Abstract
Purpose
Androgen independent prostate cancer growth and metastasis are a major cause of prostate cancer death. Aberrant androgen receptor activation due to androgen receptor mutation is an important mechanism of androgen independence. We determined the effectiveness and mechanism of 17α-estradiol (Sigma®) in blocking aberrant androgen receptor activation due to androgen receptor mutation.
Materials and Methods
We used LNCaP and MDA Pca-2b prostatic tumor cells (ATCC®) containing a mutated androgen receptor and WT estrogen receptor β to test 17α-estradiol inhibition of aberrant androgen receptor activation of prostate specific antigen gene expression and cell growth. Cotransfection analysis was used to further elucidate the mechanism of 17α-estradiol action. Xenograft animals with an LNCaP prostate tumor were prepared to study the in vivo effect of 17α-estradiol on tumor growth inhibition.
Results
In LNCaP cells 17α-estradiol produced a dose dependent inhibition of cyproterone acetate (Sigma) or dihydrotestosterone induced prostate specific antigen gene expression. In MDA Pca-2b cells 17α-estradiol inhibited cortisol (Sigma) induced prostate specific antigen expression and blocked dihydrotestosterone and cortisol induced cell proliferation in LNCaP and MDA Pca-2b cells, respectively. Cotransfection analysis showed that 17α-estradiol inhibition of aberrant androgen receptor activation of prostate specific antigen gene expression was medicated via estrogen receptors. In xenograft mice with LNCaP prostate cancer 17α-estradiol but not 17β-estradiol (Sigma) significantly inhibited tumor growth, although each estrogen tended to decrease tumor growth.
Conclusions
Results suggest that 17α-estradiol with less classic estrogenic activity is a potential therapeutic agent for androgen independent prostate cancer due to androgen receptor mutation.
Keywords: prostate, receptors, androgen, mutation, estradiol, prostatic neoplasms
Prostate cancer is the second leading cause of cancer death in American men.1 After prostate cancer has metastasized there is currently no curable therapy available. Standard therapy for metastatic prostate cancer is androgen deprivation to withdraw androgen action.2–4 Although most patients clinically respond to androgen ablation with a high rate initially, unfortunately the tumor inevitably recurs in an androgen independent form and proceeds to further growth and metastasis. Thus, it is imperative to develop new therapeutic agents for androgen independent prostate cancer.
The mechanism of androgen independent prostate tumor growth is not fully understood but it may be categorized as AR positive and AR negative.4,5 More than 80% of androgen independent tumors are AR positive and their growth may be explained by aberrant AR activation due to AR amplification, mutation, abnormal coregulator expression and ligand independent activation.4–6 As many as half of metastatic prostate tumors that escape from androgen ablation therapy have some somatic mutations in the AR gene.6 Mutated ARs have been identified in various prostatic tumor cells, such as LNCaP and MDA Pca-2b.7 AR in LNCaP cells has a point mutation at codon 877 (T877A) of the hormone binding domain, which alters AR ligand binding specificity, and can be activated by progesterone, estrogens and antiandrogens as well as androgen.7 Mutated AR in MDA Pca-2b cells has a double mutation (L701H and T877A), resulting in high affinity to glucocorticoids. 8 Such AR mutations have been found in tumor tissue in patients with androgen independent prostate cancer.6 AR signaling in androgen independent prostate cancer with AR mutations remains intact and is even broadly activated by other stimuli.6–8 Agents with the capability to block aberrant AR activation may be effective for androgen independent prostate cancer therapy.
Historically estrogen has been used as advanced prostate cancer therapy.2 However, it is limited in clinical practice due to classic estrogenic related cardiovascular side effects.9,10 The stereoisomer of βE2, αE2, is a weaker estrogen analogue with less classic estrogenic activity.11,12 It binds weakly to ERα and its receptor complex only transiently binds to the estrogen responsive element.13,14 Recently we reported that αE2 inhibited DHT induced PSA gene expression and cell proliferation in LAPC-4 prostatic tumor cells containing a WT AR. Also, αE2 but not βE215,16 effectively inhibited tumor growth in a LAPC4 xenograft animal model.16
To explore the effectiveness of αE2 for androgen independent prostate cancer we examined the effect of αE2 on inhibiting the aberrant AR activation of PSA gene expression and tumor cell growth in cell cultures and in a xenograft animal model. Results indicate that αE2 significantly inhibited the aberrant AR activation of PSA gene expression and attenuated tumor cell growth.
MATERIALS AND METHODS
Chemicals, Reagents and Plasmids
We used αE2, βE2, DHT, cyproterone acetate and cortisol dissolved in absolute ethanol at a stock concentration of 10−2 M. The PSA5.8-pGL3 reporter construct, and WT ERα and ERβ expression vectors were described previously.15,17
Cell Culture and Proliferation Assay
LNCaP cells were cultured in RPMI-1640 medium (Sigma) and MDA Pca-2b cells were cultured in BRFF-HPC1 ™ medium with supplements, as previously described. 18,19 For cell proliferation assay cells were plated in 96-well plates with the density indicated in each experiment in phenol red-free RPMI-1640 medium supplemented with 5% stripped fetal bovine serum. At 24 hours cells were treated with various hormones or vehicle controls. Medium and hormonal agents were replenished every 3 days. We determined the number of viable cells using a CellTiter One Solution Cell Proliferation Assay kit (Promega, Madison, Wisconsin).
PSA Measurement, RNA Extraction and RT-PCR
PSA in cell culture medium was measured by ELISA (Diagnostic Systems Laboratory, Webster, Texas). Total cellular RNA was extracted using TriPure® reagent. The RNA concentration was determined by ultraviolet absorbance.
RT-PCR was done using the Titan™ One Tube RT-PCR System with 1 µg total cellular RNA, as previously described. 15 We used a pair of specific primers, 5′-GATGAGGGGAAATGCGTAGA-3′ and 5′-CTTGTTACTCGCATGCCTGA-3′, for the human ERβ gene. RT-PCR conditions were 50C for 30 minutes, 94C for 2 minutes, and 40 cycles at 94C for 30 seconds, 60C for 30 seconds and 68C for 45 seconds with a final cycle at 68C for 7 minutes. PCR products were fractionated on 2% agarose gel and visualized by ethidium bromide staining. Human glyceraldehyde-3-phosphate dehydrogenase served as an internal control and yeast tRNA served as a negative control for RT-PCR.
Western Blot Analysis
Western blot was done as previously described.20 Briefly, 20 µg total cellular proteins were fractionated on sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene fluoride membrane (Amersham Pharmacia Biotech, Piscataway, New Jersey). Blots were blocked and incubated with specific primary antibody against AR (Upstate Biotech, Lake Placid, New York) or ERβ (Affinity BioReagents, Rockford, Illinois). After secondary antibody incubation the signal was visualized using the SuperSignal® West Pico Chemiluminescent kit and exposed to film (Kodak, Rochester, New York). We used α-tubulin as an internal control by incubating a specific antibody against α-tubulin (Sigma).
Cotransfection Assay
CV-1 cells (ATCC) were grown in Dulbecco’s modified Eagle’s medium (Sigma) with supplements. Cells were cotransfected using the calcium phosphate precipitation method, as described previously.15,17 Briefly, CV-1 cells seeded in 6-well plates were cotransfected with 1.5 µg PSA5.8-pGL3 luciferase reporter, 0.25 µg mutated AR expression vector with or without 0.25 µg ERα or β, or 1 µg of the empty vector pRL-null containing Renilla luciferase as an internal control with pBluescript-SK plasmid (Stratagene, LaJolla, California) to a total of 4 µg DNA per well, as indicated in experiments. At 16 hours after transfection medium was replaced and cells were treated with various hormones or vehicle controls for 48 hours. Luciferase activity was determined using a dual luciferase assay system (Promega) and expressed in rlu to indicate PSA transcription activity.
Xenograft Animal Study
An LNCaP prostatic tumor xenograft tumor model was prepared and monitored, as previously described.16 Briefly, LNCaP cells with more than 90% viability were mixed with a 50% volume of Matrigel™ to a final concentration of 5 × 106 cells per 0.2 ml. A 0.2 ml aliquot was injected subcutaneously into the left flank of adult male nude mice (Harlan™). Animals were housed aseptically at the animal facility at our institution. We assessed tumor growth, body weight and mobility change twice weekly. Tumor development was followed until the end of the experiment by caliper measurement of its length and width. Tumor volume/size was calculated using the formula, volume = (width)2 × length/2. The animal study was approved by the institutional animal care and use committee, and done in accordance with accepted standards of humane animal care.
When the tumor was approximately 350 mm3, 6 animals each were randomly assigned to 1 of the 3 treatment groups and implanted subcutaneously with a placebo (20 mg cholesterol), αE2 (2 mg αE2 plus 18 mg cholesterol) or βE2 (2 mg βE2 plus 18 mg cholesterol) pellet in the opposite flank for 4 weeks. Tumor size was recorded twice weekly after treatment.
Statistical Analysis
Data are shown as the mean ± SEM. We used 1-way ANOVA and the post hoc Student-Newman-Keuls test to determine differences among multiple groups with p <0.05 considered statistically significant.
RESULTS
Inhibition by αE2
Aberrant AR induction of PSA gene expression in LNCaP and MDA-Pca 2b cells
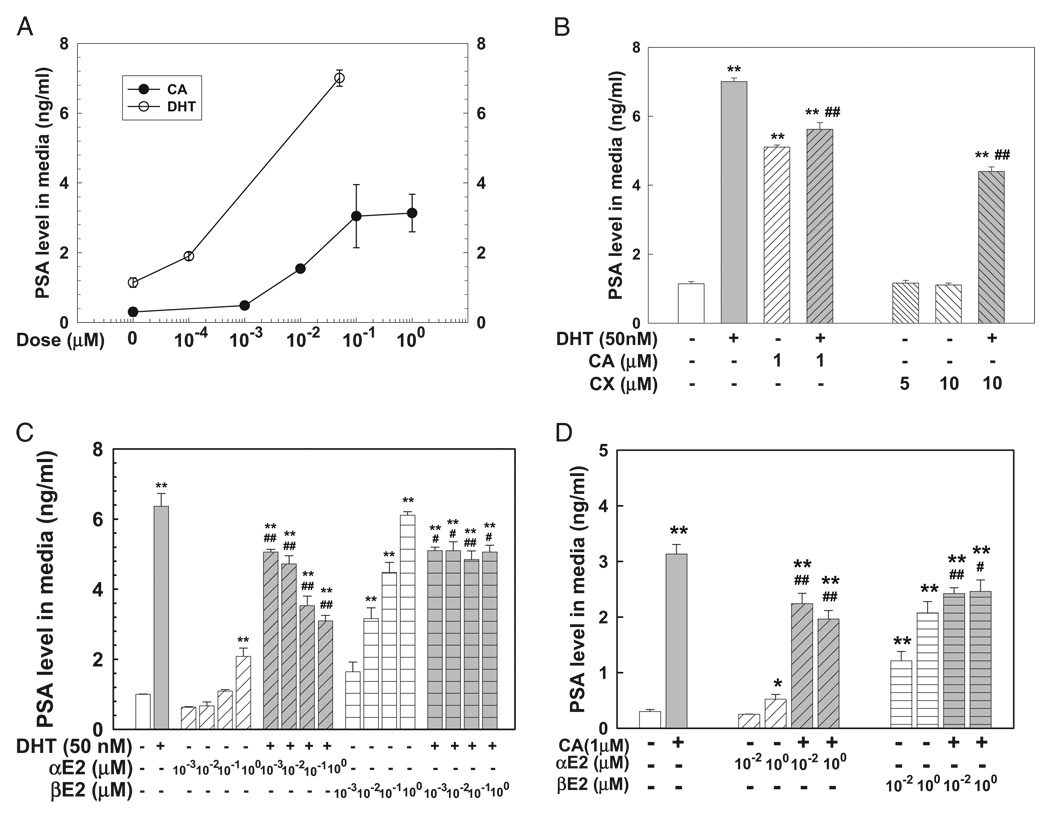
DHT and the AR antagonist cyproterone acetate produced dose dependent induction of PSA expression in LNCaP cells (fig. 1). DHT (50 nM) or cyproterone acetate (1 µM) for 72 hours produced an approximately 6-fold increase in PSA compared to the corresponding control in LNCaP cell medium while treatment with the pure AR antagonist CX (5 and 10 µM) did not alter PSA levels in LNCaP cell medium, in agreement with previous reports.7,19 DHT induced PSA expression was partially inhibited by concomitant administration of cyproterone acetate (1 µM) or CX (10 µM) in LNCaP cells (fig. 1, B). Cyproterone acetate and DHT induced PSA expression was attenuated by αE2 or βE2 (fig. 1, C and D). Notably βE2 alone produced significant, dose dependent induction of PSA expression while αE2 only moderately increased PSA at the highest dose of 1 βM (fig. 1, C and D).
Figure 1.
DHT and cyproterone acetate (CA) induced PSA expression was inhibited by concomitant αE2 or βE2 administration in LNCaP prostatic tumor cells plated in 96-well plates. Cells were treated with 0.2% ethanol as vehicle control, or various DHT or cyproterone acetate doses for 3 days (A), 50 nM DHT in presence or absence of cyproterone acetate or CX for 3 days (B), or various αE2 or βE2 doses in presence or absence of 50 nM DHT (C) or 1 µM cyproterone acetate (D) for 3 days. PSA in culture medium was determined by ELISA, as described. Data represent mean ± SEM of 6 to 9 samples in 2 or 3 independent triplicate experiments. Single asterisk indicates p <0.05 vs vehicle control. Double asterisks indicate p <0.01 vs vehicle control. Single pound sign indicates p <0.05 vs 50 nM DHT (B and C) and 1 µM cyproterone acetate (D). Double pound signs indicate p <0.01 vs 50 nM DHT (B and C) and 1 µM cyproterone acetate (D).
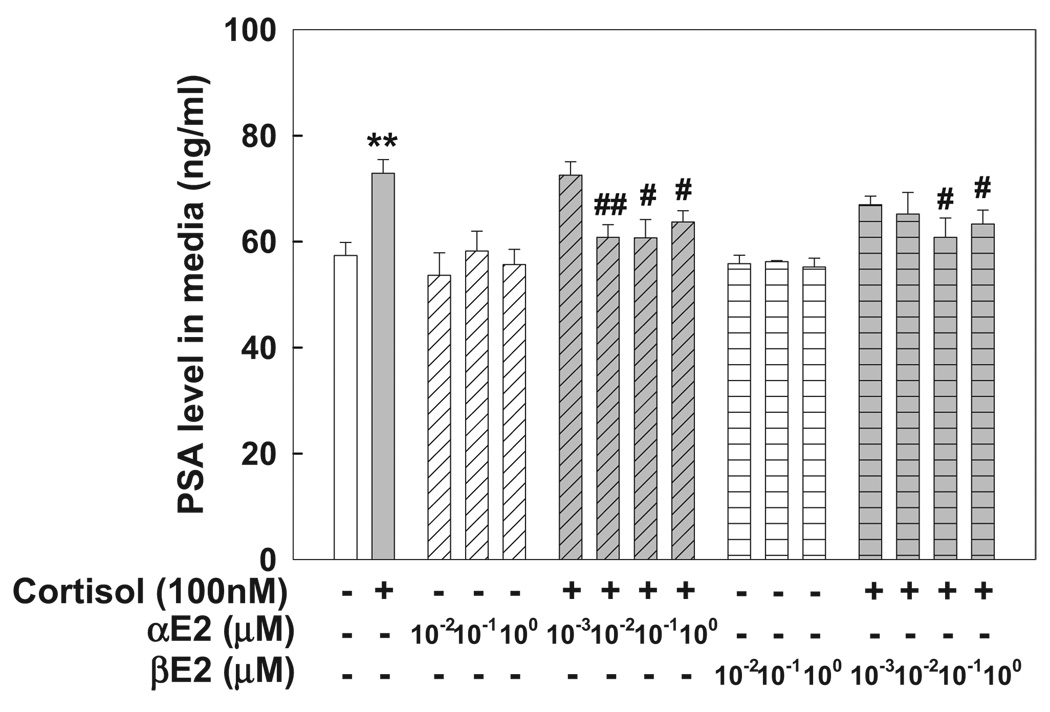
In MDA Pca-2b cells containing a double point mutation in the AR ligand binding domain with high affinity to glucocorticoid8 treatment with cortisol (100 nM) for 72 hours significantly increased PSA in cell medium. This was completely blocked by αE2 or βE2 in a dose dependent manner (fig. 2).
Figure 2.
Cortisol induced PSA expression in MDA Pca-2b cells was inhibited by concomitant αE2 or βE2 administration. Cells were plated in 96-well plates and treated with 0.2% ethanol as vehicle control, 100 nM cortisol or various αE2 and βE2 doses in absence or presence of 100 nM of cortisol for 3 days. PSA in culture medium was determined by ELISA, as described. Values represent mean ± SEM of 6 individual samples in 2 independent experiments done in triplicate. Asterisks indicate p <0.01 vs vehicle control. Single pound sign indicates p <0.05 vs 100 nM cortisol. Double pound signs indicate p <0.01 vs 100 nM cortisol.
Aberrant AR mediated transactivation of PSA expression via ERs on cotransfection analysis
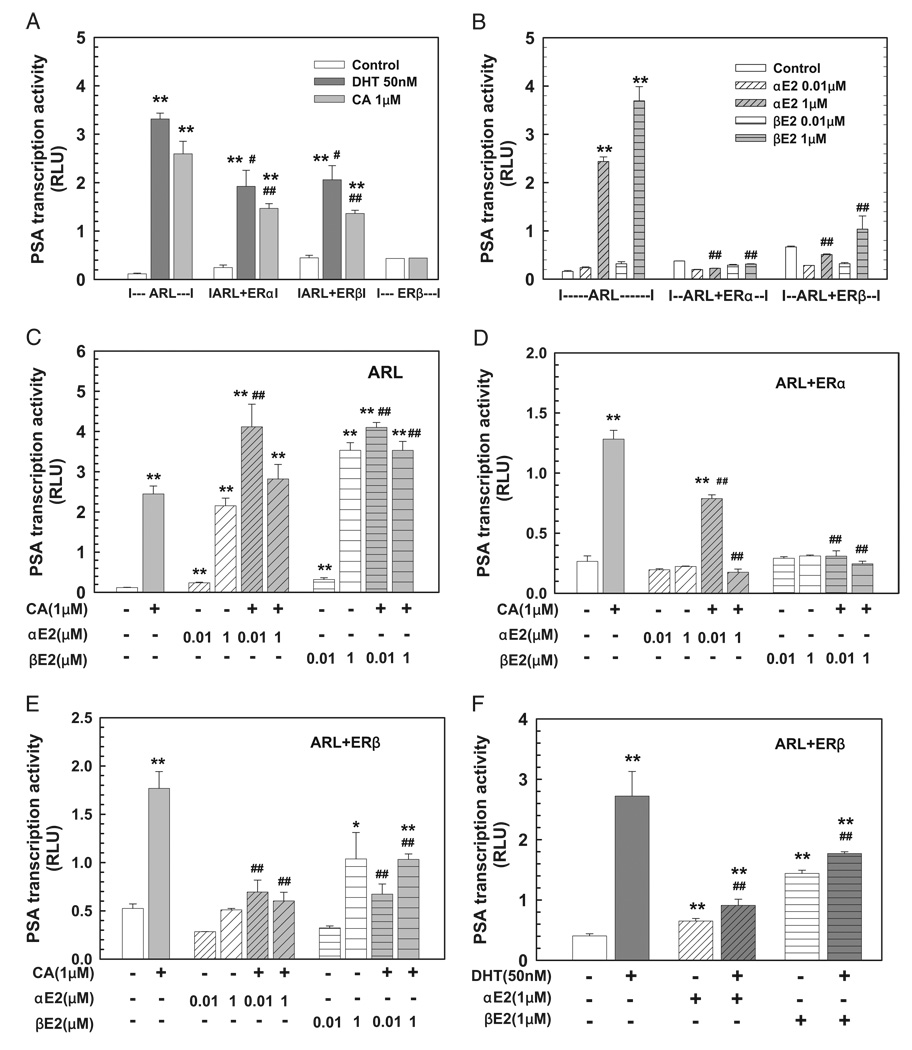
To understand the molecular mechanisms underlying the inhibitory modulation of aberrant AR activation of PSA gene expression by αE2 and βE2 we investigated the involvement of ERs in αE2 and βE2 actions using in vitro cotransfection assays in CV-1 cells. Figure 3 shows that PSA transcription activity was significantly induced by DHT (50 nM) and cyproterone acetate (1 µM) when a mutated AR (ARL) was cotransfected with a PSA promoter directed reporter construct, which was partially attenuated by the presence of ERα or ERβ compared to that in mock transfected cells (fig. 3, A). Most importantly αE2 and βE2 completely inhibited cyproterone acetate and DHT induced PSA transcription activity in a dose and ER dependent manner since αE2 and βE2 slightly potentiated the PSA transcription induced by cyproterone acetate via ARL transfected cells without ER expression (fig. 3, C to F).
Figure 3.
Aberrant ART877A activation of PSA transcription activity induced by DHT or cyproterone acetate (CA) on CV-1 cell cotransfection assay was inhibited by αE2 and βE2 via ERs. Cotransfection of PSA5.8-pGL3 reporter construct and ART877A expression vector ARL with or without ER expression vector ERα or ERβ in CV-1 cells was done as described. At 16 hours cells were treated with vehicle control, or various doses of single hormone (A and B) or hormone combination (C to F) for 48 hours. At experiment end luciferase activity was determined, normalized and shown in rlu. Data represent mean ± SEM of 4 to 8 individual samples in 2 to 4 independent experiments done in duplicate. Single asterisk indicates p <0.05 vs vehicle control. Double asterisks indicate p <0.01 vs vehicle control. Single pound sign indicates p <0.05 vs corresponding treatment in ARL transfected cells (A). Double pound signs indicate p <0.01 vs corresponding treatment in ARL transfected cells (A and B), 1 µM cyproterone acetate (C to E) and 50 nM DHT (F).
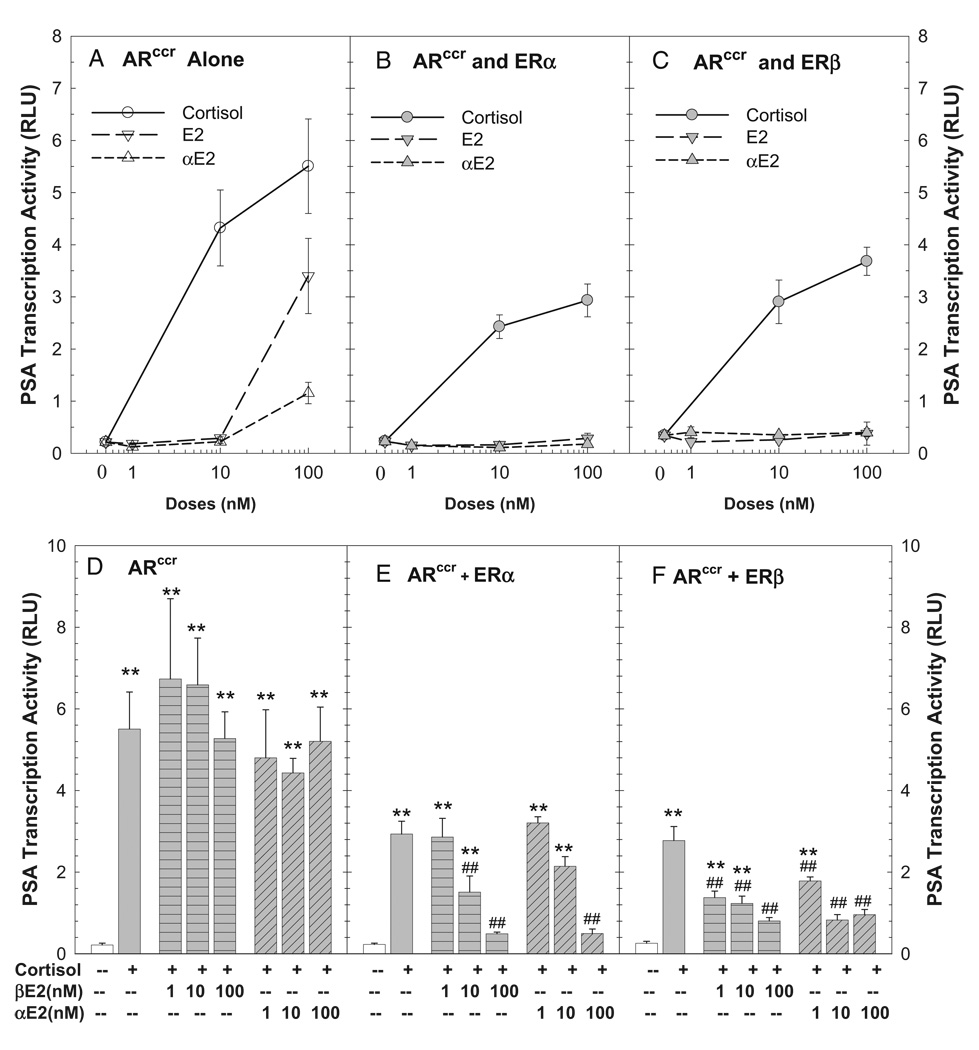
Estrogen modulation of aberrant AR activation of PSA transcription was further evaluated using the mutated AR (ARccr) detected in MDA Pca-2b prostatic tumor cells.8 Cortisol produced dose and ARccr dependent induction of PSA transcription activity (fig. 4, A). This cortisol induced ARccr transactivation was significantly inhibited by αE2 and βE2 in a dose and ER dependent manner since αE2 and βE2 failed to modulate cortisol action in the absence of ERs in mock transfected cells (fig. 4, D to F). We noted similar αE2 and βE2 inhibition of ARccr transactivation when ARccr was activated by DHT (data not shown).
Figure 4.
Aberrant ARccr induction of PSA transcription activity induced by cortisol on CV-1 cell cotransfection assay was inhibited by αE2 and βE2 acting via ERs. Cotransfection of PSA5.8-pGL3 reporter construct and ARccr expression vector (A and D) with ER expression vector ERα (B and E) or ERβ (C and F) was done as described. At 16 hours cells were treated with vehicle control, or various doses of hormones alone (A to C) or in combination (D to F) for 48 hours. At experiment end luciferase activity was determined, normalized and shown in rlu. Data represent mean ± SEM of 4 to 8 individual samples. Asterisks indicate p <0.01 vs vehicle control. Pound signs indicate p <0.01 vs 100 nM cortisol in same transfection group.
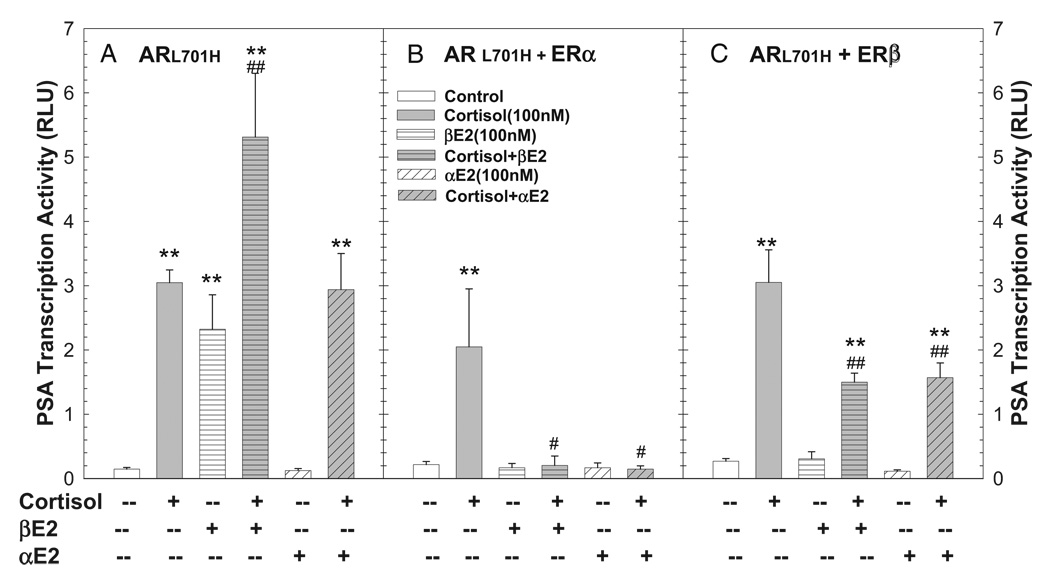
Since the point mutation of L701H in AR confers AR responsiveness to cortisol,8 we assessed the effects of estrogen on the modulation of cortisol induced ARL701H transactivation. Cortisol induced PSA transcription activity was completely inhibited by αE2 and βE2 via ERα, and attenuated by approximately 50% via ERβ (fig. 5, B and C). This estrogen effect depended on ERs since in the absence of ERs βE2 significantly potentiated the cortisol effect, while αE2 failed to modulate cortisol action (fig. 5, A).
Figure 5.
Aberrant ARL701H activation of PSA transcription induced by cortisol on CV-1 cell cotransfection assay was inhibited by αE2 and βE2 acting via ERs. Cotransfection of PSA5.8-pGL3 reporter construct and ARL701H expression vector with or without ER expression vector ERα or ERβ was done as described. At 16 hours cells were treated with vehicle control or 100 nM cortisol in presence or absence of 100 nM αE2 or βE2 for 48 hours. Luciferase activity was determined and expressed in rlu. Data represent mean ± SEM of 2 to 8 individual samples. Asterisks indicate p <0.01 vs vehicle control. Single pound sign indicates p <0.05 vs 100 nM cortisol in same transfection group. Double pound signs indicate p <0.01 vs 100 nM cortisol in same transfection group.
AR activation of LNCaP and MDA Pca-2b cell growth
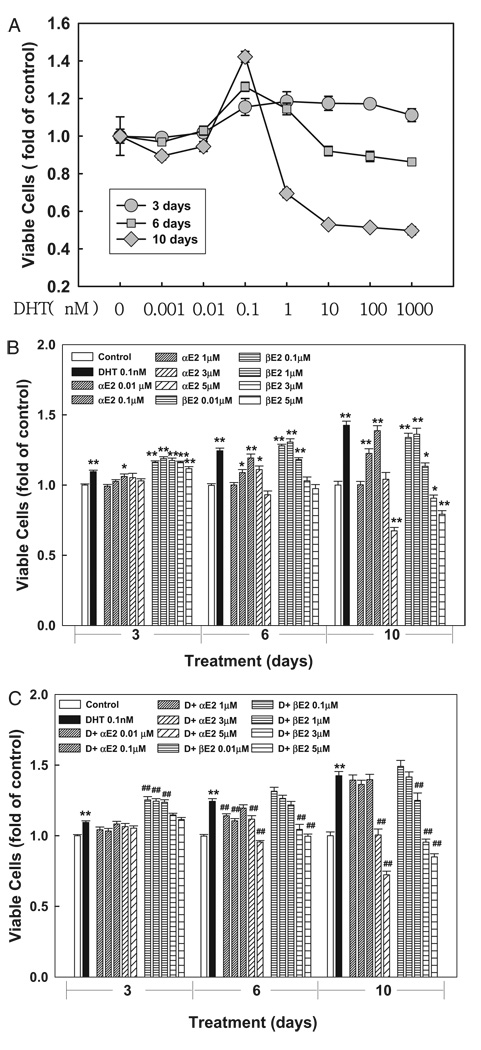
To determine the effects of αE2 and βE2 on aberrant AR activation of cell growth we treated LNCaP and MDA Pca-2b cells with various doses of DHT, cortisol, αE2 or βE2 alone or in combination for various times. In LNCaP cells DHT produced a time and dose dependent dual effect on the number of viable cells (fig. 6, A). This dose dependent, dual action of DHT on LNCaP cell growth, which was reported previously,21 is distinct from DHT induction of PSA expression for unidentified mechanisms.19
Figure 6.
In 96-well plates growth of LNCaP prostatic cells induced by DHT was attenuated by αE2. Cells were treated with 0.2% ethanol as vehicle control or various DHT doses for 3, 6 or 10 days (A). At treatment end number of viable cells was determined by cell proliferation assay and expressed as fold of vehicle control. Cells were treated with 0.2% ethanol as vehicle control, or various αE2 or βE2 doses in presence or absence of 0.1 nM DHT for 3, 6 or 10 days (B and C). Number of viable cells was determined, as described. Data represent mean ± SEM of 6 to 9 samples in 2 or 3 independent experiments done in triplicate. Single asterisk indicates p <0.05 vs vehicle control. Double asterisks indicate p <0.01 vs vehicle control. Pound signs indicate p <0.01 vs corresponding 0.1 nM DHT treatment alone at same time point.
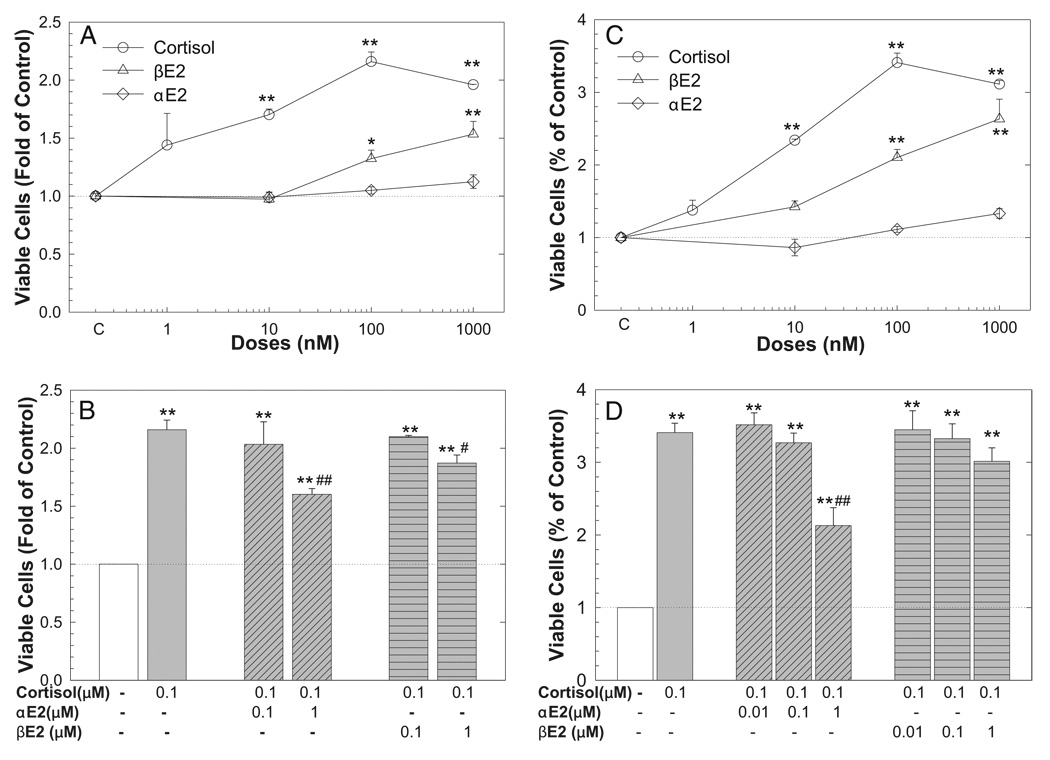
Since 0.1 nM DHT consistently stimulates cell growth, this DHT dose was used in all subsequent cell growth experiments. DHT induced cell growth was significantly inhibited by αE2 at doses of 0.01 to 5 µM. However, the effect of βE2 varied and it only inhibited DHT action at high doses (greater than 3 µM) in LNCaP cells (fig. 6, C). In MDA Pca-2b cells cortisol produced dose dependent induction of cell growth, which was significantly inhibited by αE2 but partially attenuated by βE2 in a dose dependent manner (fig. 7). Also, βE2 alone induced cell growth in dose dependent fashion in LNCaP and MDA Pca-2b cells while αE2 slightly induced cell growth in LNCaP but had no effect in MDA Pca-2b cells (figs. 6, B, and 7, A and C).
Figure 7.
Cortisol induced MDA Pca-2b cell growth was inhibited by αE2 co-administration. Cells were seeded in 96-well plates and treated with vehicle control, or various cortisol, αE2 or βE2 doses alone (A and C) or in combination (B and D) for 3 (A and B) and 6 (C and D) days. At experiment end number of viable cells was determined by cell proliferation assay and expressed as fold of vehicle control. Data represent mean ± SEM of 6 to 12 individual samples in 2 to 4 independent experiments done in triplicate. Single asterisk indicates p <0.05 vs vehicle control. Double asterisks indicate p <0.01 vs vehicle control. Single pound sign indicates p <0.05 vs 100 nM cortisol alone. Double pound signs indicate p <0.01 vs 100 nM cortisol alone.
AR and ERβ in LNCaP and MDA Pca-2b Cells
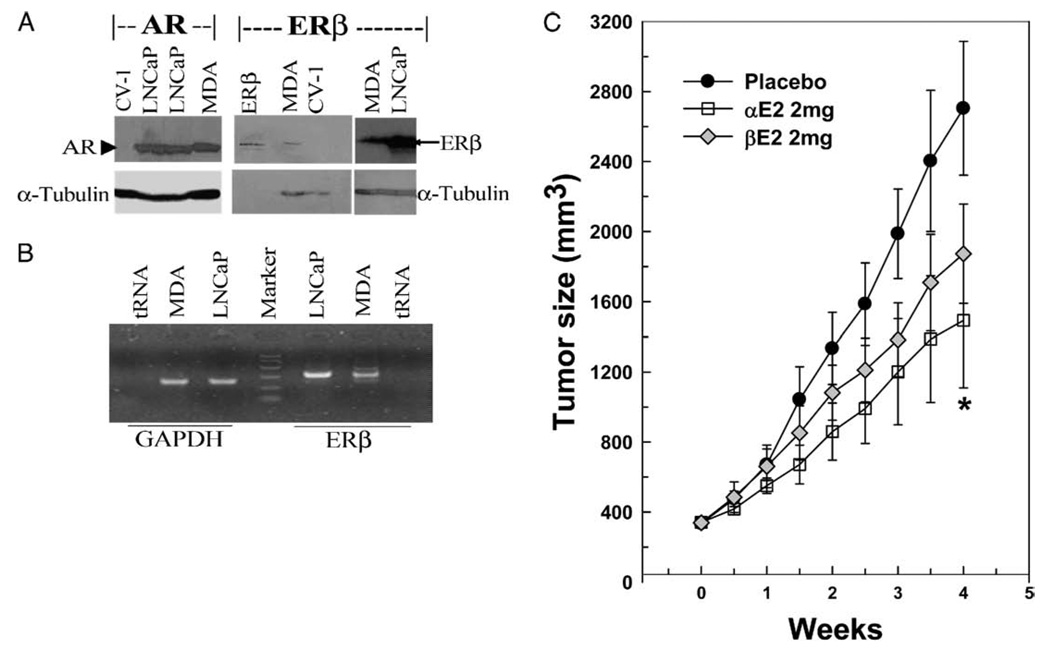
Consistent with previous reports,15,22,23 AR and ERβ were expressed in LNCaP and MDA Pca-2b cells, as shown by Western blot and RT-PCR (fig. 8, A and B). However, ERα expression was undetectable by Western blot and barely detectable by RT-PCR in LNCaP and MDA Pca-2b cells (data not shown).15
Figure 8.
AR and ERβ expressed in LNCaP and MDA Pca-2b (MDA) cells (A and B). Total cellular proteins and mRNA were extracted from LNCaP, MDA Pca-2b or CV-1 cells and analyzed. Representative Western blot shows AR and ERβ in LNCaP, MDA Pca-2b and CV-1 cell samples, and α-tubulin as internal control (A). Lane ERβ, purified ERβ. Representative RT-PCR reveals ERβ in LNCaP and MDA Pca-2b prostatic tumor cell samples with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control (B). In xenograft nude mice inoculated with LNCaP prostatic tumor cell on flank αE2 significantly inhibited tumor growth (C). When tumors were approximately 350 mm3, 6 mice each were treated subcutaneously with placebo, or 2 mg αE2 or βE2 pellet for 4 weeks. Tumor size was recorded twice weekly. Data are shown as mean ± SEM. Asterisk indicates p <0.05 vs placebo.
Tumor Growth Inhibition by αE2
In xenograft animals with LNCaP prostate cancer αE2 inhibited tumor growth. To evaluate the in vivo effectiveness of αE2 for inhibiting prostate tumor growth we treated xenograft animals with LNCaP prostate tumors with a placebo, αE2 or βE2 pellet for 4 weeks and recorded tumor size twice weekly. Initial tumor size was similar in the 3 groups at approximately 350 mm3 (fig. 8, C). Beginning 1 week after pellet implantation αE2 produced notable inhibition of tumor growth and significantly reduced tumor size at the end of the 4-week treatment (αE2 vs placebo mean 1,492 ± 383 vs 2,704 ± 382 mm3, p <0.05). However, tumor size in the βE2 treated group was not significantly different from that in the placebo group at all time points, although we noted moderate reduction. As in our previous study,16 animal body weight and mobility were not significantly altered by αE2 and βE2 vs those in the placebo group (data not shown).
DISCUSSION
Aberrant activation of AR due to AR amplification, AR mutation, coregulator dysregulation and hormone independent AR activation are often linked to prostate cancer progression and androgen independence. 4,5 However, the mechanisms of androgen independent tumor growth and PSA expression are not entirely understood. Somatic AR mutations with various characteristics have been identified in prostate cancer tissues and cell lines. Some AR mutations broaden its ligand binding specificity, such as ARL (T887A) and ARccr (T877A and L701H). ARL with a mutation of T877A in the AR ligand binding domain is stimulated strongly by βE2 and progesterone, and the AR antagonists 4-hydroxyflutamide and cyproterone acetate.24 The doubly mutated ARccr originally detected in MDA Pca-2b cells has the properties of the ARL and ARL701H mutants as well as high affinity to cortisol and cortisone.8 In agreement with previous studies8,24 we report that ARL cells were activated by DHT, cyproterone acetate and estrogen, and ARccr was strongly stimulated by cortisol, as shown by PSA expression and cell proliferation assays (figs. 1, 2, 6 and 7). These results support the concept that aberrant AR activation of cell proliferation and PSA gene expression by hormones other than androgen in LNCaP and MDA Pca-2b cells is a potential cause of androgen independence in prostate cancer cases.
Since more than 80% of androgen independent prostate cancers are AR positive and mostly AR mediated,5,25 inhibiting aberrant AR activation in tumor cells is considered a major strategy to control androgen independent prostate cancer progression. To our knowledge we report for the first time that αE2 significantly inhibits ARL activation of PSA expression induced by cyproterone acetate and DHT, LNCaP cell growth induced by DHT, and cortisol induced ARccr and ARL701H activation of PSA expression and cell growth in MDA Pca-2b cells (figs. 1, 2, 6 and 7). Furthermore, αE2 but not βE2 significantly inhibited tumor growth in a xenograft animal model of LNCaP prostatic tumors (fig. 8, C). The doses of αE2 and βE2 used in the current animal study were based on a previous study in which the circulating βE2 level attained the physiological concentration of βE2 in females.26 These doses were also used in our recent study in xenograft animals with LAPC-4 prostate tumors, in which αE2 effectively attenuated LAPC-4 xenograft tumor growth and βE2 significantly decreased PSA expression.16
Historically estrogens such as diethylstilbestrol and βE2 have been used to treat advanced prostate cancer, presumably by inhibiting testosterone biosynthesis through negative feedback of the hypothalamus-pituitary-gonadal axis.2 However, using estrogens for androgen ablation therapy is limited in clinical practice due to cardiovascular side effects. 9,10 Discovery of the ER isoform ERβ and its identification in prostate epithelial cells and prostatic tumor cells promote the concept that ligand ERs may directly modulate AR actions on prostate gene expression and cell growth. This is supported by our recent studies15,16 and a previous report.27 In agreement with reports that ERβ is the dominant ER in prostate epithelial and prostate tumor cells,22,28 we found that ERβ is expressed in LNCaP and MDA Pca-2b cells while ERα is undetectable (fig. 8, A and B).15 Our current findings that αE2 and βE2 inhibited aberrant AR activation by interacting with ER further support the direct modulation of AR action by estrogen in prostatic tumor cells.
The stereoisomer of βE2, αE2, is a weak estrogen compared to potent endogenous βE2.29 In mammalian model systems αE2 has no carcinogenic effects11,12 and is less effective in the vascular smooth muscle system.30 However, αE2 is as potent as βE2 for protecting neuronal cells29 and inhibiting DHT induced PSA expression and cell growth in LAPC-4 cells containing a WT AR.15,16 In an LAPC4 xenograft animal model αE2 but not βE2 effectively inhibited tumor growth.15,16 Our study shows that αE2 was more effective than βE2 to inhibit the aberrant AR activation of PSA expression, and cell growth in LNCaP and MDA Pca-2b cells containing mutated ARs, and αE2 but not βE2 significantly inhibited tumor growth in a xenograft animal model with LNCaP prostatic tumors. Together these data suggest that αE2 is a potential therapeutic agent for advanced prostate cancer.
CONCLUSIONS
To our knowledge we report for the first time that αE2 mediated via ERβ effectively inhibits the aberrant AR activation of PSA expression and cell growth in LNCaP and MDA Pca-2b cells, and significantly attenuates tumor growth in a xenograft animal model with LNCaP prostatic tumors. Given the efficacy of αE2 for inhibiting aberrant AR activation and the safety of αE2 with its less classic estrogenic actions, we suggest that αE2 may be a potential therapeutic agent for androgen independent prostate cancer due to AR mutation.
ACKNOWLEDGMENTS
Dr. Feldman, Stanford University School of Medicine, Stanford, California, provided AR mutants. Drs. Mosselman and Jansen, N.V. Organon, Oss, The Netherlands, provided the ERβ expression vector. Dr. Chambon, Strasbourg, France, provided ERα expression vectors. CX was obtained from Toronto Research Chemicals, North York, Canada. Hormone pellets were obtained from Hormone Pellet Press, Leawood, Kansas.
Supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK061004 (YSZ), and a KL2 Award supported by National Institutes of Health Grant KL2RR024997 of the Weill Cornell Medical College Clinical and Translation Science Center (YMQ).
Abbreviations and Acronyms
- αE2
17α-estradiol
- βE2
17β-estradiol
- AR
androgen receptor
- CX
Casodex®
- DHT
dihydrotestosterone
- ELISA
enzyme-linked immunosorbent assay
- ER
estrogen receptor
- PCR
polymerase chain reaction
- PSA
prostate specific antigen
- rlu
relative luciferase units
- RT-PCR
reverse transcriptase-PCR
Footnotes
Study received institutional animal care and use committee approval.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Carroll PR, Kantoff PW, Balk SP, et al. Overview consensus statement. Newer approaches to androgen deprivation therapy in prostate cancer. Urology. 2002;60:1. doi: 10.1016/s0090-4295(02)01559-5. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 6.Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 7.Veldscholte J, Ris-Stalpers C, Kuiper GG, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 9.Treatment and survival of patients with cancer of the prostate. The Veterans Administration Cooperative Urological Research Group. Surg Gynecol Obstet. 1967;124:1011. [PubMed] [Google Scholar]
- 10.The Coronary Drug Project: findings leading to discontinuation of the 2.5-mg day estrogen group. The Coronary Drug Project Research Group. JAMA. 1973;226:652. [PubMed] [Google Scholar]
- 11.Li JJ, Li SA, Oberley TD, et al. Carcinogenic activities of various steroidal and nonsteroidal estrogens in the hamster kidney: relation to hormonal activity and cell proliferation. Cancer Res. 1995;55:4347. [PubMed] [Google Scholar]
- 12.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed Proc. 1987;46:1858. [PubMed] [Google Scholar]
- 13.Ko YJ, Balk SP. Targeting steroid hormone receptor pathways in the treatment of hormone dependent cancers. Curr Pharm Biotechnol. 2004;5:459. doi: 10.2174/1389201043376616. [DOI] [PubMed] [Google Scholar]
- 14.Lubahn DB, McCarty KS, Jr, McCarty KS., Sr Electrophoretic characterization of purified bovine, porcine, murine, rat, and human uterine estrogen receptors. J Biol Chem. 1985;260:2515. [PubMed] [Google Scholar]
- 15.Zhu YS, Cai LQ, Huang Y, et al. Receptor isoform and ligand-specific modulation of dihydrotestosterone-induced prostate specific antigen gene expression and prostate tumor cell growth by estrogens. J Androl. 2005;26:500. doi: 10.2164/jandrol.05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao Y, Zhang ZK, Cai LQ, et al. 17Alpha-estradiol inhibits LAPC-4 prostatic tumor cell proliferation in cell cultures and tumor growth in xenograft animals. Prostate. 2007;67:1719. doi: 10.1002/pros.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu YS, Yen PM, Chin WW, et al. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc Natl Acad Sci U S A. 1996;93:12587. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao XY, Peehl DM, Navone NM, et al. 1Alpha, 25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology. 2000;141:2548. doi: 10.1210/endo.141.7.7549. [DOI] [PubMed] [Google Scholar]
- 19.Zhu YS, Cai LQ, You X, et al. Androgen-induced PSA gene expression is mediated via DHT in LNCaP cells. J Androl. 2003;24:681. doi: 10.1002/j.1939-4640.2003.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan C, Cai LQ, Wu W, et al. NSC606985, a novel camptothecin analog, induces apoptosis and growth arrest in prostate tumor cells. Cancer Chemother Pharmacol. 2009;63:303. doi: 10.1007/s00280-008-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C, Sutkowski DM, Sensibar JA, et al. Regulation of proliferation and production of prostate-specific antigen in androgen-sensitive prostatic cancer cells, LNCaP, by dihydrotestosterone. Endocrinology. 1995;136:796. doi: 10.1210/endo.136.2.7530653. [DOI] [PubMed] [Google Scholar]
- 22.Lau KM, LaSpina M, Long J, et al. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175. [PubMed] [Google Scholar]
- 23.Arnold JT, Liu X, Allen JD, et al. Androgen receptor or estrogen receptor-beta blockade alters DHEA-, DHT-, and E(2)-induced proliferation and PSA production in human prostate cancer cells. Prostate. 2007;67:1152. doi: 10.1002/pros.20585. [DOI] [PubMed] [Google Scholar]
- 24.Veldscholte J, Berrevoets CA, Ris-Stalpers C, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 25.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 26.Li SA, Weroha SJ, Tawfik O, et al. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: evidence for estrogen receptor mediation. J Endocrinol. 2002;175:297. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- 27.Kumar MV, Leo ME, Tindall DJ. Modulation of androgen receptor transcriptional activity by the estrogen receptor. J Androl. 1994;15:534. [PubMed] [Google Scholar]
- 28.Kuiper GG, Enmark E, Pelto-Huikko M, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moos WH, Dykens JA, Nohynek D, et al. Review of the effects of 17α-estradiol in humans: a less feminizing estrogen with neuroprotective potential. Drug Devel Res. 2009;70:1. [Google Scholar]
- 30.Freay AD, Curtis SW, Korach KS, et al. Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta. Role of nuclear estrogen receptor and Ca2+ uptake. Circ Res. 1997;81:242. doi: 10.1161/01.res.81.2.242. [DOI] [PubMed] [Google Scholar]