Abstract
BACKGROUND
There are many types of ovarian activity that occur in women. This review provides information on the relationship between the hormone values and the degree of biological response to the hormones including the frequency and degree of uterine bleeding. The continuous process is termed the ‘Continuum’ and is thus similar to other processes in the body.
METHODS
This review draws on information already published from monitoring ovarian activity by urinary oestrogen and pregnanediol measurements using timed 24-h specimens of urine. Much of the rationalization was derived from 5 to 6 year studies of girls progressing from childhood to adulthood, women progressing through menopause, and the return of fertility post-partum. During these times, all the reported types of ovarian activity were encountered.
RESULTS
All cycle types can be understood in terms of steps in the normal maturation of fertility at the beginning of reproductive life, its return post-partum and its demise at menopause. Each step merges into the next and therefore the sequence is termed the ‘Continuum’. Unpredictable movement from fertile to infertile types and back can occur at any time during reproductive life. Stress is a major causative factor. Hormonal definitions for each step, the relevance of the various cycle types in determining fertility and in the initiation of uterine bleeding and the roles of the pituitary hormones in causing them, are presented.
CONCLUSIONS
The findings explain the erratic fertility of women and why ovulation is not always associated with fertility. They provide an understanding of the various types of ovarian activity and their relation to pituitary function, fertility and uterine bleeding.
Introduction
The characteristics of the fertile ovulatory cycle are well documented, but much confusion exists concerning the many other, less common, types of ovarian activity in women. Because these are infertile and sometimes associated with erratic bleeding patterns, they are universally classified as ‘abnormal’. Furthermore, no unifying concept has been proposed of why they exist during reproductive life. Many gynaecologists still concentrate on regularizing the bleeding patterns without understanding the underlying hormonal environment, either endogenous (ovarian activity) or exogenous. Studies performed ∼30 years ago showed that the transitions from the amenorrhoea and infertility of childhood to the fully fertile ovulatory cycle of adulthood, during the return of fertility after child-birth and during its regression at menopause all followed the same sequence or its reverse. The sequence involved a continuous process that included all the types of ovarian activity that have been documented. Furthermore, all the types of ovarian activity could be produced during gonadotrophin therapy. As all the women being treated were aiming at pregnancy, the roles of the two pituitary hormones, follicular stimulating hormone (FSH) and luteinizing hormone (LH), contained in the gonadotrophin administered in causing each cycle type and the fertility potential of each type could be determined. The progression can now be conceptualized as steps progressing from: (i) no ovarian activity, (ii) anovulatory follicular activity with raised constant or fluctuating oestrogen levels, (iii) luteinized unruptured follicle (LUF), (iv) ovulation followed by a deficient or short luteal phase and (v) fully fertile ovulatory cycle, or the reverse of this sequence. Only Type 5 is capable of producing a continuing pregnancy. Therefore, as all the cycle types are part of a normal process they cannot be considered as abnormal. The studies also provided information on the relationship between the hormone values and the degree of biological response to the hormones, including the frequency and degree of uterine bleeding. The Steps 1–5 are actually a continuous progression from childhood to adulthood beginning with the increasing production of sufficient oestrogen to eventually cause uterine bleeding (menarche). This is followed by the gradual regularization of this process and, importantly, the gradual maturation of the ‘ovulatory mechanism’ which includes the total process in which LH is released and the subsequent events leading to ovulation. Therefore the process is termed the ‘Continuum’ and is thus similar to other processes in the body. Because it is a continuous process, each conceptualized step cannot be described in terms of means and standard deviations.
The hormone results were obtained by methods that not only gave the patterns of hormone production which allowed the times of ovulation and the cycle type to be determined, but also provided a semi-quantitative measure of ovarian hormone production rates. Because the assay procedures employed hazardous chemicals and the methods for determining accurate production rates employed the infusion of radio-active hormones, neither could be repeated today for safety reasons. As they were obtained by the best validated assay methods yet applied with minimal interference from non-specific components from the urine, it seemed appropriate to re-publish the original figures together with a re-evaluation of the results that draw them all together. Apart from rationalizing the various cycle types, most of the conclusions about follicular development, ovulation and corpus luteum function given in this survey are supported by the findings of others, (DiZerega and Hodgen, 1981; Baird et al., 1999).
Methods
Assessment of ovarian activity
Ovarian activity was assessed by measuring the cyclic outputs of the two ovarian hormones, oestrogen and progesterone by measuring their principal metabolites in urine. This measurement does not distinguish between whether oestradiol or oestrone is the primary ovarian oestrogen but it is generally considered to be oestradiol (Brown, 1957; Baird and Fraser, 1974). Because of the rapid changes in the secretion of the hormones, particularly just before and after ovulation, when daily changes of 30–50% are usual, it is necessary to monitor ovarian activity frequently, ideally daily. Most of the types of ovarian activity were documented and reported in the late 1950s and early 1960s and the long-term studies were performed during the 1970s. These provided all the results surveyed here and were obtained using the chemical methods in use at the time. As this was the first time that the assays were applied clinically, they were very carefully validated with respect to specificity, precision and applicability by all available methods including reverse isotope dilution (Gallagher et al., 1958), a point that was extensively debated at every presentation of new findings (discussion in Brown and Matthew, 1962). Application of the assays was important in the early development of assisted reproduction technologies, for example, for timing ovulation and the control of gonadotrophin therapy (Townsend et al., 1966). The hormone values obtained during these early studies are used unchanged in the present survey.
Oestrogen measurements
The oestrogen results were measured by the chemical methods developed by Brown (1955) and Brown et al. (1957, 1968, 1978). The 1955 and 1957 methods measured the three Kober-chromogenic oestrogens, oestrone, oestradiol and oestriol (the only ones known in 1955) separately by colorimetry after hydrolysis of their conjugates (method A). The 1968 method employed the highly sensitive and specific Kober–Ittrich fluorescence reaction. This measured oestrone, oestradiol and oestriol together (TE) (method B). The 1978 method measured the three oestrogens separately with the addition of tritium-labelled standards and purification to constant specific activity (method C). The methods, together with results obtained from various populations, are fully described by Brown (1976a, b). The results obtained by all the methods were compared to reference methods to ensure that conclusions drawn from one could be applied to the others. The size of the blank value, defined as the value obtained from urine (or blood) which is not due specifically to the metabolite (hormone) being measured determines the sensitivity of the assay. That produced by the colorimetric methods (A) limited the measurement to the levels excreted by post-menopausal women and men (∼3 µg/24 h). However, the fluorometric methods gave negligible urine blanks and consequently true hormonal profiles, and method C was suitable for measuring the complete range of oestrogen excretion found in the human, including women after oophorectomy and adrenalectomy and young children (∼0.1 µg/24 h). An important feature of all the methods was that they measured oestriol and oestrone, two major urinary oestrogens with different metabolic routes, oestriol on the longer route of hydroxylation before conjugation and excretion, oestrone and oestradiol on the shorter route without hydroxylation. The proportion of the primary hormone metabolized by the two different routes differs considerably among different subjects, being dependent on body weight and thyroid function (Brown and Strong, 1965) and liver function (Brown et al., 1964) but remains approximately constant for an individual. The possible large differences between individuals in the proportions of oestrone and oestriol excreted are well demonstrated by the figures shown in this survey. The sum of their excretion (oestrone plus oestradiol plus oestriol) is quantitatively related to the production rates of the primary hormones which can be calculated approximately by multiplying their sum (TE) by 6 (Brown, 1957). The results agree closely with those obtained by the infusion of radioactive steroids (Baird et al., 1969; Baird and Fraser, 1974). The average coefficients of variation judged from quality controls included with every assay run or calculated from duplicate analyses was 8%. Method C performed well in a trial conducted by the Department of Epidemiology, Harvard University, in which blind duplicates were circulated. As a consequence, it was in considerable demand for epidemiological studies relating oestrogen production and ovulation rates in populations of women with high and low risk of breast cancer (MacMahon et al., 1974).
A direct enzyme-immunoassay measuring oestrone glucuronide which women can perform themselves (the ‘Ovarian Monitor’) was developed later (Brown et al., 1988). This assay does not measure oestriol and therefore production rates cannot be calculated, however it provides the patterns of oestrogen excretion which allow ovulation and fertility to be timed and cycle type to be identified. It has greatly simplified the performance of daily assays but the urine blank restricts its application to measuring oestrogen levels during the ovulatory cycle.
Progesterone measurements
Progesterone production was assessed by endometrial biopsy or by measuring urinary pregnanediol by the colorimetric method of Klopper et al. (1955) (method A). This method produced a urine blank value equivalent to 1 mg/24 h urine. Later measurement of urinary pregnanediol excretion was performed by gas-liquid chromatography (GLC) (Barrett and Brown, 1970) (method B) which did not produce a significant urine blank value and allowed a sensitivity of assay of 0.4 mg/24 h urine. Later, an enzyme-immunoassay measuring pregnanediol glucuronide was developed (the ‘Ovarian Monitor’, Brown et al., 1988). This produced a urine blank that restricted its application to the levels found during the ovulatory cycle.
Urine collection
For the results to have quantitative meaning, the urine values must include allowance for the large daily differences in urine volume. Thus we have always expressed the oestrogen and pregnanediol values in terms of time rather than volume or creatinine concentration. Others trying to avoid this have caused much confusion. All the results presented here were obtained from complete 24 h urine collections and are expressed as excretion per 24 h. More convenient shorter-timed collection periods (minimum of 3 h or overnight) were introduced to simplify urine collection for the Ovarian Monitor. A simple calibrated jug was developed to provide a constant volume/time dilution (Blackwell et al., 2003). However, with such shorter collection periods, it is possible for an oestrogen peak to occur between the collection periods and thus be mistimed by 24 h. To counter concerns about the possibility of errors in urine collection, specimens measured after 1967 were diluted to a constant volume and the specific gravity recorded. With serial specimens from the same individual, possible errors in urine collection could be identified and investigated immediately while the woman still remembered.
Long-term studies
The studies of individuals progressing through menarche and menopause were performed using oestrogen methods B and C and the GLC pregnanediol method (B). These studies required up to 5 years of observations in the same individual. Clearly it was not possible to sample ovarian activity daily over this time. Resort was made to weekly urine sampling which always included parts of both the follicular and luteal phases of a cycle. Production of cervical mucus as assessed by the Billings Ovulation Method of Natural Family Planning (NFP) (Billings et al., 1974) was recorded daily. Mucus production is a useful self-bioassay of ovarian hormone production and the maximum score (Brown et al., 1985) correlates closely with peak oestrogen production (Billings et al., 1972). Through knowledge of the mucus scores, times of bleeding and the weekly hormone levels, it was possible to approximately identify the times of ovulation, the oestrogen peak and the pregnanediol rise and maximum.
Role of the pituitary hormones
In exploring the gonadotrophin doses required to avoid hyperstimulation and multiple pregnancies during gonadotrophin therapy, it was found that suboptimal doses of FSH and HCG/LH produced all the types of ovarian activity that had been reported (Brown, 1978). Thus, all the different cycle types are explained by the levels of the two pituitary hormones acting on the ovaries. The gonadotrophin used contained both FSH and LH and ovulation was induced with HCG (Brown, 1986).
Reporting results
In the early application of the new hormone measurements, each unexpected finding was greeted with questions about the reliability of the assay methods. When a new pattern that did not fit with current concepts was first encountered, the results were filed away until similar findings were obtained in another woman. In some cases this took years but the finding of the same pattern before became the criterion for its authenticity.
The hormone values reported in this survey are given in the units used at the time, namely µg/24 h for the oestrogens and mg/24 h for pregnanediol. No attempt has been made to convert the values to modern SI units, mainly because of difficulties in expressing them for mixtures of oestrogens with different molecular weights. Nevertheless, for comparison with other units, the important pregnanediol values of 1.6 and 3 mg/24 h urine are equivalent to 7 and 13.5 µmol/24 h urine (our values) and ∼8 and 15 nmol/l for plasma progesterone (reported values).
Assessment of ovarian activity using the urinary measurements
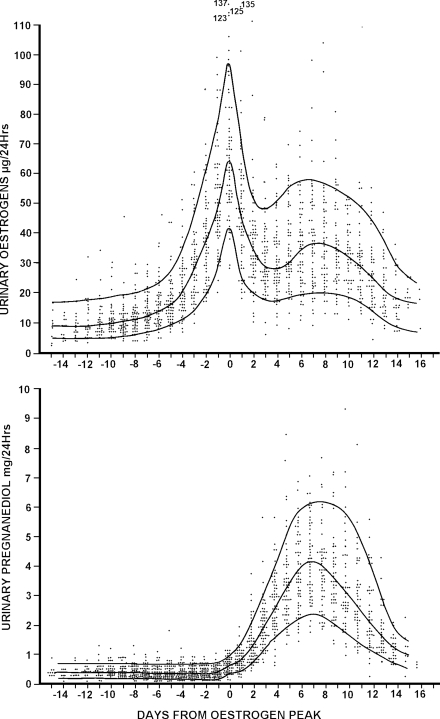
Starting at puberty, the adrenal glands produce steroids that are metabolized to the same urinary products as ovarian oestradiol or oestrone and progesterone, and this persists for the remainder of life. These are seen as low, approximately constant TE values, usually <10 µg/24 h and pregnanediol values <0.5 mg/24 h. These values are influenced by a number of factors including the weight of the subject. Growth and regression of a dominant follicle are identified as unambiguous fluctuations of oestrogen values superimposed above these figures with rises of ∼1.4 times per day over 3–5 days to a peak and falls of approximately 0.7 times per day over 2–3 days after the peak (Fig. 1). After commencing its rapid growth phase, each follicle has a limited life-span, whether its end is caused by ovulation or death by atresia. To determine which has occurred, it is necessary to test for progesterone output.
Figure 1.
Daily TE and pregnanediol values in 61 ovulatory menstrual cycles from 26 parous and 14 nulliparous women aged 20–40 years. All values are plotted and the 10th, 50th and 90th percentile lines are shown. The ovulatory oestrogen peak was identified in every cycle and the days are numbered from this day (=day 0). Reprinted with permission from Brown et al. (1981). Copyright Advocate Press and Ovulation Method Research and Reference Centre of Australia, Melbourne.
Definition of the cycle type from the pregnanediol values reached after the ovulatory oestrogen peak
In this study the ovulatory oestrogen peak was used as the cycle reference point. Others have used the LH peak (Smith et al., 1984) which usually occurs within a day of the oestrogen peak. Timing this to within a day is first required. The cycle type is then defined by the level of pregnanediol reached after this and the time elapsing between the oestrogen peak and bleeding as follows—anovulation: pregnanediol values fail to reach 1.6 mg/24 h; a LUF: pregnanediol values exceed 1.6 mg/24 h but do not reach 2 mg/24 h; ovulation has occurred when the pregnanediol values reach or exceed 2 mg/24 h (9 µM/24 h); ovulation with a deficient luteal phase: pregnanediol values exceed 2 mg/24 h but do not reach 3 mg/24 h; or short luteal phase:10 days or less calculated from the oestrogen peak designated Day 0 to the day before the ensuing bleed; and a fully fertile ovulatory cycle, pregnanediol values exceed 3 mg/24 h during the 6 days after the oestrogen peak and a luteal phase length of 11–17 days if not pregnant. The rationale for choosing these figures is given in the ‘Discussion section’. The very close relationships between the oestrogen peak, ovulation and maximum fertility were reported by Brown and Matthew (1962) either using timed laparotomy or achievement of pregnancy following timed intercourse. Their conclusions have been confirmed by (i) the use of the hormone assays for timing ovulation and egg pick-up in the early development of in vitro fertilization (IVF) (Lopata et al., 1978), (ii) timing artificial insemination (AI) (Leeton, unpublished), (iii) monitoring induction of ovulation by gonadotrophin therapy for IVF (Talbot et al., 1976) and (iv) ultrasonography (Ecochard et al., 2001; Alliende, 2002).
Results
The development of fertility at the beginning of reproductive life
Pre-puberty
The urinary oestrogen values (method C) obtained for 24 boys and 38 girls aged 2–13 years were reported (Brown et al., 1978). The values for the sum of oestrone, oestradiol and oestriol (TE) were within the range 0.1–0.5 µg/24 h in the majority between 2 and 8 years of age and no differences were found between the sexes. These values are similar to those excreted by women after oophorectomy and adrenalectomy, and are one hundredth to one twentieth of those excreted by post-menopausal women (Brown, 1976a, b; Brown et al., 1978). All children excreted the three oestrogens in the same relative proportions as those seen in adults. The ranges, mean values (in brackets) and the percentage of the total for the girls aged 2–9 years were; oestrone 0.05–0.29 (0.12) µg/24 h (33%), oestradiol 0.02–0.09 (0.04) µg/24 h (11%), oestriol, 0.04–0.59 (0.20) µg/24 h (56%) and TE 0.13–0.80 (0.36) µg/24 h. These figures are virtually identical with those reported by Girard and Nars (1976) using sensitive radioimmunoassays for measuring oestrone and oestradiol in urine. Their figures were oestrone 0.04–0.23, mean 0.10 µg/24 h and oestradiol 0.03–0.16, mean 0.10 µg/24 h. The longitudinal studies showed that the rise from the sub-microgram values of childhood was a gradual process which occurred in cyclic fluctuations and the actual rise itself could arrest at any stage for a year or more.
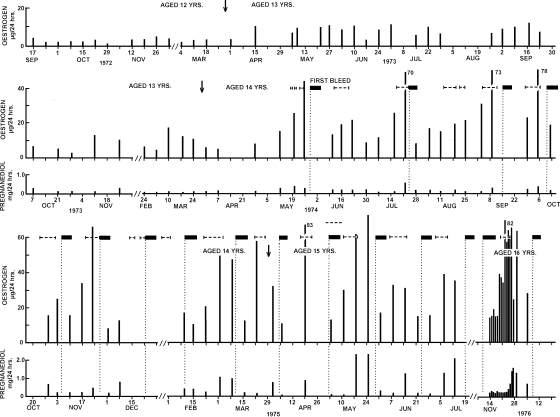
From puberty to maturity
Figure 2 (from Brown et al., 1978), shows the TE and pregnanediol values, and times of uterine bleeding recorded in a girl who provided weekly samples of urine at intervals between ages 12 and 16 years. At age 12.5 years, her TE excretion was fluctuating between 1.2 and 2.6 µg/24 h and the pregnanediol values were between none detected and 0.4 mg/24 h (not shown). During the age of 13 years the oestrogen values increased gradually with rhythmical fluctuations. At the age of 14 years the TE value peaked to 44µg/24 h and she experienced her first bleed (menarche) 3 days later. The pregnanediol values during this time remained at <0.4 mg/24 h. The TE values continued to fluctuate between 10 and 80 µg/24 h and initially, bleeding occurred immediately after the peaks. The second bleed occurred 8 weeks after the first bleed but bleeding occurred at decreasing intervals until eventually it settled to an approximately monthly rhythm. As the interval between the oestrogen peaks and bleeding increased, so did the pregnanediol values before the bleeds. The first pregnanediol value that exceeded 2 mg/24 h (the criterion for ovulation) was observed in May 1975 at age 15 years. The study ceased at age 16 years before the pregnanediol values reached 3 mg/24 h, the criterion for a fully fertile ovulation. This is therefore a pattern of follicular activity increasing until the oestrogen values reached were sufficient to initiate bleeding followed by the gradual maturation of the ovulatory mechanism as shown by the increasing pregnanediol values and the increasing interval between the oestrogen peak and bleeding. This was a continuous process which included lengthening of initially short and deficient luteal phases and increasing progesterone production. All the types of ovarian activity referred to in the introduction could be conceptualized in the process.
Figure 2.
Weekly TE and pregnanediol values in a girl measured over 4 years from age 12 to 16 years. Menarche and first ovulation were documented. Arrow denotes birthdays; filled boxes denote vaginal bleeding, vertical dotted lines show day of bleeding, horizontal dotted lines denote production of fertile type cervical mucus. [Reprinted with permission from Brown et al. (1978). Copyright Cambridge University Press].
This subject's younger sister also collected urine from age 11 years when her TE values were <0.5 µg/24 h until age 16 years. Menarche occurred at age 15 years as the TE values were peaking to 30 µg/24 h. The oestrogen values showed the same initial anovulatory pattern as her sister but the pregnanediol values remained very low during the complete study, and the interval between the oestrogen peak and bleeding showed no sign of increasing. It is concluded that the ovulatory mechanism had not begun to mature in this subject by the age of 16 years. Three other girls were studied between May and November 1972, one had her menarche during the study, one before the study commenced and the other 4 months after concluding the study. The oestrogen and pregnanediol patterns were similar to those shown in Fig. 2 except that one girl had regular bleeding cycles from the start and ovulated 2 months after her first bleed. Thus the time taken to progress from childhood to reproductive maturity is very variable among individuals. The pattern seen in the human also occurs in chimpanzees which start copulating (oestrus caused by oestrogen peaks) before menarche but may not conceive until 4 years after menarche (Short, 1978).
Approach to menopause
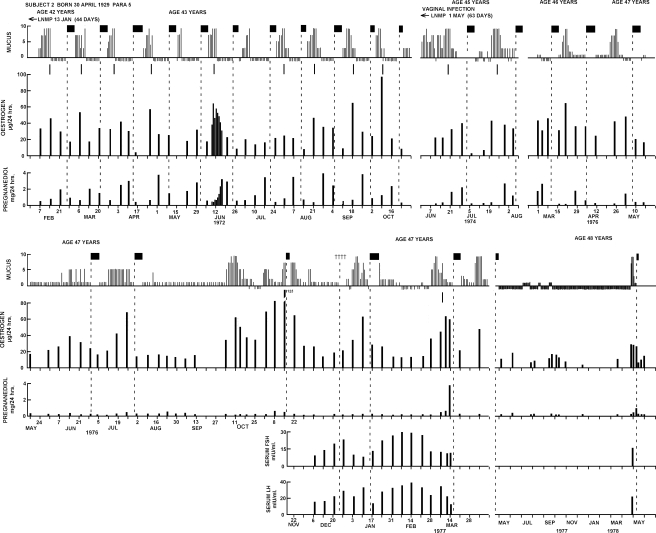
Figure 3 (from Brown et al., 1981) shows a longitudinal study provided by a woman who collected urine specimens and recorded her bleeding episodes and daily mucus scores for 6 years between 1972 and 1978 as she approached and reached menopause. At age 42 years, she recorded an unusually long period of amenorrhoea following a life-time of regular bleeding cycles and collected urine specimens to discover the cause. She had an ovulatory cycle of 44 days' duration with a deficient luteal phase (maximum pregnanediol value 2 mg/24 h) of 12 days duration. Following this she recorded regular normal ovulatory cycles for the next 8 months which were the last potentially fertile cycles recorded. At aged 45, after another period of amenorrhoea she had a long, ovulatory but deficient, cycle of 63 days followed by two further cycles of normal length but deficient luteal phases. She remained anovulatory for the next 12 months but the oestrogen values continued to fluctuate substantially and on one occasion (November 1977) reached 125 µg/24 h which is the 95th percentile for an ovulatory cycle. In her final ovulatory cycle, the pregnanediol values were consistent with ovulation but she had a short luteal phase of 4 days. Thereafter, for the majority of the time, the oestrogen values remained <20 µg/24 h (she was obese) with minor fluctuations and she experienced amenorrhoea for a year. In April 1978, at the age of 48 years, she experienced an increase in the mucus score associated with a spike in oestrogen levels and a small elevation in pregnanediol output. She bled shortly afterwards. The mucus scores paralleled the oestrogen peaks throughout the study. Measurements of FSH and LH in serum were performed from December 1976 to March 1977. The values found were intermediate between those of fertile ovulatory cycle levels and post-menopausal levels and were fluctuating in a reverse order to the oestrogen levels. This subject continued to collect specimens until she was aged 51 years. No further rises in pregnanediol levels were observed but spikes in oestrogen output from time to time, followed by bleeding up to 2 weeks later. Curettage during this time showed normal proliferative endometrium. Serum FSH and LH levels were now in the post-menopausal range. Similar studies were performed in seven other women approaching menopause with similar patterns. These studies showed that at the end of reproductive life, the ovulatory mechanism fails first before follicular activity ceases, i.e. the reverse of the sequence that occurs at the beginning of reproductive life and that follicular activity and attempted ovulation at this time can occur sporadically before it ceases entirely.
Figure 3.
Weekly TE and pregnanediol values and daily mucus scores recorded over a period of 6 years in a woman between the ages of 42 and 48 years as she approached menopause. Weekly serum LH and FSH values were measured between December, 1976 and March, 1977. The short vertical lines under the mucus scores are the best estimates of the times of ovulation. Filled boxes denote bleeding; plusses symbols denote spotting. Note that the ovulatory mechanism began to fail before the follicular activity. Reprinted with permission from Brown et al. (1981) Copyright Advocate Press and Ovulation Method Research and Reference Centre of Australia, Melbourne.
Return of fertility post-partum
Longitudinal studies were performed on 55 post-partum women (Brown et al., 1985). All the types of ovarian activity could be recognized. Their incidence was influenced by whether the woman was breast-feeding and the time interval since delivery. Amenorrhoea merging into anovulatory cycles, deficient and short luteal phases and finally fertile ovulatory cycles were the rule. The findings agreed with other studies reported at the time and assisted in the formulation of the lactational amenorrhoea method (LAM) of family planning (Kennedy et al., 1995). This states that, provided the woman is fully breast-feeding, the chances of a fertile ovulatory cycle occurring during the first 6 months post-partum or before the first bleeding episode, whichever comes first, are small enough to dispense with other contraceptive methods (calculated pregnancy rate 2 per 100 women).
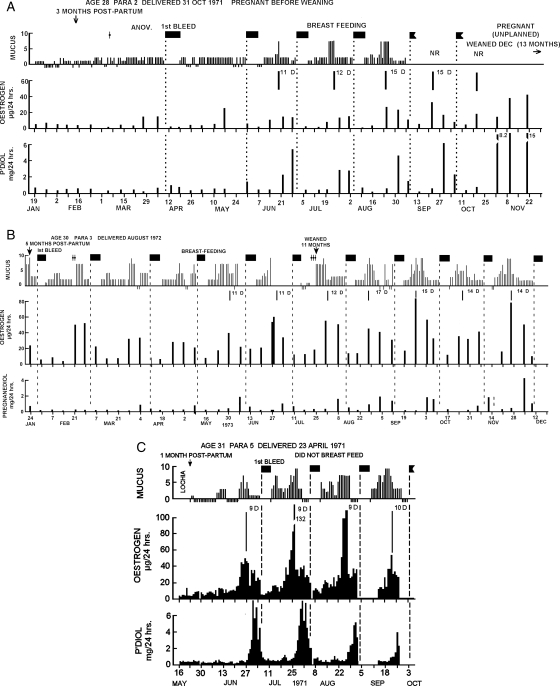
Figure 4A (from Brown et al.,1985), shows weekly hormone values, daily mucus scores and times of bleeding recorded by a woman for 14 months post-partum. The first rise in oestrogen levels occurred 5 months after delivery, followed by a rise in pregnanediol levels that did not reach 2 mg/24 h, showing that this cycle was anovulatory. Her first bleeding episode post-partum occurred 12 days later. The pregnanediol values before bleeding in subsequent cycles continued to increase and a fully fertile ovulatory cycle occurred 2 months later with pregnanediol values exceeding 3 mg/24 h and a luteal phase length of 11 days. The lengths of the luteal phases continued to increase to 15 days and the pregnanediol values before bleeding continued to exceed 3 mg/24 h. She thus had reached fertility exceptionally early and she conceived (unplanned) 12 months after delivery.
Figure 4.
Weekly TE and pregnanediol values, daily mucus scores and episodes of bleeding during the return of fertility after child-birth and during lactation. The vertical dotted lines show the day of bleeding and the solid vertical lines under the mucus represent the best estimate of the day of ovulation. Filled boxes denote bleeding; NR, no record. (A) Fertile cycles recommenced in June and conception occurred in October. (B) A similar figure showing that the return of the ovulatory mechanism came after the commencement of follicular activity as in Fig. 2. (C) This subject did not breastfeed and ovulatory cycles with short luteal phases commenced 2 months after delivery. Reprinted with permission from Brown et al. (1985) Copyright Cambridge University Press.
Figure 4B (from Brown et al., 1985), shows the results obtained in a woman who experienced her first bleeding episode 5 months post-partum following an anovulatory cycle. The next three bleeding episodes also followed anovulatory cycles but with each cycle, the intervals between the oestrogen peaks and bleeding were gradually increasing, and as this occurred so also the pregnanediol values increased from baseline levels to a fully fertile cycle with values in excess of 3 mg/24 h and a luteal phase of 14 days. The return of fertility post-partum shown in Fig. 4B closely resembles the development of fertility after menarche shown in Fig. 2 but in a shorter time span.
Figure 4C (from Brown et al., 1985), shows the results obtained from a woman who decided not to breast-feed. Ovulatory cycles returned within 2 months of delivery, the pregnanediol levels exceeded 3 mg/24 h from the start but the first three luteal phases were short (9 days) and the next was 10 days. Short and deficient luteal phases are common post-partum.
Sporadic infertile cycles during reproductive life
These studies were reported by Brown et al. (1959) and Brown and Matthew (1962).
No significant ovarian activity (amenorrhoea)
The uniformly low oestrogen and pregnanediol values obtained when no significant ovarian activity is present have been well demonstrated (Figs. 2–4). Before puberty these values are in the sub-microgram range or undetectable. After puberty the TE values are higher but usually <10 µg/24 h and the pregnanediol values are usually <0.5 mg/24 h but both may be higher in obese subjects. These levels are of adrenal origin. Amenorrhoea persists during this time unless pathology is present. In gonadotrophin therapy, the finding of continuing low oestrogen values signifies that the dose of FSH has not reached the threshold required for stimulating the growth of a dominant follicle and needs to be increased (Brown et al., 1969).
Anovulatory ovarian activity
Three patterns of oestrogen output are seen during anovulatory ovarian activity (Figs. 5–7). Characteristically, the pregnanediol values remain below 1.5 mg/24 h, the uterine endometrium shows proliferative changes only and no increase in basal body temperature occurs.
Figure 5.
(A) Daily oestriol, oestrone and oestradiol and pregnanediol values, and basal body temperature, during two anovulatory cycles of the fluctuating oestrogen type, one with a sharp TE peak and the other with a broad TE peak. The pregnanediol values were obtained by the colorimetric method of Klopper et al. (1955) and included a small urine blank. (B) Two anovulatory cycles with sharp oestrogen peaks. (C) Results from another woman showing a broad anovulatory oestrogen peak. Filled boxes denote bleeding in all diagrams. Reprinted with permission from Brown and Matthew (1962) Copyright the Endocrine Society.
Figure 7.
Daily oestriol, oestrone and oestradiol values, episodes of bleeding and results of endometrial biopsies in an anovulatory cycle of the constant oestrogen type (A) The TE values remained in the range of 20–40 µg/24 h and the biopsies showed either cystic glandular hyperplasia or late proliferative changes. (B) Two broad anovulatory oestrogen peaks with two ovulatory cycles between them and followed by another ovulatory cycle. The TE values reached 70–100 µg/24 h and were elevated for 2–3 weeks during the broad anovulatory peaks and the biopsies showed cystic glandular hyperplasia. Note that bleeding during two of the ovulatory cycles continued throughout the follicular phase and ceased at the oestrogen peaks. Filled boxes denote bleeding in all diagrams. Reprinted with permission from Brown and Matthew (1962). Copyright The Endocrine Society.
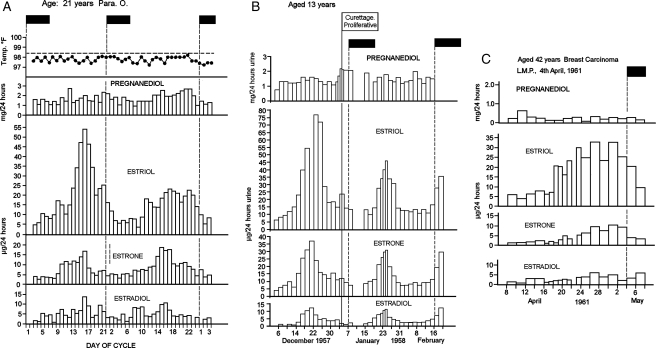
Fluctuating oestrogen values
Figure 5A (from Brown and Matthew, 1962) shows results from two cycles from a subject aged 21 years with a history of regular bleeding cycles. In the first cycle shown, a single TE peak reaching 85 µg/24 h followed by a pronounced fall was identified. This resembled the peak of an ovulatory cycle but it was not followed by an increase in pregnanediol excretion or basal body temperature. Bleeding occurred 5 days after the peak while the oestrogen levels were falling. This was therefore an anovulatory cycle with a sharp oestrogen peak that ended in oestrogen withdrawal bleeding similar to most of the anovulatory cycles shown in Figs. 2 and 3. Such patterns are produced during gonadotrophin therapy when the dose of FSH is sufficient to develop a dominant follicle but the ovulating dose of HCG is withheld or insufficient to induce ovulation (Fig. 6 in Brown et al., 1969). The second cycle from this subject showed a much broader oestrogen peak which was also anovulatory. Similarly, anovulatory oestrogen withdrawal bleeds are shown in Fig. 5B for a girl aged 13 years who had presented with continuous bleeding for 8 weeks. The bleeding ceased spontaneously on 10 December 1957 and curettage 25 days later showed proliferative endometrium only. A broad oestrogen peak followed by bleeding but without ovulation is shown in Fig. 5C for a woman aged 42 years with a history of regular bleeding cycles. Note that in these anovulatory cycles with definite oestrogen rises and falls, bleeding occurred more or less regularly and was related closely with the falls in oestrogen production.
These examples show that two types of oestrogen peaks may be encountered during anovulatory cycles, one sharp and resembling the oestrogen peak of an ovulatory cycle, the other much broader. The sharp peak may reach higher levels than those seen in ovulatory cycles (see Fig. 5B). It appears that ovulation truncates the oestrogen rise in such cycles.
Constant oestrogen values
The results shown in Fig 6A (from Brown and Matthew, 1962) are from a woman aged 19 years who, since menarche at age 14 years, had bleeding cycles that were irregular with intervals of 1–3 months. The four biopsies showed late proliferative endometrium and therefore the three bleeding episodes were anovulatory. The TE values remained approximately constant at between 12 and 20 µg/24 h throughout the study. Such a pattern occurs in gonadotrophin therapy when FSH administration is on the threshold for stimulating follicular development but not sufficient to develop a dominant follicle (Brown et al., 1969, Fig. 10, Brown, 1978). The condition in gonadotrophin therapy is rectified by slightly increasing the dose of FSH. Brown et al. (1969) noted that ovulation cannot be achieved by giving HCG while the oestrogen values remain at these constant levels but can be achieved when the dose of FSH is increased and the oestrogen values are increasing logarithmically. The phenomenon is explained on the basis that the dose of FSH has just reached the threshold required to recruit follicles that develop and regress continuously but is insufficient to recruit a dominant follicle. In the process, oestrogen is produced at a more or less constant rate. This stimulates growth of the endometrium which builds up, becomes unstable and breaks down at intervals as oestrogen breakthrough bleeding. The bleeding is analogous to that which occurs with low constant oestrogen output by oestrogen-producing tumours or low continuous administration of oestrogens. Note that the bleeding episodes were highly irregular and some were prolonged and this is a common feature of this cycle type. However, in some cases bleeding may occur regularly every 28 days. The third ‘normal’ volunteer had regular anovulatory cycles of this type and thought that she was ovulating. The finding caused much confusion in the emerging concept of normality, and a similar pattern was not encountered in another woman until 3 years later.
Figure 6.
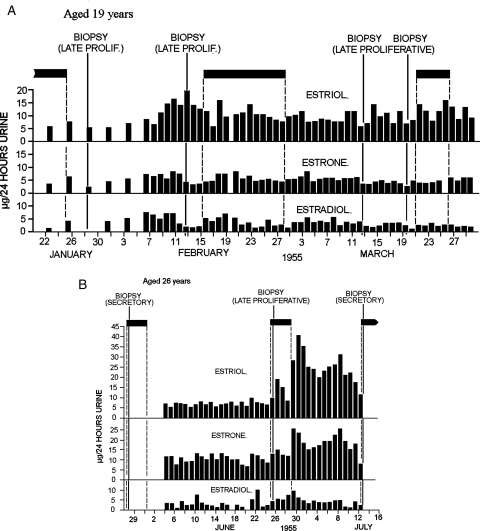
Daily oestriol, oestrone and oestradiol values, episodes of bleeding and results of endometrial biopsies during anovulatory ovarian activity of the constant oestrogen type. Filled boxes denote bleeding. (A) The TE values remained in the elevated range of 10–20 µg/24 h throughout the 3 months of the study and the biopsies showed late proliferative changes. (B) An anovulatory cycle sandwiched between two ovulatory cycles. The anovulatory bleeding occurred after the TE levels had been elevated between 15 and 20 µg/24 h for 22 days. This could be regarded as mid-cycle bleeding in an ovulatory cycle of 44 days duration. Note that the bleeding stopped as the oestrogen values rose to the ovulatory peak. Reprinted with permission from Brown and Matthew (1962). Copyright The Endocrine Society.
Anovulatory cycles and abnormal uterine bleeding including cystic glandular hyperplasia
Mid-cycle bleeding
Figure 6B (from Brown and Matthew, 1962) shows the findings from a woman aged 26 years, who from the age of 13 years had normal regular bleeding cycles. The pattern shown is that of an anovulatory cycle sandwiched between two ovulatory cycles. Anovulatory breakthrough bleeding occurred as a result of the constant oestrogen values, but the situation corrected itself, a dominant follicle emerged, and the oestrogen values rose logarithmically to an ovulatory peak. Note that the bleeding stopped as the oestrogen peak was reached. A completely normal ovulatory cycle followed. This could therefore be classified as a mid-cycle bleed. When similar situations were encountered during gonadotrophin therapy, they were corrected by increasing the dose of FSH. When this was achieved, the pregnancy rate was 31%, equal to that of normal ovulatory cycles (Fig. 10 of Brown et al., 1969). Thus, once a dominant follicle has been selected and the correct rising oestrogen profile has been achieved, the resulting ovulation is fully fertile.
Cystic glandular hyperplasia
Patients with cystic glandular hyperplasia can be divided into the two anovulatory groups, constant or fluctuating oestrogen profiles.
Constant oestrogen levels
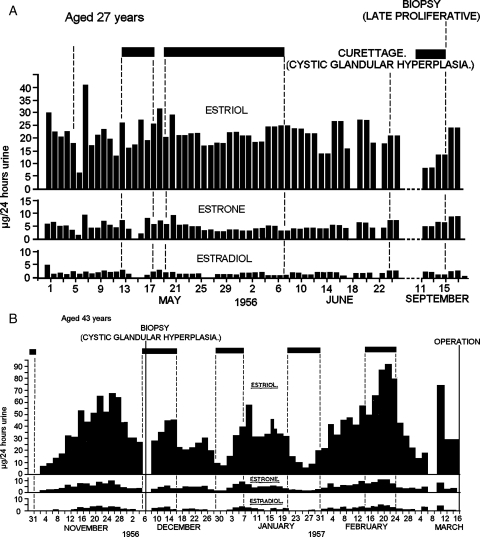
Figure 7A (Brown and Matthew, 1962) shows the results obtained in a patient aged 27 years, who had experienced irregular bleeding at intervals of 30–90 days for the past 3 years. The TE levels remained relatively constant but high, and curettage after the bleeding had stopped showed cystic glandular hyperplasia. Measurements were recommenced almost 3 months later with similar results and curettage at that time showed late proliferative changes. Therefore, oestrogen levels approximately double those seen in anovulatory cycles of the type shown in Figs. 6B and 7A are responsible for the extreme development of cystic glandular hyperplasia. The raised oestrogen values are probably caused by the follicular cysts seen on ultrasound in this condition.
Fluctuating oestrogen levels
Figure 7B (Brown and Matthew, 1962) shows the results obtained in a patient aged 43 years, para 5, who had experienced irregular bleeding for the previous 15 months. Curettage before the beginning of the study showed cystic glandular hyperplasia. Urinary oestrogen excretion was measured from November 1956 to March 1957 using 48-h specimens. A broad peak of oestrogen excretion reaching a TE value of 80 µg/24 h and being maintained above 35 µg/24 h for 20 days was seen in November. Bleeding commenced as the oestrogen levels were falling and a biopsy performed during this bleed again showed cystic glandular hyperplasia. Two ovulatory cycles followed and bleeding occurred throughout the follicular phases of these two cycles but ended at the ovulatory oestrogen peaks as in Fig. 6B and recommenced, as might have been expected, at the end of the luteal phases. The next cycle showed another broad oestrogen peak with bleeding while the oestrogen levels were still rising and ceasing as they fell. This finding was first thought to be an error in recording the dates of bleeding. This pattern showed anovulatory ovarian activity and breakthrough bleeding after the oestrogen levels had been markedly elevated for 12 days. An ovulatory cycle followed as shown by the secretory endometrium found in the hysterectomy specimen. These results show that high and fluctuating anovulatory oestrogen production with broad peaks is also a cause for cystic glandular hyperplasia. It was of interest that bleeding continued throughout the pre-ovulatory phases of the two ovulatory cycles indicating that the preceding excessive oestrogen stimulation had made the endometrium unstable. This was corrected by the progesterone produced after ovulation. This subject demonstrates that ovulatory and anovulatory cycles can occur at random in this condition.
The broad anovulatory oestrogen peaks shown in Fig. 7B are different from the sharp peak of the ovulatory cycle and the anovulatory peaks shown in Fig. 5A and B but similar to the broad peaks shown in Fig. 5A and C. The amount of TE excreted throughout the first broad peak shown in Fig. 7B was 1430 µg/24 h over 30 days and 1856 µg/24 h over 38 days in the second. The corresponding figure for the 90th percentile for the ovulatory cycle calculated from Fig. 1 was 610 µg/24 h. Thus the broad peaks represent oestrogen production 2–3 times that of the peak of the ovulatory cycle, and this together with the absence of progesterone production explains the extreme proliferation of the endometrium. Note that this woman was excreting ∼6 times more oestriol than oestrone and oestradiol combined compared with a more usual ratio of 1:1. Without the oestriol values the high production rates would not have been recognized.
The broad oestrogen peak is not common in the general population but Brown et al. (1969) reported 25 cycles of this type encountered during 222 courses of gonadotrophin therapy (11%). No pregnancy occurred in this group in spite of the use of high doses of HCG which achieved progesterone responses equivalent to ovulation. Some very high TE values exceeding 1000 µg/24 h were recorded. Important variables in achieving ovulation in such cases included rate of rise of the oestrogen values before the HCG was given and separating the last dose of FSH from the ovulating dose of HCG by an interval of 36–48 h. These findings suggested that this type of anovulatory cycle is caused by an unusual ratio of LH to FSH present in the gonadotrophin administered.
LUF, early anovulatory oestrogen peaks, multiple ovulatory oestrogen peaks and the deficient luteal phase
The hormonal characteristics of a LUF and a deficient luteal phase have already been defined. A LUF was not recognized in the early studies because the pregnanediol measurements were not sensitive enough. This was rectified by the use of GLC (method B). The existence of LUFs can be seen during the maturation of the ovulatory mechanism in Fig. 2 and its demise in Fig. 3.
Inspection of the daily hormone values obtained in 140 ovulatory cycles using the fluorometric oestrogen method (B) and the GLC pregnanediol method (B) identified 60 cycles with evanescent pregnanediol rises during the pre-ovulatory phases that satisfied the criteria for LUFs; 51of these were associated with early oestrogen peaks. It is a general rule that the longer the follicular phase the more likely that an early anovulatory oestrogen peak will occur with evidence of an associated LUF. Figure 8D provides an example of both an early oestrogen peak associated with a brief pregnanediol rise and a deficient luteal phase. The woman was experiencing an unexpected long cycle of 45 days. The oestrogen peak on Day 30 reached 40 µg/24 h which is on the 10th percentile for ovulating women (Fig. 1). The maximum pregnanediol value reached during the luteal phase was 2.2 mg/24 h on Day 41, and during the 6 days following the oestrogen peak the maximum value reached was 2.0 mg/24 h. Therefore ovulation had occurred but was followed by a deficient luteal phase. The oestrogen rise from Day 21 reaching a peak of 18 µg/24 h on Day 24 followed by a fall indicated that a follicle developed at this time but it did not progress to ovulation. The pregnanediol values doubled from 0.2 to 0.4 mg/24 h from Day 23 to 24 and this could be interpreted as being due to a LUF. Alliende (2002) described such early oestrogen peaks. It seems that a follicle which is not going to progress to ovulation is recognized and its place is immediately taken by another follicle that is ‘waiting in the wings’. This increased the chances in human evolution that a fertile ovulation occurred in every cycle and the possibility of conception in that cycle was not missed. Furthermore, when ovulation is achieved, the mechanism immediately recognizes this and positively inhibits recruitment of further follicles for ovulation.
Figure 8.
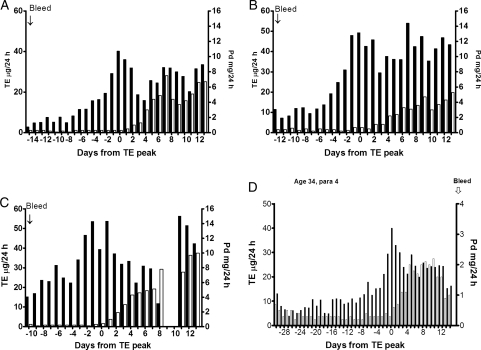
(A–C), show three conception cycles, (A) with a single oestrogen peak, which is the most common, and (B) and (C) with composite oestrogen peaks to show that such cycles are fertile. (C) Showed an early anovulatory oestrogen peak on Day-7. Note that all these conception cycles showed pregnanediol values exceeding 3 mg/24 h within 6 days of the oestrogen peak and all showed a fall by Day 10 as if the corpus luteum was regressing and was rescued by the developing pregnancies. (D) Shows a long cycle of 45 days with a deficient luteal phase, an oestrogen peak at the 10th percentile (40 µg/24 h) and an early anovulatory oestrogen peak on Day 24. The (pre-ovulatory) oestrogen peak day was Day 30.
Short luteal phase
The short luteal phase has already been defined and demonstrated in Fig. 5. Short luteal phases are common during the first cycles post-partum but they also occur sporadically throughout reproductive life. Deficient and short luteal phases can occur in the one cycle and are the most common types of infertile ovulatory cycles. An important finding was that all ovulatory cycles (pregnanediol excretion reaching or exceeding 2 mg/24 h) were followed by bleeding whether the luteal phase was normal, deficient or short, provided the endometrium was responsive to hormonal stimulation, the subject was not pregnant and no blockage was present. This differs from anovulatory cycles where bleeding may or may not follow an oestrogen peak (Fig. 13 in Brown et al., 1959).
The fertile ovulatory cycle
The hormone patterns associated with the fertile ovulatory cycle have very definite characteristics and a narrow range of possibilities. The following information is derived from the 140 ovulatory cycles already quoted in which infertility was not the reason for the study unless conception occurred in that cycle. 114 (81%) of these registered a single ovulatory oestrogen peak (Fig. 8A) indicating that a single dominant follicle was involved in the final rise to ovulation and 26 (19%) showed a composite peak indicating that two or more follicles were involved. A conception cycle with a single oestrogen peak and two conception cycles with composite peaks are shown in Fig. 8 A–C, respectively to demonstrate that such cycles are fertile. Fifty-two (37%) showed early oestrogen peaks that did not progress to ovulation (Fig. 8C and D). All of these peaks were lower than the later ovulatory peaks.
The TE rise from baseline values (mean ± SD) of 11.8 ± 5 µg/24 h to the ovulatory peak values of 63 ± 21µg/24 h was 5.8 ± 2.1 times in 5.9 ± 1.5 days. This represents a logarithmic increase in oestrogen excretion from baseline of approximately 1.4 times per day for 5 days to reach the oestrogen peak. The fall after the peak reached 0.41 ± 0.12 of the peak value in 2.9 ± 1.1 days before rising again into the luteal phase. This represents a fall of approximately 0.7 (the reciprocal of 1.4) times per day. Thus the fall after the peak is a very clear signal defining the peak, and as it represents the clearance of oestrogens which have ceased to be produced at ovulation (Baird and Fraser, 1974) it is a clear signal for timing ovulation. Its accuracy for this purpose had been repeatedly demonstrated. Nevertheless, it is possible to find this type of oestrogen peak without ovulation (Fig. 5A and B). Of the 140 cycles, 10 were conception cycles, another 10 (7%) had deficient luteal phases, the mean ± SD for the follicular phase pregnanediol levels was 0.34 ± 0.18 mg/24 h, and for the luteal phase maximum was 4.7 ± 1.1 mg/24 h. Raised progesterone values before ovulation reduce fertility (Baird et al., 1999).
Figure 1 (from Brown et al., 1981) summarizes the TE and pregnanediol excretion values found throughout 61 cycles from 26 parous and 14 nulliparous women aged 20–40 years (taken from an early analysis of the 140 cycles). All values are plotted and the 10th, 50th and 90th percentile lines are shown. The mid-cycle oestrogen peak was identified for every cycle and days were numbered from this day (=day 0). The figure allows the oestrogen levels given for the various cycle types to be compared with those found during the ovulatory cycle.
Enhanced ovulatory cycle
IVF not only performs fertilization in a petri dish but utilizes important procedures for increasing pregnancy rates. They are: (i) accurate timing of ovulation by the use of gonadotrophin therapy to cause ovulation at a predetermined time for egg pick-up: gonadotrophin therapy is also the most powerful method for converting the various types of infertile ovarian activity found in the continuum to fertile ovarian activity (Brown, 1986), (ii) enhancement of ovarian activity above the normal by gonadotrophin therapy produces more eggs for fertilization (Talbot et al., 1976), (iii) knowing the time of ovulation also provides the timing for intrauterine embryo transfer or artificial insemination (AI), which by-pass cervical problems, and also provides accurate information on the optimum time for natural intercourse and the starting time of the pregnancy, (iv) raised oestrogen production ensures adequate cervical mucus production, (v) utilization of all these IVF procedures without IVF but with AI or natural intercourse enhances pregnancy rates with only an equivalent increase in multiple pregnancy rates. (Talbot et al., unpublished), (vi) Dennerstein et al. (1993) found that women in the daily work force had lower ovarian hormone production than women at home minding children and that this also probably affected their fertility. Therefore, even for the fertile ovulatory cycle, a gradient of fertility exists that is determined by the levels of ovarian hormones produced, independent of whether more than one ovum is produced.
Stylized patterns for the steps in the continuum
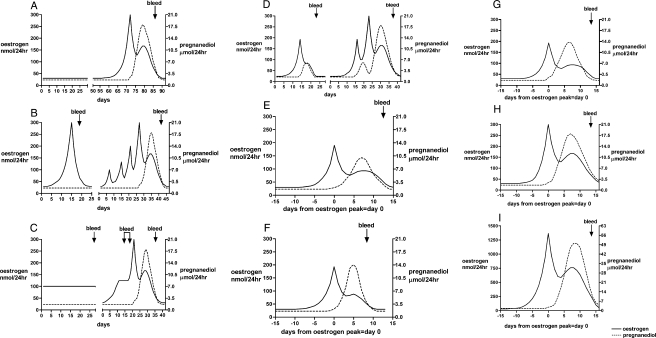
Figure 9A–I demonstrates the patterns in stylized form summarizing the various steps in the continuum. It should be appreciated that each is a step in a continuous process. Possible sequels are shown because it is important that women who are identifying their fertile periods for either pregnancy achievement or avoidance understand that continuation of current patterns of fertility can never be assumed and vigilance is always required to detect the next onset of fertility. The figures progress from no activity (A) which may be interrupted by a return of a fertile ovulation, through anovulatory activity of the fluctuating (B) or constant oestrogen (C) type and progress in that cycle to fully fertile ovarian activity, through a LUF (D) which may be followed in the same cycle by a fertile ovulation, through a deficient (E) or short (F) luteal phase which may, in the next cycle, revert to a fertile ovulatory cycle. The range of values seen during the ovulatory cycle, (G), 10th percentile and (H), 50th percentile and (I) reaching even greater fertility with gonadotrophin therapy.
Figure 9.
Conceptualized cycles of the continuum. (A) Uniformly low TE and pregnanediol values denoting no ovarian activity and amenorrhoea. A fertile ovulatory cycle may follow spontaneously. (B) Anovulatory ovarian activity with a sharp oestrogen peak followed closely by oestrogen withdrawal bleeding. Days 30–70, a possible sequel, several anovulatory oestrogen peaks not followed by bleeding which might have been interpreted as ovulatory oestrogen peaks but recognized by absence of a progesterone rise and eventually followed by a fertile ovulation. (C) Days 1–30, anovulatory ovarian activity with constantly raised oestrogen excretion and oestrogen breakthrough bleeding. Days 30–70, possible sequel, situation correcting itself and progressing to a fertile ovulatory cycle, the oestrogen breakthrough bleeding then seen as mid-cycle bleeding. (D) Days 1–25, a sharp oestrogen peak followed by a LUF follicle followed by bleeding; the pregnanediol values rose temporally but did not reach 2 mg/24 h (9 µM/24 h). Days 30–70, possible sequel, a LUF not followed by bleeding but followed by a fertile ovulatory cycle. (E) Ovulation followed by a deficient luteal phase in which the pregnanediol values exceeded 2 mg/24 h (9 µM/24 h) but did not reach 3 mg/24 h (13.5 µM/24 h). Menstruation followed. (F) Ovulation followed by a short luteal phase of 10 days or less. The pregnanediol values usually exceed 3 mg/24 h but fall prematurely. Menstruation followed. (G) A fertile ovulatory cycle with hormone values at the 10th percentiles. (see Fig. 8). (H) A fertile ovulatory cycle with hormone values at the 50th percentiles. (I) An enhanced ovulatory cycle produced by gonadotrophin therapy.
Discussion
These studies demonstrate the value of frequent monitoring of ovarian hormone production. The patterns obtained identify the various types of ovarian activity, the times of ovulation, infertility and possible fertility and clarify the bleeding patterns. The TE values obtained by the early assay methods provided an approximation to hormone production rates which correlated with the action of the ovarian hormones on the uterine endometrium and cervix. The ability to report reproductive potential and uterine bleeding in terms of the underlying ovarian activity rather than uterine bleeding would seem to be an important advance. Brown and Matthew (1962) discussed the value of the hormone assays and included examples of a range of gynaecological disorders and surgical and medical procedures showing that the hormone values and patterns correlated closely with, and confirmed, the clinical findings.
The findings reported here were obtained by measuring metabolites of the ovarian hormones excreted in urine. Before 1970, such results were the only ones available. However, in 1970, Ross et al. (1970) published their elegant studies of blood levels of estradiol, progesterone, FSH and LH throughout the ovulatory cycle using radioimmunoassays. These assays were sensitive enough to measure the 200 times lower concentrations of the hormones themselves in blood compared with their metabolites in urine. The patterns for the ovarian hormones found in blood had already been underpinned by the earlier urinary assays. After this, a situation arose where journals refused to publish further results obtained from urine, considering them to be obsolete. The author, however, in following the general change to measurements using blood, with the assistance of a UK trained scientist, set up a radioimmunoassay laboratory for the measurement of estradiol, progesterone, FSH and LH in blood. This laboratory did well in the quality control program conducted by the World Health Organization at the time. In comparing the information obtained using urine, the author found that, for serial studies, the women greatly preferred to collect daily timed specimens of urine than to present for daily blood collection. Furthermore, for some women, the stress of collecting daily blood specimens depressed the urine values compared with those that had previously been obtained without blood collection. This phenomenon was also reported by Johansson et al. (1971). As ‘stress’ was emerging as an important factor causing the change from fertile to infertile phases of the continuum, it seemed inappropriate to even risk causing the problem being studied. Another factor emerged from the childhood study that the concentrations of the hormones in blood were in most cases below the sensitivity of the best radioimmunoassays whereas they were well within the range for the urinary assays. This was also reported by Girard and Nars (1976). Therefore, the facility for performing the urine assays was continued. This was fortunate because application of the blood assays or ultrasound as the monitoring procedure in the long-term studies would have been difficult.
Another objection was that ‘women cannot be expected to collect timed specimens of urine’. This has not been our experience. From the beginning of the studies in the 1950s, women have expressed no difficulty in collecting complete 24-h specimens of urine or timed specimens of urine, provided they were instructed properly and the laboratory staff were fully aware of the problems. It was only when this objection continued to be made that it was considered necessary to institute quality control to check for the possibility. In our experience, the stated problems of timed urine collections have never been an issue. Even the childhood studies were performed with 24 h collections of urine. The youngest girl was age 2 years. The mother pooled the collections and although they could not be timed exactly to 24 h they were accurately timed and correction could be made accordingly. Because of these objections to analyses using urine, the findings obtained after 1970 have been published in reports of meetings, or in privately funded monographs or not at all. Judging from the number of publications now appearing in which large-scale hormone studies using urine are being reported, the above problems may be ended (Baird et al., 1999; Venners et al., 2006).
Ultrasonography also provides important information and the benefits of serial daily ultrasonography have been well demonstrated (Baerwald et al., 2003). However, after the report that ultrasound can visualize developing ovarian follicles and provides information complementary to hormone assays in the induction of ovulation with gonadotrophins (O'Herlihy et al., 1982), it was assumed that ultrasonography was now the preferred modern method. That the combination of ultrasonography and hormone assays, one providing anatomical information and the other providing functional information, was a powerful new tool for investigating ovarian activity, was not appreciated. The few workers who applied the combination concentrated on showing the relationship between the oestrogen peak and ovulation (Ecochard et al., 2001; Alliende, 2002). In the present study, there were many times when ultrasonography would have been most helpful in the interpretation of new hormone findings, for example in understanding the composite ovulatory oestrogen peak and the associated progesterone rises.
The continuum
The various types of ovarian activity reported here are explained as being part of the normal sequence seen during the change from the amenorrhoea of childhood through anovulatory ovarian activity at menarche and the gradual maturation of the ovulatory mechanism to the eventual establishment of the fertile ovulatory cycle. Each stage merges with the next and consequently the process is termed ‘the continuum’. The five conceptualized types are not entities and therefore cannot be grouped as such. Stress of any kind during reproductive life is the most important factor causing ovarian activity to change from the fertile to infertile types. Removal of the stress usually allows fertility to return. The sequence back is from the fertile ovulatory cycle, through the deficient or short luteal phase, LUF, anovulatory ovarian activity to no ovarian activity, the rapidity of change being dependent on the severity of the stress and the sensitivity of the woman to stress. Nothing can be predicted, stages may be skipped or reversed at random. These changes are normal responses to the environment and therefore all the types of ovarian activity in the continuum can be considered to be normal. The process was well demonstrated in a study of ovarian activity in elite women rowers carried out by Harvard University (Snow et al., 1989.) where intensity of training was the stress factor. Five oarswomen showed no change in menstrual function throughout the study (group A), and five showed changes (group B). Ovarian function was assessed by twice weekly pregnanediol measurements using GLC (method B) throughout a year divided into three phases of low, high- and low-intensity training. The figure reported by Snow et al. (1989) shows a group B oarswoman who registered the characteristic values of the fertile ovulatory cycle initially, these changed to deficient luteal phase, LUF and then anovulatory ovarian activity during the phase of intensive training and returned within months to the characteristics of the fully fertile ovulatory cycle when intensive training ceased. The phenomenon is best explained as being an evolutionary development to ensure that the added demands of pregnancy were avoided during times that were unfavourable for the survival of the mother.
This is the first time that specific pregnanediol values have been assigned to distinguishing between the fertile ovulatory cycle, deficient luteal phase, LUF and anovulation. The problem with setting criteria is that a fertile ovulatory cycle is defined as one in which a continuing pregnancy can occur. However, when this is tested by pregnancy the pregnancy rapidly alters the hormone values in the period after ovulation during the time that the luteal phase would have occurred. Thus careful monitoring is required during this period to identify a potentially fertile or infertile cycle. The Day 21 blood progesterone test is completely inadequate for this purpose. In establishing our criteria for diagnosing luteal phase deficiency, it is necessary first to have an accurate reference point. In the present survey the ovulatory oestrogen peak was used which itself may be in doubt by one day when a composite peak is present. This requires daily monitoring. The next requirement is to monitor progesterone production for the next 6 days before a pregnancy starts boosting luteal function. Our definitions are based on two observations. The first is derived from inspection of more than 50 natural conception cycles where pregnanediol excretion was measured daily for the first 6 days after the ovulatory oestrogen peak. There was no exception to the rule that the pregnanediol values should exceed a value of 3 mg/24 h (13.5µM/24 h) for at least 1 day and preferably 2 or more days by Day 6. The second criterion is derived from the rule that a good luteal phase requires first a good follicular phase (DiZerega and Hodgen, 1981). The finding of luteal phase pregnanediol values which do not reach 3–3.2 mg/24 h (13.5–14.4 µM/24 h) in a woman presenting with infertility is good reason for enhancing the hormone values with clomiphene therapy. This greatly increases the chances of pregnancy (unpublished data). That the luteal phase should last for 11 days or more after the ovulatory oestrogen peak and that a length of 10 days or less defines a short luteal phase is now generally agreed to within a day (Smith et al., 1984).
The deficient luteal phase based on these criteria is, in our experience, the most common cause of infertility. It has been encountered in every population of ‘normal’ women and in one, reached one-third of the total cycles. This phenomenon was also debated by Johansson et al. (1971). This frequency is not unexpected because it is the first step in a regression from the fertile ovulatory cycle and more severe stress and more sensitivity to stress are required to produce the more severe forms of infertile activity. Importantly, the finding of a deficient luteal phase in one cycle of an individual does not mean that the next cycle will also be deficient—the condition is sporadic and unpredictable. It is the main reason why ovulation does not necessarily confer fertility.
Conversion of infertile ovarian activity to fertile ovulatory cycles
Infertile ovarian activity that persists in women wishing to conceive is usually treatable with clomiphene. It is recommended that responses be monitored daily to ensure that the ovaries have responded and intercourse is appropriately timed. Gonadotrophin therapy may be required for women not responding to clomiphene and its use in IVF avoids the problem of identifying infertile cycle types entirely. Identification and removal of stress factors may also be successful and together with reassurance that pregnancy will be achieved in most cases, should always be applied (Townsend et al., 1966).
Hormone profiles and bleeding patterns in the various cycle types
Hormone profiles and some bleeding patterns are included in Figs. 1–9. The uterine bleeding that occurs in ovulatory cycles is the result of withdrawal of progesterone produced by the corpus luteum that has acted on an oestrogen primed endometrium to produce a secretory endometrium. The bleeding occurs 11–17 days after ovulation, is usually controlled in amount and is termed ‘normal menstruation’. This bleeding always follows ovulation provided the woman is not pregnant or no abnormality exists.
The bleeding that follows anovulatory ovarian activity depends largely on whether the underlying ovarian activity is of the constant or fluctuating oestrogen type. The constant type causes oestrogen breakthrough bleeding, and is usually associated with irregular and often uncontrolled bleeding but may very occasionally be indistinguishable clinically from true menstruation (Fig. 9 of Brown et al., 1959). The bleeding that follows fluctuating anovulatory oestrogen secretion may occur as the oestrogen values are falling (most common); as oestrogen withdrawal bleeding without an interval for the luteal phase; or while they are rising or constant (oestrogen breakthrough bleeding); or nor at all (Fig. 11, Brown et al., 1959). Whether bleeding follows an oestrogen peak usually depends on the levels of oestrogen reached but some high oestrogen values approaching 100 µg TE /24 h have been observed which have not been followed by bleeding (Fig. 13, Brown et al., 1959). Thus the bleeding shows that ovarian activity producing oestrogen has occurred but no specific pattern can be assigned to that activity.
Applications of the continuum concept
The various cycle types that occur in the continuum have been presented and explained. The concept adds to our understanding of the underlying ovarian activity associated with many gynaecological and fertility disorders (including polycystic ovarian syndrome). Besides being important in many applications of ART in explaining the phenomenon of transient infertility, understanding movements within the continuum is particularly important in the practice of NFP. The involvement of the Billings Ovulation Method Centre in providing normal volunteers with proven fertility for parts of this study is gratefully acknowledged. In return, the study has shown that the Billings Method correctly identifies fertility and infertility in all stages of the continuum, the infertile types being recognized by absence of the characteristic mucus symptoms of the fertile ovulatory cycle.
Hormone values and fertility and infertility
Firstly, fertility is associated with marked daily changes in hormone output. Any pattern that shows no changes from day to day denotes infertility. This has recently been reported by Kol and Homburg (2008) but it has been applied by the Billings' method of NFP for 40 years. Secondly, achievement of pregnancy in the current cycle is proof that the cycle is fertile. The next best proof of ovulation is provided by the post-ovulatory rise in progesterone production following an oestrogen peak and fall. In the Billings' Method this is shown by a build-up in cervical mucus production followed by a sudden change to minimal mucus (the Peak symptom). This change is caused by the post-ovulatory rise in progesterone output and its anti-estrogenic effect on the cervix. Thirdly, we have never documented more than one ovulation during a cycle. When multiple ovulations occur they do so over a very short time interval when two or more follicles are exactly synchronized (Brown, 1978). Fourthly, when ovulation occurs it is always followed after an interval by bleeding whether the luteal phase is normal, short or deficient, provided the woman is not pregnant and no abnormality exists. Fifthly, the fertile ovulatory cycle with an adequate luteal phase is the only type of ovarian activity that can produce a continuing pregnancy.
Funding
The Medical Research Council of UK, National Health and Medical Research Council of Australia, Departmental funds and the Ovulation Method Research and Reference Centre of Australia.
Acknowledgements
I thank Dr Adrian Thomas for his continued encouragement to write this survey and for his critical review of the manuscript. I thank Prof. Len Blackwell for the many discussions on ovarian function that have arisen from the work and my many clinical colleagues who provided patients and volunteers for the study. I thank Mr HAF Blair in Edinburgh and Dr Meg Smith in Melbourne and their laboratory assistants for their skill in meticulously performing the assays and maintaining strict quality control. I thank the many women who provided the urine specimens, particularly from the Billings' group who provided the majority of the fertile controls. The original manuscript for this paper was written by the author before he died. It was subsequently reviewed and submitted for publication on his behalf by Dr Thomas and Prof. Blackwell and they have made minor changes only for the purposes of clarity. They both wish to thank Mr John Smith for the preparation of the figures in electronic format.
References
- Alliende ME. Mean versus individual hormonal profiles in the menstrual cycle. Fertil Steril. 2002;78:90–95. doi: 10.1016/s0015-0282(02)03167-9. doi:10.1016/S0015-0282(02)03167-9. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson MS. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80:116–122. doi: 10.1016/s0015-0282(03)00544-2. doi:10.1016/S0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- Baird DT, Fraser IS. Blood production and ovarian secretion rates of estradiol-17ß and estrone in women throughout the menstrual cycle. J Clin Endocrinol Metab. 1974;38:1009–1017. doi: 10.1210/jcem-38-6-1009. doi:10.1210/jcem-38-6-1009. [DOI] [PubMed] [Google Scholar]
- Baird DT, Horton R, Longcope C, Tait JF. Steroid dynamics under steady state conditions. Rec Progr Horm Res. 1969;25:611–656. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Zhou H, Kamel F, McConnaughey DR, Kesner JS, Wilcox AJ. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71:40–49. doi: 10.1016/s0015-0282(98)00419-1. doi:10.1016/S0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- Barrett SA, Brown JB. An evaluation of the method of Cox for the rapid analysis of pregnanediol in urine by gas-liquid chromatography. J Endocrinol. 1970;47:471–480. doi: 10.1677/joe.0.0470471. doi:10.1677/joe.0.0470471. [DOI] [PubMed] [Google Scholar]
- Billings EL, Billings JJ, Brown JB, Burger HG. Symptoms and hormonal changes accompanying ovulation. Lancet. 1972;1:282–284. doi: 10.1016/s0140-6736(72)90291-7. [DOI] [PubMed] [Google Scholar]
- Billings EL, Billings JJ, Catarinich M. Atlas of the Ovulation Method. 2nd edn. Melbourne: Advocate Press; 1974. [Google Scholar]
- Blackwell LF, Brown JB, Vigil P, Gross B, Sufi S, d'Arcangues C. Hormonal monitoring of ovarian activity using the Ovarian Monitor, Part 1. Validation of home and laboratory results obtained during ovulatory cycles by comparison with radioimmunoassay. Steroids. 2003;68:465–476. doi: 10.1016/s0039-128x(03)00049-7. doi:10.1016/S0039-128X(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Brown JB. A chemical method for the determination oestriol, oestrone and oestradiol in human urine. Biochem J. 1955;60:185–193. doi: 10.1042/bj0600185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JB. The relationship between urinary oestrogens and oestrogens produced in the body. J Endocrinol. 1957;16:202–212. doi: 10.1677/joe.0.0160202. doi:10.1677/joe.0.0160202. [DOI] [PubMed] [Google Scholar]
- Brown JB. Determination of total estrogens in non-pregnancy and early pregnancy urine by fluorimetry, rapid method. In: Breuer H, Hamel D, Kruskemper HL, editors. Methods of Hormone Analysis. New York: Georg Thieme Verlag, Stuttgart, John Wiley & Sons; 1976a. pp. 422–434. [Google Scholar]
- Brown JB. Determination of estriol, estrone and estradiol-17ß in non-pregnancy urine by spectrophotometry or fluorimetry. In: Breuer H, Hamel D, Kruskemper HL, editors. Methods of Hormone Analysis. New York: Georg Thieme Verlag, Stuttgart, John Wiley & Sons; 1976b. pp. 446–462. [Google Scholar]
- Brown JB. Pituitary control of ovarian function; concepts derived from gonadotrophin therapy. Aust and NZ J Obstet Gynaec. 1978;18:47–54. doi: 10.1111/j.1479-828x.1978.tb00011.x. doi:10.1111/j.1479-828X.1978.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Brown JB. Chapter on Gonadotropins. In: Insler V, Lunenfeld B, editors. Infertility: Male and Female. Edinburgh, New York: Churchill Livingstone; 1986. pp. 359–396. [Google Scholar]
- Brown JB, Matthew GD. The application of urinary estrogen measurements to problems in gynecology. Recent Prog Horm Res. 1962;18:337–385. http://www.endo-society.org/custom_apps/search.cfm?q=recent+progress=in+hormone+research . [Google Scholar]
- Brown JB, Strong JA. The effect of nutritional status and thyroid function on the metabolism of oestradiol. J Endocrinol. 1965;32:107–115. doi: 10.1677/joe.0.0320107. doi:10.1677/joe.0.0320107. [DOI] [PubMed] [Google Scholar]
- Brown JB, Bulbrook RD, Greenwood FC. An additional purification step for a method for estimating oestriol, oestrone and oestradiol-17β in human urine. J Endocrinol. 1957;16:49–56. doi: 10.1677/joe.0.0160049. doi:10.1677/joe.0.0160049. [DOI] [PubMed] [Google Scholar]
- Brown JB, Kellar R, Matthew GD. Preliminary observations on urinary oestrogen excretion in certain gynaecological disorders. J Obst Gynaecol Br Empire. 1959;66:177–211. doi: 10.1111/j.1471-0528.1959.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Brown JB, Crean GP, Ginsberg J. Oestrogen metabolism and excretion in liver disease. Gut. 1964;5:56–59. doi: 10.1136/gut.5.1.56. doi:10.1136/gut.5.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JB, MacLeod SC, Macnaughtan C, Smith MA, Smyth B. A rapid method for estimating oestrogens in human urine using a semi-automatic extractor. J Endocrinol. 1968;42:5–15. doi:10.1677/joe.0.0420005. [Google Scholar]
- Brown JB, Evans JH, Adey FD, Taft HP, Townsend L. Factors involved in the induction of fertile ovulations with human gonadotrophins. J Obst Gynaecol Br Comm. 1969;76:289–307. doi: 10.1111/j.1471-0528.1969.tb05837.x. [DOI] [PubMed] [Google Scholar]
- Brown JB, Harrisson P, Smith MA. Oestrogen and pregnanediol excretion through childhood, menarche and first ovulation. J Biosoc Sci. 1978;(Suppl. 5):43–62. doi: 10.1017/s0021932000024068. [DOI] [PubMed] [Google Scholar]
- Brown JB, Harrisson P, Smith MA, Burger HG. Correlations between the Mucus Symptoms and the Hormonal Markers of Fertility Throughout Reproductive Life. Melbourne: Advocate Press and Ovulation Method Research and Reference Centre of Australia; 1981. [Google Scholar]
- Brown JB, Harrisson P, Smith MA. A study of returning fertility after childbirth and during lactation by measurement of urinary oestrogen and pregnanediol excretion and cervical mucus production. J Biosoc Sci. 1985;(Suppl. 9):5–23. doi: 10.1017/s0021932000025098. [DOI] [PubMed] [Google Scholar]
- Brown JB, Blackwell LF, Cox RI, Holmes JM, Smith MA. Chemical and homogeneous enzyme immunoasasay methods for the measurement of estrogens and pregnanediol and their glucuronides in urine. Prog Clin Biol Res. 1988;285:119–138. [PubMed] [Google Scholar]
- Dennerstein L, Brown JB, Gotts G, Morse CA, Farly TMM, Pinol A. Menstrual cycle hormonal profiles of women with and without premenstrual syndrome. J Psychosom Obstet Gynaecol. 1993;14:259–268. doi: 10.3109/01674829309084449. doi:10.3109/01674829309084449. [DOI] [PubMed] [Google Scholar]
- DiZerega GS, Hodgen GD. Folliculogenesis in the primate ovarian cycle. Endoc Rev. 1981;2:27–49. doi: 10.1210/edrv-2-1-27. doi:10.1210/edrv-2-1-27. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG. 2001;108:822–829. doi: 10.1111/j.1471-0528.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Gallagher TF, Kraychy S, Fishman J, Brown JB, Marrian GF. A comparison of methods for the analysis of estrone, estradiol and estriol in extracts of human urine. J Biol Chem. 1958;233:1093–1096. [PubMed] [Google Scholar]
- Girard J, Nars PW. Some aspects of the activity of the hypothalamo-pituitary gonadal system in children. In: James VHT, Serio M, Giusti G, editors. The Endocrine Function of the Human Ovary. London, New York, San Francisco: Academic Press; 1976. pp. 195–222. [Google Scholar]
- Johansson ED, Wide L, Gemzell C. Luteinizing hormone (LH) and progesterone in plasma and LH and oestrogens in urine during 42 normal menstrual cycles. Acta Endocrinol (Kbh) 1971;68:502–512. doi: 10.1530/acta.0.0680502. [DOI] [PubMed] [Google Scholar]
- Kennedy KI, Gross BA, Parenteau-Carreau S, Flynn AM, Brown JB, Visness CM. Breastfeeding and the symptothermal method. Stud Fam Plann. 1995;26:107–115. doi:10.2307/2137936. [PubMed] [Google Scholar]
- Klopper AI, Michie EA, Brown JB. A method for the determination of urinary pregnanediol. J Endocrinol. 1955;12:209–219. doi: 10.1677/joe.0.0120209. doi:10.1677/joe.0.0120209. [DOI] [PubMed] [Google Scholar]
- Kol S, Homberg R. Change, change, change: hormonal actions depend on change in blood levels. Hum Reprod. 2008;23:1004–1006. doi: 10.1093/humrep/den061. doi:10.1093/humrep/den061. [DOI] [PubMed] [Google Scholar]
- Lopata A, Brown JB, Leeton JF, Talbot JM, Wood C. In vitro fertilisation of preovulatory oocytes and embryo transfer in infertile patients treated with clomiphene and human chorionic gonadotrophin. Fertil Steril. 1978;30:27–35. doi: 10.1016/s0015-0282(16)43390-x. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Brown JB, Aoki K, Lin TM, Morgan RW, Woo NC. Urine estrogen profiles in Asian and North American women. Int J Cancer. 1974;14:161–167. doi: 10.1002/ijc.2910140204. doi:10.1002/ijc.2910140204. [DOI] [PubMed] [Google Scholar]
- O'Herlihy C, Evans JH, Brown JB, de Crespigny L, Robertson HP. Use of ultrasound in monitoring ovulation induction with human pituitary gonadotropins. Obstet Gynecol. 1982;60:577–582. [PubMed] [Google Scholar]
- Ross GT, Cargille CM, Lipsette MB, Rayford PL, Marshall JR, Strott CA, Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Short RV. in discussion following Brown, Harrisson and Smith. Oestrogen and pregnanediol excretion through childhood, menarche and first ovulation. J Biosoc Sci. 1978;(Suppl. 5):58–61. doi: 10.1017/s0021932000024068. [DOI] [PubMed] [Google Scholar]
- Smith SK, Lenton EA, Landgren B-M, Cooke ID. The short luteal phase and infertility. Br J Obstet Gynaecol. 1984;91:1120–1122. doi: 10.1111/j.1471-0528.1984.tb15087.x. [DOI] [PubMed] [Google Scholar]
- Snow RC, Barbieri RL, Frisch RE. Estrogen 2-hydroxylase oxidation and menstrual function among elite oarswomen. J Clin Endocrinol Metab. 1989;69:369–376. doi: 10.1210/jcem-69-2-369. doi:10.1210/jcem-69-2-369. [DOI] [PubMed] [Google Scholar]
- Talbot JM, Dooley M, Leeton J, Lopata A, Wood C, Brown JB, Evans JH. Gonadotrophin stimulation for oocyte recovery and in vitro fertilization in infertile women. Aust NZ J Obstet Gynaec. 1976;16:111–118. doi: 10.1111/j.1479-828x.1976.tb02366.x. doi:10.1111/j.1479-828X.1976.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Townsend SL, Brown JB, Johnstone JW, Adey FD, Evans JH, Taft HP. Induction of ovulation. J Obstet Gynaecol Br Comm. 1966;73:529–543. doi: 10.1111/j.1471-0528.1966.tb15531.x. [DOI] [PubMed] [Google Scholar]
- Venners SA, Liu X, Perry MJ, Korrick SA, Li Z, Yang F, Yang J, Lasley BL, Xu X, Wang X. Urinary estrogen and progesterone metabolite concentrations in menstrual cycles of fertile women with non-conception, early pregnancy loss or clinical pregnancy. Hum Reprod. 2006;21:2272–2280. doi: 10.1093/humrep/del187. doi:10.1093/humrep/del187. [DOI] [PubMed] [Google Scholar]