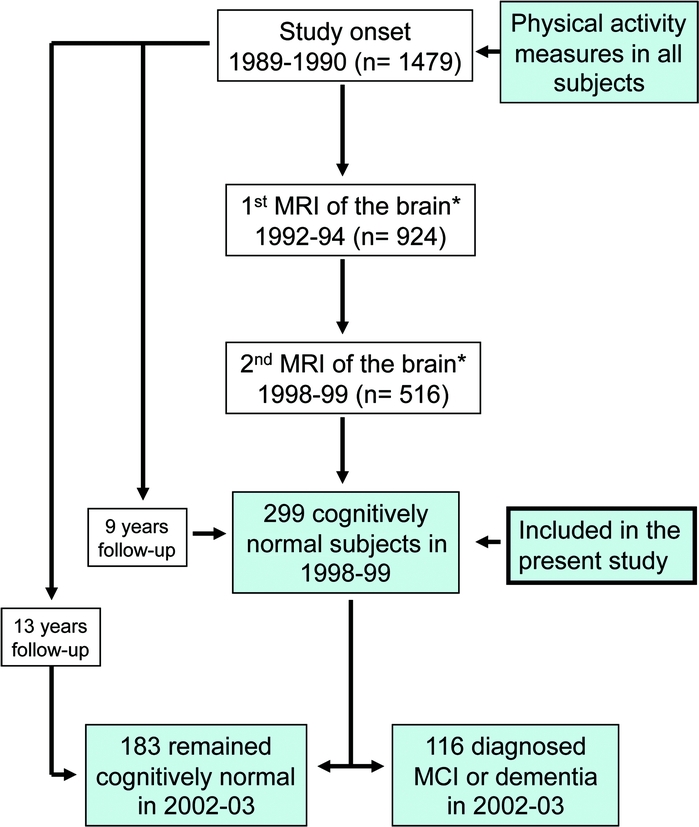
Figure 1 Subject inclusionary criteria and sample sizes
We demonstrate the longitudinal design beginning in 1989–1990 and ending with the voxel-based morphometry (VBM) analysis on high-resolution MRI data collected in 1998–1999. All participants in this sample were free of dementia and mild cognitive impairment (MCI). Originally, 1,479 individuals had physical activity assessed and 924 had a low-resolution MRI. A total of 516 of these individuals returned 5 years later for a follow-up MRI session. From these individuals, we excluded 61 with dementia, 150 with MCI, and 6 because of missing white matter grades from the first MRI assessment. Our final sample size for the VBM analysis was 299 elderly individuals between 70 and 90 years of age. *Visual rating of white matter lesions, ventricular size, atrophy, and MRI-identified infarcts.
Standard protocol approvals, registrations, and patient consents.
All participants signed an informed consent approved by the University of Pittsburgh.
Assessment of physical activity.
PA was assessed at baseline by the modified Minnesota Leisure-Time Activities Questionnaire, which evaluates the duration and frequency of PA.24,25 The total number of blocks walked over 1 week was used as the main measure of PA. Older adults report walking more than any other type of PA (e.g., tennis), and the frequency of walking is associated with greater participation in other exercises.24 Other items on the questionnaire were not used because some of them (e.g., tennis) are correlated with socioeconomic status. PA information was obtained at baseline, 9 years prior to the MRI scan.
MRI measures.
Low-resolution MRI was collected 2–3 years after baseline but was too low a quality for volumetric assessment. Instead, these data were used to quantify MRI infarcts and WM grade using previously described criteria.26
All high-resolution MRI data were acquired at the University of Pittsburgh Medical Center MR Research Center using a 1.5-T GE Signa scanner (GE Medical Systems, Milwaukee, WI, LX version). A 3-dimensional volumetric spoiled gradient recalled acquisition sequence was obtained for the whole brain (echo time/repetition time = 5/25, flip angle = 40°, number of excitations = 1, slice thickness = 1.5 mm/0 mm interslice gap) with an in-plane acquisition matrix of 256 × 256 × 124 image elements, 250 × 250 mm field of view, and an in-plane voxel size of 0.98 mm3.
Assessment of brain volume.
Voxel-based morphometry (VBM) is a technique that estimates tissue volume in a point-by-point fashion throughout the brain. This allows regionally specific conclusions about the variables of interest on differences in brain matter. Details of the VBM approach are described elsewhere,27–29 but are summarized here.
We implemented an optimized VBM approach using Statistical Parametric Mapping (SPM2) software with the addition of skull-stripping and noise reduction algorithms from the FMRI Software Library. The optimized approach reduces registration error and maintains information on tissue volumes via Jacobian-based correction.27,28 First, brain images were smoothed with a noise reduction algorithm30 and extracranial tissue was removed.31 Next, all images were transformed to a custom Pittsburgh Elderly Template32 with linear and nonlinear transformations. Images were then segmented into GM, WM, and CSF based on priors from the template. Voxel values were multiplied by the Jacobian determinant to calculate volume for each tissue type. The volumes of GM, WM, and CSF were summed to compute total intracranial volume (TIV). The final images were smoothed using a 10-mm (full width at half maximum) Gaussian kernel. Eigenvectors reflecting a weighted mean after controlling for the covariates (see below) were extracted from significant clusters and further analyzed in relation to cognitive impairment and dose response in the Statistical Package for Social Sciences (SPSS, v 16.0 SPSS Inc., Chicago, IL).
Statistical analysis.
The relationship between walking and brain volume was assessed using the General Linear Model in SPM. Voxelwise analyses were conducted with blocks walked as a continuous variable in a multiple regression model with age, TIV, gender, body mass index, race, WM grade, MRI infarcts, ventricular grade, the time taken to walk 15 feet, and education entered as covariates of no interest. Covariates were chosen based on correlations with either PA or brain volume or because correlations between these variables were reported in prior studies.2–6 Due to non-normality in the number of blocks walked, we also used log-transformed values. Values for all covariates were collected at baseline. Other potentially confounding variables such as self-report health scores, hypertension, type II diabetes, APOE genotype, subclinical vascular disease, and basic and incidental activities of daily living were unrelated to the number of blocks walked or GM volume, and were not included in the regression models. GM atrophy occurs with higher WM grade,33 thus WM and ventricular grade served as a proxy for GM integrity at baseline. WM grade and the number of MRI infarcts were quantified on the low-resolution MRI using established criteria.26 The time taken to walk 15 feet served to control for mobility differences. Statistical images were thresholded to ensure a false discovery rate of less than 5%.28 To minimize the likelihood that any cluster of significant voxels could appear by chance, we applied a cluster threshold to ensure that at minimum, significant clusters contained at least 100 contiguous voxels.
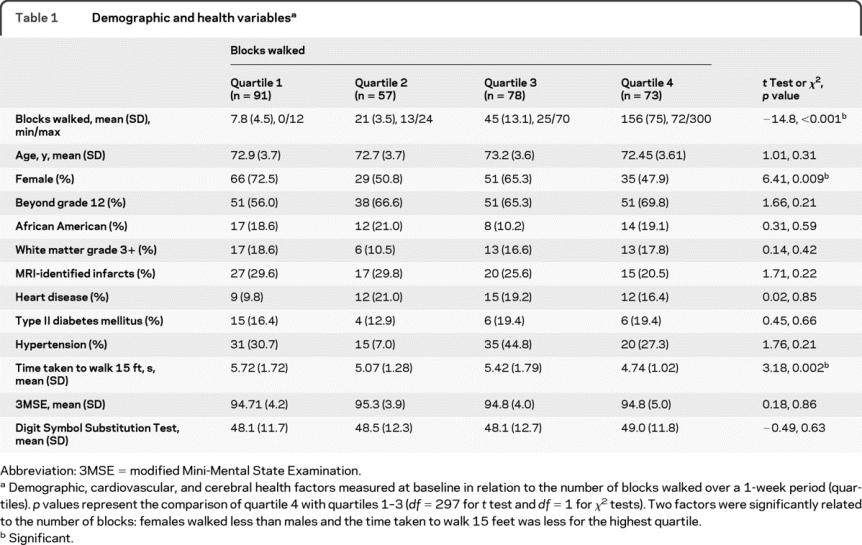
We hypothesized that a lower-bound threshold of PA is necessary to detect differences in GM volume. To explore this, we divided the participants into quartiles based on blocks walked. This resulted in groups that walked between 0 and 12 (Q1; n = 91), 13 and 24 (Q2; n = 57), 25 and 70 (Q3; n = 78), and 72 and 300 blocks (Q4; n = 73) over a 1-week period (table 1). We then examined in an analysis of covariance whether the quartiles differed in any brain region after controlling for covariates.
Table 1 Demographic and health variables
Finally, in a series of logistic regression analyses adjusting for age, gender, education, race, time taken to walk 15 feet, and APOE allele status, we examined whether PA measured at baseline was predictive of cognitive impairment 13 years later and whether the GM volume or GM volume residuals associated with greater amounts of PA would predict cognitive impairment 4 years after the MRI assessment. Cognitive impairment was determined by clinical adjudication and included individuals with either a diagnosis of dementia or MCI.22 We report odds ratios (OR) resulting from these analyses.
RESULTS
Physical activity and brain volume.
The total number of blocks walked over a 1-week period ranged from 0 to 300 (mean = 56.3; median = 25; SD = 69.7). At the baseline assessment, females reported walking less than males (r = −0.14; p < 0.01). No other variables at baseline or at the time of the MRI assessment were correlated with the number of blocks walked (table 1) including hypertension, heart disease, or WM lesions. Number of blocks walked was also unrelated to the presence of cerebral infarcts, to other measures of vascular health, and to cognitive test scores at baseline. However, individuals in the highest quartile group (see below) were faster on the time to walk 15 feet test than the other 3 quartiles. The time to walk 15 feet was included as a covariate for possible mobility confounds in all analyses.
We found that walking distance assessed at baseline predicted greater volume of GM tissue 9 years later. This effect remained significant after adjusting for age, TIV, gender, WM grade, ventricular grade, MRI infarcts, the time taken to walk 15 feet, body mass index, race, and education (figure 2A). These areas included the frontal, temporal, and occipital lobes (figure 2A). Both the entorhinal cortex and hippocampus were also related to walking distance with larger volumes associated with greater PA. These patterns were unchanged when using log-transformed values for the number of blocks walked. There were no regions showing greater volume with less PA and no significant interactions between blocks walked with age or gender (both p > 0.05).
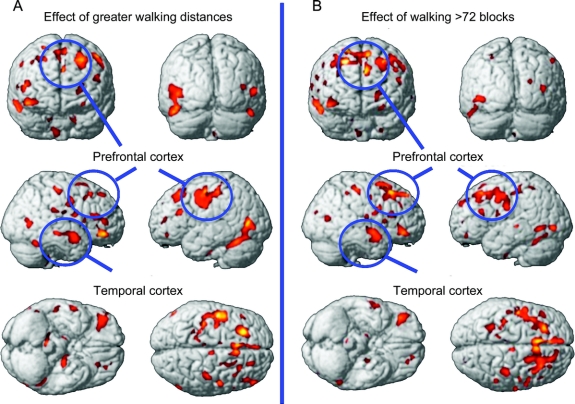
Figure 2 Brain regions associated with greater walking
(A) Brain regions showing an association between greater amounts of physical activity (blocks walked) at baseline and greater gray matter volume. Statistical map is thresholded with a false discovery rate of p = 0.05 and a minimum cluster threshold of 100 contiguous voxels. (B) Brain regions showing greater volume in the highest quartile (>72 blocks walked in 2 weeks) compared to the bottom 3 quartiles. There were no reliable differences in brain volume among the bottom 3 quartiles.
Physical activity threshold.
After splitting the participants into quartiles (Q1, Q2, Q3, Q4) based on the number of blocks walked, we found that GM volume in the highest quartile (Q4) differed from the other 3 quartiles (all p < 0.05; figure 2B). This effect was consistent in all brain regions that reached significance in the regression analyses reported above including the frontal cortex, the temporal lobes, and the hippocampal formation (figure 3 and table e-1 on the Neurology® Web site at www.neurology.org). There were no differences in GM volume among the lower 3 quartiles (all p > 0.10). Because the upper quartile was the only group that demonstrated greater GM volume than the other groups, we can assert that a large amount of PA is necessary to detect a difference in brain structure over a 9-year follow-up period.
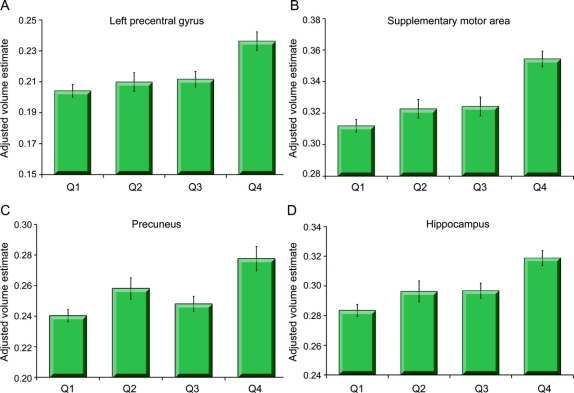
Figure 3 Threshold effects on brain volume
Mean volumes (and SEM) of 4 brain regions (precentral gyrus [A], supplementary motor area [B], precuneus [C], and hippocampus [D]) adjusted for variance due to age, total intracranial volume, gender, body mass index, race, white matter grade, presence of MRI infarcts, and education split into quartiles based on the amount of physical activity (Q1: 0–12 blocks, n = 91; Q2: 13–24 blocks, n = 57; Q3: 25–70 blocks, n = 78; Q4: 72–300 blocks, n = 73). The highest quartile group (Q4) had greater volume in all regions examined compared with the lower 3 quartiles. No significant differences were found among the lower 3 quartiles.
Physical activity and risk of cognitive impairment.
Of the original sample (n = 299), 116 developed MCI (n = 64) or dementia (n = 52), while 169 remained cognitively normal (14 were deceased before the follow-up). The individuals with MCI or dementia were combined to form a single group (see Methods) and this group was used in subsequent analyses. Using logistic regression, PA was marginally related to a reduced risk of experiencing cognitive impairment 13 years later (OR = 3.20; p < 0.07). Measures of GM volume were not predictive of cognitive impairment (appendix e-1). However, we hypothesized that the cortical effects associated with walking greater distances would be associated with a reduced risk of developing cognitive impairment. This analysis required the use of the residual GM values from the multiple regression analysis of all covariates on GM volume. This effectively removed variance associated with the covariates and left the volume of tissue in regions that was associated with PA.
Using the adjusted GM volume values in logistic regression, we found that greater volume of the inferior frontal gyrus (OR = 1.99; p < 0.01), hippocampal formation (OR = 2.01; p < 0.009), and supplementary motor area (OR = 2.24; p < 0.01) were associated with a reduced risk of developing cognitive impairment. In short, walking greater distances was associated with greater GM volume in specific regions, and greater GM volume was associated with a lower risk for experiencing cognitive impairment in later years.
DISCUSSION
Brain tissue deteriorates in late adulthood, but greater amounts of PA have been hypothesized to spare brain tissue.2,3,19 In support of this hypothesis, we report that walking greater distances was associated with greater GM volume 9 years later. This effect was significant even after controlling for measures of WM lesions and MRI infarcts at baseline as well as several factors related to general health and disease such as subclinical vascular disease, ventricular grade, and hypertension. The effect was predominant in prefrontal and temporal brain regions including the hippocampus and entorhinal cortex, regions susceptible to age-related deterioration.1
The results of this study establish 3 critical findings. First, greater amounts of PA are predictive of greater GM volume 9 years later. Second, walking relatively long distances (72 blocks or roughly 6–9 miles per week depending on the city) was necessary to detect differences in GM 9 years after the baseline evaluation of PA. Third, greater GM in the inferior frontal gyrus, the hippocampus, and the supplementary motor area was associated with a reduced risk of developing cognitive impairment (MCI or dementia).
Research in rodents provides a mechanistic explanation for the effect of exercise on brain volume and function in humans. Our results are in line with data that aerobic activity induces a host of cellular cascades that could conceivably increase GM volume. For example, running enhances learning and promotes the proliferation and survival of new neurons in the hippocampus.34 The addition of new cells requires increased nutrients, which are supplied by new vasculature.35 In a mouse model of AD, exercising animals show a reduction in β-amyloid deposits,36 reduced tau formation,37 and superior learning rates compared to sedentary animals.38
Our results are encouraging, but need to be interpreted in light of some important limitations. First, we used self-reported PA. Objective assessments of PA would help in assessing the magnitude of protection to the brain. Second, we used only a single assessment of brain volume. Future research should examine the effect of PA on brain morphology using multiple neuroimaging time points to determine whether PA moderates the rate of GM decay. Third, given the observational nature of the study, we are unable to conclude that PA causes greater GM volume. Despite controlling for several health factors that could have explained the GM-walking relationship, there remains a possibility that reduced amounts of walking is a result of ill health and that ill health leads to both reduced amounts of walking and GM volume loss. Fourth, the results from this study are restricted to survivors over the 9-year period who were free from cognitive impairment at the time of the MRI assessment. Thus, our sample was a healthier sample than those originally enrolled in the CHS study.
Strengths include the large sample size, a well-characterized cohort followed for over 13 years, and clinical adjudication of dementia. Based on our results, we can conclude that there is a relation between the amount of walking earlier in life and brain volume in later adulthood and that greater volume of tissue related to walking is associated with a reduced risk of cognitive impairment. Greater walking distances are associated with greater GM volume in a time period of life in which cortical deterioration and risk for dementia is greatest.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Erickson and Dr. Raji.
COINVESTIGATORS
Jean Olson, MD, MPH (National Heart, Lung, and Blood Institute, Project Office), Richard Kronmal, PhD (University of Washington, CHS Coordinating Center), John Robbins, MD, MHS (University of California, Davis, CHS Field Center), Paulo H. Chaves, MD, PhD (The Johns Hopkins University), Linda P. Fried (The Johns Hopkins University, CHS Field Center), Gregory Burke, MD (Wake Forest University School of Medicine, CHS Field Center), Russell Tracy, PhD (University of Vermont, CHS Collaborating Center), John Gottdiener, MD (University of Maryland, CHS Collaborating Center), Richard Prineas, MD, PhD (Wake Forest University School of Medicine, CHS Collaborating Center), Paul Enright, MD (University of Arizona, CHS Collaborating Center), Ronald Klein, MD (University of Wisconsin, CHS Collaborating Center), Daniel H. O'Leary, MD (Tufts New England Medical Center, CHS Collaborating Center).
DISCLOSURE
Dr. Erickson reports no disclosures. Dr. Raji receives research support from the American Heart Association. Dr. Lopez has served on scientific advisory boards for Pfizer Inc., Eisai Inc., and Bristol-Myers Squibb; and receives research support from the NIH (NIA P50 AG05133-24 [PI], AG20098-07 [PI], and NCCAM 5 U01 AT00162-08 [PI]). Dr. Becker serves on a scientific advisory board for and has received funding for travel from Fundacio ACE; serves on the editorial board of AIDS; and receives research support from the NIH (U01AI035041 [co-I], R03MH081723 [PI], R03MH081721 [PI], R03DA025986 [PI], P01AG05133 [co-PI], R01AG20098 [co-I], and R01MH072947 [co-I]) and the Fulbright Commission. Dr. Rosano receives research support from the NIH (RO1 AG29232 [co-I] and RO1 AG031118 [co-I]). Dr. Newman serves as an Associate Editor of the Journal of Gerontology Medical Sciences and receives research support from the NIH (R01-AG-029824 [PI], U01-AG-022376 [PI], RC2-AG-036594 [PI], U01 AG023744 [PI], R01 AG023629 [PI], N01 AG62101 [PI], N01 AR12262 [co-I], R01 AG028050 [co-I], R01 AG29232 [co-I], R01-AG-03073 [co-I], R01-HS-017695 [co-I], R01-AG-034056 [co-I], and P30 AG024827 [co-I]). Dr. Gach receives research support from the US Department of Defense and the NIH (R01 AG20098 [co-I]). Dr. Thompson serves on editorial advisory boards for IEEE Transactions on Medical Imaging, Human Brain Mapping, Medical Image Analysis, Cerebral Cortex, Current Medical Imaging Reviews, Inverse Problems and Imaging, and Translational Neuroscience; and receives research support from the NIH (R01 EB008432 [PI], NIBIB R01 EB008281 [PI], NIBIB R01 EB007813 [PI], R01 HD050735 [Subcontract PI], RC2 AG036535 [Subcontract PI], NCRR U54 RR021813 [Project PI], NCRR P41 RR013642 [Project PI], and R01 AG020098 [Subcontract PI]). A.J. Ho and Dr. Kuller report no disclosures.
Supplementary Material
Address correspondence and reprint requests to Dr. Kirk I. Erickson, Department of Psychology, 3107 Sennott Square, 210 S. Bouquet St., Pittsburgh, PA 15260 kiericks@pitt.edu
Supplemental data at www.neurology.org
e-Pub ahead of print on October 13, 2010, at www.neurology.org.
*These authors contributed equally to this work.
Study funding: Supported by the National Institute on Aging AG-023629. C.H.S. was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging, R01 HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827 and an American Heart Association predoctoral grant to C.A.R. (0815465D).
Disclosure: Author disclosures are provided at the end of the article.
Received March 15, 2010. Accepted in final form July 22, 2010.
REFERENCES
- 1.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005;15:1676–1689. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11:342–348. [DOI] [PubMed] [Google Scholar]
- 3.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 2008;9:58–65. [DOI] [PubMed] [Google Scholar]
- 4.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 2003;58:176–180. [DOI] [PubMed] [Google Scholar]
- 5.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer's disease. Neurology 2008;71:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honea RA, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer's disease. Alzheimer Dis Assoc Disord 2009;23:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009;19:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans: evidence from a randomized clinical trial. J Gerontol A Biol Sci Med Sci 2006;61:1166–1170. [DOI] [PubMed] [Google Scholar]
- 9.Andel R, Crowe M, Pederson NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci 2008;63:62–66. [DOI] [PubMed] [Google Scholar]
- 10.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 2003;51:459–464. [DOI] [PubMed] [Google Scholar]
- 11.Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol 2003;25:643–653. [DOI] [PubMed] [Google Scholar]
- 12.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–81. [DOI] [PubMed] [Google Scholar]
- 13.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005;161:639–651. [DOI] [PubMed] [Google Scholar]
- 14.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA 2004;292:1454–1461. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 2001;161:1703–1708. [DOI] [PubMed] [Google Scholar]
- 16.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 2010;67:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: the Health ABC Study. Neurology 2009;72:2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson KI, Kramer AF. Exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med 2009;43:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 21.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 22.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol 2003;60:1385–1389. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky S. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1131–1132. [DOI] [PubMed] [Google Scholar]
- 24.McPhillips JB, Pellettera KM, Barrett-Conner E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72. [PubMed] [Google Scholar]
- 25.Siscovick DS, Fried LP, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. Am J Epidemiol 1997;145:977–986. [DOI] [PubMed] [Google Scholar]
- 26.Longsreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: The Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner JA, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2001;14:1238–1241. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36. [DOI] [PubMed] [Google Scholar]
- 29.Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev 2005;11:1–9. [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23:208–219. [DOI] [PubMed] [Google Scholar]
- 31.Smith S. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spears JR, Greer PJ, Ziolko SK, et al. Evaluation of an age-specific neurological template. Annual meeting of the Organization of Human Brain Mapping; Toronto, Canada; June 2005.
- 33.Wen W, Sachdev PS, Chen X, Anstey K. Gray matter reduction is correlated with white matter hyperintensity volume: a voxel-based morphometric study in a large epidemiological sample. NeuroImage 2006;29:1031–1039. [DOI] [PubMed] [Google Scholar]
- 34.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neuroscience 1999;2:266–270. [DOI] [PubMed] [Google Scholar]
- 35.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 1990;87:5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation 2008;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res 2009;87:2561–2570. [DOI] [PubMed] [Google Scholar]
- 38.Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res 2007;184:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.