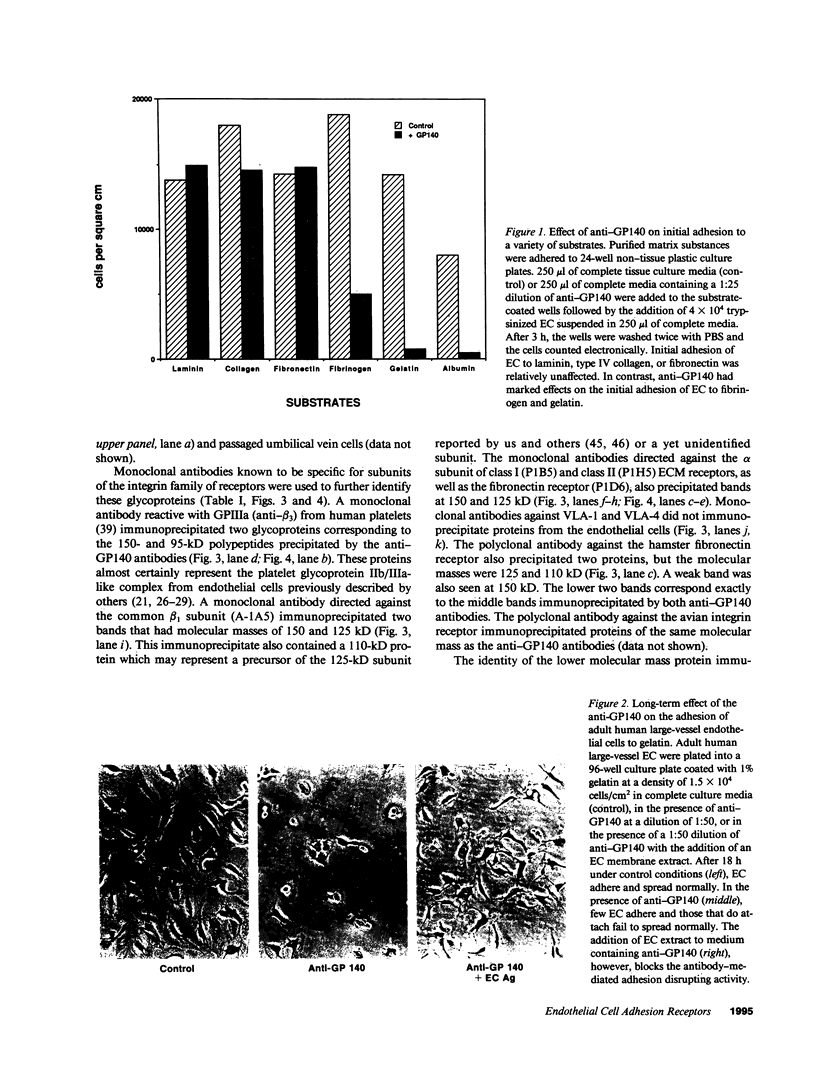
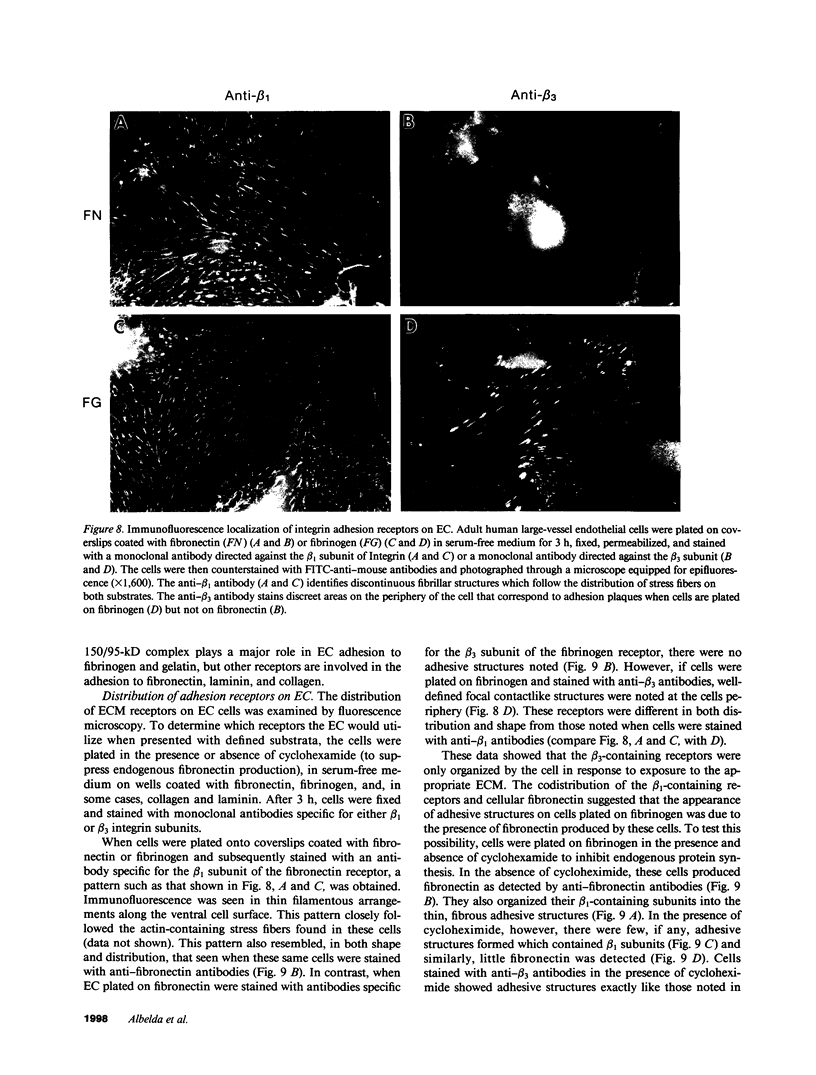
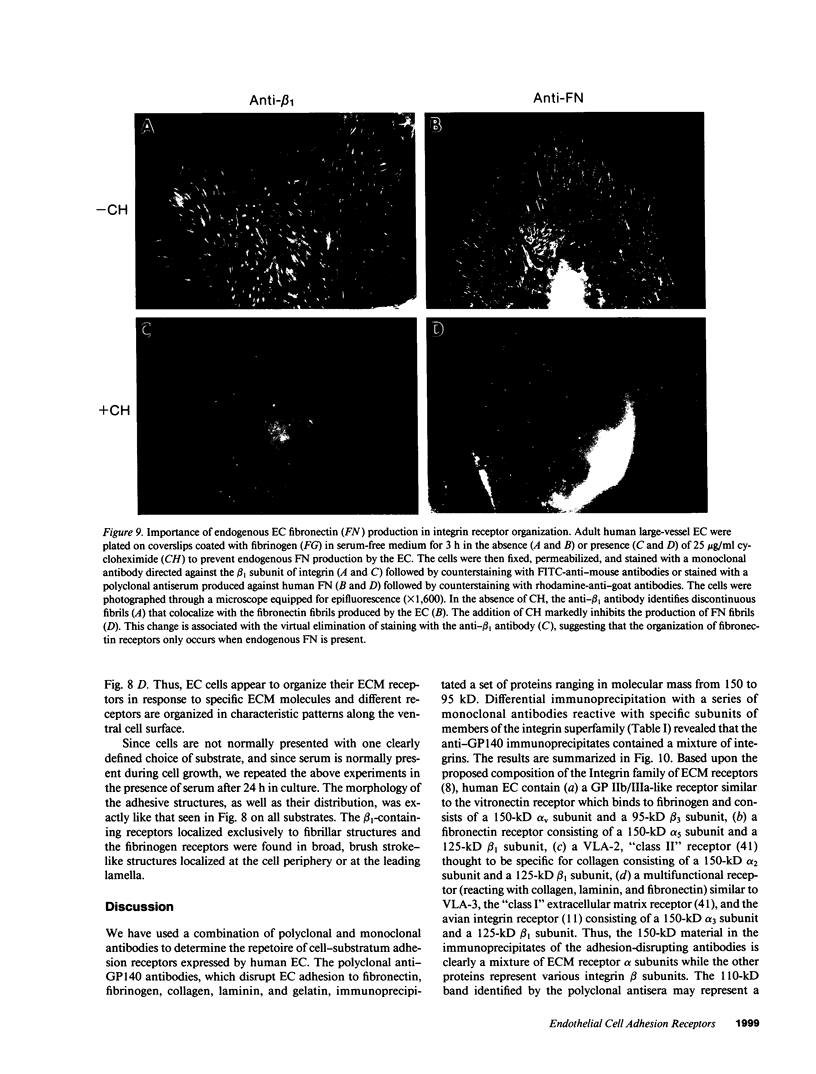
Abstract
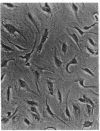
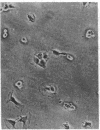
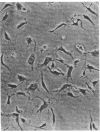
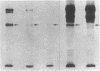
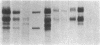
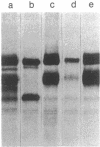
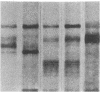
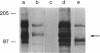
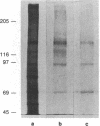
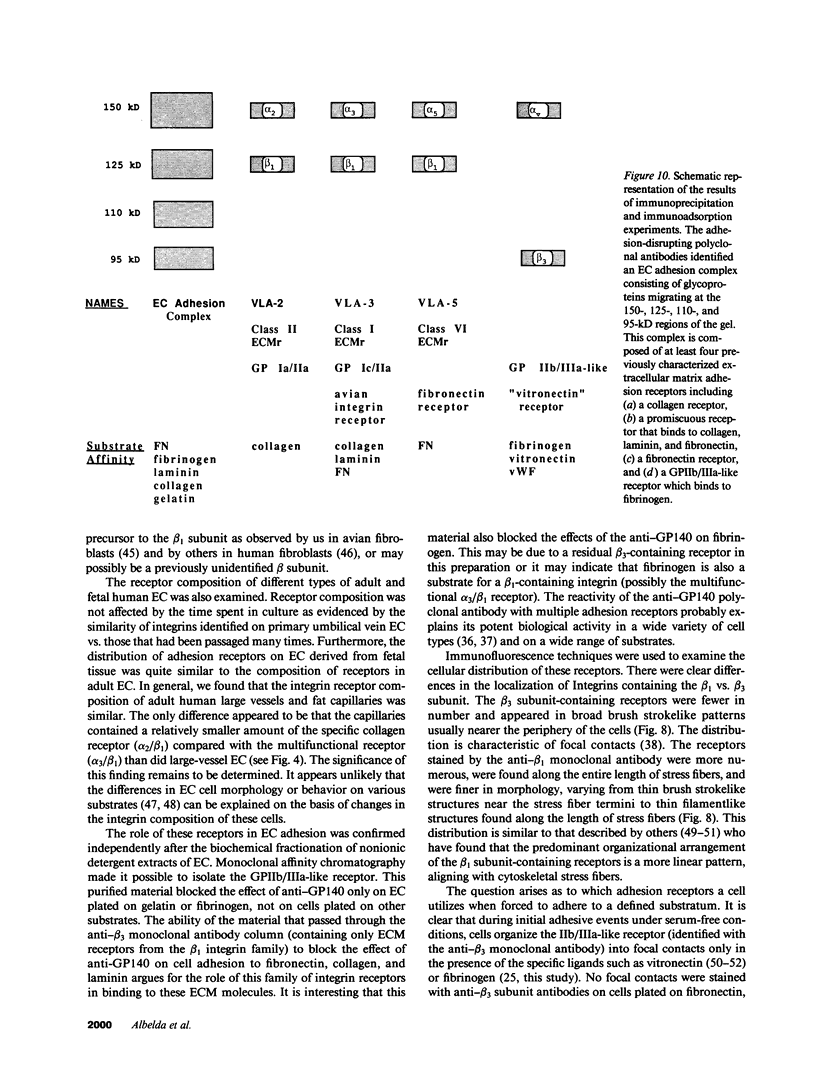
A series of immunological approaches was utilized to identify the molecules involved in cell-substratum adhesion of human endothelial cells (EC) derived from adult large vessels, fat capillaries, and umbilical veins. A polyclonal antibody prepared against partially purified extracellular matrix receptors disrupted adhesion of EC to a wide variety of substrates and identified four groups of glycoproteins migrating with apparent Mr of 150, 125, 110, and 95 kD in immunoprecipitation experiments. Specific monoclonal antibodies identified these proteins as members of the Integrin family of extracellular matrix receptors and included the alpha and beta chains of the fibronectin receptor (alpha 5/beta 1), a collagen receptor (alpha 2 beta 1), a multifunctional receptor that binds to fibronectin, collagen, and laminin (alpha 3/beta 1), as well as a receptor related to platelet IIb/IIIa (alpha v/beta 3). To directly test the importance of these molecules in cell-substratum adhesion, these proteins were purified by a combination of ion exchange, lectin affinity, and immunoaffinity chromatography and used to block the biological activity of the adhesion-disrupting polyclonal antibody. Immunofluorescence experiments further supported the role of these glycoproteins in adhesion. The GPIIb/IIIa-like receptor localized to well-formed adhesion plaques on EC plated on fibrinogen, but not on fibronectin, laminin, or type IV collagen. Receptors containing the beta 1 subunit were visualized as discontinuous fibrils which colocalized with fibronectin fibrils and actin stress fibers.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Expression and function of a putative cell surface receptor for fibronectin in hamster and human cell lines. J Cell Biol. 1986 Oct;103(4):1595–1603. doi: 10.1083/jcb.103.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J Cell Sci Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Thiagarajan P., Schwartz S. M., Harlan J. M., Heimark R. L. The platelet glycoprotein IIb/IIIa-like protein in human endothelial cells promotes adhesion but not initial attachment to extracellular matrix. J Cell Biol. 1987 Oct;105(4):1885–1892. doi: 10.1083/jcb.105.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Nielsen L. D. Either exogenous or endogenous fibronectin can promote adherence of human endothelial cells. J Cell Sci. 1986 Jun;82:263–280. doi: 10.1242/jcs.82.1.263. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Languino L. R., Balconi G., Corbascio G. C., Marchisio P. C. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987 May;104(5):1403–1411. doi: 10.1083/jcb.104.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Colella S., Corbascio G. C., Plow E., Ginsberg M., Marchisio P. C. The localization of a platelet GpIIb-IIIa-related protein in endothelial cell adhesion structures. Blood. 1988 Mar;71(3):566–572. [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Humphries M. J., Obara M., Yamada K. M., Thiery J. P. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988 Sep;7(9):2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Edgell C. J., Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989 Jan;92(Pt 1):67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Fishman A. P. Endothelium: a distributed organ of diverse capabilities. Ann N Y Acad Sci. 1982;401:1–8. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Form D. M., Pratt B. M., Madri J. A. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986 Nov;55(5):521–530. [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J., Ryckwaert J. J., Pierschbacher M., Pytela R., Ruoslahti E., Plow E. F. Immunochemical and amino-terminal sequence comparison of two cytoadhesins indicates they contain similar or identical beta subunits and distinct alpha subunits. J Biol Chem. 1987 Apr 25;262(12):5437–5440. [PubMed] [Google Scholar]
- Gospodarowicz D., Lui G. M. Effect of substrata and fibroblast growth factor on the proliferation in vitro of bovine aortic endothelial cells. J Cell Physiol. 1981 Oct;109(1):69–81. doi: 10.1002/jcp.1041090109. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdijk W. P., Sixma J. J. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985 Mar;65(3):598–604. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jarrell B., Levine E., Shapiro S., Williams S., Carabasi R. A., Mueller S., Thornton S. Human adult endothelial cell growth in culture. J Vasc Surg. 1984 Nov;1(6):757–764. doi: 10.1067/mva.1984.avs0010757. [DOI] [PubMed] [Google Scholar]
- Kaslow H. R., Groppi V. E., Abood M. E., Bourne H. R. Cholera toxin can catalyze ADP-ribosylation of cytoskeletal proteins. J Cell Biol. 1981 Nov;91(2 Pt 1):410–413. doi: 10.1083/jcb.91.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A., Rao P. E., Damsky C. H., Buck C. A. Membrane glycoproteins involved in cell--substratum adhesion. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6071–6075. doi: 10.1073/pnas.78.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeksma O. C., Zandbergen-Spaargaren J., Giltay J. C., van Mourik J. A. Cultured human endothelial cells synthesize a plasma membrane protein complex immunologically related to the platelet glycoprotein IIb/IIIa complex. Blood. 1986 Apr;67(4):1176–1180. [PubMed] [Google Scholar]
- Macarak E. J., Howard P. S. Adhesion of endothelial cells to extracellular matrix proteins. J Cell Physiol. 1983 Jul;116(1):76–86. doi: 10.1002/jcp.1041160112. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Kawai Y., Montgomery R. R., Kunicki T. J. Synthesis by cultured human umbilical vein endothelial cells of two proteins structurally and immunologically related to platelet membrane glycoproteins IIb and IIIa. J Cell Biol. 1986 Jul;103(1):81–86. doi: 10.1083/jcb.103.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara M., Kang M. S., Yamada K. M. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988 May 20;53(4):649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Rosen E. M., Noveral J. P., Mueller S. N., Levine E. M. Regulation of angiotensin I-converting enzyme activity in serially cultivated bovine endothelial cells. J Cell Physiol. 1985 Jan;122(1):30–38. doi: 10.1002/jcp.1041220106. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Rupnick M. A., Carey A., Williams S. K. Phenotypic diversity in cultured cerebral microvascular endothelial cells. In Vitro Cell Dev Biol. 1988 May;24(5):435–444. doi: 10.1007/BF02628495. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M., Gailit J., Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988 Jun;106(6):2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Sutherland A. E., Calarco P. G., Damsky C. H. Expression and function of cell surface extracellular matrix receptors in mouse blastocyst attachment and outgrowth. J Cell Biol. 1988 Apr;106(4):1331–1348. doi: 10.1083/jcb.106.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P., Shapiro S. S., Levine E., DeMarco L., Yalcin A. A monoclonal antibody to human platelet glycoprotein IIIa detects a related protein in cultured human endothelial cells. J Clin Invest. 1985 Mar;75(3):896–901. doi: 10.1172/JCI111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Damsky C. H., Reichardt L. F. Interactions of a neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J Cell Biol. 1987 Nov;105(5):2347–2358. doi: 10.1083/jcb.105.5.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]