Anti-NMDA receptor encephalitis is characterized by dyskinesias, psychosis, and seizures1 secondary to antibodies to the NR1-NR2B heteromer of the NMDA receptor.2 This syndrome, more common in women, is often related to an ovarian tumor1; the prognosis is better if the tumor is identified within 3 months of onset. Our case had nonconvulsive status epilepticus lasting 6 months, with marked improvement following removal of the ovarian tumor.
Case report.
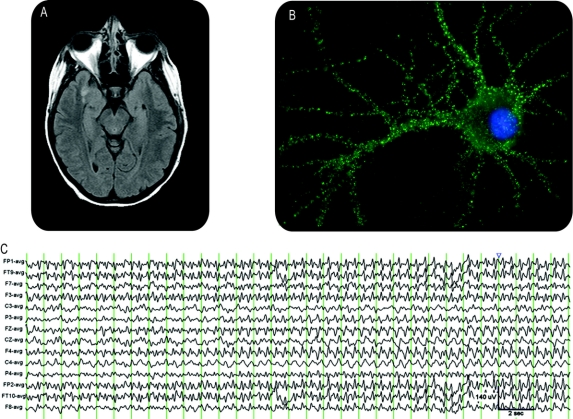
A 35-year-old woman, previously healthy and without history of psychosis, had a 3-week history of progressive headaches, short-term memory loss, and irritability. On admission, she was psychotic, requiring physical and chemical restraints. She was unable to follow commands, had echolalia, and had occasional dystonic posturing of her limbs. One week later, she became unresponsive to external stimuli. An EEG demonstrated persistent nonconvulsive status epilepticus (NCSE). Initial CSF studies were remarkable for a lymphocytic pleocytosis with 386 leukocytes. Brain MRI showed diffusion-weighted imaging hyperintensity in the right medial temporal lobe transitioning to fluid-attenuated inversion recovery hyperintensity on repeat imaging (figure 1A). Tests for multiple viral and bacterial pathogens, including herpes simplex virus, were negative. A paraneoplastic panel was negative for clinically available antibodies with the exception of a neuron-specific antibody, not otherwise identified. The CSF, sent to the University of Pennsylvania, had an antibody for the NR1/NR2B heteromer of the NMDA receptor (figure 1B).
Figure 1 Electrical, imaging, and antibody studies
(A) MRI fluid-attenuated inversion recovery sequence demonstrating left medial temporal lobe hyperintensity. (B) Cultured rat hippocampal neuron incubated with patient's CSF demonstrates antibodies to the NR1/NR2 heteromers. (C) EEG findings.
She was intubated and monitored with continuous EEG, which showed a cyclical pattern of moderate to high voltage, generalized, anteriorly biased 2-Hz alternating with 5–6 Hz sharp wave activity that lacked a triphasic morphology (figure 1C). The NCSE was refractory to phenytoin, levetiracetam, and valproic acid, and incompletely suppressed by benzodiazepines. Propofol produced abrupt cessation of this rhythmic pattern, thus supporting an epileptic etiology (figure e-1 on the Neurology® Web site at www.neurology.org). Pentobarbital was used to maintain EEG burst-suppression; she remained in this state for 5 months, with each attempt to minimize the depth of pentobarbital coma resulting in resumption of electrical status epilepticus. Three months into the course further reduction of pentobarbital produced a distinct EEG pattern with moderate voltage diffuse spindle-like rhythms and diffuse slowing consistent with sedation and independent, discrete, left and right hemispheric seizures at a rate of 3–4 per hour, lasting 30 seconds to 3 minutes. Rhythmic sharp wave activity evolved stereotypically in each hemisphere and was accompanied by subtle myoclonus and tonus.
To suppress the NMDA receptor antibody, she received IV immunoglobulin, rituximab, and cyclophosphamide sequentially, without improvement. Multiple CT scans and ultrasounds of her ovaries revealed only a hemorrhagic cyst. After 5 months in pentobarbital-induced burst suppression, she had an oopherectomy, with pathologic examination identifying an ovarian teratoma.
Phenobarbital was weaned. Five weeks after oopherectomy, her EEG showed reemergence of irregular faster frequencies without periodic or epileptiform features; later, recognizable sleep-wake cycles and normal waking organization emerged. Two weeks postoperatively she awakened, shaking her head in response to questions, and within 4 weeks she was alert and conversant. She had depressive symptoms and intermittent hallucinations that resolved over several months. Neuropsychiatric testing 6 months following resolution of status epilepticus revealed mild deficits on naming and memory tests without functional impairments. She has not had recurrent seizures.
Discussion.
Our case is typical in many respects of the anti-NMDA antibody syndrome, with a prodrome of headaches, confusion, and psychosis prior, but which progressed to prolonged NCSE. There was dramatic improvement with removal of the ovarian teratoma.
Prolonged NCSE carries a poor prognosis, with a mortality rate of 56%.3 The ictal EEG showed continuously evolving diffuse, rhythmic slow and sharp waves, an unusual ictal pattern for a late phase of prolonged or refractory status epilepticus.4 Many rhythmic and periodic patterns may mimic status epilepticus but are primarily encephalopathic including treatment-responsive rhythmic triphasic waves5 and barbiturate-induced rhythmic triphasic wave patterns.6 In our case, the initial EEG pattern of sharp rhythms cyclically evolving in frequency and spatial distribution, its cessation with sufficient antiseizure treatment, and later development of more overt independent bihemispheric electrical seizures on lightening of sedation argue for a genuinely epileptic etiology, further supported by resolution with resection of her teratoma.
Anti-NMDA receptor encephalitis may respond to IV immunoglobulin, cyclophosphamide, or rituximab.7 However, this patient's seizures were refractory to all immunomodulatory therapy. Despite imaging studies to the contrary, it was only upon removal of her ovaries that a teratoma was identified. Perhaps in cases where anti-NMDA receptor encephalitis proves refractory to immunomodulatory therapies, oopherectomy may be a prudent therapeutic approach. Finally, this case illustrates the need to consider anti-NMDA receptor encephalitis as a cause of refractory status epilepticus should associated features be present, as this diagnosis carries a favorable prognosis.
Supplementary Material
e-Pub ahead of print on September 8, 2010, at www.neurology.org.
Disclosure: Dr. Johnson reports no disclosures. Dr. Henry receives research support from Schwarz Pharma, UCB, King Pharmaceuticals, GlaxoSmithKline, NeuroPace, Inc., Supernus Pharmaceuticals, Inc., Sepracor Inc., and Eisai Inc. Dr. Fessler has served on a scientific advisory board and a speakers' bureau for UCB and receives research support from Supernus Pharmaceuticals, Inc., Sepracor Inc., GlaxoSmithKline, NeuroPace, Inc., Schwarz Pharma, UCB, Eisai Inc., King Pharmaceuticals, Marinus Pharmaceuticals, Inc., Lundbeck Inc. (Ovation), and Ortho-McNeil-Janssen Pharmaceuticals, Inc. Dr. Dalmau receives research support from Euroimmun and the NIH/NCI (RO1CA107192 [PI] and RO1CA89054-06A2 [PI]); has received license fee payments from Euroimmun for a patent re: NMDA receptor autoantibody test; and has received royalty payments and may accrue revenue for a patent re: Ma2 autoantibody test.
Received January 25, 2010. Accepted in final form May 17, 2010.
Address correspondence and reprint requests to Dr. Nicholas Johnson, 601 Elmwood Ave., Box 673, Rochester, NY 14642; Nicholas_Johnson@urmc.rochester.edu
Supplemental data at www.neurology.org
&NA;
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Tuzun E, Wu H, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drislane FW, Blum AS, Lopez MR, et al. Duration of refractory status epilepticus and outcome: Loss of prog-nostic utility after several hours. Epilepsia 2009;50:1566–1571. [DOI] [PubMed] [Google Scholar]
- 4.Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res 1990;5:49–60. [DOI] [PubMed] [Google Scholar]
- 5.Lancman ME, Marks S, Mahmood K, et al. Atypical triphasic waves associated with the use of pentobarbital. Electroencephalogr Clin Neurophysiol 1997;102:175–177. [DOI] [PubMed] [Google Scholar]
- 6.Fountain NB, Waldman WA. Effects of benzodiazepines on triphasic waves: implications for nonconvulsive status epilepticus. J Clin Neurophysiol 2001;18:345–352. [DOI] [PubMed] [Google Scholar]
- 7.Ishiura H, Matsuda S, Higashihara M, et al. Response of Anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology 2008;71:1921–1923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.