Abstract
Studies support the concept that irritable bowel syndrome (IBS) is a biopsychosocial disorder that can be explained by a neurobiological model which postulates stress-induced alterations in central stress and arousal circuits and activation of parallel motor outputs from brain regions that can affect bodily function and behavior. Sustained stress can result in chronic overactivity or underactivity of allostatic (or adaptive) systems, including the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system, metabolic, and immune systems, can occur. Animal and human studies have demonstrated that chronic or sustained stress is associated with the onset and exacerbation of symptoms of IBS. Chronic stress is also an independent predictor of developing post-infectious IBS. IBS patients specifically show stress-induced alterations in gastrointestinal motility, rectal perception, autonomic tone and HPA axis responses, although these findings are not entirely consistent among studies. This can be in part due to differences in study methodology or to various factors that can affect these physiologic responses. A greater recognition and understanding of the effects of stress in IBS may help identify targets for future drug development and also help guide more effective management of IBS symptoms.
Keywords: irritable bowel syndrome, stress, early adverse life events, corticotropin-releasing factor, hypothalamic-pituitary-adrenal (HPA) axis, cortisol, autonomic nervous system
Introduction
Stressors can be acute or chronic and range from daily hassles to life-threatening situations, such as natural disasters and violence that trigger the “fight or flight” response. Over time, chronic or recurrent stress results in an increase demand on physiologic systems. The wear and tear on the body, termed “allostatic load,” set in motion long-term behavioral patterns, physiological reactivity, and other changes in the body that can lead to disease and health-damaging behaviors.1 Chronic overactivity or underactivity of allostatic (or adaptive) systems can occur.2 Allostatic systems include the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system (ANS), and cardiovascular, metabolic, and immune systems. Allostatic load clinically manifests as undue fatigue, irritability, and feelings of demoralization3 among other visceral and somatic symptoms that are often reported in patients with irritable bowel syndrome (IBS) and other functional pain syndromes that commonly overlap with IBS.4
Increasing evidence supports a prominent role of stress in the pathophysiology and clinical presentation of IBS. It has been postulated that in the predisposed individual, sustained stress can result in persistent increased responsiveness of central stress circuits and vulnerability to develop functional and affective disorders (Figure A, supporting document).5 The role of stress may be particularly important in altering brain-gut interactions, resulting in the development and/or exacerbation of IBS symptoms. A conceptual pathophysiologic model for IBS can take into account the reported relationship of IBS symptoms with central factors such as stressful6–8 or traumatic9 life events, the frequently reported co-morbidity with anxiety disorders,10 and peripheral factors such as gut inflammation, motility, and sensation. Understanding the biologic mechanisms of stress hyperresponsiveness in IBS patients may help identify targets for drug development. Recognition of IBS patients with stress-related IBS symptoms can help guide management approaches, including earlier and more effective treatment interventions which can potentially result in a decrease in the disproportionately high healthcare costs and economic burden associated with IBS. The objectives of this review are to: 1) discuss the role of stress in the clinical presentation and course of IBS and 2) the physiologic effects of chronic stress on gastrointestinal (GI) function and central stress response systems. While this review will focus on human studies conducted in IBS, relevant preclinical and translational studies are also discussed.
Effect of chronic stressors on the development of IBS and health outcomes
The association of early adverse life events (EALs) and IBS
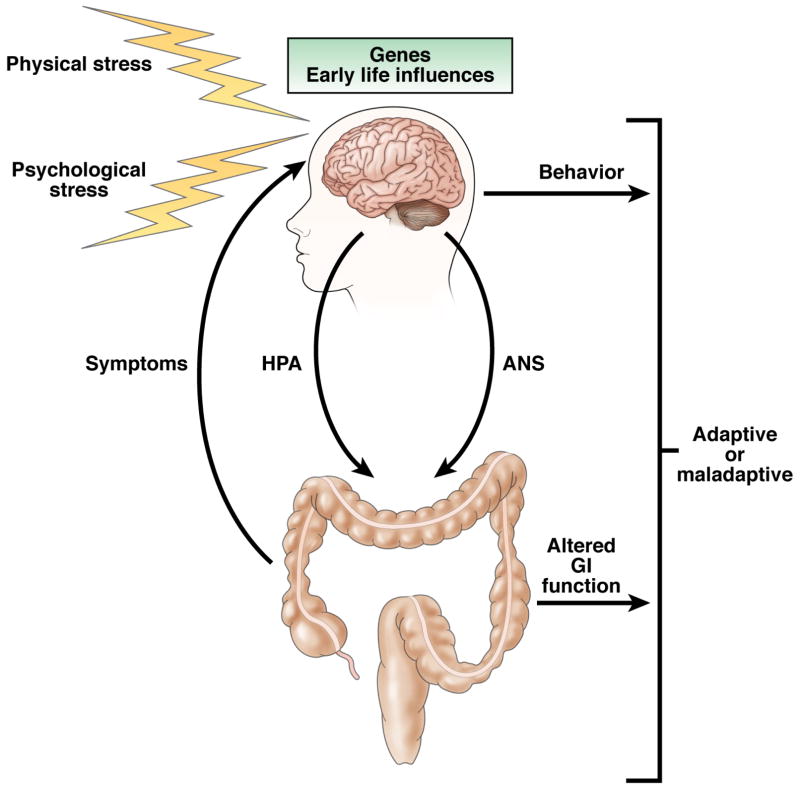
EALs refer to traumatic experiences during childhood including, but not limited to, maladjusted relationships with a parent or primary caregiver, severe illness or death of a parent, and physical, sexual or emotional abuse. Converging neurobiology and epidemiology studies suggest that EALs, such as abuse and other traumatic events, cause sustained brain dysfunction resulting in alterations in stress-responsive neurobiological systems, which in turn, increase the vulnerability of developing long-term health, behavioral and social problems (Figure 1).11, 12 Medical conditions associated with EALs include autoimmune disorders, chronic obstructive lung disease, asthma, obesity, mood disorders, substance abuse, and prescription drug use.11, 13, 14
Figure 1. Physiologic and pathologic responses to stress.
An adult’s resilience or vulnerability to stress can be determined by genetic inherence and early life experiences. Activation of the central stress response sets in motion the HPA axis, ANS and other adaptive systems. Stress-induced changes in GI function occur and these can in turn result in perceived symptoms of IBS. (Adapted from Lightman, 200884)
Previous studies demonstrated that IBS patients have experienced a greater prevalence of physical, verbal, and sexual abuse compared to healthy individuals and patients with organic GI conditions.15, 16, 17, 18, 19 While other types of EALs have not been well studied in IBS, studies suggest that perturbations during the pre- and perinatal period might contribute to the development of IBS in adults.15
A recent preliminary study conducted in 233 IBS patients and 353 healthy controls showed that IBS was associated with a higher total early life trauma score, including general trauma, and physical, emotional and sexual abuse events under the age of 18, even after controlling for anxiety and depression.20 However, somatization was the strongest predictor of IBS and mediated the association of early life trauma score and IBS, suggesting that factors associated with the presence of widespread non-GI symptoms mediate the relationship between childhood trauma or abuse and development of IBS. Previous studies have demonstrated that somatization is associated with a history of sexual and/or physical abuse in patients with functional gastrointestinal disorders, and that it impacts health related quality of life (HRQOL).9, 14, 21
There is evidence to support that stress affects the clinical outcome in IBS. In a prospective study, Bennett et al.7 showed that the presence of at least one chronic life stressor during the first six months of the observational period was highly predictive of symptom intensity at 16 months, explaining 97% of the variance. All patients who experienced symptom improvement did not report a current chronic life stressor. Drossman et al.22 found that an abuse history was associated with greater pain, bed disability days, psychological distress and daily function in GI clinic patients independent of their diagnosis.
Chronic stress and psychological symptoms independently predict PI - IBS
Studies implicate chronic stress as playing a significant role in increasing the vulnerability to develop IBS.23 Gwee and colleagues8 found that stressful life events in the one year preceding gastroenteritis and the hypochondriasis score independently predicted PI-IBS even after controlling for age, sex, marital and employment status, and stool culture results. A subsequent study by Dunlop et al.24 found that rectal mucosal enterochromaffin cell count and depression score were independently associated with PI-IBS. These studies suggest that both biological and psychological factors are important in the development of PI-IBS.
Bidirectional brain -gut interactions and central stress response
Studies support the concept that IBS is a biopsychosocial disorder which can be explained by a neurobiological model which postulates stress-induced alterations in central stress and arousal circuits, referred to as the emotional motor system (EMS), which is comprised of parallel motor outputs from the brain regions involved in emotional function.23, 25 These outputs include the sympathetic and parasympathetic nervous systems, HPA axis, endogenous pain modulation systems, and ascending aminergic pathways. A variety of stressors can activate these output systems and affect bodily functions and behavior.23
Stress results in increased corticotropin releasing factor (CRF) and noradrenergic release and activation of behavioral and autonomic responses. The principal branches of the general stress response are the HPA axis and the locus coeruleus-noradrenergic (LC-NE) systems.1 In response to stress, CRF activates the HPA axis and the autonomic nervous system (ANS), including activation of noradrenergic-containing sites, e.g., LC-NE system, and other neurotransmitters that are involved in maintaining homeostasis. Release of CRF from the paraventricular nucleus (PVN) of the hypothalamus is under excitatory input from the central nuclei of the amygdala and inhibitory input from the hippocampus (Figure B, supporting documents).26 In addition to its major role in regulating the endocrine response to stress, CRF is crucially involved in mediating stress-related autonomic, immune, behavioral, and visceral responses.27–29
Stress-induced alterations in GI function in IBS
Although the effects of stress on gut function are universal, patients with IBS appear to have greater reactivity to stress compared with healthy individuals in terms of gut motility, visceral perception, emotional ratings and HPA axis hormone levels (Table 1).30–33
Table 1.
Summary of stress -induced physiologic changes in IBS
| Function | Findings in IBS vs. controls |
|---|---|
| GI motility | Suppressed antral and small bowel motor activity and enhanced colonic motor activity |
| Visceral perception | Decreased rectal non-painful and pain thresholds to distension and electrostimulation during psychological stress in IBS but not in controls Higher stress, anxiety and anger ratings higher in IBS vs. controls |
| Intestinal permeability and secretion | Increased small intestinal and colonic permeability demonstrated in IBS but not measured in response to stress Net water flux was significantly lower in healthy women with moderate stress compared to those with low stress. Chloride secretion was lower and albumin was higher in moderate stress vs. low stress but not statistically significant |
| Autonomic tone | Increases in blood pressure and heart rate and shift to lower cardiosympathetic/vagal balance after mental stress in IBS and controls but no group differences |
| HPA axis | Increased basal levels of cortisol in IBS vs. controls Two studies show increased HPA axis response and one shows blunted response to hormone stimulation in IBS vs. controls Most studies report lack of a response to a meal and/or mental stressor in IBS HPA axis response varies depending on the type of physical stressor |
GI motility
Experimental stress can provoke alterations in GI motility. Over 20 year ago, Welgan and colleagues demonstrated that IBS patients had higher colonic motor activity at the resting state compared to healthy controls and showed greater increases with various psychological and physical stressors compared to baseline, while controls did not.30, 34 In one of Welgan’s studies, IBS patients exhibited both greater increases in colonic motor and spike potential activity and anger ratings during anger stressors than controls.30 Several other studies have demonstrated alterations in upper gut (antrum and proximal small bowel) to stress in IBS patients.35–38 These studies support the impact of stress on alterations in upper and lower gut motility in IBS patients.
Visceral perception
In experimental studies, stress has been associated with an increase in visceral perception. Posserud and colleagues33 demonstrated that while healthy controls had higher sensory thresholds (decreased perception) to non-painful rectal distensions during mental stress, IBS patients showed no change. Furthermore, sensory thresholds significantly decreased (increased perception) in IBS patients but not controls during subsequent distensions whether or not mental stress was given. In addition, IBS patients reported significantly higher ratings of stress during the rectal distensions compared to controls. Dickhaus et al.31 reported significantly higher intensity and unpleasantness sensory ratings to phasic 45 mmHg rectal distensions in IBS patients during dichotomous listening auditory stress but not during the control condition of relaxing sounds. Emotional ratings of stress, anger, and anxiety were significantly higher during the stressful vs. relaxation condition in IBS patients but not in controls. Murray and colleagues39 found that lower non-painful and painful thresholds to rectal electrostimulation during a physical (cold water hand immersion) or psychological stressor (dichotomous listening) in IBS patients but not in controls.
Although these studies support a greater visceral perceptual and emotional responsiveness to stress in IBS patients, the significant findings were detected by within-group comparisons rather than between-group comparisons with controls. Group differences may be due to smaller than required sample sizes to detect significant stress-induced perceptual differences between IBS patients and controls.
Intestinal secretion and permeability
There is evidence that stress and IBS are associated with changes in intestinal secretion and permeability, but there are no definitive data that stress induces alterations in epithelial secretion and permeability in IBS patients. Two studies reported increased small intestinal permeability in some patients with IBS-D compared to healthy controls.40, 41 Barbara and colleagues demonstrated that colonic biopsies from IBS patients had increased paracellular permeability42 and release of mediators including tryptase, histamine and prostaglandin E243 compared to that from healthy controls. Furthermore, the supernatant from colonic biopsy cultures from IBS patients reduced transepithelial resistance and ZO-1 mRNA expression and increased permeability of human intestinal epithelial Caco-2 cells.42 Animal studies suggest that stress-induced increases in paracellular and transcellular permeability in the ileum and colon are mediated via CRF1 receptors.44
A recent study conducted in healthy young women showed that the physical stressor of cold water hand immersion was associated with changes in net water flux and chloride and albumin outputs from the jejunum.45 This study suggests that acute stress can alter small intestinal epithelial secretion in healthy women particularly in the presence of underlying chronic stress, but further studies are needed, particularly in patients with IBS.
Alterations in the central stress response in IBS
Autonomic nervous system (ANS) tone
The sympathetic and parasympathetic branches of the ANS mediate brain-gut communication largely through modulation of the third ANS branch, the enteric nervous system, and alterations in ANS output and interactions may play a role in IBS. 46, 47 Through its three divisions, the ANS modulates and coordinates GI motility, secretion, and immune function.48, 49
In addition to the alterations in gut motility and transit that have been reported in IBS,50 studies measuring cardioautonomic tone in IBS further support that dyregulations in ANS exist in IBS.51–53 Increased SNS activity and decreased PNS activity are the most frequently noted differences when IBS patients are compared to healthy controls. 54–58 Enhanced cardiosympathetic and decreased cardiovagal tone have been demonstrated in subgroups of IBS patients during 24-hour monitoring51, 52 and during rectosigmoid distension.53
Measurements of cardioautonomic responses to stress are very limited in IBS. Elsenbruch et al.32, 59 compared evaluated autonomic responses to stressful mental tasks in female IBS patients and controls. A significant shift to a lower cardiosympathetic/vagal balance was only seen in IBS and control subjects who perceived the mental task as at least moderately stressful. They also noted increases in systolic and diastolic blood pressure and heart rate to a public speaking task in IBS and control subjects, but there were again no group differences.59 Two studies found elevated basal plasma levels of norepinephrine in IBS patients vs. controls but no significant changes in response to a psychological stressor.31, 33 Thus, these studies suggest that while cardioautonomic tone and catecholamines may be altered in some patients with IBS, a significantly different ANS response to experimental psychological stressors may be challenging to provoke in IBS patients.
HPA axis function
In response to stress, CRF is released from the PVN of the hypothalamus and results in the secretion of adrenocorticotropin hormone (ACTH) from the pituitary gland via CRF1 receptors, which in turn, stimulates the adrenal cortex causing it to release cortisol (Figure B, supporting documents). CRF synthesis and release are subsequently inhibited through a glucocorticoid negative feedback system mediated by both glucocorticoid and mineralocorticoid receptors in the PVN, pituitary gland and hippocampus.26
Although dysregulations in HPA axis activity have also been reported in IBS,33, 53, 54, 60–68 there are conflicting reports with respect to both basal and stress or hormone stimulated responses (Table A, supporting documents). Studies have reported increased basal cortisol levels,60, 63, 65 and enhanced responses to physical61, 67 or psychological33, 66 stressors and hormone stimulation,38, 62 but also blunted HPA axis responses64, 68 or no difference between IBS and control groups.32, 59, 69 These differences may be due to differences in study methodology and/or the patient populations (e.g., psychological co-morbidity) studied.61 Blunted basal ACTH levels were found in the presence of elevated cortisol levels over 24 hours, which is more likely a reflection of enhanced negative feedback of cortisol and/or downregulation of CRF1 receptors at the pituitary gland level.60 Overall, the current literature suggests that there is enhanced basal activity of the HPA axis and to hormone challenge, a lack of a significant response to a meal and/or mental stressor, and varying HPA axis response depending on the type of physical stressor in IBS patients compared to controls.
One confounding factor that can affect HPA axis function and may be more prevalent in the IBS patient population is the presence EALs. Increased HPA axis response to hormone challenge or mental stress has been reported in individuals with history of childhood sexual abuse,70, 71 parental loss during childhood,72 and EALs and high levels of chronic stress.73 Videlock and colleagues61 demonstrated that IBS patients and controls with EALs had a greater cortisol response to a visceral stressor than individuals without EALs. There was also a faster rise and a slower return of stimulated cortisol to basal levels in IBS, which was associated with increased IBS symptom intensity and lower HRQOL. These findings support a role for EALs in the development and life-long functioning of the HPA axis.
In the setting of EALs, increased stress responsiveness due to a decrease in negative feedback at the level of the glucorticoid receptor (GR) in the hippocampus has been demonstrated in both animal and human studies. Exposure to perinatal stress (i.e. maternal separation) predisposes adult rats to develop stress-induced visceral hypersensitivity, enhanced defecation, intestinal mucosal dysfunction, increased HPA axis responses and anxiety-like behavior.74–76 77 These phenotypic features are similar to those seen in IBS patients, and thus maternal separation has served as an animal model of IBS.5 Perinatal stress is associated with decreased hippocampal GR expression, increased hypothalamic CRF mRNA expression and basal and stress-induced corticosterone levels.78, 79 Szyf and colleagues80 found a link between maternal licking and grooming in early life and epigenetic alterations at the GR gene locus in the adult offspring, namely increased methylation of the GR exon 17 promotor. These findings provide a possible mechanism linking early life psychosocial exposure and long-lasting programming of gene expression elicited by maternal care in rats. Their group also recently reported strong evidence to support a similar mechanism in humans by demonstrating decreased hippocampal GR expression and increased methylation of the GR NR3C1 promoter in suicide victims with a history of childhood abuse compared to suicide victims without abuse and to controls without abuse who died of unrelated causes.81
Summary and Future Directions
There is strong evidence to support that IBS is a stress-sensitive disorder. In a predisposed individual, sustained stress can result in enhanced responsiveness of central stress circuits, dysregulation of adaptive systems, and an increased vulnerability to develop functional disorders including IBS. EALs have been shown to be associated with multiple medical illnesses and negative health behaviors. Animal models and, more recently, human studies support the impact of early life stress on the subsequent development of IBS symptoms and on persistent stress hyperresponsive which may be mediated by epigenetic programming. Chronic stress has been also shown to affect the clinical course and health outcome in IBS patients. While both central and peripheral mechanisms are likely to play important roles in the initiation of maintenance of IBS symptoms, it is the interactions between brain and gut that appear to play a key role in the stress-induced changes of GI function, autonomic and neuroendocrine responses, and pain modulation. Earlier recognition of stress-related symptoms may help to institute more effective treatment of IBS, such as comprehensive stress management and cognitive behavioral therapy and improve global outcome. Future studies are needed to determine if genetic factors predispose an individual to be more susceptible to the damaging effects of stress and predisposition to IBS.
Supplementary Material
Acknowledgments
Grant support: Dr. Chang is supported by NIH grants P50 DK64539 and AR46122.
Abbreviations
- IBS
irritable bowel syndrome
- CRF
corticotropin releasing factor
- PI-IBS
post-infectious irritable bowel syndrome
Footnotes
Disclosures: There is nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 3.Appels A. Mental precursors of myocardial infarction. British Journal of Psychiatry. 1990;156:465–471. doi: 10.1192/bjp.156.4.465. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Palsson O, Jones KR. Systemic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA, Naliboff BD, Chang L, Coutinho SV. Stress and the gastrointestinal tract: V. Stress and irritable bowel syndrome. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drossman DA. Abuse, Trauma, and GI Illness: Is There a Link? American Journal of Gastroenterology. doi: 10.1038/ajg.2010.453. [DOI] [PubMed] [Google Scholar]
- 10.Creed F. The relationship between psychosocial parameters and outcome in the irritable bowel syndrome. American Journal of Medicine. 1999;107:74S–80S. doi: 10.1016/s0002-9343(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 11.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 13.Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34:396–403. doi: 10.1016/j.amepre.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creed F, Tomenson B, Guthrie E, Ratcliffe J, Fernandes L, Read N, Palmer S, Thompson DG. The relationship between somatisation and outcome in patients with severe irritable bowel syndrome. Journal of Psychosomatic Research. 2008;64:613–20. doi: 10.1016/j.jpsychores.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. American Journal of Gastroenterology. 2008;103:765–74. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, Mitchell CM. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Annals of Internal Medicine. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: Association with irritable bowel-type symptoms. American Journal of Gastroenterology. 1995;90:366–371. [PubMed] [Google Scholar]
- 18.Salmon P, Skaife K, Rhodes J. Abuse, dissociation, and somatization in irritable bowel syndrome: towards an explanatory model. J Behav Med. 2003;26:1–18. doi: 10.1023/a:1021718304633. [DOI] [PubMed] [Google Scholar]
- 19.Ross CA. Childhood sexual abuse and psychosomatic symptoms in irritable bowel syndrome. J Child Sex Abus. 2005;14:27–38. doi: 10.1300/J070v14n01_02. [DOI] [PubMed] [Google Scholar]
- 20.Videlock EJ, Mayer EA, Naliboff BD, Chang L. Childhood trauma and abuse is associated with an increased vulnerability for multiple somatic symptoms including irritable bowel syndrome (IBS) Gastroenterology. 2010;138(Suppl) [Google Scholar]
- 21.Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Demyttenaere K, Tack J. Risk factors for impaired health-related quality of life in functional dyspepsia. Alimentary Pharmacology and Therapeutics. doi: 10.1111/j.1365-2036.2010.04510.x. [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA, Li Z, Leserman J, Toomey TC, Hu YJ. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi: 10.1053/gast.1996.v110.pm8613034. [DOI] [PubMed] [Google Scholar]
- 23.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Holstege G. The emotional motor system. European Journal of Morphology. 1992;30:67–79. [PubMed] [Google Scholar]
- 26.Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 27.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Reviews. 1991;43:425–473. [PubMed] [Google Scholar]
- 28.Kiank C, Tache Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain, Behavior, and Immunity. 24:41–8. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Current Pharmaceutical Design. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 30.Welgan P, Meshkinpour H, Beeler M. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology. 1988;94:1150–1156. doi: 10.1016/0016-5085(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 31.Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, Fass R, Chang L, Mayer M, Naliboff BD. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. American Journal of Gastroenterology. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 32.Elsenbruch S, Lovallo WR, Orr WC. Psychological and physiological responses to postprandial mental stress in women with the irritable bowel syndrome. Psychosomatic Medicine. 2001;63:805–810. doi: 10.1097/00006842-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosomatic Medicine. 1985;47:139–149. doi: 10.1097/00006842-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Welgan P, Meshkinpour H, Ma L. Role of anger in antral motor activity in irritable bowel syndrome. Digestive Diseases and Sciences. 2000;45:248–251. doi: 10.1023/a:1005487821063. [DOI] [PubMed] [Google Scholar]
- 36.Lind CD. Motility disorders in the irritable bowel syndrome. Gastroenterology Clinics of North America. 1991;20:279–295. [PubMed] [Google Scholar]
- 37.Kellow JE, Langeluddecke PM, Eckersley GM, Jones MP, Tennant CC. Effects of acute psychologic stress on small-intestinal motility in health and the irritable bowel syndrome. Scandinavian Journal of Gastroenterology. 1992;27:53–58. doi: 10.3109/00365529209011167. [DOI] [PubMed] [Google Scholar]
- 38.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1999;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 40.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. American Journal of Gastroenterology. 2006;101:1288–94. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 43.Barbara G, Wang B, Stanghellini V, De Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 44.Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 (Suppl 7):33–46. [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antolin M, Martinez C, Rezzi S, Saperas E, Kochhar S, Santos J, Malagelada JR. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172. e1. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 46.Mayer EA, Naliboff BD, Chang L. Evolving pathophysiological model of functional gastrointestinal disorders: Implications for treatment. European Journal of Surgery Supplement. 2002;168:3–9. [PubMed] [Google Scholar]
- 47.Crowell MD, Harris L, Jones MP, Chang L. New insights into the pathophysiology of irritable bowel syndrome: Implications for future treatments. Current Gastroenterology Reports. 2005;7:272–279. doi: 10.1007/s11894-005-0019-8. [DOI] [PubMed] [Google Scholar]
- 48.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52:585–638. [PubMed] [Google Scholar]
- 49.Hansen MB. The enteric nervous system I: organisation and classification. Pharmacology and Toxicology. 2003;92:105–113. doi: 10.1034/j.1600-0773.2003.t01-1-920301.x. [DOI] [PubMed] [Google Scholar]
- 50.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–81. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterology and Motility. 2007;19:110–8. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 52.Heitkemper M, Burr RL, Jarrett M, Hertig V, Lustyk MK, Bond EF. Evidence for autonomic nervous system imbalance in women with irritable bowel syndrome. Digestive Diseases and Sciences. 1998;43:2093–2098. doi: 10.1023/a:1018871617483. [DOI] [PubMed] [Google Scholar]
- 53.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex-specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heitkemper M, Jarrett M, Cain KC, Burr R, Levy RL, Feld A, Hertig V. Autonomic nervous system function in women with irritable bowel syndrome. Digestive Diseases and Sciences. 2001;46:1276–1284. doi: 10.1023/a:1010671514618. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 56.Thompson JJ, Elsenbruch S, Harnish MJ, Orr WC. Autonomic functioning during REM sleep differentiates IBS symptom subgroups. American Journal of Gastroenterology. 2002;97:3147–3153. doi: 10.1111/j.1572-0241.2002.07112.x. [DOI] [PubMed] [Google Scholar]
- 57.Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of autonomic nervous system indices based on abdominal pain reports in women with irritable bowel syndrome. Biological Research for Nursing. 2000;2:97–106. doi: 10.1177/109980040000200203. [DOI] [PubMed] [Google Scholar]
- 58.Waring WS, Chui M, Japp A, Nicol EF, Ford MJ. Autonomic cardiovascular responses are impaired in women with irritable bowel syndrome. Journal of Clinical Gastroenterology. 2004;38:658–663. doi: 10.1097/01.mcg.0000135362.35665.49. [DOI] [PubMed] [Google Scholar]
- 59.Elsenbruch S, Lucas A, Holtmann G, Haag S, Gerken G, Riemenschneider N, Langhorst J, Kavelaars A, Heijnen CJ, Schedlowski M. Public speaking stress-induced neuroendocrine responses and circulating immune cell redistribution in irritable bowel syndrome. American Journal of Gastroenterology. 2006;101:2300–7. doi: 10.1111/j.1572-0241.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 60.Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–62. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalmic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential marker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Patacchioli FR, Angelucci L, Dellerba G, Monnazzi P, Leri O. Actual stress, psychopathology and salivary cortisol levels in the irritable bowel syndrome (IBS) Journal of Endocrinological Investigation. 2001;24:173–177. doi: 10.1007/BF03343838. [DOI] [PubMed] [Google Scholar]
- 64.Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosomatic Medicine. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- 65.Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21:1148–e97. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. American Journal of Gastroenterology. 2001;96:460–466. doi: 10.1111/j.1572-0241.2001.03526.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Q, Fillingim RB, Riley JL, 3rd, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 148:454–61. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FitzGerald LZ, Kehoe P, Sinha K. Hypothalamic--pituitary-- adrenal axis dysregulation in women with irritable bowel syndrome in response to acute physical stress. West J Nurs Res. 2009;31:818–36. doi: 10.1177/0193945909339320. [DOI] [PubMed] [Google Scholar]
- 69.Elsenbruch S, Holtmann G, Oezcan D, Lysson A, Janssen O, Goebel MU, Schedlowski M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? American Journal of Gastroenterology. 2004;99:703–10. doi: 10.1111/j.1572-0241.2004.04138.x. [DOI] [PubMed] [Google Scholar]
- 70.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 71.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 72.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–6. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 75.Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2007;293:G198–203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 76.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–8. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- 77.Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–8. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 78.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 79.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 80.McGowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Research. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, Walker E. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. American Journal of Gastroenterology. 1996;91:906–913. [PubMed] [Google Scholar]
- 83.Walter SA, Aardal-Eriksson E, Thorell LH, Bodemar G, Hallbook O. Pre-experimental stress in patients with irritable bowel syndrome: high cortisol values already before symptom provocation with rectal distensions. Neurogastroenterology and Motility. 2006;18:1069–77. doi: 10.1111/j.1365-2982.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 84.Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 85.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.